Summary
Many common renal insults such as ischemia and toxic injury primarily target the tubular epithelial cells especially the highly metabolically active proximal tubular segment. Tubular epithelial cells are particularly dependent on autophagy to maintain homeostasis and respond to stressors. The pattern of autophagy in the kidney has a unique spatial and chronological signature. Recent evidence shows that there is a complex crosstalk between autophagy and various cell death pathways. The purpose of this review is to specifically discuss the interplay between autophagy and cell death in the renal tubular epithelia. It is imperative to review this topic because recent discoveries have improved our mechanistic understanding of the autophagic process and have highlighted its broad clinical applications, making autophagy a major target for drug development.
Introduction
Autophagy is an essential process responsible for maintaining homeostasis and is observed in all eukaryotic organisms 1-4. This highly conserved lysosomal degradation pathway generates energy and basic components for reuse by degrading toxic or damaged proteins, lipids, and organelles and it is highly activated during nutrient shortage. Autophagy is mainly responsible for the degradation of long-lived proteins while short-lived proteins are able to be degraded by the ubiquitin-proteasome pathway5-7. In general, autophagy is especially important for quality control of organelles, proteins and other cellular components in differentiated, post-mitotic cells. Every cell in the human body exhibits basal autophagy, however, at vastly different levels in diverse cell types 5,8-11. This difference in the level of autophagy is also observed when autophagy is maximally induced by intra- or extracellular stressors, such as starvation. During embryonic development, autophagy is also activated as a crucial component of organism remodeling 10. Autophagy has been considered an adaptive mechanism that enables cells and organisms, along with several other pro-survival mechanisms, to withstand various stresses including nutrient deprivation, hypoxia, and exposure to toxins or infective agents. Recently, the identification of the specific components in the autophagic machinery and the link between abnormalities in autophagy and certain human diseases transformed this field from relative obscurity to a major field of research 5,12-15.
Autophagy is clearly necessary to maintain the health of the kidney (see below). Several common renal insults have been shown to cause alterations in autophagy including ischemia, toxic injury, and inflammation. Dysregulated, excessive, and defective autophagy are all implicated in a variety of disease states. For example, excessive autophagy contributes to the expansion of malignant cell colonies in renal cell cancer 16,17; insufficient autophagy, a result of renal ischemia, facilitates cell death 18-20; and dysregulated autophagy leads to chronic inflammation, autoimmune diseases, and modifies the intrarenal inflammatory milieu, potentially preventing immune cell infiltration21,22. Many common renal insults, for example, ischemia and toxic injury, primarily target the tubular epithelial cells especially the highly metabolically active proximal tubular segment 19,23-27. Tubules are responsible for reabsorption and secretion of various solutes and the damage to this part of the nephron is a key mediator of acute kidney injury, defined as a rapid decline in renal function 19,23-28.
Over the last half-decade, several insightful reviews on the role of autophagy in the kidney have been published 4,7,11,29-33. The purpose of this review is to specifically discuss the interplay between autophagy and tubular cell death in the kidney. It is imperative to review this topic because recent discoveries have improved our mechanistic understanding of the autophagic process and have highlighted its broad clinical applications, resulting in autophagy becoming a major target for drug development.
Key players and regulation of autophagy
Three main forms of autophagy have been described: 1) macroautophagy, 2) microautophagy and 3) chaperon-mediated autophagy 3,7,10,11,33-37. During macroautophagy (herein referred to as “autophagy”), a double membrane vesicle, called an autophagosome, is formed around cytosolic components and organelles destined for degradation. These autophagosomes fuse with the lysosomes and their content is degraded to yield basic building blocks that the cell can then reutilize. Microautophagy is a process where cytosolic cargo is directly engulfed and degraded by lysosomes and is thought to be, with a few exceptions, a nonselective process. The components and its regulation are poorly understood in mammalian cells. Chaperone-mediated autophagy involves a complex and specific pathway where soluble cytosolic proteins are translocated across the lysosomal membrane for degradation. The proteins that are substrates of this process have a specific amino acid sequence that is recognized by a cytosolic chaperone, heat shock cognate protein of 70 kDa (hsc70). The protein-hsc70 complex binds to a lysosome associated membrane protein (LAMP-2) which, acting in concert with other co-chaperones, facilitates its translocation into the lysosome. After the discovery of autophagy related genes, genetic knockout studies of these genes helped us to understand the importance of this pathway and expanded our knowledge of the different players and their functions.
An increasing number of genes and proteins (Atg=Autophagy-related) have been identified to be involved in the autophagy pathway. Atg proteins have specific roles in various steps of the autophagic machinery including autophagosomal membrane formation, maturation, and fusion with the lysosome. A key regulator of autophagy is the mechanistic (or mammalian) target of rapamycin (mTOR), a nutrient sensing protein kinase that inhibits autophagy. Rapamycin (sirolimus) is an mTOR kinase inhibitor that has been used as an anti-rejection medication in renal transplant recipients and also widely used in the laboratory to induce autophagy, even in nutrient rich conditions. We defer to other excellent reviews for the details of this process 1,5-7,12,13,15,18,38-44.
The development of various reliable methods for monitoring autophagy has greatly contributed to the expansion of our knowledge about this process. Nevertheless, there continues to be a debate about the acceptable methods of measuring autophagy, especially in live animals and humans. There are several guidelines published for the use and correct interpretation of assays we currently employ in monitoring autophagy 2,45,46. Most of the current assays measure the steady-state level of different autophagy proteins as a measure of the level of activation. However, the steady-state protein content of LC3-II, visualization of fluorescent LC3-labeled autophagosomes, or identification of double-membrane vesicles on electron microscopy (EM) does not give information about autophagic flux. LC3-II content could be increased due to up-regulation of autophagy or because of decreased clearance of the autophagosomes by the lysosomes. The inclusion of autolysosome inhibitors (e.g. chloroquine, bafilomycin) may gain information about autophagic flux. Also, using these techniques is much more challenging in the kidney in vivo. In this regard, the newly established RFP-GFP-LC3 mouse model greatly facilitates autophagy monitoring in vivo in mice47 (Livingston M et al., Autophagy, in press). In addition, measuring mRNA levels may be used for monitoring autophagosome formation in a wide range of cell types48.
As our knowledge about autophagy is being solidified, we still have several controversies to resolve. In some disease processes autophagy has been reported as being both protective and deleterious. This could be the result of problems with the experimental design, such as the use of chemical inhibitors with their numerous off-target effects that led to some confusing results. For example, the PI3K inhibitor 3-methyladenine (3MA) has an effect on both class I PI3K-AKT pathway and class III PI3K Vps43, which inhibit and activate autophagosome biogenesis, respectively 49-51. Similarly, using RNA interference technology to block the expression of autophagic proteins does not fully block expression; therefore, the results can be misleading. Despite these challenges the publications with new discoveries about autophagy in the kidney is growing exponentially from year-to-year.
Pathological role of autophagy in human diseases – A brief summary of important findings in non-renal diseases
Autophagy has been implicated in various human disease states including neurodegenerative disorders, cancer, metabolic diseases, infections, inflammatory bowel disease, cardiovascular diseases, and renal diseases 5,9,15,52-54. Numerous laboratories both in industry and academics are focusing on the development of stimulators and inhibitors of autophagy. There are several commonly used medications that were later discovered to also target autophagy; some of these are already in clinical trials for cancer, amyotrophic lateral sclerosis (ALS), and cystic fibrosis (chroloquine, sirolimus, epigallocatechin gallate, etc.) 53. In the cancer biology literature, autophagy is described, depending on the scenario, as either a potential facilitator of tumor progression or an inhibitor of oncogenesis 53. There is mounting evidence that autophagy is important in the inflammatory and immune response promoting the clearance of intracellular organisms, mediating anti-inflammatory reactions and antigen presentation 22,55. The most definitive role for autophagy in a human disease process comes from the neurodegenerative literature. Defective autophagy contributes to Alzheimer's disease, Parkinson's disease, and familial ALS 5,12. For example, the gene mutations in autosomal recessive juvenile Parkinson's disease affect mitophagy, the autophagic degradation of dysfunctional, damaged, toxic mitochondria 5. Recent research has also identified autophagy as having a prominent role in aging. The long-lived proteins and damaged organelles that are normally cleared by functional autophagy are implicated in aging. There is an age-dependent decline in the level of autophagy observed in several organs and organisms 15,56 and studies have shown that modulation of autophagy can influence the life span of organisms, ranging from yeast to mammals 54. Autophagy also has a significant role in the immune system and this is underscored by the fact that several viruses, bacteria and parasites are degraded by autophagy 11,57. The innate immunity, antigen presentation and lymphocyte development also require specific contributions from the autophagic pathway 11. The elimination of auto-reactive T cells in the thymus doesn't happen without properly functioning autophagy. Additionally, autophagy is essential for the delivery of certain antigens to MHC II molecules, for clearance of mitochondria in the developing T cells and development and survival of certain B cells 10.
Autophagy and cell death pathways
In healthy adult organisms, cell death and division are in balance to maintain cell populations in tissues, but under stress conditions excessive or inappropriate cell death leads to diseases. Our understanding of the molecular mechanisms and different phenotypes of cell death has been expanding so that we now distinguish between an increasing number of cell death pathways including apoptosis, necrosis, programmed necrosis and autophaghic cell death 42,58-61.
The most well-studied and best-characterized form of cell death is apoptosis, a pathway of highly orchestrated signaling events. Apoptosis is triggered by intrinsic stimuli via the mitochondria or by extrinsic stimuli through cell surface receptors interacting with FAS, tumor necrosis factor-α (TNF-α), and TNF-related weak inducer of apoptosis (TWEAK) 42. During apoptosis, specific biochemical events lead to characteristic morphological changes in the cell culminating in controlled death 59. Biochemical changes include a proteolytic cascade initiated by caspases and changes in BCL-2 family proteins 42,59,60,62. Classical morphological changes include plasma membrane blebbing, loss of cell membrane asymmetry, cell shrinkage, chromosomal DNA fragmentation, chromatin condensation, and surface expression of opsonic receptors that allow neighboring cells or macrophages to rapidly phagocytose cell fragments referred to as “apoptotic bodies”.
Necrosis, traditionally thought to be unregulated and passive, results in rapid disintegration of the plasma membrane allowing toxic substances to be released into the extracellular media that stimulate further damage in the surrounding tissue. Apoptosis and necrosis were initially considered mutually exclusive but recent findings question this dogma. The intrinsic and extrinsic variables that determine whether a cell dies by apoptosis or necrosis are incompletely characterized 42. Several mediators of both cell death types have been described and shown to work together, demonstrating the need for further research in this area. New evidence also suggests that necrosis can be regulated and is not just an accidental form of cell death 63. This new form of cell death is referred to as programmed necrosis or regulated necrosis, of which the most well understood form is necroptosis 63,64.
Excessive accumulation of autophagosomes is often observed in dying cells, but it is still debated if autophagy, per se, can cause cell death 58. Some experimental evidence indicates that autophagy is primarily a pro-survival rather than a pro-death mechanism, 3,58 and the observed autophagy up-regulation is a protective mechanism that is overwhelmed by the cell death machinery in dying cells. In contrast, some scientists believe that autophagy is essential for cell death in certain stress conditions and cell types 65. These scientists point to the fact that an autophagy knockdown model prevented cell death and that other forms of cell death pathways were not affected 66. Nevertheless, the data supporting this are mainly from experiments done in cell lines and current data are completely lacking regarding the effect of autophagy knockdown on human diseases.
Several renal insults cause necrosis, apoptosis, necroptosis, or autophagic cell death in the kidney, including ischemia, toxic injury, sepsis, nutrient deprivation, heat, radiation, and ureteral obstruction 1,4,25,39,44,67-70. There is an expanding body of literature regarding the interaction between autophagy and various cell death pathways including necrosis and necroptosis63,71,72. While both apoptosis and autophagy occur under normal physiological conditions, playing an important role in maintaining tissue homeostasis, cell turnover, embryogenesis, and immune tolerance, they can also lead to the cell's demise under stress conditions 60. They, along with the other cell death pathways mentioned above, can be triggered by intra- or extracellular stressors and when the process reaches an irreversible state, it leads to death of the cell, organ, or organism. In some instances, both autophagy and apoptosis are triggered by certain stimuli, but in other cases one of these processes is inhibited while the other is upregulated. Several experiments concluded that autophagy is protective in various tubular injury models in the kidney (see below), but there is also data supporting an injurious effect. Furthermore, autophagy has been shown to either suppress or induce apoptosis depending on the cell type and experimental situation. Both apoptosis and autophagic cell death are programmed cell death pathways 60,61 and the interconnections between these two processes are extremely complex and we are still in the process of discovering key details (Figure 1).
Figure 1. Crosstalk between autophagy and apoptosis.
Overview of the molecular mechanisms of the autophagy–apoptosis crosstalk. Lines denote interactions or processes. Stimulatory interactions are depicted in blue, whereas inhibitory interactions are depicted in red.
Recent work has shed light on the close, intricate interplay between apoptosis, necrosis, necroptosis, and autophagy. There are several signal transduction molecules that have multiple roles: they are players in both the apoptotic and the autophagic pathway or they can trigger either necrosis or apoptosis depending on the specific stress condition. For example, the tumor suppressor protein p53 integrates multiple stress signals into a series of responses: it activates apoptosis both through transcription-dependent and -independent mechanisms, and at the same time, it regulates the autophagy gene network leading to enhanced autophagy 73. Furthermore, we now have compelling evidence that it also regulates necrosis 72. Another protein that controls both autophagy and apoptosis is Beclin-1. This protein has a critical role in autophagosome formation and sits at the crossroads between autophagy and apoptosis, interacting with several key players in these pathways. For example, c-Jun N terminal kinase-1 (JNK1) phosphorylates Beclin1 and Bcl-2, an anti-apoptotic protein, inhibiting the interaction between these two proteins to activate autophagy74. Several BH3-only proteins and BH3 mimetics interfere with Beclin 1-Bcl-2/Bcl-KL interaction and induce autophagy 75. In addition, several other BCL-2 family members have been shown to promote or prevent both apoptosis and necrosis 71. Autophagy could also inhibit apoptosis by degrading mitochondria, the key source of apoptotic proteins, or caspase-8, an executor of apoptosis76. The next important crosstalk point between autophagy and cell death is the AMPK pathway. Activation of the LKB1–AMPK pathway by starvation or growth factor deprivation increases the stability of cyclin-dependent kinase inhibitor p27kip1 and promotes cell survival through induction of autophagy, while knockdown of p27kip1 under these conditions activates apoptosis77. Another important example for the crosstalk between autophagy and cell death is endoplasmic reticulum (ER) stress that induces autophagy as an adaptive response, but it can also lead to cell death by apoptosis or necrosis78-83. DAPK, a calcium/calmodulin regulated Ser/Thr kinase that is induced by ER stress, has been linked to both apoptosis and autophagic cell death possibly via Beclin1 84. At this point most of the data is coming from cell culture experiments and it is not entirely clear if these interactions are important in vivo in tissues or if they contribute in any way to various human disease states.
Basal autophagy in the tubular epithelium in the kidney
There is a high level of constitutive basal autophagy observed in proximal tubular cells in a wide type of animals and when this autophagy is inhibited, such as in proximal tubule specific ATG5 knockout mice, the animal then develops interstitial fibrosis and renal failure with aging 19,85, proving that autophagy is indispensable in this part of the kidney. On the other hand, the lack of autophagy in the distal tubule and the collecting duct does not lead to obvious pathologies or histological changes in the mouse without added insults 27.
Nephrologists see an increasing incidence of clinically relevant chronic kidney disease in older patients with a higher percentage of glomerulosclerosis, vascular changes, interstitial fibrosis and tubular atrophy in kidney biopsies from these patients 86. Although alteration in autophagy is not the only change observed, the accumulation of toxic protein aggregates, increase in the oxidized protein load and damaged mitochondria are almost uniformly observed in the aging kidney in various cell types. This evidence points towards a universal role for autophagy in the aging kidney. The release of reactive oxygen species (ROS) from damaged mitochondria is a catalyst for widespread cellular damage. Halting or slowing this process by upregulating autophagy might lead to improved renal function in these patients. This seems to be the case, at least in mice, where increased expression of Sirt1, a member of the sirtuin family of proteins, attenuates renal damage by restoring autophagy 87.
It is not the focus of this review, but it is important to mention that autophagy also plays an important role in several other cell types in the kidney, including podocytes. Podocytes are terminally differentiated post-mitotic cells and they rely heavily on autophagy to remove toxic, damaged organelles and proteins. Experiments consistently showed that basal autophagy is a protective mechanism in glomerular health. Autophagy deficient podocytes don't have a major effect on glomerular development but with aging they lead to albuminuria, podocyte loss, late onset glomerular sclerosis, and accumulation of protein aggregates 6,7,88. Under stress conditions, inhibition of autophagy leads to worsening renal failure in several animal models of glomerular diseases 6,89. Podocyte loss is the hallmark of progressive glomerular diseases, however, the exact type of cell death pathways involved is not clearly defined but autophagic cell death has been observed in podocytes 6,7,89.
Several common renal insults cause alterations in autophagy in tubular epithelial cells including ischemia, toxic injury and inflammation. In the next part of this review, we will discuss the role of autophagy in the most common forms of kidney injury affecting the tubules and the crosstalk between autophagy and cell death in these models of renal stress.
The role of cell death and autophagy in acute ischemic kidney injury
In clinical practice, transient ischemia due to hypovolemia, hypotension, or heart failure commonly causes acute kidney injury (AKI) and accounts for nearly one-third of patients requiring acute renal replacement therapy 42. Proximal tubular epithelial cells (PTCs) are highly susceptible to acute ischemia. Extensive evidence suggests that detachment, dysfunction, and death of these cells are primarily responsible for the pathophysiological and clinical aspects of ischemic AKI, but inflammation is also prominent and may partly mediate its duration and long-term consequences 42,90,91 . Under physiological conditions, proximal tubule cells are columnar in shape with highly polarized basal-lateral and apical surface membrane domains that drive solute reabsorption or excretion. Ischemia causes some cells to die by various death mechanisms, whereas other cells undergo sublethal injury 90. Loss of tubular cells leads to unregulated paracellular diffusion of water, ions, and macromolecules, thereby causing increased backleak, which reduces glomerular filtration rate (GFR). Both lethally and sublethally damaged cells enter the lumen along with apical membrane material and other cellular debris and can aggregate to form casts, resulting in tubular obstruction. Tubular obstruction further compromises organ function by increasing intratubular pressures to levels that are inconsistent with filtration. Together, loss of cell surface area and tubular obstruction decrease GFR. Reductions in proximal tubule ion and water reabsorption result in high distal delivery of Na+, K+, Cl−, and high distal flow rates. In turn, tubuloglomerular feedback produces afferent arteriole constriction and lowers GFR. PTCs release cytokines and chemokines in response to cell injury, and these agents have direct effects on endothelial function. Peritubular capillaries are seen congested with infiltrating leukocytes that impair local renal blood flow, aggravating tubular ischemia. Many investigators believe that dysfunctional, overactive or insufficient autophagy also significantly contributes to ischemic renal dysfunction. The relative contribution of each specific mechanism to loss of organ function in AKI is uncertain and may depend on the type and severity of the insult as well as the host's inherent susceptibility to stress 42,90.
It has been shown in numerous experimental systems, including various animal models as well as in humans, that ischemia–reperfusion induces autophagy in PTCs 23,26,85,92,93. For example, ischemia resulted in increased expression of LC3 and other autophagy-related proteins in mice and rats and also increased the number of LC3-GFP positive autophagosomes in cell lines especially after treatment with a lysosomal inhibitor that prevented LC3 degradation 23,92-94. It was also documented that there was increased co-localization between LC3 and LAMP2, a lysosomal protein, in vacuolar structures both in human kidney proximal tubular cell line (HK-2) and in intact kidneys after ischemia. The observed increase in co-localization between LC3 and LAMP2 suggested that autophagic flux was increased and more autophagosomes fused with the lysosomes for degradation 92,93. Hypoxia during in utero development also led to autophagy induction and apoptosis in the kidney with alteration in Beclin 1, hypoxia inducible factor 1alpha (HIF-1α), AKT, and mTOR signaling 95. The mechanism by which ischemia up-regulates autophagy is under intense investigation.
The list of molecules that play crucial roles in regulation of tubular epithelial cell autophagy has been expanding. Autophagy seems to be induced in these cells by several independent pathways. For example, p53-sestrin-2 and HIF-1alpha-BNIP3 are both activated by stress leading to autophagy up-regulation to protect the renal tubules 96. Mitochondrial permeability transition, oxidative stress and ROS can also directly promote autophagy in the ischemic kidney 27,97,98. ULK1, a kinase with an important role in autophagy initiation, is also induced by hypoxia in kidney tissue and the lack of this protein suppresses autophagy in ischemic models 99-102. Hypoxia causes dissociation of mTOR and ULK1 leading to autophagy induction99-101. In non-renal cell culture models, ULK1 interacts with mTORC1, AMPK, JNK and Beclin 1 to intervene at several steps in the autophagic and apoptotic pathways48,99-102. Yet another recently discovered mechanism, clusterin, a chaperone-like protein was shown to be required for autophagy induction 103. Both in cell culture and in mouse models, autophagy was clusterin-dependent and correlated positively with cell survival103. In the kidney, ER stress is well known to regulate autophagy and cell death in various cells including the tubules during kidney injury104 and likely plays a central role during ischemia. New evidence shows that both the pro-apoptotic and anti-apoptotic members of the BCL-2 family proteins can interact with the autophagic pathway in the tubules. For example, Bcl-xl up-regulation inhibits autophagy induction after hypoxia 92 possibly through an interaction with Beclin 175,95.
Mounting evidence supports the notion that autophagy has a protective role in renal tubular cells during ischemia–reperfusion injury partially by inhibiting apoptosis. Autophagy is an early response to ischemia and proceeds the appearance of apoptotic cells 23,94,105,106. In mice, suppression of autophagy leads to apoptosis during reperfusion and to worse renal outcome including more severe renal dysfunction and histologic injury (Figure 2) 23,94,107. Atg5- and Atg7-deficient mice have more protein aggregates, p62 and damaged mitochondria in their tubular epithelium even at baseline and are more susceptible to ischemic renal injury. They exhibit more pronounced tubular damage, apoptosis and renal failure at early time points after stress 23,26,27,85. Interestingly, 30 days after ischemia-reperfusion injury mice with a conditional deletion of Atg5 in the proximal tubular S3 segment present with significantly less tubular senescence, reduced interstitial fibrosis and better renal function. Collectively, this data raises the possibility that autophagy is beneficial for early survival but if too many severely compromised tubular cells survive they can hinder recovery and lead to inflammation and renal disease 108. To further support the protective role of autophagy in this segment of the kidney during ischemia, pharmacological autophagy inducers were used in mice. Rapamycin, an MTORC1 inhibitor, partially prevented renal ischemic injury and restored autophagic activity in an ischemic AKI model109. Another common clinically relevant injury, where oxidative stress plays a significant role, is contrast induced nephropathy. Similarly to ischemia, there was increased autophagy and apoptosis observed in a rat model of contrast induced nephropathy and autophagy inhibition increased oxidative stress, apoptosis and tubular cell injury 110,111.
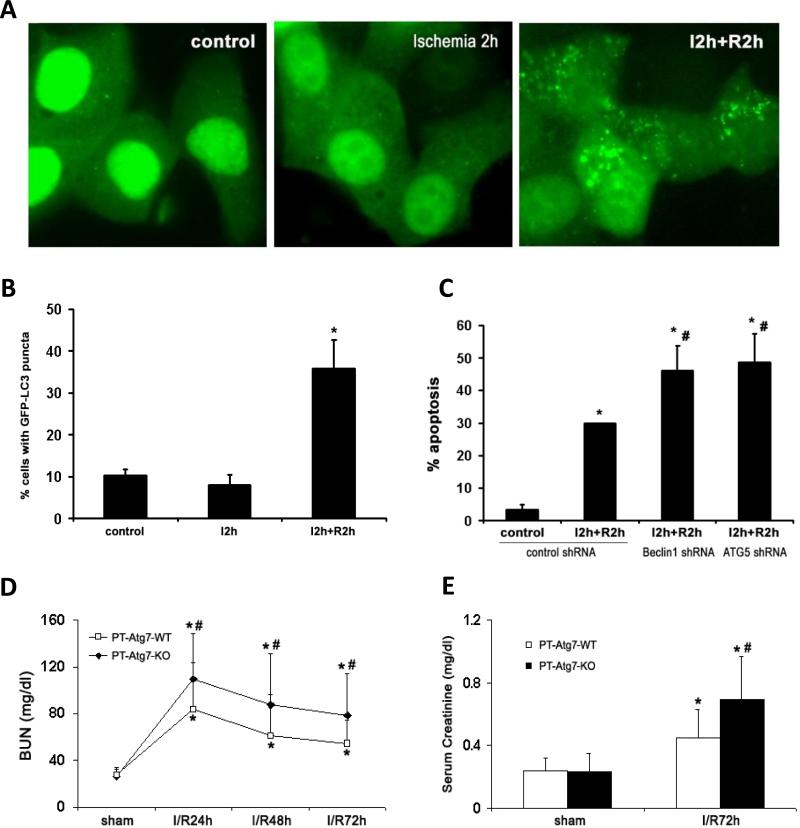
Figure 2. Autophagy protects against in vitro (A-C) and in vivo (D-E) ischemia-reperfusion injury.
A-B: Induction of autophagy and its cytoprotective effect against cell apoptosis in response to in vitro ischemia−reperfusion. (A) Representative images (×60) of rat kidney proximal tubular cells (RPTC) that were transiently transfected with GFP-LC3. For in vitro ischemia, the transfected cells were incubated in a glucose-free buffer in an anaerobic chamber for 2 hours (I2 hours); for reperfusion, the cells were transferred back to full culture medium with oxygen for another 2 hours (I2 hours +R2 hours). The formation of GFP-LC3 puncta was analyzed immediately after ischemia or following reperfusion. (B) Percentage of cells with punctuate GFP-LC3. Data are expressed as mean ± SD (n = 3). *P < 0.05. (C) RPTC cells were transiently transfected with GFP-tagged control shRNA, Beclin-1 shRNA, or ATG5 shRNA. The transfected cells were treated with in vitro ischemia−reperfusion as described above (I2 hours+R2 hours). After treatment, the cells were stained with Hoechst33342 and apoptosis percentage in transfected (green) cells was determined by nuclear morphology. Data are expressed as mean ± SD (n = 4). *P < 0.05, significantly different from the control group. **P < 0.05, significantly different from the ischemia− reperfusion treated control shRNA group. D-E: Atg7 KO mice are more sensitive to renal ischemia-reperfusion injury. Wild-type (n=14) and PT-Atg7-KO littermate mice (n=14) were subjected to sham operation or 25 minutes of bilateral renal ischemia followed by 0 to 72 hours of reperfusion. Blood samples were collected for measurements of BUN (D) and serum creatinine (E). Data are expressed as mean ± SD. * P < 0.05, significantly different from the sham control groups; # P < 0.05, significantly different from the wild-type group. Figure is adopted from Jiang et al. 23,94
The idea that autophagy is an important stress response in the transplanted kidney is supported by expanding experimental and clinical evidence. The transplant kidney is affected by both acute and chronic ischemia. The renal tubular epithelium in the allograft is not only one of the main sites of injury but also the source of inflammatory and pro-fibrotic cytokines that leads to tubular atrophy and interstitial fibrosis. Prolonged cold storage followed by rewarming and ischemia during kidney transplantation causes acute kidney injury termed delayed graft function. In animal models of cold storage and rewarming there is increased autophagy and apoptosis observed and inhibition of autophagy leads to worse renal injury68. Furthermore, increased number of autophagic vacuoles were observed in tubules of transplanted human kidneys by electron microscopy 112. Targeting autophagy in humans with ischemic renal injury has not been attempted yet and it is too early to say if up-regulation of autophagy for AKI would be of any benefit.
Autophagy and cell death in toxic renal injury
Nephrotoxic medications (cisplatin, cyclosporine) and heavy metals (arsenic, cadmium) mainly target the proximal tubules in the kidney and up-regulate autophagy in tissue culture experiments and murine models of toxic renal injury 25,113-121. Autophagy is activated within the first hours of injury in models using cisplatin, cyclosporine, heavy metals or aristolochic acid measured by LC3-II accumulation and visualization of autophagosomes 119,122-124. Amongst toxins, cisplatin is the most widely studied and a large portion of the data presented here is derived from experiments using this chemotherapeutic agent. How toxic injury causes autophagy induction is still not clear but there are several candidate pathways. For example, oxidative stress caused by toxins could be the main inducer. Cisplatin induces both heme oxygenase 1 and autophagy. Overexpression of heme oxygenase 1 reduces oxidative stress induced cell death and impedes autophagy induction while lack of this protein increases ROS level and tubular apoptosis suggesting that heme oxygenase 1 regulates autophagy 97. The main toxic effect of cisplatin is DNA damage. DNA damage is followed by p53 nuclear translocation resulting in the induction of downstream target genes that regulate cell cycle progression and initiation of apoptosis. Interestingly, chemical inhibition of p53 hinders the autophagic response in the renal tubular cells suggesting that p53 not only regulates cell death but also autophagy 72,73,115. In a mouse model of cisplatin-induced acute kidney injury, renal proximal tubule-specific Atg7-knockout mice had more renal damage including renal dysfunction, tissue damage and cell death by apoptosis (Figure 3) suggesting that autophagy is protective against toxin-induced injury23. Consistently, autophagy up-regulation by rapamycin alleviated proximal tubule toxicity induced by cisplatin and gentamicin 23,125.
Figure 3.
AKI caused by cisplatin treatment is aggravated in PT-Atg7-KO mice. Wild-type (n=15) and PT-Atg7-KO littermate mice (n=21) were injected with 25mg/kg cisplatin or saline as control. (A) and (B) Blood samples were collected for measurements of BUN and serum creatinine. Data are expressed as mean ± SD. * P < 0.05, significantly different from the control (or day 0) groups; # P < 0.05, significantly different from the relevant wild-type group. (C) Representative histology of kidney cortex (H-E staining, ×200). (D) Pathological score of tubular damage in cisplatin-treated wild-type and PT Atg7-KO mice. (E) Quantification of TUNEL-positive cells in cisplatin-treated wild-type and PT-Atg7-KO groups. (F) Representative images of TUNEL staining (×200). Data in (D-E) are expressed as mean ± SD. * P < 0.05, significantly different from the wild-type group. Figure is adopted from Jiang et al. 23
During nephrotoxic injury, similarly to the ischemic injury models mentioned above, autophagy seems to precede apoptosis. During the initial injury phase autophagy might try to clear the damaged proteins and organelles thereby reducing oxidative stress but after the autophagic pathway is overwhelmed there is nothing to stop apoptosis117. Cyclosporine, a commonly used immunosuppressant, has similar effects on autophagy as cisplatin including causing ER stress, oxidative stress and tubular cell death. Autophagy is activated by cyclosporine-induced cellular stress and autophagy alleviates injury both in acute and chronic cyclosporine injury models 69,120,121,126. Furthermore, cyclosporine induces hemodynamic changes in the kidney, possibly leading to ischemia, and also increases ROS production, apoptosis and ER stress in the tubules. Finally, cyclosporine induces chronic metabolic stress via effects on mitochondrial respiration that is worse in autophagy deficient cells 26. In the cyclosporine toxic injury model, TMBIM6 (transmembrane Bax inhibitor motif containing 6) was shown to regulate autophagy and lysosomal function both in vitro and in vivo. As the above examples show, in ischemic and toxic renal injury models autophagy and apoptosis have very similar roles hence therapeutics that work in one disease might also work in the other.
Autophagy and cell death in polycystic kidney disease
Polycystic kidney disease (PKD) is the most common genetic form of CKD and causes about 5% of all end-stage renal diseases. In PKD, healthy kidney tissue is slowly replaced by growing cysts, resulting in renal failure. The primary defect is in cilia-mediated signaling activity in the tubules. It has been widely accepted that apoptosis in tubular epithelial cells has a central role in cyst formation and now a growing body of research shows that autophagy might also play a role in PKD, potentially by altering apoptosis. At this point, however, it is not well understood how the crosstalk between apoptosis and autophagy affects tubular cell fate in PKD. However, there are several signaling pathways affecting both autophagy and apoptosis that are dysregulated in PKD. For example, the transcription factor, signal transducer and activator of transcription 1 (STAT1), and MAPK8 (JUNK1) are present in cysts and can potentially mediate cell death by apoptosis or necrosis, and autophagy 127,128. A recent study has further demonstrated a reciprocal regulation between cilia and autophagy 129. Essentially, the cells with shorter cilia had lower levels of autophagy and, conversely, inhibition of autophagy reduced cilia growth. Notably, shorter cilia were shown in proximal tubule cells in atg7-knockout mice. MTOR and the proteasome pathways may mediate this reciprocal regulation. Interestingly, cilia may affect the sensitivity of renal tubular cells to apoptosis 130.
Apoptosis inhibition by pharmacological agents or genetic manipulations slows or prevents cyst formation and growth 127,128,131-135. Apoptotic or anti-apoptotic BCL2 family members, for example BIM or BAD, seem to regulate autophagy and could play a role in PKD 7,136. Surprisingly, in a rat PKD model, apoptosis is more prevalent at early stages of PKD while autophagy seems to be more prominent later in the disease course 132. This is the reverse of what was found in ischemic and toxic renal injury models where autophagy seems to precede apoptosis 23,94. Tubular cells lining the cysts seem to have a lower level of autophagy than cells in healthy tubules. Short treatment with Bafilomycin A1, an inhibitor of lysosomal acidification, resulted in an increase in the LC3-II level in wild type, but not in PKD kidneys, suggesting that baseline autophagic flux is lower in PKD cells 137.
The lower level of autophagy in PKD could be the result of mTOR activation. In fact, MTOR signaling is upregulated in murine models of PKD and in kidneys from patients with PKD127,131-135,137. Furthermore, MTORC1 inhibition with rapamycin or sirolimus prevented PKD progression in animal models 7,131,138,139. These studies suggested that the mTOR signaling pathway may modulate disease progression, but, disappointingly, 2 years of treatment with sirolimus had no clear beneficial effect in patients with autosomal dominant PKD 133,135. Additionally, there was a high rate of discontinuation of therapy in the treatment group because of relatively frequent side effects. In these studies, there was a small reduction in rate of increase of kidney volume, but it did not translate into functional benefit. Sirolimus was started at a stage where there were already large cysts in the kidney with clinical CKD. Therefore, the question still remains if starting mTOR inhibition at an earlier stage will prevent cyst formation and CKD. Critiques of the study point out that it is also possible that the achieved drug level might have been too low in the cysts. Others suggested that kidney-specific delivery systems might have more effect on the cyst growth. Similarly to sirolimus, metformin, a frequently used antidiabetic agent, also has an effect on autophagy and might be beneficial in PKD. Metformin is a 5′ AMP-activated protein kinase (AMPK) activator that increases autophagy and slows cyst formation both in vitro and in vivo 134. Unfortunately, we have not yet found a cure for PKD, but no doubt, there will be more clinical trials targeting autophagy in PKD with the hope that new therapies will lead to better clinical outcome in these patients.
Tubular autophagy in other renal diseases
There are several clinically important kidney diseases not mentioned above where tubular cell autophagy might play a role. One of the important findings is that in diabetic kidney models mTOR is activated in the proximal and distal tubules with down regulation of the AMP-activated protein kinase pathway resulting in autophagy inhibition 140. In addition, prolonged exposure to high glucose levels induced apoptosis and autophagy in tubular epithelial cells in vitro 141. This is not unexpected because in the diabetic kidney there is elevated ROS production and ER stress both of which can up-regulate autophagy. Autophagy is also induced by lipotoxicity in tubular cells via modulating fatty acid β-oxidation142.
One of the few disease processes where autophagy inhibition seems to be beneficial is AKI due to sepsis. In a mouse model of lipopolysaccharide (LPS) induced-AKI, autophagy was induced in the renal cortex that is mainly consists of tubules and autophagy inhibition protected against LPS-induced acute kidney injury and inflammation143. However, the latest study by Mei S. et al. showed that pharmacological and genetic suppression of autophagy worsens LPS-induced acute kidney injury in mice (Mei S., Dong Z., Scientific Reports, in press).
Tubular atrophy and interstitial fibrosis is the hallmark of chronic kidney disease. In animal models of renal fibrosis using unilateral ureteral obstruction (UUO), autophagy, apoptosis and necrosis are all augmented. Again, autophagy seems to precede apoptosis and interstitial fibrosis in the injury process. Pharmacological inhibition of autophagy in the UUO model enhanced cell death and worsened renal outcome28,144,145. However, autophagy-deficiency in proximal tubules suppressed tubular cell death and inflammation, resulting in attenuation of renal fibrosis in UUO (Livingston M., Dong Z., Autophagy, in press). Autophagy might be regulated by transforming growth factor-β1 (TGF-β1), a cytokine that has been established as a central mediator of kidney fibrosis 146-149.
In renal cancer cells, hypoxia-inducible factor 2alpha (HIF2alpha) is partially degraded by autophagy and suppresses renal tumorigenesis. Inhibition of autophagy increases HIF2alpha, whereas induction of autophagy decreases HIF2alpha 17. In the clear cell renal cell carcinoma a frequent finding is a loss or mutation of ATG7 and this correlates with progression17. Modulating autophagy might have therapeutic benefits in this form of cancer.
Therapeutic implications
Tubular epithelial cells are highly dependent on autophagy to maintain homeostasis and respond to stressors. Autophagy and the various cell death pathways, in particular apoptosis, are interconnected and their complexity affords new targets for effective interventions. The ability to influence aging, life or death by targeting autophagy and apoptosis has an immense therapeutic potential. Despite the current absence of human trials, several therapeutic interventions targeting the apoptotic and/or the autophagic pathway have shown beneficial effects in animal models. Anti-apoptosis therapies have not been successfully used in human AKI but selective targeting to the relevant renal cell types most susceptible to apoptosis and improved understanding of the potential “double-edged apoptotic sword” that affords both beneficial and untoward consequences will lead us closer to the solution 24,25,42,49,59-62,71,77,118,141,150,151. Meanwhile, targeting autophagy as therapeutic intervention has uncovered unexpected challenges. The picture is complicated because autophagy, similar to apoptosis, can both play a protective or damaging role depending on the specific disease process, cell type and also the stage of the disease. The challenge for clinicians will be to selectively turn on or turn off the autophagic pathway dependent on the clinical situation.
The most commonly used autophagy activators are mTOR inhibitors but induction of autophagy can be also achieved by various other ways, for example, by activating AMPK or sirtuin 1, activators of autophagy, or by acetyl coenzyme A depletion mimicking the biochemical starvation milieu. Yet another strategy for autophagy induction is the deployment of various sublethal stresses. Autophagy inhibition is the goal in various cancers where autophagy enhances cancer growth. There are multiple clinical trials where autophagy inhibition is attempted by chloroquine or 3-hydroxychloroquine targeting the lysosomes but these medications have numerous off target effects.
We are still far from developing the preferred product profile for a drug that targets the autophagic pathway to treat AKI, but we already gained crucial knowledge from using certain medications in clinical practice that at least partially target autophagy. For example, medications that inhibit mTOR (rapamycin or sirolimus) have been in clinical practice for years. The problem is that they not only upregulate autophagy but also alter the function of several other mTOR substrates. Disappointingly, clinical studies with sirolimus in PKD have not resulted in breakthrough (see above). MTOR inhibitors might increase lifespan in animals 152 but their side effect profile is not favorable. For example, sirolimus delays wound healing after surgery and also delays renal recovery after an ischemic insult153. Furthermore, sirolimus has been associated with worsening proteinuria, glomerulosclerosis, diabetes mellitus and dyslipidemia154-156. The same problem with off target effects and adverse reactions applies to most of the currently used autophagy modulating chemicals and the net biological affect might be unfavorable as a result. Clearly, a more specific approach is required to precisely manipulate autophagy without unwanted effects. Site specific targeting to the tubular cells might solve some of the problems. The non-specificity of drugs could be mitigated by gene therapeutic approaches that have been successfully used for autophagy modulation in rodents. Another hurdle is that at this point we are not sure what level of autophagy induction or inhibition would be the most beneficial. The timing and duration of treatment needs to be also considered because the level of autophagic flux seems to be changing with time during stress and can be variable in different disease processes. Autophagy up-regulation might be required at a certain time point while autophagy inhibition would be more beneficial at another point. Given the complex role autophagy plays in various organs and disease processes it will be crucial to develop cell type or organ specific modulators of autophagy. In spite of all of these difficulties, we expect that novel, increasingly more specific inhibitors or inducers of autophagy will become available for clinical trials. We are confident that manipulation of known key steps in the autophagy pathway will inevitably improve the outcome of human kidney diseases.
Conclusions
Autophagy and tubular cell death represent an intricately intertwined process and we are just starting to understand their effects on each other and on the fate of the cell and organism. Accumulating evidence shows that apoptosis and autophagy can either cooperate or antagonize each other. Autophagy can promote cell survival by inhibiting apoptosis, inflammation and oxidative stress. Recent discoveries leading to improved mechanistic understanding of the autophagy process in the kidney and its broad clinical applications have generated important new therapeutic targets for drug development. However, challenges still remain. We have techniques to monitor autophagy flux reliably in cell lines and in some animal models but we are lacking the tools to do the same in human kidneys or kidney samples. Consequently, a defect or dysregulation in autophagy has not yet been conclusively shown to cause or contribute to any known human kidney diseases. There are also many controversies in the literature concerning the role of autophagy in disease. Everyone agrees though that the evidence indicates a leading role for autophagy in adaptive stress responses and in the maintenance of cellular homeostasis. The saying “Not too much, not too little, but just right” even holds true for autophagy. Insufficient autophagy clearly leads to pathologies but too much autophagy can also cause excessive self-digestion of cellular components that is detrimental to cells. Hopefully, the rapidly expanding understanding of alterations in autophagy will ultimately lead to the development of new therapeutic strategies to prevent or slow down chronic kidney disease progression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Rechter S, Decuypere JP, Ivanova E, et al. Autophagy in renal diseases. Pediatr Nephrol. 2015 doi: 10.1007/s00467-015-3134-2. [DOI] [PubMed] [Google Scholar]
- 2.Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–88. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Periyasamy-Thandavan S, Jiang M, Schoenlein P, Dong Z. Autophagy: molecular machinery, regulation, and implications for renal pathophysiology. Am J Physiol Renal Physiol. 2009;297:F244–56. doi: 10.1152/ajprenal.00033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghavami S, Shojaei S, Yeganeh B, et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol. 2014;112:24–49. doi: 10.1016/j.pneurobio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Hartleben B, Wanner N, Huber TB. Autophagy in glomerular health and disease. Semin Nephrol. 2014;34:42–52. doi: 10.1016/j.semnephrol.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Huber TB, Edelstein CL, Hartleben B, et al. Emerging role of autophagy in kidney function, diseases and aging. Autophagy. 2012;8:1009–31. doi: 10.4161/auto.19821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fougeray S, Pallet N. Mechanisms and biological functions of autophagy in diseased and ageing kidneys. Nat Rev Nephrol. 2015;11:34–45. doi: 10.1038/nrneph.2014.201. [DOI] [PubMed] [Google Scholar]
- 9.Goligorsky MS. SIRTing out the link between autophagy and ageing. Nephrol Dial Transplant. 2010;25:2434–6. doi: 10.1093/ndt/gfq348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12:823–30. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takabatake Y, Kimura T, Takahashi A, Isaka Y. Autophagy and the kidney: health and disease. Nephrol Dial Transplant. 2014;29:1639–47. doi: 10.1093/ndt/gft535. [DOI] [PubMed] [Google Scholar]
- 12.Aki T, Funakoshi T, Unuma K, Uemura K. Impairment of autophagy: from hereditary disorder to drug intoxication. Toxicology. 2013;311:205–15. doi: 10.1016/j.tox.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Bizargity P, Schroppel B. Autophagy: basic principles and relevance to transplant immunity. Am J Transplant. 2014;14:1731–9. doi: 10.1111/ajt.12743. [DOI] [PubMed] [Google Scholar]
- 14.Cecconi F, Jaattela M. Targeting ions-induced autophagy in cancer. Cancer Cell. 2014;26:599–600. doi: 10.1016/j.ccell.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:1845–6. doi: 10.1056/NEJMc1303158. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee P, Basu A, Wegiel B, et al. Heme oxygenase-1 promotes survival of renal cancer cells through modulation of apoptosis- and autophagy-regulating molecules. J Biol Chem. 2012;287:32113–23. doi: 10.1074/jbc.M112.393140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu XD, Yao J, Tripathi DN, et al. Autophagy mediates HIF2alpha degradation and suppresses renal tumorigenesis. Oncogene. 2015;34:2450–60. doi: 10.1038/onc.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Decuypere JP, Ceulemans LJ, Agostinis P, et al. Autophagy and the Kidney: Implications for Ischemia-Reperfusion Injury and Therapy. Am J Kidney Dis. 2015;66:699–709. doi: 10.1053/j.ajkd.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Isaka Y, Kimura T, Takabatake Y. The protective role of autophagy against aging and acute ischemic injury in kidney proximal tubular cells. Autophagy. 2011;7:1085–7. doi: 10.4161/auto.7.9.16465. [DOI] [PubMed] [Google Scholar]
- 20.Livingston MJ, Dong Z. Autophagy in acute kidney injury. Semin Nephrol. 2014;34:17–26. doi: 10.1016/j.semnephrol.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen WT, Hung KC, Wen MS, et al. Impaired leukocytes autophagy in chronic kidney disease patients. Cardiorenal Med. 2013;3:254–64. doi: 10.1159/000356212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leventhal JS, He JC, Ross MJ. Autophagy and immune response in kidneys. Semin Nephrol. 2014;34:53–61. doi: 10.1016/j.semnephrol.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82:1271–83. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaushal GP. Autophagy protects proximal tubular cells from injury and apoptosis. Kidney Int. 2012;82:1250–3. doi: 10.1038/ki.2012.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaushal GP, Kaushal V, Herzog C, Yang C. Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity. Autophagy. 2008;4:710–2. doi: 10.4161/auto.6309. [DOI] [PubMed] [Google Scholar]
- 26.Kimura T, Takahashi A, Takabatake Y, et al. Autophagy protects kidney proximal tubule epithelial cells from mitochondrial metabolic stress. Autophagy. 2013;9:1876–86. doi: 10.4161/auto.25418. [DOI] [PubMed] [Google Scholar]
- 27.Liu S, Hartleben B, Kretz O, et al. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy. 2012;8:826–37. doi: 10.4161/auto.19419. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y, Ruan S, Wu X, Chen H, Zheng K, Fu B. Autophagy and apoptosis in tubular cells following unilateral ureteral obstruction are associated with mitochondrial oxidative stress. Int J Mol Med. 2013;31:628–36. doi: 10.3892/ijmm.2013.1232. [DOI] [PubMed] [Google Scholar]
- 29.Kume S, Uzu T, Maegawa H, Koya D. Autophagy: a novel therapeutic target for kidney diseases. Clin Exp Nephrol. 2012;16:827–32. doi: 10.1007/s10157-012-0695-2. [DOI] [PubMed] [Google Scholar]
- 30.Kume S, Yamahara K, Yasuda M, Maegawa H, Koya D. Autophagy: emerging therapeutic target for diabetic nephropathy. Semin Nephrol. 2014;34:9–16. doi: 10.1016/j.semnephrol.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Pallet N. Emerging roles of autophagy in the stressed kidney allograft. Semin Nephrol. 2014;34:34–41. doi: 10.1016/j.semnephrol.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Pallet N, Livingston M, Dong Z. Emerging functions of autophagy in kidney transplantation. Am J Transplant. 2014;14:13–20. doi: 10.1111/ajt.12533. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Choi ME. Autophagy in kidney health and disease. Antioxid Redox Signal. 2014;20:519–37. doi: 10.1089/ars.2013.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oczypok EA, Oury TD, Chu CT. It's a cell-eat-cell world: autophagy and phagocytosis. Am J Pathol. 2013;182:612–22. doi: 10.1016/j.ajpath.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turcotte S, Giaccia AJ. Targeting cancer cells through autophagy for anticancer therapy. Curr Opin Cell Biol. 2010;22:246–51. doi: 10.1016/j.ceb.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bejarano E, Cuervo AM. Chaperone-mediated autophagy. Proc Am Thorac Soc. 2010;7:29–39. doi: 10.1513/pats.200909-102JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massey A, Kiffin R, Cuervo AM. Pathophysiology of chaperone-mediated autophagy. Int J Biochem Cell Biol. 2004;36:2420–34. doi: 10.1016/j.biocel.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Czyzyk-Krzeska MF, Meller J, Plas DR. Not all autophagy is equal. Autophagy. 2012;8:1155–6. doi: 10.4161/auto.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Decuypere JP, Pirenne J, Jochmans I. Autophagy in renal ischemia-reperfusion injury: friend or foe? Am J Transplant. 2014;14:1464–5. doi: 10.1111/ajt.12717. [DOI] [PubMed] [Google Scholar]
- 40.Dong Z. Introduction: autophagy in kidneys. Semin Nephrol. 2014;34:1. doi: 10.1016/j.semnephrol.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Franch HA. Chaperone-mediated autophagy in the kidney: the road more traveled. Semin Nephrol. 2014;34:72–83. doi: 10.1016/j.semnephrol.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011;80:29–40. doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He L, Livingston MJ, Dong Z. Autophagy in acute kidney injury and repair. Nephron Clin Pract. 2014;127:56–60. doi: 10.1159/000363677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia G, Sowers JR. Autophagy: a housekeeper in cardiorenal metabolic health and disease. Biochim Biophys Acta. 2015;1852:219–24. doi: 10.1016/j.bbadis.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jangamreddy JR, Panigrahi S, Los MJ. Monitoring of autophagy is complicated--salinomycin as an example. Biochim Biophys Acta. 2015;1853:604–10. doi: 10.1016/j.bbamcr.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 46.Weihl CC. Monitoring autophagy in the treatment of protein aggregate diseases: steps toward identifying autophagic biomarkers. Neurotherapeutics. 2013;10:383–90. doi: 10.1007/s13311-013-0180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Wang ZV, Hill JA, Lin F. New autophagy reporter mice reveal dynamics of proximal tubular autophagy. J Am Soc Nephrol. 2014;25:305–15. doi: 10.1681/ASN.2013040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuyuki S, Takabayashi M, Kawazu M, et al. Detection of WIPI1 mRNA as an indicator of autophagosome formation. Autophagy. 2014;10:497–513. doi: 10.4161/auto.27419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H, Jin X, Zhang Z, Xing Y, Kong X. Inhibition of autophagy enhances apoptosis induced by the PI3K/AKT/mTor inhibitor NVP-BEZ235 in renal cell carcinoma cells. Cell Biochem Funct. 2013;31:427–33. doi: 10.1002/cbf.2917. [DOI] [PubMed] [Google Scholar]
- 50.Makhov P, Golovine K, Teper E, et al. Piperlongumine promotes autophagy via inhibition of Akt/mTOR signalling and mediates cancer cell death. Br J Cancer. 2014;110:899–907. doi: 10.1038/bjc.2013.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou J, Liao W, Yang J, et al. FOXO3 induces FOXO1-dependent autophagy by activating the AKT1 signaling pathway. Autophagy. 2012;8:1712–23. doi: 10.4161/auto.21830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kimura T, Takabatake Y, Takahashi A, Isaka Y. Chloroquine in cancer therapy: a double-edged sword of autophagy. Cancer Res. 2013;73:3–7. doi: 10.1158/0008-5472.CAN-12-2464. [DOI] [PubMed] [Google Scholar]
- 53.Kroemer G. Autophagy: a druggable process that is deregulated in aging and human disease. J Clin Invest. 2015;125:1–4. doi: 10.1172/JCI78652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–95. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 55.Anders HJ, Schlondorff DO. Innate immune receptors and autophagy: implications for autoimmune kidney injury. Kidney Int. 2010;78:29–37. doi: 10.1038/ki.2010.111. [DOI] [PubMed] [Google Scholar]
- 56.Yu J, Parkhitko A, Henske EP. Autophagy: an ‘Achilles’ heel of tumorigenesis in TSC and LAM. Autophagy. 2011;7:1400–1. doi: 10.4161/auto.7.11.17652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knodler LA, Celli J. Eating the strangers within: host control of intracellular bacteria via xenophagy. Cell Microbiol. 2011;13:1319–27. doi: 10.1111/j.1462-5822.2011.01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–10. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 60.Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 62.Tavassoly I, Parmar J, Shajahan-Haq AN, Clarke R, Baumann WT, Tyson JJ. Dynamic Modeling of the Interaction Between Autophagy and Apoptosis in Mammalian Cells. CPT Pharmacometrics Syst Pharmacol. 2015;4:263–72. doi: 10.1002/psp4.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–47. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 64.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–20. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 65.Liu Y, Levine B. Autosis and autophagic cell death: the dark side of autophagy. Cell Death Differ. 2015;22:367–76. doi: 10.1038/cdd.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luciani MF, Giusti C, Harms B, et al. Atg1 allows second-signaled autophagic cell death in Dictyostelium. Autophagy. 2011;7:501–8. doi: 10.4161/auto.7.5.14957. [DOI] [PubMed] [Google Scholar]
- 67.Anbalagan S, Pires IM, Blick C, et al. Radiosensitization of renal cell carcinoma in vitro through the induction of autophagy. Radiother Oncol. 2012;103:388–93. doi: 10.1016/j.radonc.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 68.Jain S, Keys D, Nydam T, Plenter RJ, Edelstein CL, Jani A. Inhibition of autophagy increases apoptosis during re-warming after cold storage in renal tubular epithelial cells. Transpl Int. 2015;28:214–23. doi: 10.1111/tri.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pallet N, Anglicheau D. Autophagy: a protective mechanism against nephrotoxicant-induced renal injury. Kidney Int. 2009;75:118–9. doi: 10.1038/ki.2008.537. author reply 9. [DOI] [PubMed] [Google Scholar]
- 70.Sato S, Adachi A, Sasaki Y, Dai W. Autophagy by podocytes in renal biopsy specimens. J Nippon Med Sch. 2006;73:52–3. doi: 10.1272/jnms.73.52. [DOI] [PubMed] [Google Scholar]
- 71.Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013;1833:3448–59. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 72.Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149:1536–48. doi: 10.1016/j.cell.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kenzelmann Broz D, Spano Mello S, Bieging KT, et al. Global genomic profiling reveals an extensive p53-regulated autophagy program contributing to key p53 responses. Genes Dev. 2013;27:1016–31. doi: 10.1101/gad.212282.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–88. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maiuri MC, Criollo A, Tasdemir E, et al. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L). Autophagy. 2007;3:374–6. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- 76.Hou W, Han J, Lu C, Goldstein LA, Rabinowich H. Autophagic degradation of active caspase-8: a crosstalk mechanism between autophagy and apoptosis. Autophagy. 2010;6:891–900. doi: 10.4161/auto.6.7.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang J, Shao SH, Xu ZX, et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–24. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 78.Chandrika BB, Yang C, Ou Y, et al. Endoplasmic Reticulum Stress-Induced Autophagy Provides Cytoprotection from Chemical Hypoxia and Oxidant Injury and Ameliorates Renal Ischemia-Reperfusion Injury. PLoS One. 2015;10:e0140025. doi: 10.1371/journal.pone.0140025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cybulsky AV. The intersecting roles of endoplasmic reticulum stress, ubiquitin- proteasome system, and autophagy in the pathogenesis of proteinuric kidney disease. Kidney Int. 2013;84:25–33. doi: 10.1038/ki.2012.390. [DOI] [PubMed] [Google Scholar]
- 80.Ding WX, Ni HM, Gao W, et al. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem. 2007;282:4702–10. doi: 10.1074/jbc.M609267200. [DOI] [PubMed] [Google Scholar]
- 81.Moon SY, Kim HS, Nho KW, Jang YJ, Lee SK. Endoplasmic reticulum stress induces epithelialmesenchymal transition through autophagy via activation of c-Src kinase. Nephron Exp Nephrol. 2014;126:127–40. doi: 10.1159/000362457. [DOI] [PubMed] [Google Scholar]
- 82.Pallet N, Anglicheau D, Thervet E. Autophagy is an adaptative mechanism against endoplasmic reticulum stress. Nephrol Dial Transplant. 2009;24:3891. doi: 10.1093/ndt/gfp518. author reply. [DOI] [PubMed] [Google Scholar]
- 83.Yuan Y, Xu X, Zhao C, et al. The roles of oxidative stress, endoplasmic reticulum stress, and autophagy in aldosterone/mineralocorticoid receptor-induced podocyte injury. Lab Invest. 2015;95:1374–86. doi: 10.1038/labinvest.2015.118. [DOI] [PubMed] [Google Scholar]
- 84.Inbal B, Bialik S, Sabanay I, Shani G, Kimchi A. DAP kinase and DRP-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death. J Cell Biol. 2002;157:455–68. doi: 10.1083/jcb.200109094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kimura T, Takabatake Y, Takahashi A, et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol. 2011;22:902–13. doi: 10.1681/ASN.2010070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou XJ, Rakheja D, Yu X, Saxena R, Vaziri ND, Silva FG. The aging kidney. Kidney Int. 2008;74:710–20. doi: 10.1038/ki.2008.319. [DOI] [PubMed] [Google Scholar]
- 87.Kume S, Uzu T, Horiike K, et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010;120:1043–55. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hartleben B, Godel M, Meyer-Schwesinger C, et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest. 2010;120:1084–96. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weide T, Huber TB. Implications of autophagy for glomerular aging and disease. Cell Tissue Res. 2011;343:467–73. doi: 10.1007/s00441-010-1115-0. [DOI] [PubMed] [Google Scholar]
- 90.Molitoris BA. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J Clin Invest. 2014;124:2355–63. doi: 10.1172/JCI72269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lu CY, Hartono J, Senitko M, Chen J. The inflammatory response to ischemic acute kidney injury: a result of the ‘right stuff’ in the ‘wrong place’? Curr Opin Nephrol Hypertens. 2007;16:83–9. doi: 10.1097/MNH.0b013e3280403c4e. [DOI] [PubMed] [Google Scholar]
- 92.Chien CT, Shyue SK, Lai MK. Bcl-xL augmentation potentially reduces ischemia/reperfusion induced proximal and distal tubular apoptosis and autophagy. Transplantation. 2007;84:1183–90. doi: 10.1097/01.tp.0000287334.38933.e3. [DOI] [PubMed] [Google Scholar]
- 93.Wu HH, Hsiao TY, Chien CT, Lai MK. Ischemic conditioning by short periods of reperfusion attenuates renal ischemia/reperfusion induced apoptosis and autophagy in the rat. J Biomed Sci. 2009;16:19. doi: 10.1186/1423-0127-16-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang M, Liu K, Luo J, Dong Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol. 2010;176:1181–92. doi: 10.2353/ajpath.2010.090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xia S, Lv J, Gao Q, et al. Prenatal exposure to hypoxia induced Beclin 1 signaling-mediated renal autophagy and altered renal development in rat fetuses. Reprod Sci. 2015;22:156–64. doi: 10.1177/1933719114536474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ishihara M, Urushido M, Hamada K, et al. Sestrin-2 and BNIP3 regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. Am J Physiol Renal Physiol. 2013;305:F495–509. doi: 10.1152/ajprenal.00642.2012. [DOI] [PubMed] [Google Scholar]
- 97.Bolisetty S, Traylor AM, Kim J, et al. Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J Am Soc Nephrol. 2010;21:1702–12. doi: 10.1681/ASN.2010030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu WJ, Luo MN, Tan J, et al. Autophagy activation reduces renal tubular injury induced by urinary proteins. Autophagy. 2014;10:243–56. doi: 10.4161/auto.27004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alers S, Loffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chan EY, Kir S, Tooze SA. siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J Biol Chem. 2007;282:25464–74. doi: 10.1074/jbc.M703663200. [DOI] [PubMed] [Google Scholar]
- 101.Russell RC, Tian Y, Yuan H, et al. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15:741–50. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shukla S, Patric IR, Patil V, et al. Methylation silencing of ULK2, an autophagy gene, is essential for astrocyte transformation and tumor growth. J Biol Chem. 2014;289:22306–18. doi: 10.1074/jbc.M114.567032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alnasser HA, Guan Q, Zhang F, Gleave ME, Nguan CY, Du C. Requirement of clusterin expression for prosurvival autophagy in hypoxic kidney tubular epithelial cells. Am J Physiol Renal Physiol. 2015:ajprenal 00304. doi: 10.1152/ajprenal.00304.2015. 2015. [DOI] [PubMed] [Google Scholar]
- 104.Kawakami T, Inagi R, Takano H, et al. Endoplasmic reticulum stress induces autophagy in renal proximal tubular cells. Nephrol Dial Transplant. 2009;24:2665–72. doi: 10.1093/ndt/gfp215. [DOI] [PubMed] [Google Scholar]
- 105.Guan X, Qian Y, Shen Y, et al. Autophagy protects renal tubular cells against ischemia / reperfusion injury in a time-dependent manner. Cell Physiol Biochem. 2015;36:285–98. doi: 10.1159/000374071. [DOI] [PubMed] [Google Scholar]
- 106.Wang IK, Sun KT, Tsai TH, et al. MiR-20a-5p mediates hypoxia-induced autophagy by targeting ATG16L1 in ischemic kidney injury. Life Sci. 2015;136:133–41. doi: 10.1016/j.lfs.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 107.Zhang YL, Zhang J, Cui LY, Yang S. Autophagy activation attenuates renal ischemia-reperfusion injury in rats. Exp Biol Med (Maywood) 2015 doi: 10.1177/1535370215581306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baisantry A, Bhayana S, Rong S, et al. Autophagy Induces Prosenescent Changes in Proximal Tubular S3 Segments. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014111059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cheng H, Fan X, Lawson WE, Paueksakon P, Harris RC. Telomerase deficiency delays renal recovery in mice after ischemia-reperfusion injury by impairing autophagy. Kidney Int. 2015;88:85–94. doi: 10.1038/ki.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Buyuklu M, Kandemir FM, Ozkaraca M, et al. Benefical effects of lycopene against contrast medium-induced oxidative stress, inflammation, autophagy, and apoptosis in rat kidney. Hum Exp Toxicol. 2015;34:487–96. doi: 10.1177/0960327114542964. [DOI] [PubMed] [Google Scholar]
- 111.Ko GJ, Bae SY, Hong YA, Pyo HJ, Kwon YJ. Radiocontrast-induced nephropathy is attenuated by autophagy through regulation of apoptosis and inflammation. Hum Exp Toxicol. 2015 doi: 10.1177/0960327115604198. [DOI] [PubMed] [Google Scholar]
- 112.Suzuki C, Isaka Y, Takabatake Y, et al. Participation of autophagy in renal ischemia/reperfusion injury. Biochem Biophys Res Commun. 2008;368:100–6. doi: 10.1016/j.bbrc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 113.Domitrovic R, Cvijanovic O, Pernjak-Pugel E, Skoda M, Mikelic L, Crncevic-Orlic Z. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem Toxicol. 2013;62:397–406. doi: 10.1016/j.fct.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 114.Herzog C, Yang C, Holmes A, Kaushal GP. zVAD-fmk prevents cisplatin-induced cleavage of autophagy proteins but impairs autophagic flux and worsens renal function. Am J Physiol Renal Physiol. 2012;303:F1239–50. doi: 10.1152/ajprenal.00659.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Periyasamy-Thandavan S, Jiang M, Wei Q, Smith R, Yin XM, Dong Z. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int. 2008;74:631–40. doi: 10.1038/ki.2008.214. [DOI] [PubMed] [Google Scholar]
- 116.Rovetta F, Stacchiotti A, Consiglio A, et al. ER signaling regulation drives the switch between autophagy and apoptosis in NRK-52E cells exposed to cisplatin. Exp Cell Res. 2012;318:238–50. doi: 10.1016/j.yexcr.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 117.Takahashi A, Kimura T, Takabatake Y, et al. Autophagy guards against cisplatin-induced acute kidney injury. Am J Pathol. 2012;180:517–25. doi: 10.1016/j.ajpath.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 118.Yang C, Kaushal V, Shah SV, Kaushal GP. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol. 2008;294:F777–87. doi: 10.1152/ajprenal.00590.2007. [DOI] [PubMed] [Google Scholar]
- 119.Chargui A, Zekri S, Jacquillet G, et al. Cadmium-induced autophagy in rat kidney: an early biomarker of subtoxic exposure. Toxicol Sci. 2011;121:31–42. doi: 10.1093/toxsci/kfr031. [DOI] [PubMed] [Google Scholar]
- 120.Pallet N, Bouvier N, Legendre C, et al. Autophagy protects renal tubular cells against cyclosporine toxicity. Autophagy. 2008;4:783–91. doi: 10.4161/auto.6477. [DOI] [PubMed] [Google Scholar]
- 121.Yadav RK, Lee GH, Lee HY, et al. TMBIM6 (transmembrane BAX inhibitor motif containing 6) enhances autophagy and reduces renal dysfunction in a cyclosporine A-induced nephrotoxicity model. Autophagy. 2015;11:1760–74. doi: 10.1080/15548627.2015.1082021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kimura A, Ishida Y, Wada T, et al. The absence of interleukin-6 enhanced arsenite-induced renal injury by promoting autophagy of tubular epithelial cells with aberrant extracellular signal-regulated kinase activation. Am J Pathol. 2010;176:40–50. doi: 10.2353/ajpath.2010.090146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pallet N. Response letter to “autophagy in renal ischemia-reperfusion injury: friend or foe?”. Am J Transplant. 2014;14:1466–7. doi: 10.1111/ajt.12720. [DOI] [PubMed] [Google Scholar]
- 124.Zeng Y, Li S, Wu J, et al. Autophagy inhibitors promoted aristolochic acid I induced renal tubular epithelial cell apoptosis via mitochondrial pathway but alleviated nonapoptotic cell death in mouse acute aritolochic acid nephropathy model. Apoptosis. 2014;19:1215–24. doi: 10.1007/s10495-014-0996-x. [DOI] [PubMed] [Google Scholar]
- 125.Cui J, Bai XY, Sun X, et al. Rapamycin protects against gentamicin-induced acute kidney injury via autophagy in mini-pig models. Sci Rep. 2015;5:11256. doi: 10.1038/srep11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lim SW, Hyoung BJ, Piao SG, Doh KC, Chung BH, Yang CW. Chronic cyclosporine nephropathy is characterized by excessive autophagosome formation and decreased autophagic clearance. Transplantation. 2012;94:218–25. doi: 10.1097/TP.0b013e31825ace5c. [DOI] [PubMed] [Google Scholar]
- 127.Takakura A, Nelson EA, Haque N, et al. Pyrimethamine inhibits adult polycystic kidney disease by modulating STAT signaling pathways. Hum Mol Genet. 2011;20:4143–54. doi: 10.1093/hmg/ddr338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu W, Kong T, Beaudry S, et al. Polycystin-1 protein level determines activity of the Galpha12/JNK apoptosis pathway. J Biol Chem. 2010;285:10243–51. doi: 10.1074/jbc.M109.070821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang S, Livingston MJ, Su Y, Dong Z. Reciprocal regulation of cilia and autophagy via the MTOR and proteasome pathways. Autophagy. 2015;11:607–16. doi: 10.1080/15548627.2015.1023983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang S, Wei Q, Dong G, Dong Z. ERK-mediated suppression of cilia in cisplatin-induced tubular cell apoptosis and acute kidney injury. Biochim Biophys Acta. 2013;1832:1582–90. doi: 10.1016/j.bbadis.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Belibi FA, Edelstein CL. Novel targets for the treatment of autosomal dominant polycystic kidney disease. Expert Opin Investig Drugs. 2010;19:315–28. doi: 10.1517/13543781003588491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ecder T, Melnikov VY, Stanley M, et al. Caspases, Bcl-2 proteins and apoptosis in autosomal-dominant polycystic kidney disease. Kidney Int. 2002;61:1220–30. doi: 10.1046/j.1523-1755.2002.00250.x. [DOI] [PubMed] [Google Scholar]
- 133.Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–9. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 134.Takiar V, Nishio S, Seo-Mayer P, et al. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci U S A. 2011;108:2462–7. doi: 10.1073/pnas.1011498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Walz G, Budde K, Mannaa M, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830–40. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 136.Lin HH, Yang TP, Jiang ST, Yang HY, Tang MJ. Bcl-2 overexpression prevents apoptosis-induced Madin-Darby canine kidney simple epithelial cyst formation. Kidney Int. 1999;55:168–78. doi: 10.1046/j.1523-1755.1999.00249.x. [DOI] [PubMed] [Google Scholar]
- 137.Belibi F, Zafar I, Ravichandran K, et al. Hypoxia-inducible factor-1alpha (HIF-1alpha) and autophagy in polycystic kidney disease (PKD). Am J Physiol Renal Physiol. 2011;300:F1235–43. doi: 10.1152/ajprenal.00348.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Inoki K. mTOR signaling in autophagy regulation in the kidney. Semin Nephrol. 2014;34:2–8. doi: 10.1016/j.semnephrol.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu M, Wahl PR, Le Hir M, Wackerle-Men Y, Wuthrich RP, Serra AL. Everolimus retards cyst growth and preserves kidney function in a rodent model for polycystic kidney disease. Kidney Blood Press Res. 2007;30:253–9. doi: 10.1159/000104818. [DOI] [PubMed] [Google Scholar]
- 140.Barbosa Junior Ade A, Zhou H, Hultenschmidt D, Totovic V, Jurilj N, Pfeifer U. Inhibition of cellular autophagy in proximal tubular cells of the kidney in streptozotocin-diabetic and uninephrectomized rats. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;61:359–66. doi: 10.1007/BF02890439. [DOI] [PubMed] [Google Scholar]
- 141.Zhao X, Liu G, Shen H, et al. Liraglutide inhibits autophagy and apoptosis induced by high glucose through GLP-1R in renal tubular epithelial cells. Int J Mol Med. 2015;35:684–92. doi: 10.3892/ijmm.2014.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xin W, Zhao X, Liu L, et al. Acetyl-CoA carboxylase 2 suppression rescues human proximal tubular cells from palmitic acid induced lipotoxicity via autophagy. Biochem Biophys Res Commun. 2015;463:364–9. doi: 10.1016/j.bbrc.2015.05.070. [DOI] [PubMed] [Google Scholar]
- 143.Wu Y, Zhang Y, Wang L, Diao Z, Liu W. The Role of Autophagy in Kidney Inflammatory Injury via the NF-kappaB Route Induced by LPS. Int J Med Sci. 2015;12:655–67. doi: 10.7150/ijms.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chung SD, Lai TY, Chien CT, Yu HJ. Activating Nrf-2 signaling depresses unilateral ureteral obstruction-evoked mitochondrial stress-related autophagy, apoptosis and pyroptosis in kidney. PLoS One. 2012;7:e47299. doi: 10.1371/journal.pone.0047299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim WY, Nam SA, Song HC, et al. The role of autophagy in unilateral ureteral obstruction rat model. Nephrology (Carlton) 2012;17:148–59. doi: 10.1111/j.1440-1797.2011.01541.x. [DOI] [PubMed] [Google Scholar]
- 146.Ding Y, Choi ME. Regulation of autophagy by TGF-beta: emerging role in kidney fibrosis. Semin Nephrol. 2014;34:62–71. doi: 10.1016/j.semnephrol.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ding Y, Kim S, Lee SY, Koo JK, Wang Z, Choi ME. Autophagy regulates TGF-beta expression and suppresses kidney fibrosis induced by unilateral ureteral obstruction. J Am Soc Nephrol. 2014;25:2835–46. doi: 10.1681/ASN.2013101068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim SI, Na HJ, Ding Y, Wang Z, Lee SJ, Choi ME. Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-beta1. J Biol Chem. 2012;287:11677–88. doi: 10.1074/jbc.M111.308460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xu Y, Yang S, Huang J, Ruan S, Zheng Z, Lin J. Tgf-beta1 induces autophagy and promotes apoptosis in renal tubular epithelial cells. Int J Mol Med. 2012;29:781–90. doi: 10.3892/ijmm.2012.911. [DOI] [PubMed] [Google Scholar]
- 150.Bonegio R, Lieberthal W. Role of apoptosis in the pathogenesis of acute renal failure. Curr Opin Nephrol Hypertens. 2002;11:301–8. doi: 10.1097/00041552-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 151.Turkmen K, Martin J, Akcay A, et al. Apoptosis and autophagy in cold preservation ischemia. Transplantation. 2011;91:1192–7. doi: 10.1097/TP.0b013e31821ab9c8. [DOI] [PubMed] [Google Scholar]
- 152.Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lieberthal W, Fuhro R, Andry CC, et al. Rapamycin impairs recovery from acute renal failure: role of cell-cycle arrest and apoptosis of tubular cells. Am J Physiol Renal Physiol. 2001;281:F693–706. doi: 10.1152/ajprenal.2001.281.4.F693. [DOI] [PubMed] [Google Scholar]
- 154.Letavernier E, Bruneval P, Mandet C, et al. High sirolimus levels may induce focal segmental glomerulosclerosis de novo. Clin J Am Soc Nephrol. 2007;2:326–33. doi: 10.2215/CJN.03751106. [DOI] [PubMed] [Google Scholar]
- 155.Letavernier E, Pe'raldi MN, Pariente A, Morelon E, Legendre C. Proteinuria following a switch from calcineurin inhibitors to sirolimus. Transplantation. 2005;80:1198–203. doi: 10.1097/01.tp.0000185200.17589.74. [DOI] [PubMed] [Google Scholar]
- 156.Pallet N, Legendre C. Adverse events associated with mTOR inhibitors. Expert Opin Drug Saf. 2013;12:177–86. doi: 10.1517/14740338.2013.752814. [DOI] [PubMed] [Google Scholar]