Abstract
Maillard reaction products (MRPs) of soybean protein isolate (SPI) and sugars (glucose and maltose) were prepared by heating in the aqueous dispersion at 95 °C for 15 min with ultrasonic pretreatment (ultrasonic power of 200 W) for 20 min. Effect of ultrasonic pretreatment on physicochemical characteristics and rheological properties of SPI/sugar MRPs was investigated. SPI/sugar MRPs prepared with ultrasonic pretreatment had higher degree of glycation (DG), lower browning and less compact tertiary conformation than that with non-ultrasonic pretreatment. Surface hydrophobicity (H0), particle size and rheological properties were measured by fluorescence spectrophotometry, laser particle size analysis and dynamic oscillatory rheometry, respectively. Glycation reduced H0 and particle size as well as weaken the gel network formed by the acidification of GDL. However, ultrasound increased H0 and decreased particle size. This is desirable for the formation of acid-induced gel structure. The ultrasonic pretreatments reduced/eliminate the weakening effect of glycation on the gel network of SPI/sugar MRPs, and even improved the gel properties.
Keywords: Ultrasonic pretreatment, Soybean protein, Sugar, Maillard reaction, Rheological properties
Introduction
Soy protein isolate (SPI) has high nutrition as well as various functional properties such as solubility, emulsifying, gelling and foaming attributes, which makes SPI extensively used in the food industry (Chi et al. 2008). However, functional properties of SPI need to be further improved through appropriate modifications that contain physical, chemical and enzymatic treatments (Wang et al. 2008).
Recently, some efforts are made to improve the functional properties of proteins through glycation reactions between proteins and sugars, which are based on Maillard reactions (MR) between the ε-amino groups of proteins and the reducing-end carbonyl groups of sugars (Wang et al. 2013). MR is a spontaneous reaction without adding chemicals, involving a complex network of non-enzymatic reactions in certain conditions of temperature and water activity, which is one of the major food protein modifying reactions occurring during thermal food processing (Spotti et al. 2014). The pigment and aroma produced by MR can influence the food sensory quality, which lead to wide application of MR in the food industry (Amarowicz 2009). In the previous researches, the glycation of different protein such as soy proteins (Gu et al. 2009), rice proteins (Li et al. 2009) and milk proteins (Pinto et al. 2014) with various reducing sugars by MR have been investigated, which mainly focused on the studies of solubility, emulsification, thermal stability and antioxidant properties (Jiménez-Castaño et al. 2005; Zhu et al. 2008). However, there are fewer studies on physicochemical characteristics and rheological properties of glycoconjugates of soy proteins with reducing sugars.
Application of ultrasound technology in food industry has increased recently, due to its positive effects on physical and functional properties of proteins in food (Jambrak et al. 2009). The effects of ultrasound on proteins are mainly attributed to the cavitation effect that is generated at low frequency and higher power in liquid systems (Gülseren et al. 2007). The micro-bubbles are rapidly formed during sonication and violently implode, resulting in extreme high temperatures (5000 K) and pressures (1000 atm), which can produce very high shear energy waves and turbulence (Soria and Villamiel 2010). Ultrasound is capable of changing material properties through high temperature and pressure as well as turbulence. Ultrasound treatment can modify secondary structure of proteins, which leads to the increase in surface hydrophobicity (Stanic-Vucinic et al. 2012) and the aggregation of proteins (Chandrapala et al. 2011). Moreover, ultrasound treatment could also accelerate the MR in the aqueous solution between a single amino acid and a reducing sugar, such as glycine and glucose or maltose (Guan et al. 2011). Nevertheless, to the best of our knowledge, there is little study about the effects of ultrasound on the MR between SPI and reducing sugar, and even less physicochemical and rheological properties of SPI/sugar MRPs.
In this research, the glycation between SPI and reducing sugars by MR in aqueous systems with ultrasonic pretreatment was studied. The effect of ultrasonic pretreatment on physicochemical and structural characteristics of SPI/sugar MRPs as well as rheological properties of glycoconjugates acid-induced gels has been investigated using laser particle size analysis, fluorescence spectrophotometer and dynamic oscillatory rheometry.
Materials and methods
Materials and chemicals
Soybean protein isolate (SPI) with protein content of 92.4 % (dry wt) was provided by Harbin High-Tech Ltd. (Harbin, China). Glucose (G), maltose (M), o-phthaldialdehyde (OPA) and 1-anilino-8-naphtalene-sulfonate (ANS) were purchased from Sigma-Aldrich Chemical Company, (St Louis, MO, USA) and other reagents were purchased from local reagent company. All reagents were analytical grade and used without further purification.
Preparation of SPI/sugar MRPs
SPI dispersions were prepared by dispersing the SPI powder in phosphate buffer (0.1 M, pH 7.0) to give a protein concentration of 8 % (w/w). The sugars (glucose and maltose) were mixed in the SPI dispersions to give the sugar concentrations of 2 %, 4 %, 8 % and 16 % (w/v). The SPI/sugar dispersions were stirred for 2 h at ambient temperature with a magnetic stirrer and stored at 4 °C overnight to ensure that SPI was fully dissolved and mixed adequately with sugars. The ultrasonic pretreatment of mixture dispersions were performed with constant cooling in the ice bath by ultrasonic equipment model JY92-2D (NingBo Scientz Biotechnology Co. Ltd., Ningbo, Zhejiang, China) with a 0.54 cm diameter titanium probe. The dispersions were treated at the ultrasonic power of 200 W (138.26 W/cm2) for 20 min by ultrasound with pulse duration of on-time 3 s and off-time 1 s. Thereafter, the dispersions were heated in the water bath at 95 °C for 15 min, cooled down to ambient temperature with the ice bath and dialyzed at 4 °C for 24 h. Finally, the samples were lyophilized to obtain ultrasonic SPI/sugar MRPs. Furthermore, the same mixture dispersions were heated in the water bath at 95 °C for 15 min without ultrasonic pretreatment and the subsequent procedure was the same to the ultrasonic SPI/sugar MRPs to obtain non-ultrasonic SPI/sugar MRPs. As control, SPI samples without addition of sugars were also heated under the same conditions with ultrasonic or non-ultrasonic pretreatment and SPI native was not treated. All samples were stored at −20 °C until analysis.
Degree of glycation (DG)
The method for determination of degree of glycation was based on a spectrophotometric assay using OPA for determination of free amino groups (Vigo et al. 1992). 80 mg OPA was dissolved in 2 ml 95 % ethanol and mixed with 50 ml of 10 mM sodium tetraborate buffer solution (pH 9.7), 5 ml of 20 % (w/w) SDS and 200 μl of β-mercaptoethanol. The solution was mixed well and diluted to a final volume of 100 ml with distilled water to form the OPA reagent. 200 μl of sample dispersions (2 mg/ml) were incubated with 4 ml of OPA reagent for 5 min at room temperature. The absorbance was measured at 340 nm using a UV-VIS spectrophotometer in order to obtain the free amino groups. The blank was 200 μl distilled water in 4 ml OPA reagent. The DG was calculated as following equation:
Where Ab was absorbance of the blank and As was absorbance of the sample.
Browning intensity
The sample dispersions were diluted with distilled water to a final concentration of 1 % (w/w). Browning intensity as the marker at the final stages of the reactions (Kim and Lee 2008) was evaluated by the absorbance at 420 nm measured in a spectrophotometer (UNICO UV-2100, Shanghai, China).
Surface hydrophobicity (H0)
Surface hydrophobicity (H0) of soluble proteins in samples at pH 7.0 was measured according to the method of Kato and Nakai (1980), using 1-anilino-8-naphthalene-sulfonate (ANS) as the hydrophobicity fluorescence probe. The sample dispersions of 1 mg/ml were prepared by phosphate buffer (0.01 M, pH 7.0), with stirring for 1 h at room temperature, centrifuged at 10,000 g for 15 min and soluble protein concentration was determined in the supernatants according to the method of Lowry. Serial dilutions of the supernatant were made with the same buffer at protein concentrations ranging from 0.05 to 1 mg/ml. Then, 20 μl of ANS solution (8.0 mM in 0.01 M phosphate buffer, pH 7.0) was added to 2 ml of the sample. Fluorescence intensity was measured at wavelengths of 390 nm (excitation) and 470 nm (emission) with a Perkin-Elmer 2000 fluorescence spectrophotometer. The initial slope of fluorescence intensity versus protein concentration was taken as an index of H0.
Particle size
The sample dispersions of 8 % (w/w) were prepared with distilled water and stirred using a magnetic stirrer at 25 °C for 2 h. The samples were stored at 4 °C overnight to ensure that the samples were completely dissolved. The particle sizes of samples were measured by light scattering using a Mastersizer 2000 equipped with a Hydro 2000 MU dispersion unit (Malvern Instruments Ltd., Worcestershire, UK). The pump speed was set at 1900 rpm, and the refractive index and absorption parameter of the disperse phase were 1.46 and 0.1, respectively. Particle size was reported as the average of three readings of the volume-mean diameter (D43) (Arzeni et al. 2011).
Intrinsic fluorescence spectroscopy
The sample dispersions were prepared with phosphate buffer (10 mM, pH 7.0) to give a final concentration of 1 % (w/w). The intrinsic fluorescence spectra were obtained by a Hitachi F-2000 fluorescence spectrophotometer (Hitachi, Ltd., Tokyo, Japan). The excitation wavelength was 280 nm (Jiménez-Castaño et al. 2005), and the emission spectra was recorded from 300 to 450 nm with a slit width of 5 nm.
Rheological properties
Dynamic oscillatory rheological measurements were carried out in a Bohlin CVO rotational rheometer (Malvern Instruments, UK) using parallel plates (40 mm diameter, 1 mm gap), according to the method of Kontogiorgos et al. (2006) with a slight modification. The dispersions prepared by SPI (8 %, w/w) and sugar (8 %, w/w) were added with GDL (0.8 %, w/w) after heating at 95 °C for 15 min under the condition of non-ultrasonic and ultrasonic pretreatment. Following addition of GDL, the sample dispersions were placed into the configuration of rheometer immediately. Exposed edges of the dispersions were covered with a few drops of paraffin oil to prevent evaporation. The dispersions were acidified with GDL at 25 °C for 5 h and the pH was measured at different time. The continuous decrease in the pH of the dispersions had stabilized after 3 h. The storage modulus (G′) and loss modulus (G″) were measured continuously during the entire acidification treatment at a frequency of 1 Hz and a strain of 0.1 %. Preliminary strain sweep experiments showed that the rheological measurements were within the linear viscoelastic region.
Statistical analysis
All measurements were performed in triplicate. Analysis and graphic presentations were performed using OriginPro 8.5 software (OriginLab Corporation, Northampton, USA). Standard deviation was calculated for each measurement by Excel software (Microsoft Office 2003). For statistical treatment of data, significant differences (p < 0.05) among the data were determined by analysis of variance (ANOVA) using SPSS software version 17.0 (SPSS Inc., Chicago, IL).
Results and discussion
Degree of glycation (DG)
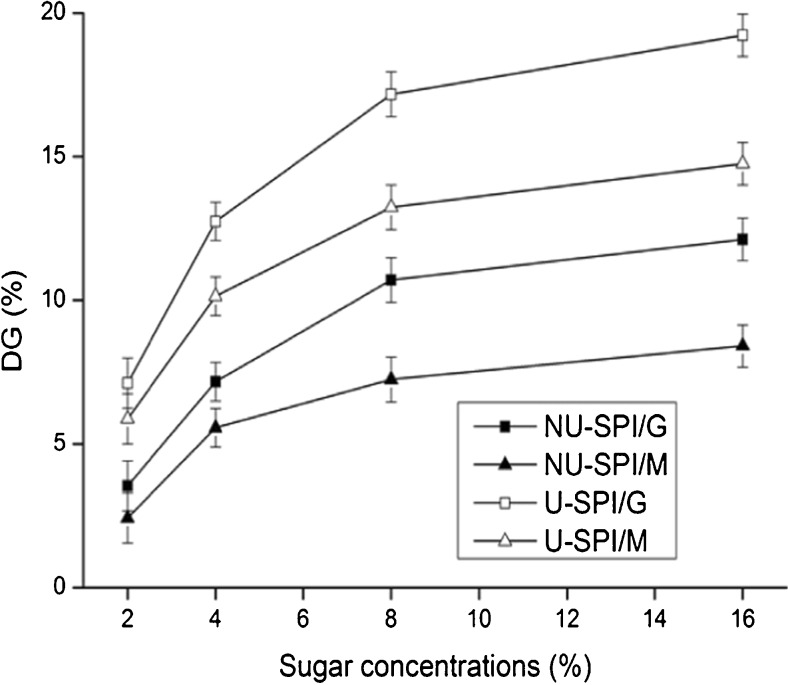
DG of SPI heated with glucose or maltose with and without ultrasonic pretreatment is shown in Fig. 1. DG value of SPI with or without ultrasonic pretreatment heated in the presence of glucose (SPI/G MRPs) was higher than that with maltose (SPI/M MRPs). The Maillard reaction rate was related to the size of reducing sugars involved in the reaction. Chevalier et al. (2001) showed that monosaccharides were more reactive with the amino groups of protein than disaccharides in Maillard reaction, which also explained why the DG value of SPI/G MRPs was higher than that of SPI/M MRPs.
Fig. 1.
Degree of glycation (DG) of non-ultrasonic and ultrasonic SPI/sugar MRPs. (■) represents non-ultrasonic SPI/G MRPs, (▲) represents non-ultrasonic SPI/M MRPs, (□) represents ultrasonic SPI/G MRPs, (△) represents ultrasonic SPI/M MRPs
Moreover, the DG value of SPI/sugar MRPs with ultrasonic pretreatment was higher than that without ultrasonic pretreatment. This result indicated that the Maillard reaction with ultrasonic pretreatment developed much faster than reaction without ultrasonic pretreatment. It is very likely that there was rapid molecule movement due to cavitation and unfolding of protein chains during ultrasonic treatment, leading to reactive groups to be brought into closer proximity (Kardos and Luche 2001), which were beneficial to the Maillard reaction between protein and sugars.
In addition, the DG value increased with the glucose or maltose concentration. The DG value of SPI/G and SPI/M MRPs increased rapidly in the range of sugar concentrations from 2 % to 8 %, whereas the increase in DG value was relatively slower at sugar concentrations higher than 8 %. It is possible that more sugars were accessible for interaction with ε-amino groups of lysine in protein as the increase of sugar concentrations, which would result in an improvement of the DG value. However, the glycation reaction reaches a saturation point due to the reduction of the number of ε-amino groups as the further increase in sugar concentrations (Achouri et al. 2005), leading to slower increase in DG value. Therefore, sugar concentration at 8 % was carried out in the subsequent experiments.
Browning intensity
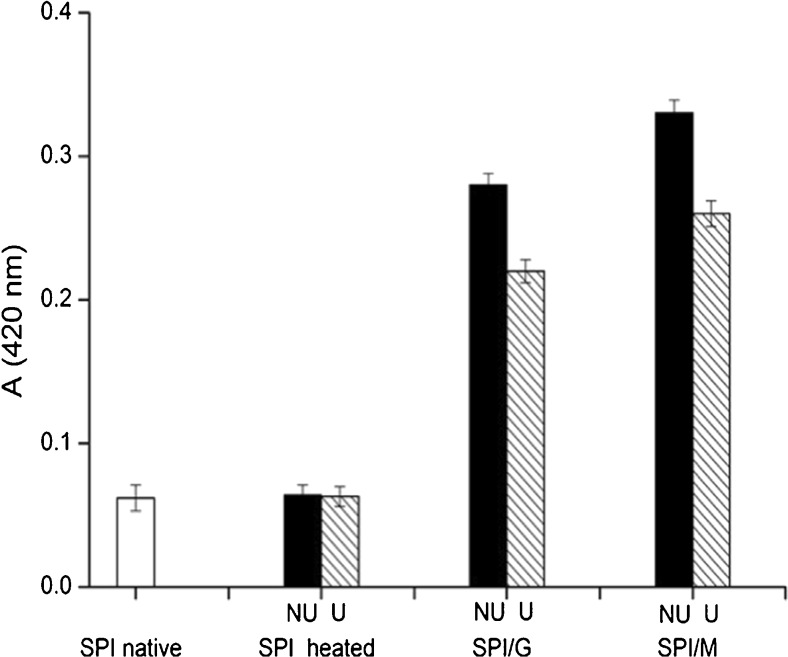
Browning intensity was measured by the absorbance at 420 nm and often used as an indicator of the Maillard reaction progress (Sun et al. 2011), which symbolized an advanced stage of the Maillard reaction.
Figure 2 shows the absorbance at 420 nm of SPI and SPI/sugar MRPs with and without ultrasonic pretreatment. Compared with SPI native, whether adopted ultrasonic pretreatment or not, the heat treatment of SPI without sugars (SPI heated) did not result in absorbance increase. However, absorbance of SPI/sugar MRPs increased and browning colour appeared after SPI was heated with sugars (glucose or maltose) due to formation of chromophores (Ajandouz et al. 2001), which indicated the occurrence of glycation. Moreover, except for the Maillard reaction, caramelisation of sugar could also occur during heat treatment, which contributed to non-enzymatic browning (Ajandouz et al. 2008).
Fig. 2.
Browning intensity of SPI and SPI/sugar MRPs under the condition of non-ultrasonic and ultrasonic pretreatment. Black represents non-ultrasonic (NU) pretreatment, hatched represents ultrasonic (U) pretreatment
The browning intensity of SPI/M MRPs was greater than that of SPI/G MRPs. It was possible that disaccharide formed more acids than monosaccharide during Maillard reaction with protein, leading to the decrease in pH, which had a positive effect on the Maillard reaction progress (Brands and Van Boekel 2002). This was similar to the results of Li et al. (2009), which studied the Maillard reaction products of rice protein with sugar.
UV-absorbing intermediate compounds were formed prior to generation of brown pigments during Maillard reaction. Browning pigments (melanoidins) were formed by polymerization of intermediate products (Wang et al. 2011). However, despite the higher DG value (Fig. 1), ultrasonic pretreatment decreased browning intensity of SPI/sugar MRPs, which revealed that ultrasonication might interfere with the formation of late MRPs melanoidins through inhibiting polymerization of intermediate products during Maillard reaction. The result indicated that ultrasonic pretreatment could prepare SPI/sugar MRPs with higher degree of glycation and lower browning.
Surface hydrophobicity (H0)
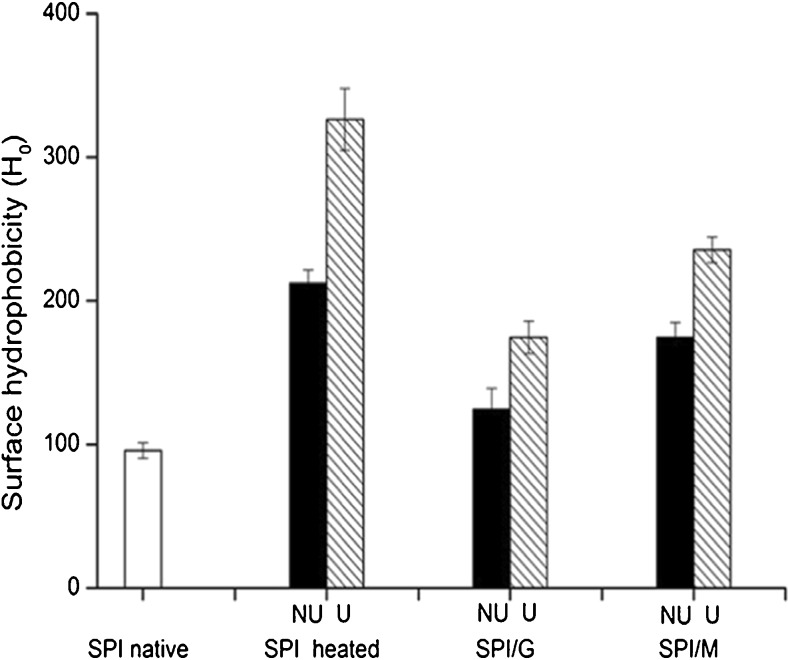
H0 is an index of the number of hydrophobic groups on the surface of a protein molecule in contact with the polar aqueous environment (Chandrapala et al. 2011). H0 increased as a function of exposing hydrophobic groups induced by denaturation of protein, which was an important factor influencing the functional properties of proteins.
The H0 of SPI and SPI/sugar MRPs under the condition of non-ultrasonic and ultrasonic pretreatment was presented in Fig. 3. Compared with SPI native, the H0 of non-ultrasonic heated SPI was increased. Most of hydrophobic residues were buried in the interior of the compact globular proteins (Hayakawa and Nakai 1985), leading to inaccessibility of the fluorescence probe (ANS) to the hydrophobic residues. However, the heat treatment could expose the hydrophobic residues from the interior to the surface of the molecule due to the heat denaturation of the protein (Kohyama et al. 1995), resulting in the increase of the H0.
Fig. 3.
Surface hydrophobicity (H0) of SPI and SPI/sugar MRPs under the condition of non-ultrasonic and ultrasonic pretreatment. Black represents non-ultrasonic (NU) pretreatment, hatched represents ultrasonic (U) pretreatment
The H0 decreased in SPI/sugar MRPs with respect to heated SPI, which suggested glycation had the protective effects against protein denaturation during heat treatment, reducing the exposure of hydrophobic residues to the surface of the protein molecule. The H0 of SPI/G MRPs was lower than that of SPI/M MRPs, indicating that a higher degree of glycation resulted in decreased hydrophobicity, as DG of SPI/G MRPs was higher than that of SPI/M MRPs (Fig. 1), which is consistent with the finding of Achouri et al. (2005) who suggested the decrease in hydrophobicity of 11S glycinin was connected with the increase in degree of glycation by glucose. Moreover, previous study also suggested that surface hydrophobic environment reduced more quickly as more polysaccharides were grafted with the protein (Mu et al. 2010).
In addition, the H0 of ultrasonic systems was higher than that of non-ultrasonic systems, revealing ultrasonic pretreatment could significantly increase the H0 of heated SPI and SPI/sugar MRPs, which was in agreement with the studies of Chen et al. (2011) who showed that ultrasonic treatment could lead to the increase in surface hydrophobicity of SPI. The increase in the H0 by ultrasonic pretreatment may be due to the cavitation and micro-streaming forces induced by ultrasound, resulting in the exposure of the hydrophobic regions that were originally buried within the molecular interior to the surface of the SPI molecule, according to the results of Hu et al. (2013a).
For the heated SPI, glycation would reduce the exposure of hydrophobic residues to the surface of the SPI molecule by protecting protein against heat denaturation, while ultrasonic pretreatment before the glycation could weaken this protection effect (SPI/G MRPs with higher DG) or expose more hydrophobic groups to the surface of the SPI molecule (SPI/M MRPs with lower DG). This finding indicated that appropriate ultrasonic treatment had more prominent influence on unfolding of heated SPI than glycation.
Particle size
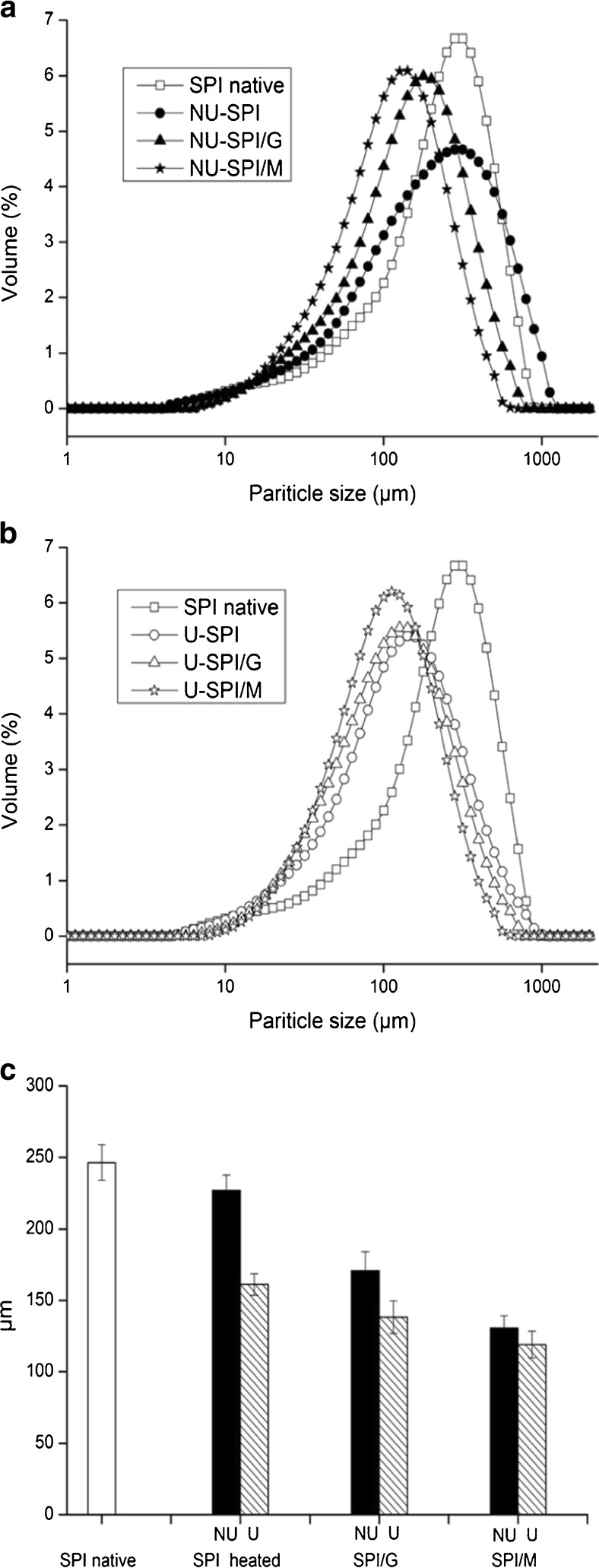
Particle size characterization of SPI and SPI/sugar MRPs was illustrated in Fig. 4. Glycation and ultrasonic treatment had a certain effect on particle size distributions of SPI. It was observed from Fig. 4a and b that SPI and SPI/sugars heated under the condition of non-ultrasonic and ultrasonic pretreatment showed a unimodal distribution of particles as the same to SPI native, and had more particles with smaller size than SPI native. Ultrasonic pretreatment changed the particle size distribution of heated SPI, which changed from broad distributions (Fig. 4a) to narrow distributions (Fig. 4b). However, the particle size distributions of ultrasonic SPI/sugar MRPs had no obvious changes with respect to non-ultrasonic SPI/sugar MRPs. This agrees with the results of Arzeni et al. (2011).
Fig. 4.
Particle size characterization of SPI and SPI/sugar MRPs. a: Particle size distribution of SPI and SPI/sugar MRPs under the condition of non-ultrasonic pretreatment. (□) represents SPI native, (●) represents non-ultrasonic heated SPI, (▲) represents non-ultrasonic SPI/G MRPs, (★) represents non-ultrasonic SPI/M MRPs. b: Particle size distribution of SPI and SPI/sugar MRPs under the condition of ultrasonic pretreatment. (□) represents SPI native, (○) represents ultrasonic heated SPI, (△) represents ultrasonic SPI/G MRPs, (☆) represents ultrasonic SPI/M MRPs. c: Effect of ultrasonic pretreatment on D43 (volume-mean diameter, μm) of SPI and SPI/sugar MRPs. Black represents non-ultrasonic (NU) pretreatment, hatched represents ultrasonic (U) pretreatment
In agreement with the particle distributions, heat treatment reduced the volume-mean diameter (D43) of SPI, from 246.40 ± 12.47 μm for SPI native to 226.91 ± 10.81 μm for heated SPI (Fig. 4c). It is reported that heat treatment had a significant effect on particle size distribution and average particle size of soymilks. It is possible that heat treatment might solubilize the large protein aggregates, resulting in the changes of particle size characterization (Nik et al. 2009).
No matter under the condition of non-ultrasonic or ultrasonic pretreatment, the D43 of SPI/sugar MRPs was lower than that of heated SPI. It is very likely that glycation might reduce the aggregation of protein molecules due to the protective effects of sugars against protein denaturation during the heat treatment, according to the results of Gu et al. (2009), which resulted in the decrease of SPI particle size. There were smaller size particles (D43) in SPI/M MRPs compared with SPI/G MRPs, revealing the molecular size of sugars would affect the particles size of SPI-glycoconjugates.
Ultrasonic pretreatment resulted in more remarkable reduction of the D43 for all systems. The decrease of particle size after ultrasonic pretreatments might be attributed to the cavitation and micro-streaming forces resulting from the ultrasound, which could disrupt noncovalent bonds between SPI molecules Hu et al. (2013c). It also might be probably ascribed to the dissociation of macromolecular protein by ultrasound during heating (Dong et al. 2011). This was in accord with the results of Jambrak et al. (2009) who had already reported that ultrasonic pretreatments could change the particle size of proteins.
Intrinsic fluorescence spectroscopy
The intrinsic fluorescence spectra were measured for the evaluation of conformational changes around tryptophan (Trp) residues in the proteins (Broersen et al. 2004).
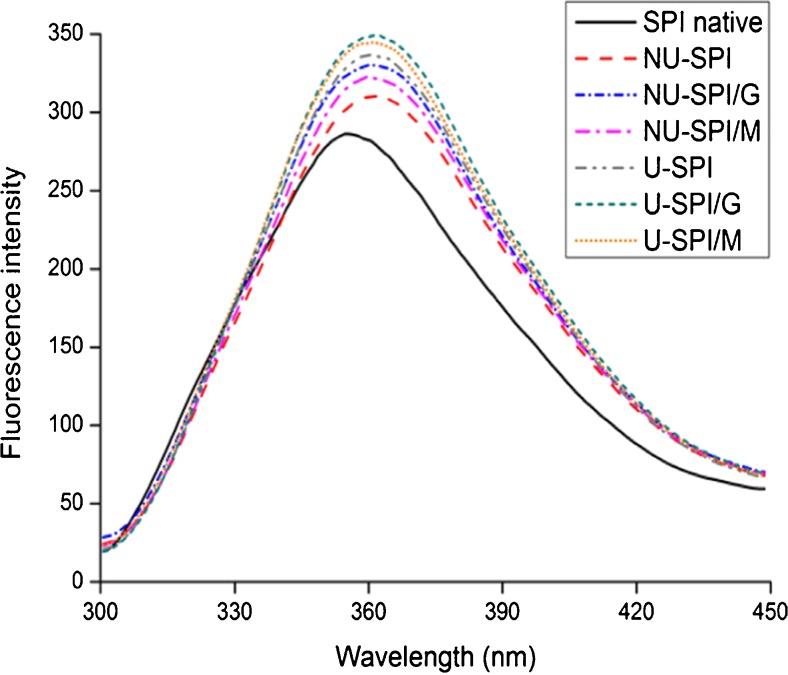
The intrinsic fluorescence spectra of SPI heated with glucose or maltose used in non-ultrasonic and ultrasonic pretreatment was presented in Fig. 5. Whether adopted ultrasonic pretreatment or not, the heat treatment of SPI without sugars (NU-SPI and U-SPI) increased fluorescence intensity with respect to SPI native. The changes in structure around the Trp residues induced by denaturation of protein during heating resulted in the increase of fluorescence intensity (Jiménez-Castaño et al. 2007). The fluorescence intensity of U-SPI was higher than that of NU-SPI, indicating ultrasonic pretreatment could promote thermal denaturation of protein, leading to the further changes of structure.
Fig. 5.
The intrinsic fluorescence spectra of SPI and SPI/sugar MRPs under the condition of non-ultrasonic and ultrasonic pretreatment. NU-SPI: non-ultrasonic heated SPI; NU-SPI/G: non-ultrasonic SPI/G MRPs; NU-SPI/M: non-ultrasonic SPI/M MRPs; U-SPI: ultrasonic heated SPI; U-SPI/G: ultrasonic SPI/G MRPs; U-SPI/M: ultrasonic SPI/M MRPs
It has been reported that development of fluorescent compounds might be related to Maillard reaction induced by heating (Jing and Kitts 2002). For the SPI/sugar MRPs, the fluorescence intensity of non-ultrasonic and ultrasonic SPI/sugars MRPs was higher than that of non-ultrasonic and ultrasonic heated SPI respectively, which indicated that glycosylation with sugars enhanced the content of fluorescent compounds. The increase of fluorescent compounds was more prominent in SPI/sugar MRPs used in ultrasonic pretreatment as compared to that used in non-ultrasonic pretreatment, which may be the result of high degree of glycation (Fig. 1). Additionally, compared with SPI/M MRPs, the higher fluorescence intensity could be obtained in SPI/G MRPs, which was related to spectrophotometric properties and fluorescent chemical structures of different glycoconjugates (Morales and van Boekel 1997). This is consistent with the results of Dragana et al. (2013).
Maximum wavelength (λmax) of the fluorescence emission for SPI without sugars was 354.4 nm (SPI native), 358.1 nm (NU-SPI) and 360.3 nm (U-SPI), respectively. While the λmax for SPI/sugar systems was 359.6 nm (NU-SPI/G), 358.7 nm (NU-SPI/M), 361.2 nm (U-SPI/G) and 360.6 nm (U-SPI/M), respectively. Compared with SPI native, the λmax of all systems increased, which indicated the occurrence of bathochromic shift of the λmax. This phenomenon could be attributed to conformational changes of protein and the polarity of the environment surrounding Trp residues, which would affect λmax (Jiménez-Castaño et al. 2005).
The λmax of U-SPI was greater than that of NU-SPI, revealing ultrasonic pretreatment could accentuate conformational changes of SPI. The bathochromic shift of the λmax for SPI/sugar MRPs suggested that the glycation has been changed the conformation of SPI (Kobayashi et al. 2001), and tertiary conformation in SPI became less compact after glycation. Moreover, SPI/sugar MRPs used in ultrasonic pretreatment had much less compact tertiary conformation than SPI/sugar MRPs used in non-ultrasonic pretreatment (Liu et al. 2011).
Rheological properties
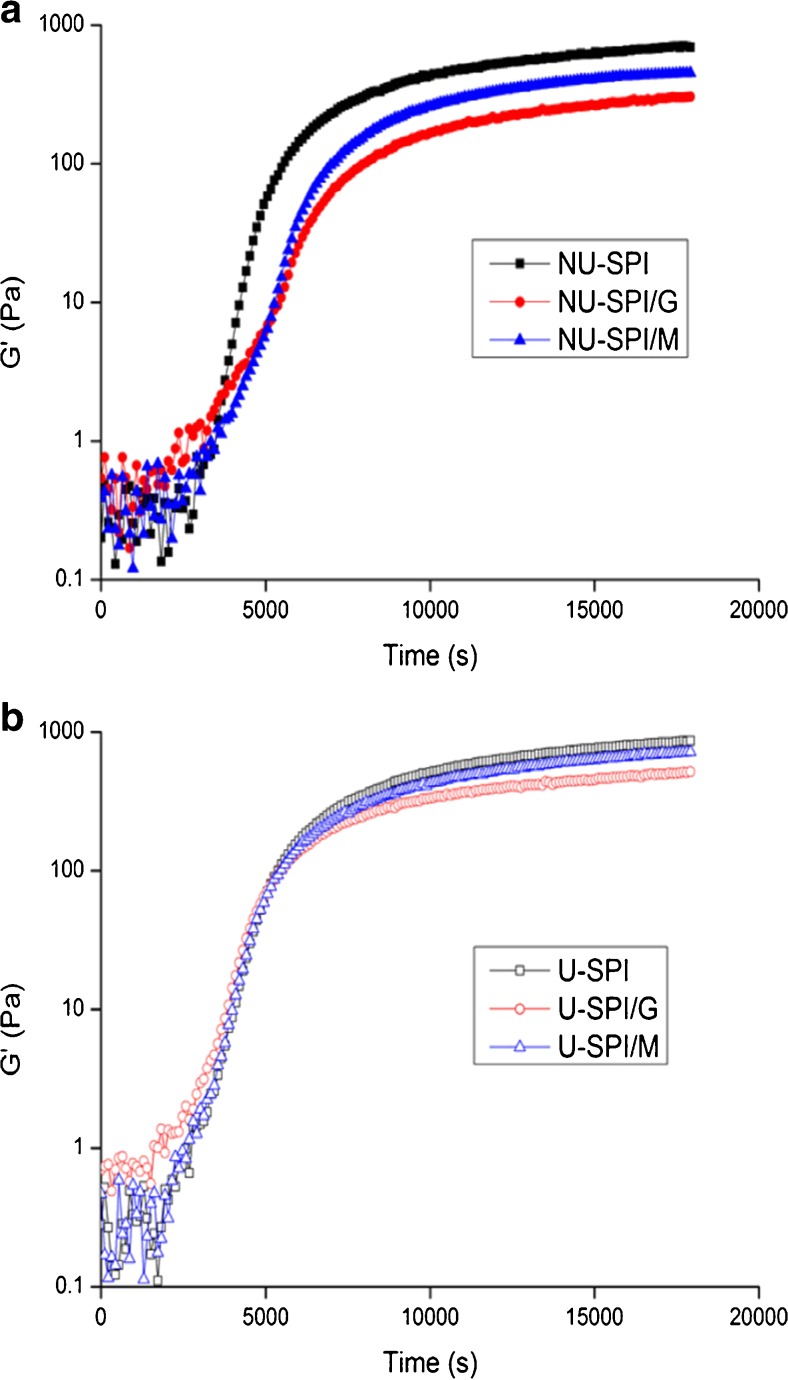
Change in storage modulus (G′) of SPI and SPI/sugar MRPs under the condition of non-ultrasonic and ultrasonic pretreatment during acidification was given in Fig. 6. Whether with or without ultrasonic pretreatment the development patterns of the storage modulus (G′) of SPI/sugar MRPs during acidification was similar to that of heated SPI, which was the same as the finding of Kontogiorgos et al. (2006). The G′ of all systems increased gradually with the extension of acidification time, reaching an equilibrium value eventually, which was related to the formation of acid gel by protein aggregation due to the reduction of pH near to the isoelectric point of SPI during acidification (Lazaridou et al. 2008).
Fig. 6.
Change in storage modulus (G′) of SPI and SPI/sugar MRPs under the condition of non-ultrasonic (a) and ultrasonic (b) pretreatment during acidification. (■) represents non-ultrasonic heated SPI, (●) represents non-ultrasonic SPI/G MRPs, (▲) represents non-ultrasonic SPI/M MRPs; (□) represents ultrasonic heated SPI, (○) represents ultrasonic SPI/G MRPs, (△) represents ultrasonic SPI/M MRPs
At the beginning of the acidification, under the condition of non-ultrasonic pretreatment, the G′ of SPI/sugar MRPs was higher than heated SPI. On the contrary, the G′ of SPI/sugar MRPs after acidification was lower than heated SPI (Fig. 6a), indicating the growth rate of G′ for SPI/sugar MRPs was slower than that for heated SPI, which was in agreement with the finding of Gu et al. (2009). Although the growth trend of G′ for heated SPI and SPI/sugar MRPs with ultrasonic pretreatment was similar to that without ultrasonic pretreatment, the differences in growth rate of G′ between heated SPI and SPI/sugar MRPs could be reduced by ultrasonic pretreatment (Fig. 6b).
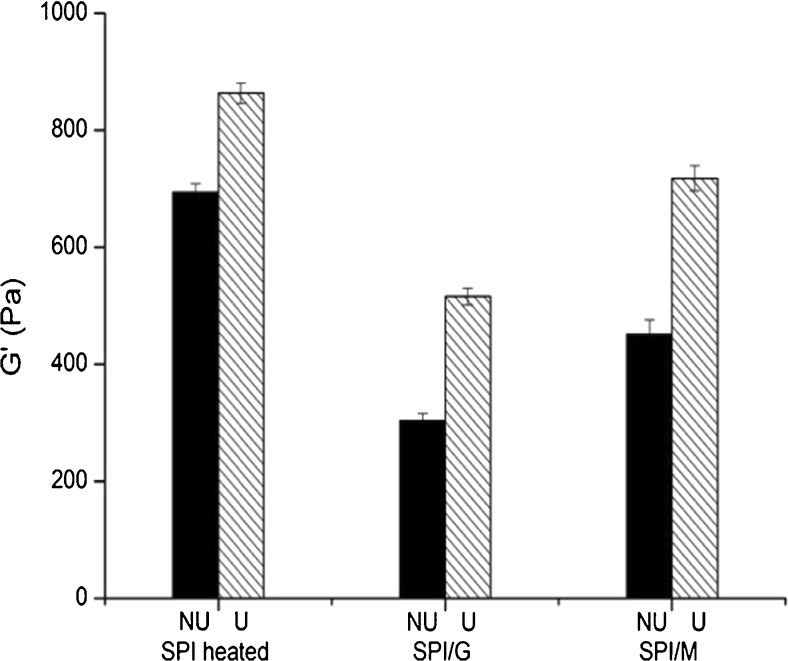
Storage modulus (G′) values of SPI and SPI/sugar MRPs under the condition of non-ultrasonic and ultrasonic pretreatment after acidification were illustrated in Fig. 7. With or without adopted ultrasonic pretreatment the G′ decreased in SPI/sugar MRPs with respect to heated SPI, which suggested glycation would weaken the gel network. It could be due to the protective effects of sugars against protein denaturation by increasing the initial temperature of heat denaturation during glycation, which decreased protein precipitation upon GDL acidification leading to softer gels induced by altering bond formation during gelation (Gu et al. 2009). However, Shevkani et al. (2015) showed that proteins from kidney bean and field pea with higher thermal denaturation temperature could produce stronger heat-induced gels, which was different from the results in this research. Similar results were obtained in a recent study on amaranth protein isolates (Shevkani et al. 2014). This difference could be caused by the difference between acid-induced and heat-induced gel mechanism. Moreover, the G′ of SPI/G MRPs was lower than that of SPI/M MRPs, which was associated with the degree of glycation (Fig. 1). It is possible that the protective effects against protein denaturation of glucose with higher DG were more obvious than that of maltose, resulting in the weakening of gel network. This is also consistent with the results of surface hydrophobicity (H0) (Fig. 3) in this study, which illustrated glucose had more remarkable protective effects against protein denaturation than maltose.
Fig. 7.
Storage modulus (G′) values of SPI and SPI/sugar MRPs under the condition of non-ultrasonic and ultrasonic pretreatment after acidification. Black represents non-ultrasonic (NU) pretreatment, hatched represents ultrasonic (U) pretreatment
However, the G′ of heated SPI and SPI/sugar MRPs with ultrasonic pretreatment was higher than that without ultrasonic pretreatment respectively, suggesting reinforce of gel network after ultrasonic pretreatment. In spite of higher degree of glycation (Fig. 1), ultrasonic pretreatment exposed the hydrophobic regions from the interior to the surface of the SPI molecules, as ultrasonic pretreatment increased surface hydrophbility of heated SPI and SPI/sugar MRPs (Fig. 3), which might be beneficial to the formation of gel network by hydrophobic interactions. In addition, the molecules of the ultrasonic systems were smaller than that of the non-ultrasonic systems, as ultrasonic pretreatments reduced the particle size of heated SPI and SPI/sugar MRPs (Fig. 4), which could make a contribution to acid-induced gel of SPI. This is in agreement with the results of Hu et al. (2013b), who demonstrated the reduction of particle size of SPI by ultrasonic pretreatments would strengthen the gel network. Furthermore, compared with non-ultrasonic heated SPI, although the gel network was weakened by glycation, ultrasonic pretreatments could reduce (ultrasonic SPI/G MRPs) or eliminate (ultrasonic SPI/M MRPs) this weakening effect of glycation on the gel network, and even improve the gel properties.
Conclusions
Ultrasonic pretreatment could accelerate the glycation between SPI and sugars (glucose and maltose) by Maillard reaction, which prepared SPI/sugar MRPs with higher degree of glycation and lower browning. Intrinsic fluorescence indicated that SPI/sugar MRPs prepared with ultrasonic pretreatment had less compact tertiary conformation than that with non-ultrasonic pretreatment. In addition, glycation was able to reduce H0 and particle size due to the protective effects of sugars against thermal denaturation of protein, which weakened the gel network formed by GDL. However, the cavitation and micro-streaming forces induced by ultrasound resulted in the increase of H0 and further decrease of particle size, which might be beneficial to the formation of acid-induced gel network, suggesting that ultrasonic pretreatments could reduce or eliminate the weakening effect of glycation on the gel network of SPI/sugar MRPs, and even improve the gel properties.
Acknowledgments
The authors are thankful to the financial support provided by Northeast Agricultural University Innovation Foundation for Postgraduate (Grant No. yjscx14059).
Footnotes
Highlights 1. SPI/sugar MRPs prepared using ultrasound had higher degree of glycation and lower browning
2. Ultrasound had more remarkable effects on structural changes of SPI/sugar MRPs
3. Glycation was able to reduce H0 and particle size of SPI
4. Ultrasound could increase H0 and further decrease particle size of glycated SPI
5. Ultrasound could reduce or eliminate the weakening effect of glycation on the gel network
References
- Achouri A, Boye JI, Yaylayan VA, Yeboah FK. Functional properties of glycated soy 11S glycinin. J Food Sci. 2005;70:C269–C274. doi: 10.1111/j.1365-2621.2005.tb07172.x. [DOI] [Google Scholar]
- Ajandouz EH, Tchiakpe LS, Dalle Ore F, Benajiba A, Puigserver A. Effects of pH on caramelization and Maillard reaction kinetics in fructose-lysine model systems. J Food Sci. 2001;66(7):926–931. doi: 10.1111/j.1365-2621.2001.tb08213.x. [DOI] [Google Scholar]
- Ajandouz EH, Desseaux V, Tazi S, Puigserver A. Effects of temperature and pH on the kinetics of caramelisation, protein cross-linking and Maillard reactions in aqueous model systems. Food Chem. 2008;107(3):1244–1252. doi: 10.1016/j.foodchem.2007.09.062. [DOI] [Google Scholar]
- Amarowicz R. Antioxidant activity of Maillard reaction products. Eur J Lipid Sci Technol. 2009;111(2):109–111. doi: 10.1002/ejlt.200900011. [DOI] [Google Scholar]
- Arzeni C, Martínez K, Zema P, Arias A, Pérez O, Pilosof A. Comparative study of high intensity ultrasound effects on food proteins functionality. J Food Eng. 2011;108(3):463–472. doi: 10.1016/j.jfoodeng.2011.08.018. [DOI] [Google Scholar]
- Brands CMJ, Van Boekel MAJS. Kinetic modeling of reactions in heated monosaccharide-casein systems. Food Chem. 2002;23:13–26. doi: 10.1021/jf011164h. [DOI] [PubMed] [Google Scholar]
- Broersen K, Voragen AGJ, Hamer RJ, de Jongh HHJ. Glycoforms of β-lactoglobulin with improved thermostability and preserved structural packing. Biotechnol Bioeng. 2004;86:78–87. doi: 10.1002/bit.20030. [DOI] [PubMed] [Google Scholar]
- Chandrapala J, Zisu B, Palmer M, Kentish S, Ashokkumar M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason Sonochem. 2011;18(5):951–957. doi: 10.1016/j.ultsonch.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Chen L, Chen J, Ren J, Zhao M. Effects of ultrasound pretreatment on the enzymatic hydrolysis of soy protein isolates and on the emulsifying properties of hydrolysates. J Agric Food Chem. 2011;59(6):2600–2609. doi: 10.1021/jf103771x. [DOI] [PubMed] [Google Scholar]
- Chevalier F, Chobert J-M, Popineau Y, Nicolas MG, Haertle T. Improvement of functional properties of β-lactoglobulin glycated through the Mailard reaction is related to the nature of the sugar. Int Dairy J. 2001;11:145–152. doi: 10.1016/S0958-6946(01)00040-1. [DOI] [Google Scholar]
- Chi YJ, Zhu XQ, Ling WB. Soy protein processing technology. Beijing: Science press; 2008. [Google Scholar]
- Dong X-Y, Guo L-L, Wei F, Li J-F, Jiang M-L, Li G-M, Zhao Y-D, Chen H. Some characteristics and functional properties of rapeseed protein prepared by ultrasonication, ultrafiltration and isoelectric precipitation. J Sci Food Agric. 2011;91(8):1488–1498. doi: 10.1002/jsfa.4339. [DOI] [PubMed] [Google Scholar]
- Dragana S-V, Ivana P, Danijela A, Milan N, Tanja CV. Structure and antioxidant activity of β-lactoglobulin-glycoconjugates obtained by high-intensity-ultrasound-induced Maillard reaction in aqueous model systems under neutral conditions. Food Chem. 2013;138:590–599. doi: 10.1016/j.foodchem.2012.10.087. [DOI] [PubMed] [Google Scholar]
- Gu X, Campbell LJ, Euston SR. Influence of sugars on the characteristics of glucono-δ-lactone-induced soy protein isolate gels. Food Hydrocoll. 2009;23:314–326. doi: 10.1016/j.foodhyd.2008.01.005. [DOI] [Google Scholar]
- Guan YG, Zhang BS, Yu SJ, Wang XR, Xu XB, Wang J. Effects of ultrasound on a glycin-glucose model system—A means of promoting Maillard reaction. Food Bioprocess Technol. 2011;4(8):1391–1398. doi: 10.1007/s11947-009-0251-6. [DOI] [Google Scholar]
- Gülseren I, Güzey D, Bruce BD, Weiss J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason Sonochem. 2007;14(2):173–183. doi: 10.1016/j.ultsonch.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Hayakawa S, Nakai S. Relationships of hydrophobicity and net charge to the solubility of milk and soy proteins. J Food Sci. 1985;50:486–491. doi: 10.1111/j.1365-2621.1985.tb13433.x. [DOI] [Google Scholar]
- Hu H, Wu JH, Li-Chan ECY, Zhu L, Zhang F, Xu XY, Fan G, Wang LF, Huang XJ, Pan SY. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013;30:647–655. doi: 10.1016/j.foodhyd.2012.08.001. [DOI] [Google Scholar]
- Hu H, Fan X, Zhou Z, Xu XY, Fan G, Wang LF, Huang XJ, Pan SY, Zhu L. Acid-induced gelation behavior of soybean protein isolate with high intensity ultrasonic pre-treatments. Ultrason Sonochem. 2013;20:187–195. doi: 10.1016/j.ultsonch.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Hu H, Li-Chan ECY, Wan L, Tian M, Pan SY. The effect of high intensity ultrasonic pre-treatment on the properties of soybean protein isolate gel induced by calcium sulfate. Food Hydrocoll. 2013;32:303–311. doi: 10.1016/j.foodhyd.2013.01.016. [DOI] [Google Scholar]
- Jambrak AR, Lelas V, Mason TJ, Kresic G, Badanjak M. Physical properties of ultrasound treated soy proteins. J Food Eng. 2009;93(4):386–393. doi: 10.1016/j.jfoodeng.2009.02.001. [DOI] [Google Scholar]
- Jiménez-Castaño L, López-Fandiño R, Olano A, Villamiel M. Study on β-lactoglobulin glycosylation with dextran: effect on solubility and heat stability. Food Chem. 2005;93:689–695. doi: 10.1016/j.foodchem.2004.09.050. [DOI] [Google Scholar]
- Jiménez-Castaño L, Villamiel M, Lopez-Fandiño R. Glicosilation of individual whey proteins by Maillard reaction using dextran of different molecular mass. Food Hydrocoll. 2007;21:433–443. doi: 10.1016/j.foodhyd.2006.05.006. [DOI] [Google Scholar]
- Jing H, Kitts DD. Chemical and biochemical properties of casein-sugar Maillard reaction products. Food Chem Toxicol. 2002;40(7):1007–1015. doi: 10.1016/S0278-6915(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Kardos N, Luche J-L. Sonochemistry of carbohydrate compounds. Carbohydr Res. 2001;332:115–131. doi: 10.1016/S0008-6215(01)00081-7. [DOI] [PubMed] [Google Scholar]
- Kato A, Nakai S. Hydrophobicity determined by a fluorescence probe methods and its correlation with surface properties of proteins. Biochim Biophys Acta. 1980;624:13–20. doi: 10.1016/0005-2795(80)90220-2. [DOI] [PubMed] [Google Scholar]
- Kim J-S, Lee Y-S. Effect of reaction pH on enolization and racemisation reactions of glucose and fructose on heating with amino acid enantiomers and formation of melanoidins as result of the Maillard reaction. Food Chem. 2008;108(2):582–592. doi: 10.1016/j.foodchem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Hirano A, Ohta A, Yoshida T, Takahashi K, Hattori M. Reduced immunogenicity of β-lactoglobulin by conjugation with carboxymethyl dextran differing in molecular weight. J Agric Food Chem. 2001;49:823–831. doi: 10.1021/jf000926q. [DOI] [PubMed] [Google Scholar]
- Kohyama K, Sano Y, Doi E. Rheological characteristics and gelation mechanism of tofu (soybean curd) J Agric Food Chem. 1995;43(7):1808–1812. doi: 10.1021/jf00055a011. [DOI] [Google Scholar]
- Kontogiorgos V, Ritzoulis C, Biliaderis CG, Kasapis S. Effect of barley β-glucan concentration on the microstructural and mechanical behavior of acid-set sodium caseinate gels. Food Hydrocoll. 2006;20:749–756. doi: 10.1016/j.foodhyd.2005.07.008. [DOI] [Google Scholar]
- Lazaridou A, Vaikousi H, Biliaderis CG. Impact of mixed-linkage (1-3, 1-4) β-glucans on physical properties of acid-set skim milk gels. Int Dairy J. 2008;18:312–322. doi: 10.1016/j.idairyj.2007.08.005. [DOI] [Google Scholar]
- Li Y, Lu F, Luo CR, Chen ZX, Mao J, Shoemaker C, Zhong F. Functional properties of the Maillard reaction products of rice protein with sugar. Food Chem. 2009;117:69–74. doi: 10.1016/j.foodchem.2009.03.078. [DOI] [Google Scholar]
- Liu Y, Zhao G, Ren J, Zhao M, Yang B. Effect of denaturation during extraction on the conformational and functional properties of peanut protein isolate. Innovative Food Sci Emerg Technol. 2011;12:375–380. doi: 10.1016/j.ifset.2011.01.012. [DOI] [Google Scholar]
- Morales FJ, van Boekel MAJS. A study on advanced Maillard reaction in heated casein/sugar solutions: fluorescence accumulation. Int Dairy J. 1997;7(11):675–683. doi: 10.1016/S0958-6946(97)00071-X. [DOI] [Google Scholar]
- Mu L, Zhao M, Yang B, Zhao H, Cui C, Zhao Q. Effect of ultrasonic treatment on the graft reaction between soy protein isolate and gum acacia and on the physicochemical properties of conjugates. J Agric Food Chem. 2010;58:4494–4499. doi: 10.1021/jf904109d. [DOI] [PubMed] [Google Scholar]
- Nik AM, Tosh SM, Woodrow L, Poysa V, Corredig M. Effect of soy protein subunit composition and processing conditions on stability and particle size distribution of soymilk. LWT Food Sci Technol. 2009;42(7):1245–1252. doi: 10.1016/j.lwt.2009.03.001. [DOI] [Google Scholar]
- Pinto MS, Léonil J, Henry G, Cauty C, Carvalho AF, Bouhallab S. Heating and glycation of β-lactoglobulin and β-casein: aggregation and in vitro digestion. Food Res Int. 2014;55:70–76. doi: 10.1016/j.foodres.2013.10.030. [DOI] [Google Scholar]
- Shevkani K, Singh N, Rana JC, Kaur A. Relationship between physicochemical and functional properties of amaranth (Amaranthus hypochondriacus) protein isolates. Int J Food Sci Technol. 2014;49:541–550. doi: 10.1111/ijfs.12335. [DOI] [Google Scholar]
- Shevkani K, Singh N, Kaur A, Rana JC. Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocoll. 2015;43:679–689. doi: 10.1016/j.foodhyd.2014.07.024. [DOI] [Google Scholar]
- Soria AC, Villamiel M. Effect of ultrasound on the technological properties and bioactivity of food: a review. Trends Food Sci Technol. 2010;21(7):323–331. doi: 10.1016/j.tifs.2010.04.003. [DOI] [Google Scholar]
- Spotti MJ, Martinez MJ, Pilosof AMR, Candioti M, Rubiolo AC, Carrara CR. Influence of Maillard conjugation on structural characteristics and rheological properties of whey protein/dextran systems. Food Hydrocoll. 2014;39:223–230. doi: 10.1016/j.foodhyd.2014.01.014. [DOI] [Google Scholar]
- Stanic-Vucinic D, Stojadinovic M, Atanaskovic-Markovic M, Ognjenovic J, Grönlund H, van Hage M. Structural changes and allergenic properties of β-lactoglobulin upon exposure to high-intensity ultrasound. Mol Nutr Food Res. 2012;56(12):1894–1905. doi: 10.1002/mnfr.201200179. [DOI] [PubMed] [Google Scholar]
- Sun W, Yu S, Yang X, Wang J, Zhang J, Zhang Y. Study on the rheological properties of heat-induced whey protein isolate-dextran conjugate gel. Food Res Int. 2011;44:3259–3263. doi: 10.1016/j.foodres.2011.09.019. [DOI] [Google Scholar]
- Vigo MS, Malec LS, Gomez RG, Llosa RA. Spectrophotometric assay using o-phthaldialdehyde for determination of reactive lysine in dairy products. Food Chem. 1992;44:363–365. doi: 10.1016/0308-8146(92)90269-8. [DOI] [Google Scholar]
- Wang XS, Tang CH, Li BS, Yang XQ, Li L, Ma CY. Effects of high-pressure treatment on some physicochemical and functional properties of soy protein isolates. Food Hydrocoll. 2008;22:560–567. doi: 10.1016/j.foodhyd.2007.01.027. [DOI] [Google Scholar]
- Wang HY, Qian H, Yao WR. Melanoidins produced by the Mail lard reaction: structure and biological activity. Food Chem. 2011;128(3):573–584. doi: 10.1016/j.foodchem.2011.03.075. [DOI] [Google Scholar]
- Wang W-Q, Bao Y-H, Chen Y. Characteristics and antioxidant activity of water-soluble Maillard reaction products from interactions in a whey protein isolate and sugars system. Food Chem. 2013;139:355–361. doi: 10.1016/j.foodchem.2013.01.072. [DOI] [PubMed] [Google Scholar]
- Zhu D, Damodaran S, Lucey JA. Formation of whey protein isolate (WPI)-dextran conjugates in aqueous solutions. J Agric Food Chem. 2008;56:7113–7118. doi: 10.1021/jf800909w. [DOI] [PubMed] [Google Scholar]