Abstract
Ozonated water and peracetic acid were tested as sanitizers to enhance the storability of fresh-cut melon cubes. Sanitizers were also combined with suitable packaging materials (polypropylene and polylactic acid based plastic films). Fresh-cut melon cubes were stored at 4 °C for up to 7 days. Ozonated water and peracetic acid treatments were given by dipping cubes into 0.8 ppm O3 and 100 ppm Tsunami 100™ solutions, respectively, for 3 min. Both sanitizers exhibited efficiency in reducing the total microbial counts on melon cubes (< 2 log CFU g−1). Respiratory activity and ethylene production were both affected by the interaction between the sanitizer and the packaging used. Carbon dioxide and oxygen reached 9.89 kPa and 12.20 kPa partial pressures, respectively, using peracetic acid treatment in combination with polypropylene film packaging, consequently developing off-odors starting from day 3. Strong color changes were noted in cubes stored in polylactic acid packaging after 7 days of storage, affecting the sensory quality of the melon cubes. Sensory evaluation (overall visual quality) indicated loss in flavor in the polypropylene packaging. The overall visual quality started to decline on 3rd day because of the development of translucency.Overall, the use of ozone in combination with polypropylene packaging provided the best solution to maintain the quality of melon cubes for up to 5 days of storage at 4 °C.
Keywords: Cucumis melo, Fourth range product, Quality maintenance, Ozone, Tsunami, Plastic film
Introduction
Freshly cut fruits and vegetables are highly perishable (Massantini and Salcini 2003), including fresh-cut melon both in mixed salad fruits or as loose pieces. Melon can become contaminated during preharvest and postharvest handling and packaging, transportation, distribution and/or immediately before consumption (Beuchat 1996). Cutting the melon may cause important changes in quality attributes such as color, sweetness, texture (Portela and Cantwell 1998), ethylene production, respiration rate (Aguayo et al. 2007), microbial growth (Aguayo et al. 2003) and aromatic volatile compounds (Beaulieu 2006).
The washing process, in which physical and chemical treatments are used to eliminate, or at least reduce, the population of pathogenic and spoilage microorganisms, is one of the most important processing operations (Moscetti et al. 2013). Chlorine-based washing systems have been widely used by the majority of fresh produce manufactures to reduce microbial contamination of fresh-cut vegetables with good efficiency (Sapers et al. 2001). In recent years, concern has been raised about potentially toxic and carcinogenic by-products that can be formed when chlorine reacts with organic compounds (Villanueva et al. 2004a). The use of hypochlorite-based systems for washing fresh products is already prohibited in various European Union countries (or especially Denmark and Germany) (Betts and Everis 2005).
Another effective system to sanitize fresh-cut produce is the use of a peracetic acid-based sanitizer. This sanitizing agent does not react with proteins to produce toxic or carcinogenic compounds (Holah et al. 1990). Peracetic acid has been suggested to act primarily on lipoproteins in the cell membrane (Leaper 1984), and it may be equally effective against outer-membrane lipoproteins, facilitating its action against Gram-negative cells. Due to its tolerance to many factors such as temperature, pH, water hardness and contamination from the soil, it is highly useful in treating horticultural produce (Artés et al. 2007). Park and Beuchat (1999) observed that 40 to 80 ppm peracetic acid was effective in significantly reducing Salmonella and Escherichia coli O157:H7 on the melon surface.
The use of ozonated water has been suggested as an interesting alternative to traditional sanitizers due to its efficacy at low concentrations and short contact times as well as its breakdown into non-toxic products (Carletti et al. 2013; Botondi et al. 2015b). The efficacy of ozonated water is closely related to ozone solubility, which increases as the temperature of water decreases (Bablon et al. 1991). The flow rate of ozone and contact time affect the transference of ozone to water, and appropriate mixing or turbulence increases bubble contact and solubilization in water. The purity and pH of water greatly affect the rate of ozone solubilization. Disinfection byproduct residues from oxidative reactions are generally considered to be innocuous (Suslow 2004). In 1997, the Electric Power Research Institute (EPRI) supported a generally recognized as safe (GRAS) classification of ozone as a disinfectant for foods when used at levels and for methods of application in accordance with good manufacturing practices. In 2001, the Food and Drug Administration (FDA 2001) approved the application of ozone as a direct food additive for the treatment, storage, and processing of foods in gas and aqueous phases.
Packaging technology is an efficient mechanism of reducing postharvest deteriorative processes in fresh products. Packaging using polymer films has been cited as one of the most suitable methods available to provide physical protection against mechanical damage and contamination, maintain the gaseous concentration in the produce surroundings and, thus, to control or moderate the processes responsible for quality degradation in fresh-cut produce. Currently, many types of plastic films are available for fruits and vegetables; it is important to select the most appropriate packaging film because a bad material shortens the shelf life of this type of food. Generally speaking, polyethylene (PE) or polypropylene (PP) bags and polyvinylchloride (PVC) trays are used for packaging purposes.
UE regulation no. 1580/2007 (European Commission 2007) introduces the use of eco-friendly packages for fruits and vegetables. When evaluating novel plastic polymers, it is important to consider polylactic acid (PLA), which is biodegradable and compostable, in accordance with EN 13432/2002 (UNI 2002), and is derived from renewable sources. PLA has been considered one of the best solutions for packaging materials (Rhim et al. 2007). Oriented PLA can be used as fresh food service containers (Auras et al. 2005). In spite of the major environmental advantages of biopolymers, the actual cost of these materials is a great obstacle to their use. However, EU policy on environmental protection, with incentives for companies using biodegradable materials, opens the potential to reduce the cost of this material. Passively modified atmospheric packaging in combination with active packaging using PLA-coated cardboard trays wrapped with a thin, low-density PE film significantly extends the shelf life of fresh tomatoes stored at 20 °C for 30 d (García-García et al. 2011). Furthermore, PE and PLA polymeric trays maintained the overall quality of fresh cut spinach stored in a cold room for 6 d at 4 °C, but spinach stored in PLA trays maintained its flavor longer (Botondi et al. 2015a).
The aim of this research is to evaluate ozone treatment compared to peracetic acid as standard treatment to preserve fresh-cut melons. In addition, storage in a modified atmosphere using PP and PLA bags, which are biodegradable and compostable and, are derived from renewable sources is evaluated.
Materials and methods
Sample preparation
Muskmelon fruits (Cucumis melo L. cv. Marcelo) were grown with no chemicals and were harvested from a farm in Central Italy in late June 2014. The fruits were immediately taken to the laboratory in appropriate thermal boxes, and a low temperature (4 ± 0.5 °C) was maintained throughout transportation. Muskmelons were preliminarily selected by size (13.0 ± 1.5 cm diameter), weight (920 ± 60 g) and external skin color (75 % yellowness), and only sound fruits were used. For each melon, the soluble solid content (SSC) was evaluated to obtain a uniform batch. Muskmelons were washed by dipping in chlorinated water (150 ppm) for 5 min and vigorous scrubbing with a brush. Fruits were manually peeled and cut into cubes (2.5 × 2.5 cm; corresponding to 20.0 ± 2.2 g per cube) using a stainless steel sharpened knife. Peeling and cutting were conducted under sanitary conditions. Cubes were randomly selected and assigned to 6 treatments of 3 replications each (approximately 200 g) and then maintained at 4 ± 0.5 °C for 30 min before sanitizing treatments. Tsunami-treated samples (TS) were dipped into a 100 ppm Tsunami 100™ solution (15.2 % peracetic acid and 11.2 % hydrogen peroxide) (Ecolab, Mendota Heights, MN) for 3 min at room temperature (20 ± 1 °C). Ozone-treated samples (O3) were dipped into 0.8-ppm ozonated water for 3 min at room temperature. An ozone generator YX-1000 (BNP, Guangzhou, China) was used for ozonation. Control samples were dipped into 50 ppm NaOCl solution at pH 6.0 for 3 min at room temperature.
After sanitizing treatments, samples were left to dry for 30 min at room temperature prior to packaging and storing at 4 ± 0.5 °C for 7 d in a dark cell. Thus, each sample was packaged using a low-permeability commercial plastic film (polypropylene, PP) and a fully biodegradable and compostable film (polylactic acid, PLA). PP and PLA were characterized by water vapor transmission rates (WVTR) of 23 g/m2 (23 °C, 60/70 % R.H.) and 40 g/m2 (23 °C, 85 % R.H.), respectively, and an oxygen permeability (OP) of 63 cm3/m2, 24 h, 1 bar (38 °C, 90 % R.H.) and 370 cm3/m2, 24 h, 1 bar (23 °C, 75 % R.H.), respectively.
Analytical measurements
All analyses were performed immediately prior to treatment (0 d) and at 1, 3, 5 and 7 d of storage. Measurements were performed on a total of 12 trays for each storage duration (3 replications per treatment).
The oxygen, carbon dioxide and ethylene partial pressures (kPa) in the tray headspace were monitored at 20 °C according to Massantini et al. 2009a. Oxygen and carbon dioxide partial pressures were monitored with an Oxycarb analyzer (Isolcell, Bolzano, Italy). The ethylene partial pressure was measured with a gas chromatograph (Fractovap 4200, Carlo Erba, Milan, Italy) equipped with a flame ionization detector (FID) and a 1-m long activated alumina column (80/100 mesh). A nitrogen carrier flow of 20 mL min−1 was used. Ethylene measurement was performed isothermally at 100 °C.
Flesh color was measured with a CM-2600d colorimeter (Konica Minolta, Osaka, Japan). Results were expressed according to the CIELab color space through the L* (luminance), h (hue angle, h = tang−1b*/a*) and C* (saturation index or Chroma, C* = [a*2 + b*2]1/2) coordinates. We also measured the color difference CIE 1976 (ΔE* = [ΔL*2 + Δa*2 + Δb*2]1/2) among the color data for each sample collected every sampling day from the beginning of the test (0 d). The value of ΔE* expresses the difference between two colors numerically as follows: imperceptible (ΔE* < 1), minimal (1 ≤ ΔE* < 2), just perceptible (2 ≤ ΔE* < 3), perceptible (3 ≤ ΔE* < 5), strong difference (5 ≤ ΔE* < 12) and different color (ΔE* ≥ 12) (Massantini et al. 2009b).
Flesh firmness was determined with a digital penetrometer model 53,205 (TR Turoni & Co., Forlì, Italy) provided with an 8-mm tip; results are expressed as the maximum force (N) required the 7-mm probe to penetrate the melon pulp.
The soluble solid content was measured as Brix degrees with a WM-7 digital refractometer (Atago, Tokyo, Japan) and official ‘Refractometer Method for fruit and fruit products’ (AOAC 932.12) was followed (Horwitz and Latimer 2005).
To determine microbial growth during storage, samples were homogenized in 90 mL of sterile peptone-buffered water in a sterile bag with a Stomacher model 4153–50 (International PBI, Milan, Italy) for 2 min. Serial dilutions were prepared in peptone saline solution, as needed, for plating. Mesophilic aerobic bacteria, mold and yeast were quantified. The following media and incubation conditions were used for each microbial group: plate count agar (Merck, Darmstadt, Germany) for mesophilic aerobic bacteria, incubated at 30 °C for 48 h and 7 °C for 10 d, respectively, and potato dextrose agar (Merck, Darmstadt, Germany) with oxytretracycline (0.1 g L1) for yeast and mold, incubated at 22 °C for 3 and 7 d, respectively. Microbial counts were expressed as log CFU g−1. The microbial quality of the product was evaluated based on a maximum of 7 log CFU g−1 for mesophilic bacteria, 5 log CFU g−1 for yeast, and 3 log CFU g−1 for mold (Silveira et al. 2008).
Sensory evaluation was performed by a group of 10-trained judges. The members of the panel (6 women and 4 men; aged 20–35) were trained to recognize and score the quality attributes of fresh and stored processed melon samples. Melon cubes were randomly selected from each package. The following sensory parameters were assessed: overall visual quality (OVQ), flavor, translucency and off-odor. OVQ and flavor were assessed using a 10-point scale, where 0 = inedible, 3 = poor, 5 = fair, 7 = good and 9 = excellent. Off-odor and translucency were scored on a 0–5 scale, where 0 = none, 3 = moderate and 5 = severe.
Statistical analyses
A three-factor analysis of variance (ANOVA) was performed to evaluate the main effect of sanitizer, packaging and storage period, and the interaction of factors. The least significant difference (LSD) was calculated for an appropriate level of interaction (p ≤ 0.05). Results are reported as the mean and standard error of the mean. R statistics software ver. 3.0.2 with package ‘agricolae’ ver. 1.1–6 was used. Sensorial data were subjected to hierarchical clustering using the Wards method following the objective of identifying agglomerates by treatment and packaging used as well as days of storage.
Results and discussion
Microbial analysis
Fresh-cut melon is a fertile environment for pathogenic and spoilage microorganisms, so the sanitizing process is one of the most important procedures ensuring the microbial safety of the product. In our experiment, all sanitizing treatments reduced the initial total count of mesophilic bacteria, mold and yeast at counts significantly lower than 2.2, 2.5 and 1.0 log CFU g−1, respectively. However, none of the adopted sanitizers or packages hindered the microbial growth more than another, except for control samples (chlorine treatment). Moreover, the trend in microbial count during storage was consistent with results reported by several authors on fresh-cut melon (Luna-Guzmán and Barrett 2000; Silveira et al. 2008), confirming the effectiveness and robustness of both ozonated water and Tsunami 100™ as sanitizers.
Specifically, the microbial load of both mold and yeast was not affected by ozonated water, Tsunami 100™ and type of packages because no significant differences were observed on different days of storage. Specifically, at 0 d the counts for both sanitizers were lower than 0.8 log CFU g−1 and at 7 d the values ranged between 1.1 and 1.5 log CFU g−1, which were under the limits allowed by the European Community legislation (European Commission 2005). On the contrary, control samples (chlorine treatment) did not fit the market at day 5 (data not shown).
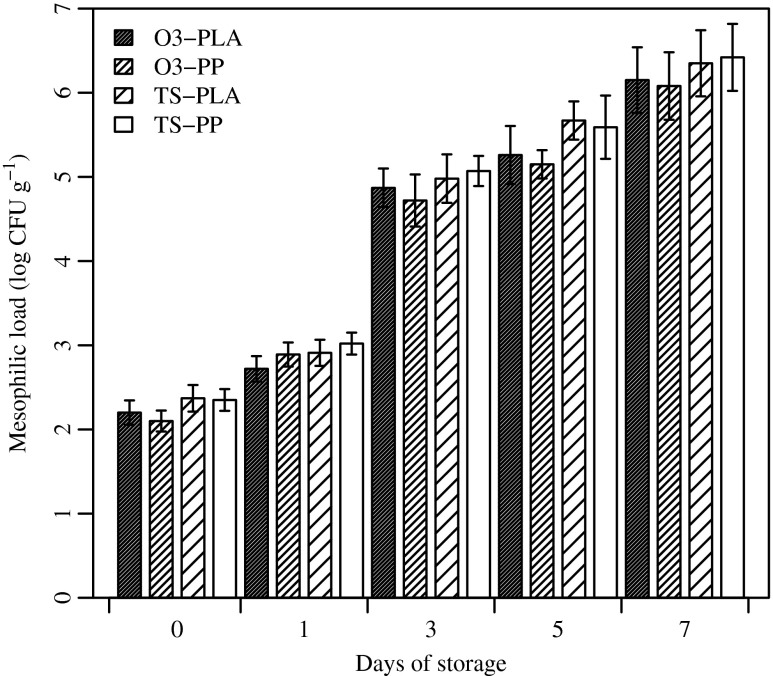
Figure 1 shows the growth of aerobic mesophilic bacteria on fresh-cut melon up to 7 d of storage. On day 3, mesophilic counts were approximately 5 log CFU g−1 in all samples, except for control samples (chlorine treatment) that showed a count higher than 6.0 log CFU g−1 (data not shown). Toxins may be metabolized at a microbial count higher than 6.0 log CFU g−1 (Wu et al. 2012). Thus, after a 5-d storage period, only fresh-cut melon cubes treated by ozonated water in both PLA and PP packages were not significantly over 6 log CFU g−1 (p ≤ 0.01). In contrast, on day 7, the mesophilic count exceeded 6.0 log CFU g−1 in all samples, resulting in potential health risks for the consumer.
Fig. 1.
Mesophilic load (log CFU g-1) of fresh-cut melon sanitized with ozonated water (O3) and Tsunami 100TM (TS) and packaged with polylactic acid (PLA) or polypropylene (PP) films. Vertical bars represent the standard error of the means (n = 5)
Package atmosphere composition
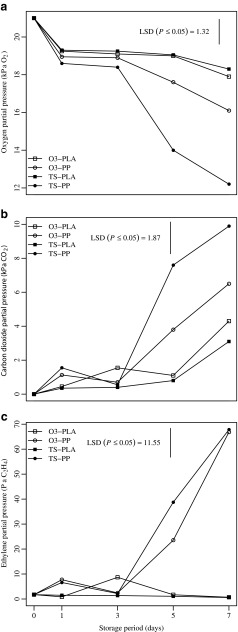
The trends of oxygen and carbon dioxide partial pressures in the tray headspace are shown in Fig. 2a and 2b. After a 1-d period of adjustment between the respiration and permeation rates, a steady state was established inside the package. In several treatments, the headspace composition started to change starting at day 5, as also observed by Silveira et al. (2011), due to a significant interaction between main factors (i.e., ‘sanitizer’ and ‘packaging’) (p ≤ 0.05). As expected, the physiological activity of melon cubes and microbial count both rising contributed to the headspace composition (Luna-Guzmán and Barrett 2000), which was also affected by the gas permeability of the package. The oxygen and carbon dioxide partial pressures progressively decreased and increased, respectively, with larger changes in the PP-wrapped trays.
Fig. 2.
Evolution of oxygen (a), carbon dioxide (b) and ethylene (c) partial pressures in the headspace of packages containing fresh-cut melon cubes dipped in ozonated water (O3) or Tsunami 100TM (TS) and sealed using polylactic acid (PLA) or polypropylene (PP) films. Samples were stored at 4 °C up to 7 d. Vertical bars indicate the Least Significant Difference (LSD) (n = 5)
In detail, the oxygen concentration was significantly reduced to below 18 kPa after 3 d of storage in the PP package, which also showed the lowest partial pressure when combined with TS sanitizer (Fig. 2a). In contrast, the oxygen level was maintained above 18 kPa for the PLA package regardless of the sanitizing treatment used. The highest carbon dioxide concentrations (between 6 and 10 kPa) were observed after 3 d of storage in the PP packages (Fig. 2b). The carbon dioxide level never exceeded partial pressures of 15 kPa, which was indicated by Bai et al. (2003) as a safe threshold to prevent fermentation in fresh-cut melon.
In fresh-cut melon, oxygen consumption and carbon dioxide production may be affected by microbial growth and senescence of tissue (Villanueva et al. 2004b). In our experiment, samples exhibited major changes in oxygen and carbon dioxide partial pressures when treated with the TS sanitizer and PP packaging. TS-PP samples had an average headspace composition of 12.20 kPa O2 and 9.89 kPa CO2 during storage at 4 °C up to 7 d. These results led us to speculate that the microbial count was related to increased respiration during storage; however, both the sanitizer and package were also involved in changes in physiological activity.
Ethylene production confirmed the aforementioned hypothesis by showing that fresh-cut melon stored in PP packaging accumulated the highest amount of ethylene after day 3, thereby indicating possible increases in physiological activity (Fig. 2c). This finding could be related to possible deterioration of the tissue due to senescence, in accordance to the results reported by Aguayo et al. (2004). However, PLA samples did not show significant changes in ethylene partial pressure during storage. Moreover, the sanitizer used did not significantly affect the evolution of ethylene concentration in the headspace throughout the storage period.
Finally, PLA packaging exhibited low carbon dioxide and ethylene concentrations into the package, probably due to the high gas permeability of the plastic film.
In this study, the use of alternative sanitizers to chlorine paired with MAP and low temperature allowed to extend shelf life of fresh-cut Marcelo melon.
Soluble solid content (SSC)
There was no significant difference among the samples initial values of the SSC of melon cubes (approximately 13.56° Brix). A very slight increase in SSC was noted during storage (p ≤ 0.05), probably as consequence of water loss (data not shown). Changes in SSC were affected by package material, with the highest average value of 13.93 °Brix for PLA samples (p ≤ 0.05), which were characterized by a high WVTR. Finally, the sanitizing treatment showed no significant effect on SSC (p = 0.74).
Color measurement
Appearance is a primary criterion for the quality evaluation of food (Kays 1999). In this context, color perception represents a discriminant factor for food choice, preference and acceptability. Moreover, color may have a fundamental role in the perception of flavor and texture of food (Meilgaard et al. 2006).
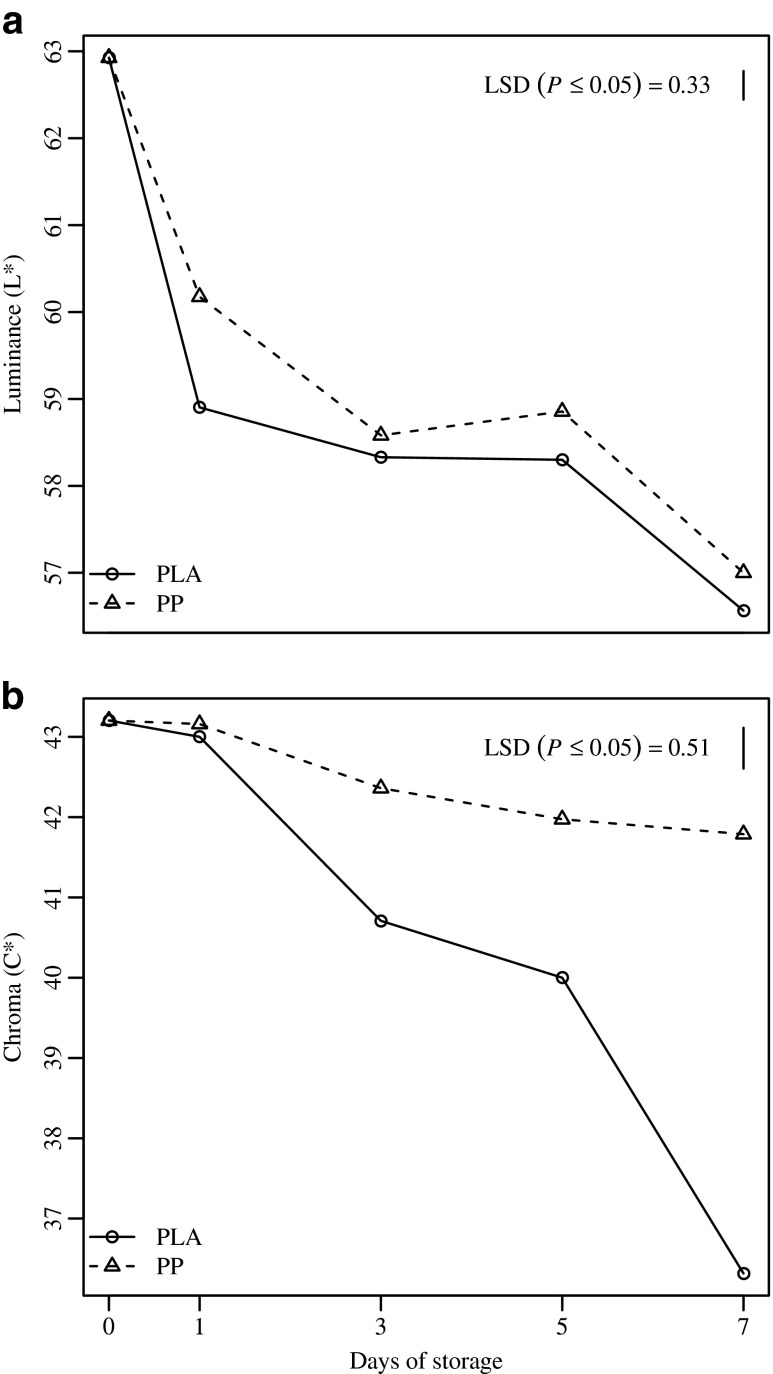
In terms of melon cube color, Fig. 3 shows that the L* and C* values of all samples decreased with increasing storage time. No differences in color were observed between sanitizer treatments (data not shown), while both L* and C* exhibited a significant interaction between ‘storage time’ and ‘packaging’ factors (p ≤ 0.05), showing the lowest values for the PLA samples. At day 0, luminance data were of 62.93 ± 0.69, dropping noticeably to 61.84 ± 0.62 and 56.56 ± 0.53 at the end of the storage for PP and PLA packages, respectively. Chroma data ranged from 43.20 ± 0.27 to 41.79 ± 0.64 and 36.31 ± 0.79 for PP and PLA samples, respectively. A significant decrease in luminance and Chroma in melon cubes could be a clear symptom of translucency development, the water-soaked aspect of the cubes, and therefore a loss in quality appearance (Beaulieu et al. 2011).
Fig. 3.
Luminance (a) and chroma (b) trends in fresh-cut melon cubes packaged using polylactic acid (PLA) or polypropylene (PP) films. Samples were stored at 4 °C up to 7 d. Vertical bars indicate the Least Significant Difference (LSD) (n = 5)
Hue angle (h) can be a symptom of ripening process or browning appearance. In our experiment, the hue angle did not show any changes during storage (data not shown). The sanitizers used in this study are strong oxidizing agents and thus are capable of interfering with the activity of enzymes related to the browning of the tissue, such as polyphenol oxidase (Silveira et al. 2008).
The CIELab color difference (∆E*) allows precise measurement and better understanding of the color development of melon cubes that occurred during storage. The results show that samples with ∆E* values over the ‘strong’ color difference threshold on the seventh day of storage were in PLA packaging. In fact, the ∆E* of PLA samples was 9.44 (a ‘strong difference’ in color), while PP samples had a value of 2.52 (a ‘perceptible difference’ in color). Results confirmed that packages with higher vapor transmission rate and gas permeability caused the higher loss in the typical color of melon cubes.
Firmness
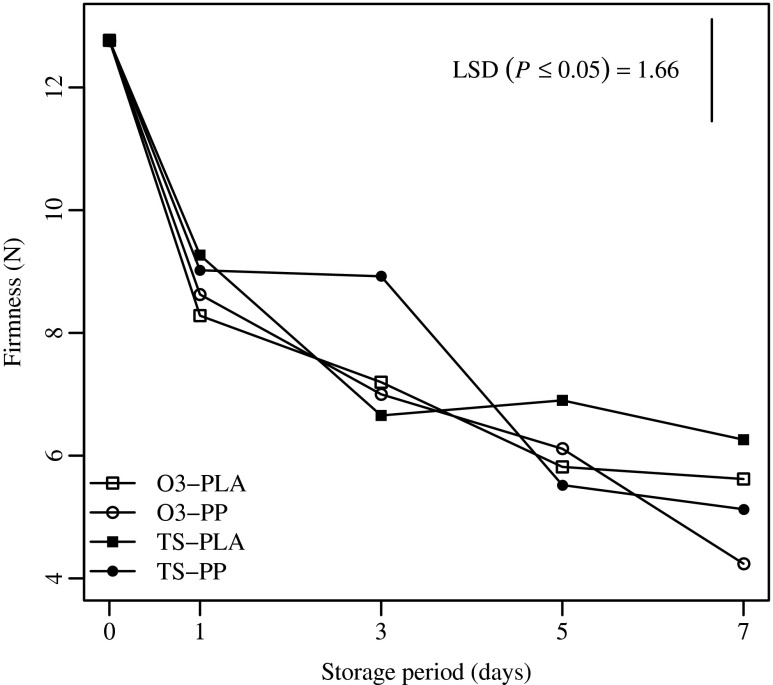
Firmness significantly declined during storage for all treatments (p ≤ 0.01). No ‘sanitizer’ or ‘packaging’ main effects were observed (p = 0.15 and p = 0.85, respectively). As shown in Fig. 4, the firmness gradually decreased by 58.34 % at the end of the storage, reaching the minimum average value of 5.38 ± 0.45 N. The change in firmness was higher than that observed by Silveira et al. (2008) and Aguayo et al. (2003), who reported losses of firmness of approximately 43.57 % and 21.7 % in fresh-cut ‘Galia’ and ‘Amarillo’ melon cultivars, respectively, stored at 5 °C for 10 d. Thus, the firmness trend of fresh-cut melon is also affected by cultivar.
Fig. 4.
Firmness of fresh-cut melon cubes dipped in ozonated water (O3) or Tsunami 100TM (TS) and packaged using polylactic acid (PLA) or polypropylene (PP) films. Samples were stored at 4 °C up to 7 d. Vertical bars indicate the Least Significant Difference (LSD) (n = 40)
In general, the internal tissue in fresh-cut melon lacks the protection of the typical net or waxy pericarp of whole melon, making it highly susceptible to softness (Aguayo et al. 2004). In addition, loss of firmness in fresh-cut melon is strictly related to modifications in pectic and hemicellulosic polysaccharides as well as net loss of non-cellulosic neutral sugars. Softening is usually associated with enzymatic activity, such as galactosidase, endo-polygalacturonase, and/or exo-galactosidase (Botondi et al. 2000). Specifically, endo-polygalacturonase plays an important role in postharvest fruit decay caused by microorganisms. Microbial growth is affected by the gas composition in a modified atmosphere packaging (MAP) system. Thus, under inappropriate gas composition, spoilage might be associated with undesirable changes in firmness. MAP techniques have the potential to delay and arrest microbial spoilage and consequently may reduce the softening of fresh-cut melon (Aguayo et al. 2004). Nevertheless, several authors (Amaro et al. 2012; Botondi et al. 2014) reported controversial results on the use of MAP for the storage of fresh-cut melon, with no effect on delaying the natural softening of the product.
Finally, the sanitizers and packaging tested in this study did not produce different changes in firmness among samples, probably because of the lack of difference in microbial growth. However, PP samples tended to have lower firmness at the end of the storage period.
Sensory analysis
Shelf life based on OVQ is mainly affected by the development of translucency, while the end of aroma quality is affected by the development of a rancid off-odor (Bai et al. 2003). In our experiment, remarkable changes in the sensory parameters of melon pieces were observed as consequence of storage (Table 1). Moreover, the results indicate that the overall visual quality and translucency were not affected by the sanitizer or packaging, while the flavor intensity and off-odor showed a statistically significant effect due to for packaging (p ≤ 0.05) and sanitizer × packaging × storage time interaction (p ≤ 0.01), respectively. It is important to note that no significant difference in sensory profile was observed immediately after sample preparation (0 d).
Table 1.
Effect of sanitization treatments, packaging and storage on sensory attributes of fresh-cut melon stored at 4 °C for 7 d. Results are reported as the mean ± standard deviation of the mean
| Main factors | OVQ | Flavor | Translucency | Off-odor | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sanitized (S) | |||||||||||||||
| Ozone | 7.08 | ± | 1.94 | 6.13 | ± | 2.05 | 1.91 | ± | 0.22 | 1.78 | ± | 0.72 | |||
| Tsunami | 7.07 | ± | 1.98 | 6.47 | ± | 1.88 | 1.85 | ± | 0.32 | 1.84 | ± | 0.74 | |||
| Packaging (P) | |||||||||||||||
| PLA | 7.03 | ± | 2.02 | 6.73 | ± | 1.05 | a | 1.94 | ± | 0.19 | 1.76 | ± | 0.79 | ||
| PP | 7.13 | ± | 1.89 | 5.87 | ± | 0.97 | b | 1.82 | ± | 0.15 | 1.87 | ± | 0.66 | ||
| Storage Time (T) | |||||||||||||||
| 0 | 9.00 | a1 | 9.00 | a | 1.00 | a | 1.00 | ||||||||
| 1 | 8.75 | ± | 0.40 | a | 6.75 | ± | 0.95 | b | 1.20 | ± | 0.45 | a | 1.50 | ± | 0.58 |
| 3 | 6.71 | ± | 0.10 | b | 5.66 | ± | 0.98 | c | 2.23 | ± | 0.62 | b | 1.71 | ± | 0.34 |
| 5 | 5.76 | ± | 0.31 | c | 5.55 | ± | 0.43 | c | 1.98 | ± | 0.67 | b | 2.05 | ± | 0.51 |
| 7 | 4.93 | ± | 0.17 | d | 4.54 | ± | 0.76 | d | 3.21 | ± | 0.46 | c | 2.81 | ± | 0.24 |
| Interaction | |||||||||||||||
| S x P x T | NS | NS | NS | ***2 | |||||||||||
1Different letters indicate significant differences at p ≤ 0.05
2*** indicate significance at p ≤ 0.01
Melon cubes had excellent overall visual quality up to day 1. Subsequently, the OVQ gradually declined starting from day 3 because of translucent areas. Translucency development was previously reported by Bai et al. (2003). They observed a relationship between translucency and oxygen partial pressure inside packages greater than 15 kPa. Translucency might also be due to an increase in microbial activity, although all melon pieces were free of decay at day 5 of storage. The OVQ score approached the limit of marketability (5 = fair) at day 7 at a value of 4.93 ± 0.17.
Flavor was notably lower in PP-packaged samples (5.87 ± 0.97) than in PLA-packaged samples (6.73 ± 1.05). PP wrapping may be responsible for low biosynthesis in esters and poor flavor intensity as consequence of the lower oxygen level in the PP package (Amaro et al. 2012). The sanitizer did not affect the loss of flavor, while the score decreased within the first few days of storage in all samples. The trend in flavor loss was similar to that reported for OVQ. In fact, the flavor score was close to the marketability threshold of 5 at day 7 of storage (4.54 ± 0.76). However, the flavor was compromised before visual quality in accordance with Beaulieu and Baldwin (2002). Moreover, the Tsunami 100™ sanitizer in combination with polypropylene wrapping (TS-PP) developed off-odors starting from day 3 (2.76 ± 0.63), while other samples showed a slight production of off-odor up to the end of storage (ranged between 2.0 and 2.2). The cause of the off-odor development was not identified but was often associated with samples subjected to the Tsunami-dipping treatment.
Fresh-cut melon may develop loss of freshness within few days of refrigerated storage (Beaulieu 2006). Thus, as expected, the shelf life limit for fresh-cut melon needs to take into account the synergistic interaction between post cutting treatment and packaging on flavor and sensory acceptability. Flavor quality is a major driving force within the fresh-cut industry, so flavor deterioration should be minimized. The flavor of fresh produce does not improve during postharvest life. Therefore, it is also necessary to consider the initial product variability expected from various melon cultivars growing in different regions, crop years and agro-pedo-climatic conditions.
Finally, cluster analysis showed 4 primary groups that were mainly distinguished by days of storage (Fig. 5), thus demonstrating that the Tsunami treatment in combination with the polypropylene significantly affected the storability of the melon cubes. In fact, the pattern showed that the TS-PP samples at both 3 and 7 d of storage were uniquely preserved by clustering with the sample group belonging to the 1 d of storage (Cluster 2).
Fig. 5.
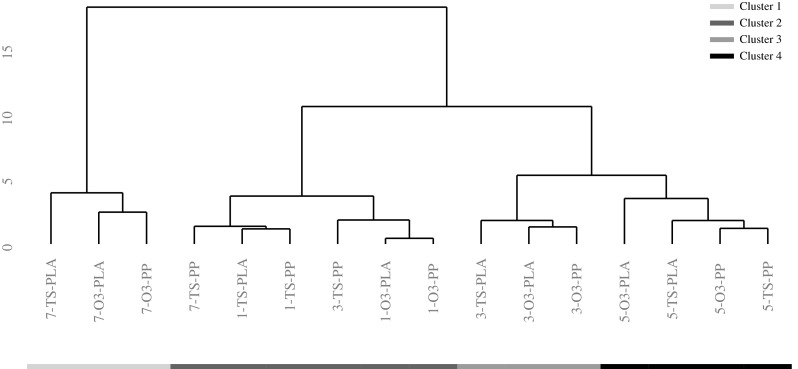
Dendrogram plot of fresh-cut melon samples dipped in ozonated water (O3) or Tsunami 100TM (TS), packaged using polylactic acid (PLA) or polypropylene (PP) films and stored up to 7 d at 4 °C. The numeral prefix represents the day of storage (1, 3, 5 or 7) while the grey-colored bottom bar represents the clustering groups
Conclusions
Both sanitizers effectively achieved the same results in terms of initial microbial load, outperforming control treatments with chlorine. However, after 5-days storage period, the microbial count exceeded 6.0 log CFU g−1 in all TS samples, creating potential health risks to the consumer. Other significant differences among samples were noted as a consequence of the use of different packaging, where PLA had a light barrier effect against gas produced during the respiratory activity. Melon cubes stored using the PLA exhibited higher luminance and Chroma loss, corresponding to the development of a ‘strong difference’ in color after 7-d storage. PP packaging was used in combination with ozonated water of melon cubes showed minor loss in flavour.
References
- Aguayo E, Allende A, Artés F. Keeping quality and safety of minimally fresh processed melon. Eur Food Res Technol. 2003;216:494–499. [Google Scholar]
- Aguayo E, Escalona VH, Rtés FA. Metabolic behavior and quality changes of whole and fresh processed melon. J Food Sci. 2004;69:148–155. [Google Scholar]
- Aguayo E, Escalona VH, Artés F. Quality of minimally processed Cucumis melo var. saccharinus as improved by controlled atmosphere. Eur J Hortic Sci. 2007;72:39–45. [Google Scholar]
- Amaro AL, Beaulieu JC, Grimm CC, et al. Effect of oxygen on aroma volatiles and quality of fresh-cut cantaloupe and honeydew melons. Food Chem. 2012;130:49–57. doi: 10.1016/j.foodchem.2011.06.052. [DOI] [Google Scholar]
- Artés F, Gomez P, Artés-Hernández F, et al. Improved strategies for keeping overall quality of fresh-cut produce. Acta Hortic. 2007;746:245–258. doi: 10.17660/ActaHortic.2007.746.27. [DOI] [Google Scholar]
- Auras RA, Singh SP, Singh JJ. Evaluation of oriented poly(lactide) polymers vs. existing PET and oriented PS for fresh food service containers. Packag Technol Sci. 2005;18:207–216. doi: 10.1002/pts.692. [DOI] [Google Scholar]
- Bablon G, Belamy W, Billen G, et al. Practical applications of ozone: principles and case studies. In: Langlais G, Reckhow DA, Brink DR, et al., editors. Ozone in water treatment: application and engineering. Chelsea, MI, USA: Lewis Publisher; 1991. pp. 133–316. [Google Scholar]
- Bai J, Saftner RA, Watada AE. Characteristics of fresh-cut honeydew (Cucumis xmelo L.) available to processors in winter and summer and its quality maintenance by modified atmosphere packaging. Postharvest Biol Technol. 2003;28:349–359. doi: 10.1016/S0925-5214(02)00209-0. [DOI] [Google Scholar]
- Beaulieu JC (2006) Effect of cutting and storage on acetate and nonacetate esters in convenient, ready-to-eat fresh-cut melons and apples. HortScience:65–73
- Beaulieu JC, Baldwin EA. Fresh-cut fruits and vegetables. Science, technology, and market. In: Lamikanra O, editor. Fresh-cut fruits and vegetables. Science, technology, and market. London: CRC Press LLC; 2002. pp. 391–426. [Google Scholar]
- Beaulieu JC, Ingber BF, Lea JM. Physiological, volatile, and SEM surface effects resulting from cutting and dipping treatments in cantaloupe. J Food Sci. 2011;76:S415–S422. doi: 10.1111/j.1750-3841.2011.02308.x. [DOI] [PubMed] [Google Scholar]
- Betts G, Everis L. Improving the safety of fresh fruit and vegetables. USA: Elsevier; 2005. [Google Scholar]
- Beuchat LR. Pathogenic microorganisms associated with fresh produce. J Food Prot. 1996;59:204–216. doi: 10.4315/0362-028X-59.2.204. [DOI] [PubMed] [Google Scholar]
- Botondi R, Cardarelli MT, Mencarelli F. The role of ethylene in regulating cell wall-degrading enzyme activity using antisense ACC-oxidase in cantaloupe melons. Acta Hortic. 2000;510:471–477. doi: 10.17660/ActaHortic.2000.510.74. [DOI] [Google Scholar]
- Botondi R, Bartoloni S, Forniti R, Mencarelli F (2014) Caratterizzazione qualitativa di frutti di melone di IV gamma. Ind Aliment 544:20–23
- Botondi R, Bartoloni S, Baccelloni S, Mencarelli F. Biodegradable PLA (polylactic acid) hinged trays keep quality of fresh-cut and cooked spinach. J Food Sci Technol. 2015;52:5938–5945. doi: 10.1007/s13197-014-1695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botondi R, De Sanctis F, Moscatelli N, et al. Ozone fumigation for safety and quality of wine grapes in postharvest dehydration. Food Chem. 2015;188:641–647. doi: 10.1016/j.foodchem.2015.05.029. [DOI] [PubMed] [Google Scholar]
- Carletti L, Botondi R, Moscetti R, et al. Use of ozone in sanitation and storage of fresh fruits and vegetables. J Food Agric Environ. 2013;11:585–589. [Google Scholar]
- European Commission Commission regulation (EC) No. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off J Eur Union. 2005;338:1–26. [Google Scholar]
- European Commission Commission regulation (EC) No. 1580/2007 laying down implementing rules of council regulations (EC) No. 2200/96, (EC) No. 2201/96 and (EC) No. 1182/2007 in the fruit and vegetable sector. Off J Eur Union. 2007;350:1–98. [Google Scholar]
- FDA Secondary direct food additives permitted in food for human consumption. Rules Regul. 2001;64:44122–44123. [Google Scholar]
- García-García I, Taboada-Rodríguez A, López-Gomez A, Marín-Iniesta F. Active packaging of cardboard to extend the Shelf life of tomatoes. Food Bioprocess Technol. 2011;6:754–761. doi: 10.1007/s11947-011-0759-4. [DOI] [Google Scholar]
- Holah JT, Higgs C, Robinson S, et al. A conductance-based surface disinfection test for food hygiene. Lett Appl Microbiol. 1990;11:255–259. doi: 10.1111/j.1472-765X.1990.tb00175.x. [DOI] [Google Scholar]
- Horwitz W, Latimer GW (eds) (2005), 18th Edition. Official methods of analysis of AOAC international, 18th edn
- Kays S. Preharvest factors affecting appearance. Postharvest Biol Technol. 1999;15:233–247. doi: 10.1016/S0925-5214(98)00088-X. [DOI] [Google Scholar]
- Leaper S. Synergistic killing of spores of bacillus subtilis by peracetic acid and alcholo. J Food Technol. 1984;19:355–360. doi: 10.1111/j.1365-2621.1984.tb00359.x. [DOI] [Google Scholar]
- Luna-Guzmán I, Barrett D. Comparison of calcium chloride and calcium lactate effectiveness in maintaining shelf stability and quality of fresh-cut cantaloupes. Postharvest Biol Technol. 2000;19:61–72. doi: 10.1016/S0925-5214(00)00079-X. [DOI] [Google Scholar]
- Massantini R, Salcini MC. New technologies in minimally processed product preparation. Ind Alimetari. 2003;42:953–958. [Google Scholar]
- Massantini R, Moscetti R, Contini M, Bellincontro A. Zucchini (cucurbita pepo L.) minimally processed packed in plastic film. Ind Aliment. 2009;493:26–37. [Google Scholar]
- Massantini R, Moscetti R, Monarca D, et al. The influence of cover crops and double harvest on storage of fresh hazelnuts (Corylus Avellana L.) Adv Hortic Sci. 2009;23:231–237. [Google Scholar]
- Meilgaard MC, Carr BT, Civille G V (2006) Sensory evaluation techniques, Fourth Edition. CRC Press; 4 edition, New York
- Moscetti R, Carletti L, Monarca D, et al. Effect of alternative postharvest control treatments on the storability of “golden delicious” apples. J Sci Food Agric. 2013;93:2691–2697. doi: 10.1002/jsfa.6086. [DOI] [PubMed] [Google Scholar]
- Park CM, Beuchat LR. Evaluation of sanitizers for killing Escherichia coli O147:H7, salmonella and naturally occurring microorganisms on cantaloupes, honeydew melons, and asparagus. Dairy, Food, Environ Sanit. 1999;19:842–847. [Google Scholar]
- Portela SI, Cantwell MI. Quality changes of minimally processed honeydew melons stored in air or controlled atmosphere. Postharvest Biol Technol. 1998;14:351–357. doi: 10.1016/S0925-5214(98)00052-0. [DOI] [Google Scholar]
- Rhim J-W, Lee J-H, Hong S-I. Increase in water resistance of paperboard by coating with poly(lactide) Packag Technol Sci. 2007;20:393–402. doi: 10.1002/pts.767. [DOI] [Google Scholar]
- Sapers GM, Miller RL, Pilizota V, Mattrazzo AM. Antimicrobial treatments for minimally processed cantaloupe melon. J Food Sci. 2001;66:345–349. doi: 10.1111/j.1365-2621.2001.tb11344.x. [DOI] [Google Scholar]
- Silveira AC, Conesa A, Aguayo E, Artes F. Alternative sanitizers to chlorine for use on fresh-cut “galia” (Cucumis melo var. catalupensis) melon. J Food Sci. 2008;73:405–411. doi: 10.1111/j.1750-3841.2008.00939.x. [DOI] [PubMed] [Google Scholar]
- Silveira, AC, Aguayo E, Escalona VH, Artés F (2011) Hot water treatment and peracetic acid to maintain fresh-cut galia melon quality. Innov Food Sci Emerg Technol 12:569–576.
- Suslow T V (2004) Minimizing the risk of foodborne illness associated with cantaloupe production and handling in California. In: University of California D (ed) California cantaloupe advisory board. Agricultural and Natural Resources Publication, Davis
- UNI (2002) EN-13432 - packaging - requirements for packaging recoverable through composting and biodegradation - test scheme and evaluation criteria for the final acceptance of packaging
- Villanueva CM, Cantor KP, Cordier S, et al. Disinfection byproducts and bladder cancer: a pooled analysis. Epidemiology. 2004;15:357–367. doi: 10.1097/01.ede.0000121380.02594.fc. [DOI] [PubMed] [Google Scholar]
- Villanueva MJ, Tenorio MD, Esteban MA, Mendoza MC. Compositional changes during ripening of two cultivars of muskmelon fruits. Food Chem. 2004;87:179–185. doi: 10.1016/j.foodchem.2003.11.009. [DOI] [Google Scholar]
- Wu ZS, Zhang M, Wang S. Effects of high pressure argon treatments on the quality of fresh-cut apples at cold storage. Food Control. 2012;23:120–127. doi: 10.1016/j.foodcont.2011.06.021. [DOI] [Google Scholar]