Abstract
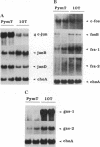
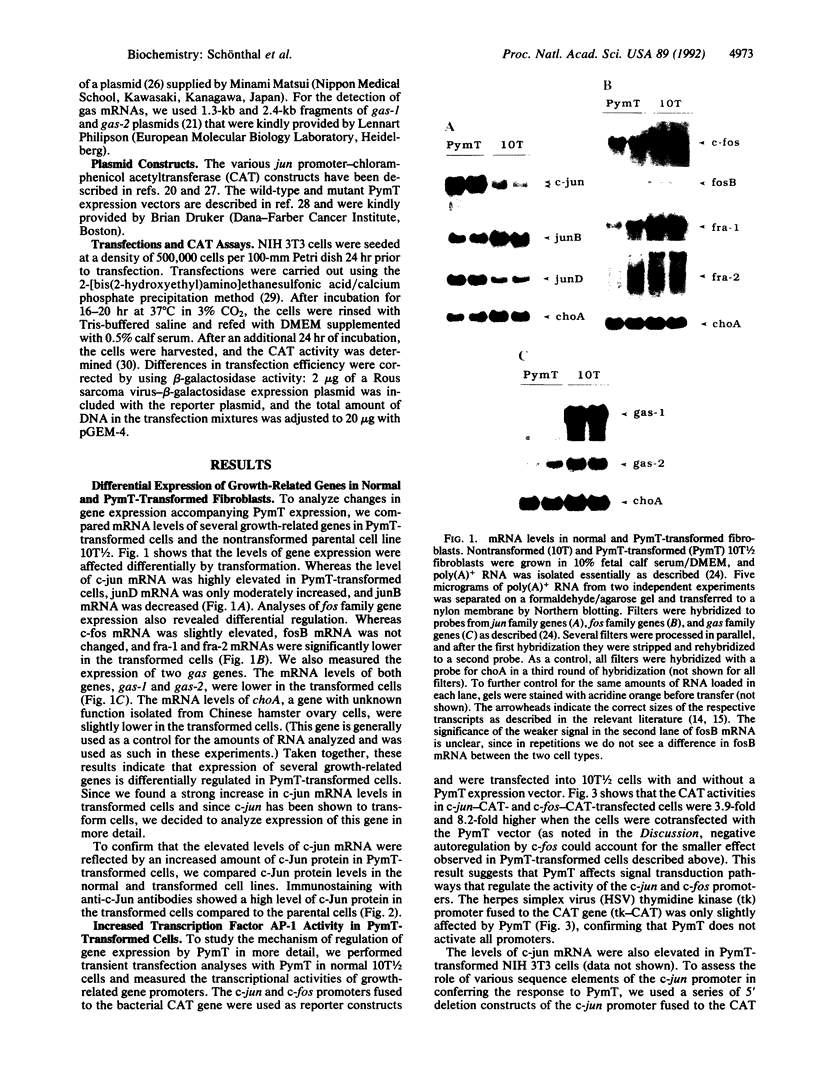
Polyoma virus middle-sized tumor (PymT) antigen is required for neoplastic cell transformation by polyoma virus. We studied changes in gene expression accompanying expression of PymT in murine fibroblasts. These experiments showed that PymT differentially affects several growth-related genes. c-jun protooncogene expression was highly increased, whereas the expression of two growth arrest-specific genes (gas) was reduced, in cells transformed by PymT. Cotransfection experiments showed that the increase in c-jun expression resulted from elevated activity of the transcription factor AP-1 and was mediated through the phorbol 12-tetradecanoate 13-acetate response element in the c-jun promoter. The degree of c-Jun/AP-1 activation by different PymT mutants correlated with their transforming capability, suggesting that regulation of c-Jun/AP-1 activity may play a role in cell transformation by polyoma virus.
Full text
PDF
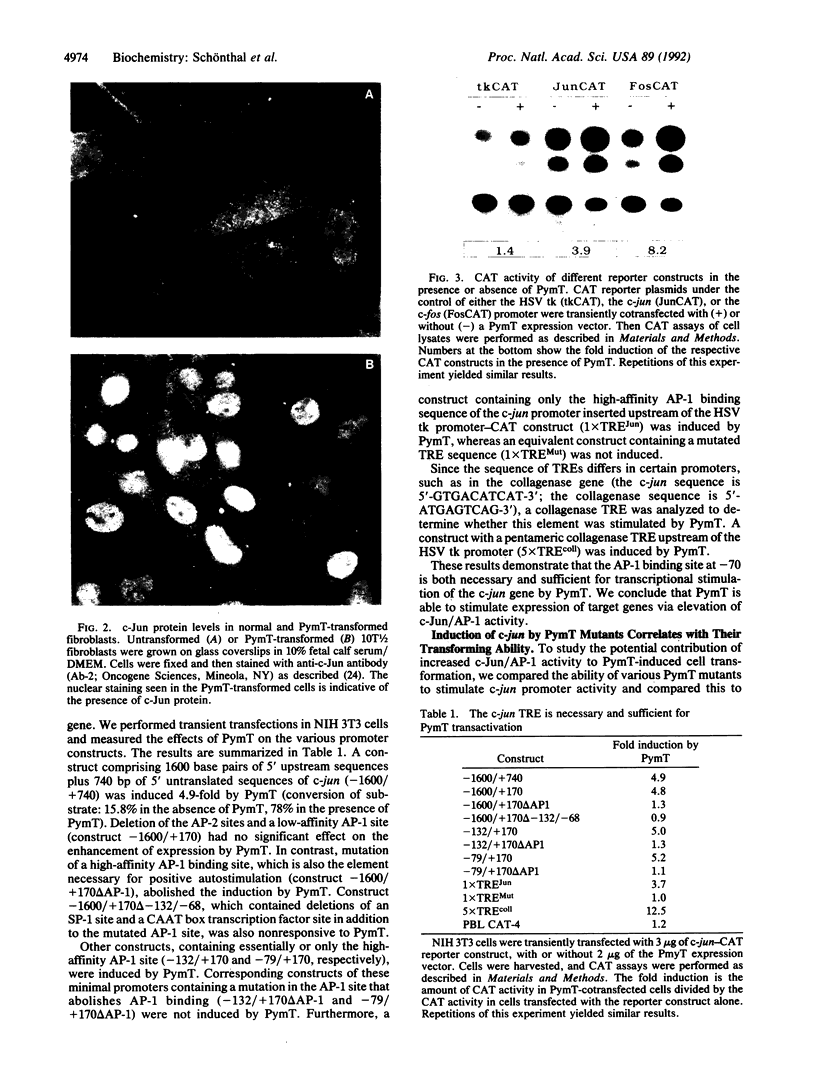
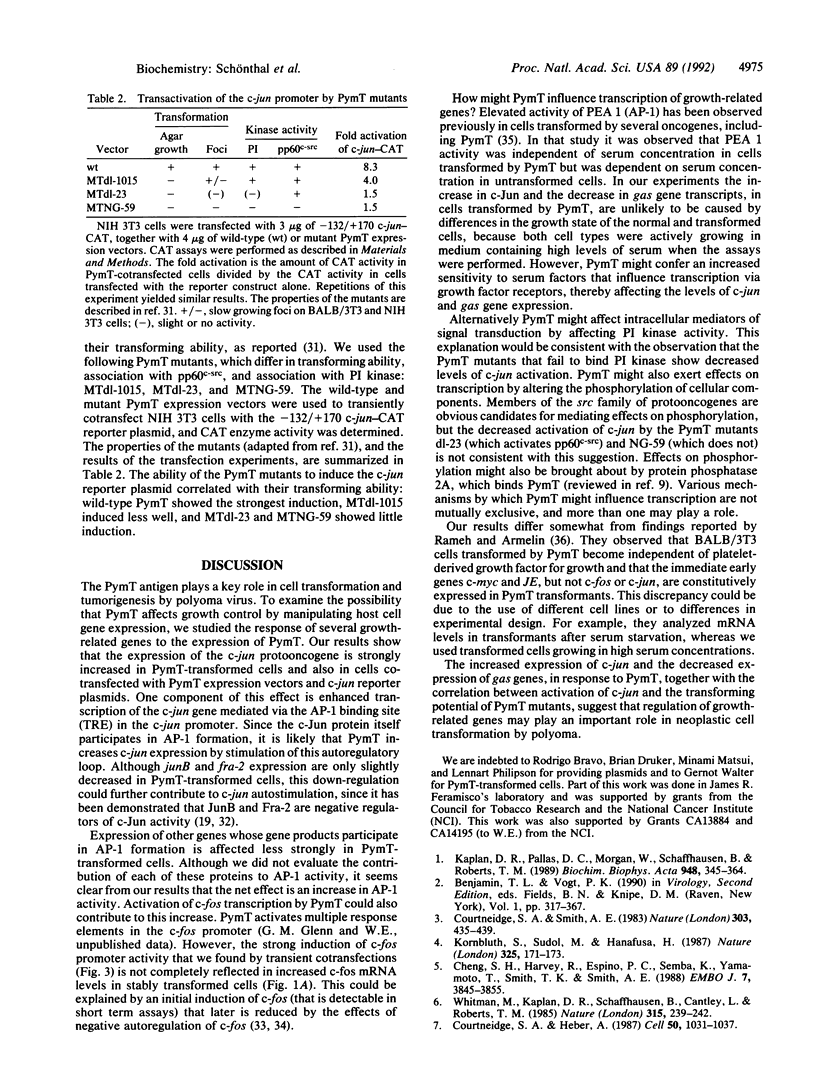

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Bos T. J., Monteclaro F. S., Mitsunobu F., Ball A. R., Jr, Chang C. H., Nishimura T., Vogt P. K. Efficient transformation of chicken embryo fibroblasts by c-Jun requires structural modification in coding and noncoding sequences. Genes Dev. 1990 Oct;4(10):1677–1687. doi: 10.1101/gad.4.10.1677. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Harvey R., Espino P. C., Semba K., Yamamoto T., Toyoshima K., Smith A. E. Peptide antibodies to the human c-fyn gene product demonstrate pp59c-fyn is capable of complex formation with the middle-T antigen of polyomavirus. EMBO J. 1988 Dec 1;7(12):3845–3855. doi: 10.1002/j.1460-2075.1988.tb03270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu R., Angel P., Karin M. Jun-B differs in its biological properties from, and is a negative regulator of, c-Jun. Cell. 1989 Dec 22;59(6):979–986. doi: 10.1016/0092-8674(89)90754-x. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Heber A. An 81 kd protein complexed with middle T antigen and pp60c-src: a possible phosphatidylinositol kinase. Cell. 1987 Sep 25;50(7):1031–1037. doi: 10.1016/0092-8674(87)90169-3. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Druker B. J., Ling L. E., Cohen B., Roberts T. M., Schaffhausen B. S. A completely transformation-defective point mutant of polyomavirus middle T antigen which retains full associated phosphatidylinositol kinase activity. J Virol. 1990 Sep;64(9):4454–4461. doi: 10.1128/jvi.64.9.4454-4461.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn G. M., Eckhart W. Transcriptional regulation of early-response genes during polyomavirus infection. J Virol. 1990 May;64(5):2193–2201. doi: 10.1128/jvi.64.5.2193-2201.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Pallas D. C., Morgan W., Schaffhausen B., Roberts T. M. Mechanisms of transformation by polyoma virus middle T antigen. Biochim Biophys Acta. 1989 Feb;948(3):345–364. doi: 10.1016/0304-419x(89)90006-1. [DOI] [PubMed] [Google Scholar]
- Kornbluth S., Sudol M., Hanafusa H. Association of the polyomavirus middle-T antigen with c-yes protein. Nature. 1987 Jan 8;325(7000):171–173. doi: 10.1038/325171a0. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Matsui M., Tokuhara M., Konuma Y., Nomura N., Ishizaki R. Isolation of human fos-related genes and their expression during monocyte-macrophage differentiation. Oncogene. 1990 Mar;5(3):249–255. [PubMed] [Google Scholar]
- Morgan W. C., Kaplan D. R., Pallas D. C., Roberts T. M. Recombinant retroviruses that transduce middle T antigen cDNAs derived from polyomavirus mutants: separation of focus formation and soft-agar growth in transformation assays and correlations with kinase activities in vitro. J Virol. 1988 Sep;62(9):3407–3414. doi: 10.1128/jvi.62.9.3407-3414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby M. C., Walter G. Protein phosphatases and DNA tumor viruses: transformation through the back door? Cell Regul. 1991 Aug;2(8):589–598. doi: 10.1091/mbc.2.8.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas D. C., Cherington V., Morgan W., DeAnda J., Kaplan D., Schaffhausen B., Roberts T. M. Cellular proteins that associate with the middle and small T antigens of polyomavirus. J Virol. 1988 Nov;62(11):3934–3940. doi: 10.1128/jvi.62.11.3934-3940.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameh L. E., Armelin M. C. T antigens' role in polyomavirus transformation: c-myc but not c-fos or c-jun expression is a target for middle T. Oncogene. 1991 Jun;6(6):1049–1056. [PubMed] [Google Scholar]
- Ryseck R. P., Bravo R. c-JUN, JUN B, and JUN D differ in their binding affinities to AP-1 and CRE consensus sequences: effect of FOS proteins. Oncogene. 1991 Apr;6(4):533–542. [PubMed] [Google Scholar]
- Sassone-Corsi P., Sisson J. C., Verma I. M. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988 Jul 28;334(6180):314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- Schneider C., King R. M., Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988 Sep 9;54(6):787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- Schönthal A., Alberts A. S., Frost J. A., Feramisco J. R. Differential regulation of jun family gene expression by the tumor promoter okadaic acid. New Biol. 1991 Oct;3(10):977–986. [PubMed] [Google Scholar]
- Schönthal A., Büscher M., Angel P., Rahmsdorf H. J., Ponta H., Hattori K., Chiu R., Karin M., Herrlich P. The Fos and Jun/AP-1 proteins are involved in the downregulation of Fos transcription. Oncogene. 1989 May;4(5):629–636. [PubMed] [Google Scholar]
- Schönthal A., Feramisco J. R. Different promoter elements are required for the induced expression of c-fos and c-jun proto-oncogenes by the v-mos oncogene product. New Biol. 1990 Feb;2(2):143–150. [PubMed] [Google Scholar]
- Schönthal A., Tsukitani Y., Feramisco J. R. Transcriptional and post-transcriptional regulation of c-fos expression by the tumor promoter okadaic acid. Oncogene. 1991 Mar;6(3):423–430. [PubMed] [Google Scholar]
- Schütte J., Viallet J., Nau M., Segal S., Fedorko J., Minna J. jun-B inhibits and c-fos stimulates the transforming and trans-activating activities of c-jun. Cell. 1989 Dec 22;59(6):987–997. doi: 10.1016/0092-8674(89)90755-1. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Okuno H., Yoshida T., Endo T., Nishina H., Iba H. Difference in transcriptional regulatory function between c-Fos and Fra-2. Nucleic Acids Res. 1991 Oct 25;19(20):5537–5542. doi: 10.1093/nar/19.20.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk C., Imler J. L., Wasylyk B. Transforming but not immortalizing oncogenes activate the transcription factor PEA1. EMBO J. 1988 Aug;7(8):2475–2483. doi: 10.1002/j.1460-2075.1988.tb03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M., Kaplan D. R., Schaffhausen B., Cantley L., Roberts T. M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985 May 16;315(6016):239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- Zullo J., Stiles C. D., Garcea R. L. Regulation of c-myc and c-fos mRNA levels by polyomavirus: distinct roles for the capsid protein VP1 and the viral early proteins. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1210–1214. doi: 10.1073/pnas.84.5.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]