Abstract
The effects of different pHs (4, 5 and 6), temperatures (4, 20 and 80 °C) and storage (up to 12 days) on differential tristimulus colorimetry and betalain content related to the colour of yellow pitaya (Selenicereus megalanthus) have been investigated. The peel of the yellow pitaya was extracted with different solvents to see its colorant capacity. Highly-acidic extracts (pH 4) showed the lowest betalain content, chroma (C*ab = 60 against 70) and the yellow component of the colour (b*). Storage temperature manifested a great influence on CIELAB parameters when yellow pitaya peel was added to highly-acidic foodstuffs, with a tendency towards red hues (hab, from 100° to 85°) and remarkable changes on lightness (L*, from 90 to 75) as temperature increased. However, low-acidic extracts (pH 5 and 6) were superior from a colour stability standpoint, not being influential the temperature of storage. All colour changes according to pH and temperature were visually appreciable by human eyes (∆E*ab > 3). New opportunities for diversification of colorant market could be possible by employing yellow pitaya peel as natural resource.
Keywords: Yellow pitaya (Selenicereus megalanthus), Differential tristimulus colorimetry, Betalains, Stability
Introduction
The interest about the displacement of synthetic substances in favour of coloured natural products is continuously growing. In fact, the search for new coloured plant materials is been recently stimulated, predicting an upward annual growth rates of 10–15 % in the European markets (Stintzing and Carle 2004).
In that view, carotenoids, chlorophylls, anthocyanins and betalains, present in a high number of fruits and vegetables, are responsible for a wide range of colours. Therefore, natural resources containing them are being deeply considered as possible natural colorants, above all carotenoids-, chlorophylles- and anthocyanins-rich products. There is another group of pigments called betalains, which could represent a potential source of natural colorant. However, up to the present, betalains have been scientifically studied in a lesser extent.
Up to now, red beet (Beta vulgaris L.) (De Azeredo et al. 2009; Nemzer et al. 2011; Pavokovi and Krsnik-Rasol 2011) and cactus pear (Opuntia sp.) (Castellanos-Santiago and Yahia 2008; Cejudo-Bastante et al. 2014a) are the most investigated source of betalains, but there are a large number of betalain-rich products potentially usable as natural colorants.
On that concern, pitaya (Hylocereus sp.), also known as dragon fruit, is an orchard crop and a member of the Cactaceae family. It is native to Central South America and is distributed throughout Colombia, Ecuador, Peru, and Bolivia (Weiss et al. 1995). The fruit is a medium-size oblong berry bearing tubercles and thorns that are shed during ripening. It contains numerous small digestible black seeds and the pulp and skins adopt different colourations pursuant to the specie (Nerd and Mizrahi 1997). Accordingly, among all the existing species of Hylocereus, Hylocereus polyrhizus (red-purple pitaya), Hylocereus undatus (white pitaya) and Hylocereus or Selenicereus megalanthus (yellow pitaya) are the most widespread.
Some scientific studies of characterization of different species of Hylocereus have been developed. In that way, some researches about the chemical characterization of red-purple pitaya from different countries have been reported on the basis of several perspectives. Thus, Stintzing et al. (2002, 2004) firstly characterized the betalain profile of red-purple pitaya (Hylocereus polyrhizus (Weber) Britton & Rose) by HPLC-MS and RMN, as well as Wybraniec and Mizrahi (2002) of several red and white species of Hylocereus from Israel. Moreover, Esquivel et al. (2007b, 2007c) studied the pigment profiles, colour, betalain contents and antioxidant activity of fruits of five different Hylocereus sp. genotypes from Costa Rica during ripening (Esquivel et al. 2007a). In addition, recently, Lee et al. (2014) defined the metabolite profiling of Korean red and white pitayas (Hylocereus polyrhizus and Hylocereus undatus) by comparing betalain biosynthesis and antioxidant activity. However, scarce studies of chemical characterization of other species of Hylocereus (such as yellow pitaya, Selenicereus megalanthus) has been previously conducted. That is the case of the researches about phenolics (Jiang et al. 2011), amino acids (Kugler et al. 2006) and fatty acids of seeds (Chemah et al. 2010), among others. Also, Nerd and Mizrahi (1998, 1999) observed the effect of the ripening stage after storage on fruit quality (peel content, weight loss, water content and titratable acidity) but only Kugler et al. (2007) deepened on the content of total polyphenols, and their relationship with the antioxidant activity.
Despite of the potential use as natural colorant of the different species of Hylocereus, scarce technological scientific studies about this fruit have been conducted; that is it, the behaviour of pitaya as colorant under different commercial or industrial processes. In that way, some advances have been published in red-purple pitaya, considering the possible circumstances that colorant could suffer when it is added to foodstuffs (Herbach et al. 2004a, b; Woo et al. 2011) and also by developing and optimizing an enzyme-assisted liquefaction treatment under low temperature to produce a colorant of purple pitaya (Schweiggert et al. 2009). However, despite such possible broad agro-alimentary implications as natural colorant, none research study about colorimetry and betalain content of yellow pitaya has been found in bibliography yet.
Therefore, the present work was performed on yellow pitaya (Selenicereus megalanthus) cultivated in Colombia, and the interest was focused on the colour characterization and betalain content, not previously reported in that raw material. Only the peel of the fruit was taken into account because of its yellow colour and location of betalains, being the flesh purple of a white colour and devoid of betalains. Furthermore, not only a colour characterization of yellow pitaya is needed, but also even to spotlight the behaviour in terms of colour and betalains when it acts as colorant of other agro-alimentary foodstuffs. As a result, stability assays at different pHs and temperatures over time have been also carried out. It is highlighted that this research is the first endeavour to tend to reproduce commercial conditions of processing and nature of the food upon the yellow pitaya to be added as colorant.
Materials and methods
The effect of acidity and temperature over time on colour and betalain content was scrutinized. Several temperatures (4, 20 and 80 °C) and pHs (4, 5 and 6) were selected as representative of several processing and storage conditions, and acidities of sources where yellow pitaya was added as natural colorant. A follow-up over time (0 and 5 h, and 1, 2, 6, 8 and 12 days) of CIELAB colour parameters, tristimulus differential colorimetry and betalain content have been evaluated. In order to a better understanding, the behaviour of temperature and acidities on yellow pitaya peel was separately discussed.
Sample preparation
Yellow pitayas (Selenicereus megalanthus) (code 45,988) were collected in San Juan de Pasto (Nariño, Colombia). A random sampling of 60 shrubs distributing in an area of approximatively 2000 m2 (1°35´15.2″N latitude y 77°11´19.1´´O longitude, at 1920 m above sea level) was carried out. Visual characteristics such as size or colour were used at harvesting, and a total of 8 Kg of fruits were collected. According the NTC 3554 regulation, the samples were picked at a maturity stage of 5, based on the yellow colour and the slight greenish tonality at the end of the fruit.
The quartering method was employed to reach a representative sample of the set. Previous homogenization, the fruits were distributed into four quadrants. Two fruits of opposite quadrants was randomly selected. The remainder fruits were mixed again and the process was repeated twice more. As a result, six fruits were considered for the analysis, with approximately 1126 g of total weight. They were washed and dried, and prickles were manually removed. The white pulp and black seeds were also discarded.
Preparation of extracts
Yellow peels were only considered for the analysis, with around a 33 % of total yield. They were cut into small pieces (1 cm2) and macerated with 1 L of methanol:water (60:40) for 1 day at 10 °C, according to the method described by Cejudo-Bastante et al. (2014b). Once filtration, the organic solvent was removed at 35 °C using a rotary evaporator (Heidolph, Schwabach, Germany) and re-dissolved with distilled water. The extract was lyophilized (Labconco, MO, USA) and stored at 4 °C until their analysis.
Lyophilized peel (3g) was used for the extractions, with the addition of 9.5 mL of methanol and water (50:50), containing 50 mM of sodium ascorbate. Samples were then stirred at 225 rpm for 10 min in darkness, to avoid possible degradation by light. Afterwards, a centrifugation at 12,000 x g at 10 °C and 5 min was carried out, according to the method proposed by Cejudo-Bastante et al. (2014a). Supernatants were separated from the plant tissue and, in order to achieve the complete discolouration of the plant material, the procedure was developed once more with the extraction solution and finally with 100 % methanol. Later, the extract obtained was concentrated in vacuum (30 °C) and re-suspended in 15 mL with purified water. All experiences were carried out in duplicate.
Technological treatments of the extracts
Extracts were adjusted to different acidities pH (pH 4, 5 and 6) using hydrochloride acid 0.1 and 1 M. Subsequently, each sample were submitted to three different temperatures: 4 °C, and 20° and 80 °C, putting samples into a refrigerator and an oven JP Selecta, S.A. (Barcelona, Spain), respectively. At each time-point, an aliquot of each sample were taken and used for further analyses.
The samples kept at 4 and 20 °C were analysed at the beginning of the treatment (0 days), after 5 h (0.21 days) and after 1, 2, 6, 8 and 12 days, whereas only the first three points were considered for samples underwent 80 °C.
Colorimetric measurements
A Hewlett-Packard UV-vis HP8452 spectrophotometer (Palo Alto, CA) was used for to develop colour measurements. The whole visible spectrum (380–770 nm) was recorded at constant intervals (Δλ = 2 nm) using 2 mm path length glass cells and distilled water as reference. The original software CromaLab© was used for determining the CIELAB parameters (Heredia et al. 2004) following the Commission Internationale de L’Eclairage’s recommendations: (CIE 2004) the CIE 1964 10° Standard Observer and the Standard Illuminant D65. Euclidean distance between two points in the three-dimensional space define by L* (lightness), a* and b* (red and yellow component of the colour, respectively) were used for calculating colour differences (ΔE*ab): ΔE*ab = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2. All experiences were carried out in duplicate.
Spectrophotometric quantification of betalains
The UV-vis spectra were recorded from 360 to 800 nm in order to develop a photometric quantification of betalains. Maximum absorbance of 484 nm was reported to quantify betaxanthins (B), following the equation:
where Abs is the value of maximum absorbance at 484 nm, D is the dilution factor, V is the final volume (mL) of the extracts, MW and ε are the molecular weight and the molar extinction coefficient of indicaxanthin (308 g mol−1 and 48,000 L (mol cm)−1 in H2O), L is the path-length (2 mm) and W the dried weight of the sample (g). All measurements were carried out in duplicate.
Statistical analysis
Statistical analysis was carried out by using Statistica version 8.0 software (Statistica 2007). Univariate analyses of variance (Tukey test and ANOVA) (p < 0.05) were applied to discriminate among the means of chemical data.
Results and discussion
Colorimetric characteristics
The monitoring of the evolution of CIELAB colour parameters (L*, C*ab, hab) were carried out along 12 days under different conditions (Fig. 1). The behaviour of the extracts exposed to 80 °C was separately discussed.
Fig. 1.
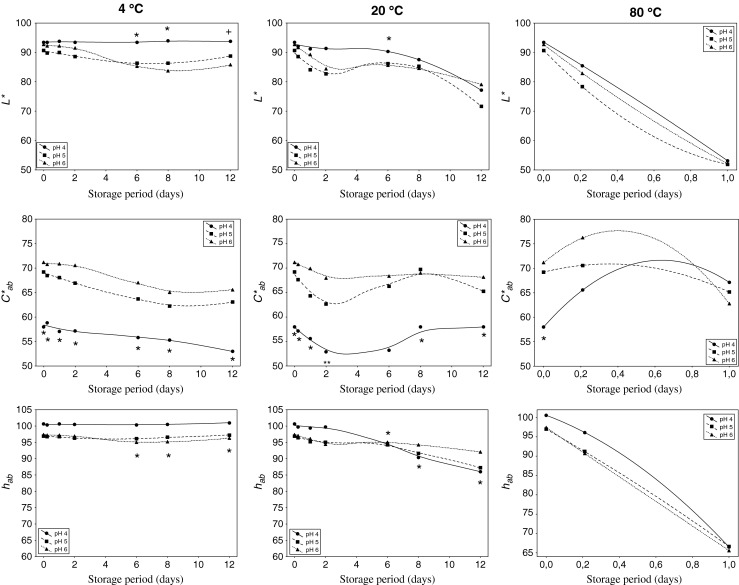
Effect of pH on CIELAB parameters of yellow pitaya peel at different temperatures over time. Significant differences (p < 0.05) according to the Tukey test among pH 4 (*), and among 80 °C (+), and the rest of temperatures
Consequences of the acidity
The effect of pH on the colorimetric parameters at different temperatures over time was evaluated.
It is worth to mention the great stability of the extracts over time, above all in terms of chroma (C*ab), because the initial values only slightly dropped at the end of the treatment (attenuation of only 4–5 CIELAB units) (Fig. 1). However, the behaviour of hue (hab) and lightness (L*) was greatly influenced by temperature.
As it can be observed, an important grouping according to pH took place at the beginning of the treatment (0 h). Significant (p < 0.05) differences were manifested among pHs above all in chroma (C*ab), situating the more acidic extracts (pH 4) with significant lower values (58 against around 70) (Fig. 1). The tonality (hab) of the extracts experimented a similar behaviour, with a slightly more contribution to yellow colour the extracts adjusted to pH 4. As a result, high-acidic extracts contributed with a huge yellow colour but lower chromaticity, whereas low-acidic samples provide higher colorant intensity of a lower yellowish colour. Thus, the colour intensity was strongly dependent on the pH, attributing the extracts with the acidities compressed between 5 and 6 to a more saturated colour. Similar results were found by Woo et al. (2011) in a study about red-purple pitaya (Hylocereus polyrhizus), affirming that pH 5 was the acidity that reach a higher remaining colour during the storage. However, any scientific study in that matter has been reported in yellow pitaya.
When ANOVA was applied to the set of data in order to study variances, significant (p < 0.05) differences was observed in chroma (C*ab) according to pH (p = 0.000, F = 54.02). With the intention of refining the statistical analysis, Tukey test was applied by pairs of samples (pairs of pHs), obtaining that all pHs showed significant (p < 0.05) differences among them, increasing significantly the colour intensity (C*ab) with pH, regardless of temperature. If each sequential point was taken into account, it could be asserted that chroma manifested significant (p < 0.05) differences among pH 4 and the rest of pHs in all points of the time sequence. Only punctual differences manifested lightness and hue along the time according to pH, above all in the last steps of the treatment.
When heating conditions was taken into account (80 °C), any significant (p > 0.05) difference were detected according to pH over time (Fig. 1).
The effect of the time provoked continuous higher values of a*, similarly to demonstrated Cejudo-Bastante et al. (2014b), whereas the effect of pH was mainly observed in the b* component of the colour (data not shown). As pH rose, the yellow component of the colour (b*) also experimented an increase, standing together the less acidic extracts (pH 5 and 6) and separated from those adjusted to pH 4. Besides, the extracts evolved in a lesser extent over time concerning to a* as pH decreased. According to several authors (Castellar et al. 2003; Vaillant et al. 2005), although betalains are stable over a broad pH range from 3 to 7, the optimal stability of betalains is reached between pH 5–6. Thus, the initial yellow colorant of extracts was greatly maintained in the pH 5 and 6 extracts, more than in those adjusted to pH 4.
In resume, the fact that extracts were adjusted to one or another pH (depending on the food on which they could be added) mainly influenced on the values of colour intensity (C*ab), not changing significantly the typology of the colour (hab) neither the lightness (L*) of the extracts. Thus, when yellow pitaya peel extracts were adjusted to an acidic pH (result of being added as colorant to fruit-based salads, for example), the provided colorant intensity was significantly lower, and therefore it could be added more quantity of extract to counteract this deficiency. Once exceeded this acidity, yellow pitaya could be indistinctly added to foods in an interval of pH between 5 and 6 (for example ready-to-serve vegetable-meals) without affecting colour, because as lightness, hue as chroma had similar values among them.
Consequences of the temperature
Temperature exerted an important effect on the CIELAB colour parameters, being more marked as temperature increased (Fig. 2). In order to see the possible significant differences provoked by the application of temperature to a wide range of acidities, statistical analysis was conducted.
Fig. 2.
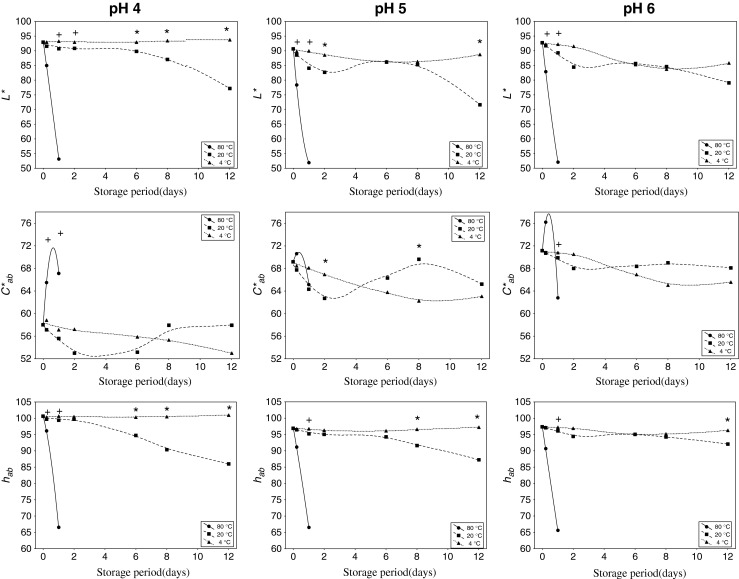
Effect of temperature on CIELAB parameters of yellow pitaya peel at different pHs over time. Significant differences (p < 0.05) according to the Tukey test among pH 4 (*), and among 80 °C (+) and the rest of pHs
When extracts were heated at (80 °C), only one day storage was enough to drastically and significantly (p < 0.05) reduce the hue (hab) and lightness (L*), reaching 1.5-fold lower values (95° to 65°, and 90 to 50 CIELAB units, respectively). As a consequence, a total degradation of the colour and a subsequent forfeit of colour stability were produced, similarly to that affirmed Herbach et al. (2004a, b ) after studying colour and pigment profiles red beet under thermal treatments.
When comparing among 4 °C and 20 °C conditions, and according ANOVA applied the effect of temperature was more marked at the last steps of the treatment (6–12 days). Lightness (L*) and hue (hab) showed remarkable and significant (p < 0.05) decreases as temperature increased. That tendency become accentuated as pH decreased. In terms of chroma (C*ab), significant (p < 0.05) differences were observed over time, contrarily to that observed by Woo et al. (2011), who displayed a significant loss of colour in only one week when purple pitaya extracts were stored at 25 °C, in comparison with the practically invariable values after storage at low temperatures (4 °C).
The significant (p < 0.05) colorimetric differences among temperatures (4 °C and 20 °C) were higher as pH decreased and after six days of storage. Thereby, at pH 4, lightness and hue were strongly influenced by the storage conditions, whereas only significant differences were appreciated in the tonality of the extracts adjusted to pH 5. Any distinction manifested pH 6-extracts.
For all pHs, the red component of the colour (a*) increased with temperature, and, consequently, the temperature favoured the change from yellow to reddish tonalities (towards positive values of a*) (data not shown). Similar results were also observed in other raw materials with similar tonalities such as white wines (Fernández-Zurbano et al. 1995) or apple-based foods (Palazón et al. 2009) under different storage temperatures. However, extracts stored under refrigeration did not practically suffer changes in that parameter, but only slight diminutions of the yellow component of the colour (b*).
Hence, temperature exerted an important effect on CIELAB colour parameters of the extracts, above all lightness and hue, and their results varied on the basis of pH. So, the possible natural colorant could maintain its initial colour properties when stored at room temperature for at least 6 days, no matter the pH. If the time of storage was long lasting, refrigeration conditions could be needed in order to avoid a change of tonality towards more reddish dyes and less brightness, above all for acidic foodstuffs. Extreme thermal treatment supposed a radical change towards those conditions regardless of pH.
Differential colorimetry
Effect of pH
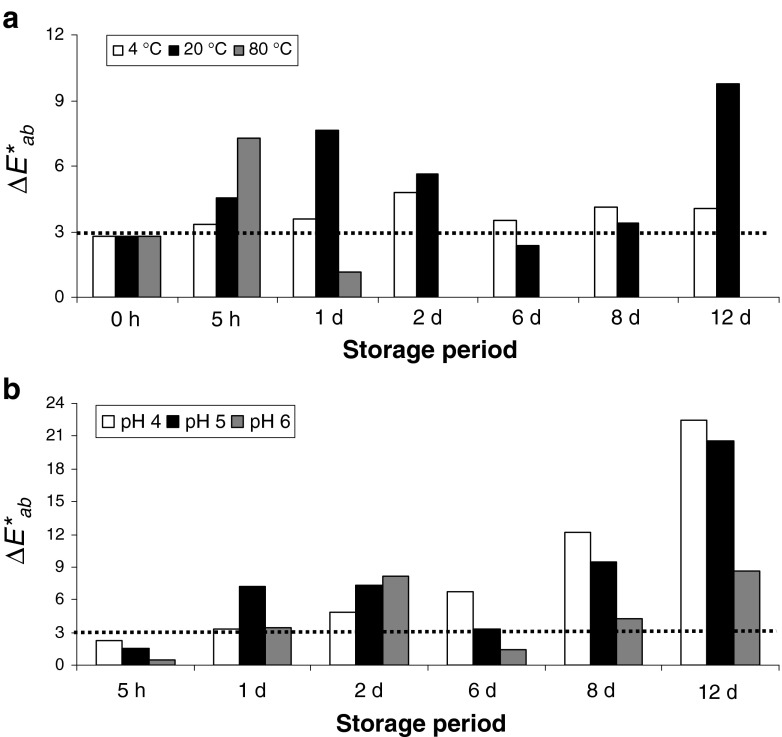
ΔE*ab was calculated by pairs of pHs to get an insight into if appreciable colour differences were produced as a consequence of the acidity change.
The comparison of pHs was carried out to highlight the possible visual colour differences among them. Thus, the higher visually colour differences were highlighted when extracts adjusted to pH 4 were compared to the rest of pHs (pH 4 against pH 5 and pH 6) (ΔE*ab ~ 10–14, data not shown). After calculating the percentage of the quadratic parameters (as proposed Gordillo et al. 2012), the differences were mainly ascribed to variations of chroma (Δ2C*ab) (data not shown).
Extracts adjusted to pH 5 and 6 were also compared to highlight the possible appreciable colour variations among them. Thus, although any visually appreciated colour difference was observed at the beginning of the treatment (0 h) (Fig. 3a) (in the light of ΔE*ab situated below 3 CIELAB units) (Martínez et al. 2001), those colour variations became visually noticeable as time passed. Those differences were mainly due to chroma and lightness (Δ2C*ab and Δ2L*; data not shown).
Fig. 3.
Colour variation (ΔE*ab) of yellow pitaya peel between pH 5 and 6 at different temperatures (a) and between 4 °C and 20 °C at different pHs (b)
Overall, the colour differences of yellow pitaya extracts were pH-dependent in a range of 4–6 at any temperature applied.
Effect of temperature
As expected, colour variations among samples subjected to cooking (80 °C) and the rest of temperatures exceeded by far the limit to which colour differences are visually noticeable (ΔE*ab > > 3) (data not shown). Those differences were both qualitative and quantitative, caused by hue (Δ2H) and lightness (Δ2L*). Similar results were also manifested by Fernández-López et al. (2013) in some natural red extracts submitted to heating.
However, the question was whether colour would be influenced by storage conditions. Hence, the pattern evolution of the colour differences over time among 4 °C and 20 °C at each pH was calculated (Fig. 3b). Thereby, when room (20 °C) and cold (4 °C) storage conditions were compared, it was evidenced that not only visually colour differences were appreciated among them (ΔE*ab > 3), but that they were increasing over time. Therefore, colour differences of yellow pitaya extracts were temperature-dependent at any pH.
Overall, although betalains are stable in a wide range of pHs, appreciable differences on colour intensity was observed depending on the acidity that yellow pitaya undergoes. Moreover, colour visually changes was also observed depending on the thermal process that this fruit suffers.
Betaxanthin content
Impact of pH
Figure 4a shows the influence of pH on betaxanthin content over time at different temperatures. At the beginning of the treatment, the initial content of betaxanthins was not dependent on pH, in the light of the absence of significant differences (p < 0.05).
Fig. 4.
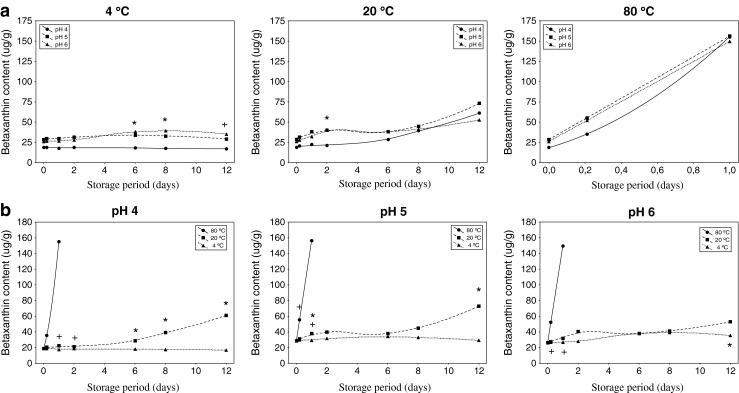
Betaxanthin content of yellow pitaya peel: effect of pH at different temperatures over time a (significant differences (p < 0.05) according to the Tukey test among pH 4 and the rest of pHs (*), and among all pHs (+)), and effect of temperature at different pHs over time b (significant differences (p < 0.05) according to the Tukey test among 4 °C and 20 °C (*), and among 80 °C and the rest of temperatures (+))
It could be remarked that the absorbance at 484 nm increased as time passed, whose behavior was greatly influenced by the temperature rather than by pH.
At 4 °C, although with the lowest values of betaxanthin content, the high-acidic samples (pH 4) remained stable during storage, being significant different (p < 0.05) from the low-acidic samples. Thus, as temperature increased (20 °C and 80 °C), the content of betaxanthins (or the absorbance at 484 nm) increased during storage, and independent of the pH. Furthermore, those results were in agreement with Havlíková et al. (1983) who affirmed that raising the temperature, samples tended to resemble pH 6 conditions, regardless of the initial pH.
In conclusion, it is highlighted the scarce significant differences among pHs at any temperature, concluding that the content of betaxanthin remain invariable whatever the pH of the food where yellow pitaya was added.
Impact of temperature
Fig. 4b, shows the content of betaxanthin greatly depended on the temperature. Changes on betaxanthin concentration began to be noticeable (> 15 %) after six days of treatment (and only 5 h when cooking).
It could be clearly observed how temperature made the content of betaxanthins increased overmuch, appreciating significantly diverse among temperatures (ANOVA, p < 0.05). However, low temperatures palliated this behaviour, observing a scarce or lower evolution as time progressed when extracts were stored at 4 °C. Similar results were also reported by Cejudo-Bastante et al. (2014a) when ulluco extracts were submitted to different thermal storage conditions.
Upon heating (80 °C), yellow pitaya extracts suffered a pronounced degree of degradation, being a thermosensitive raw material at those conditions. The very high content of betaxanthins at the end of the cooking (80 °C) may had to accelerated browning. The formed compounds could be characterized by decarboxylation of betacyanins at C-17 and/or C-2, and dehydrogenation at C-14/C-15, which could modify appearance and stability of the genuine pigments (Herbach et al. 2006).
Conclusions
Yellow pitaya peel is a potential and promising natural source with multiple possibilities as colorant. This fruit is suitable for being added as natural colorant in a range of 4–6 pHs (from vegetables- or fruit-based dishes to yoghurts, for example) taking into account the behaviour of colorimetric parameters and betaxanthins, assuming some restrictions of temperature to protect as possible the initial properties. Extracts adjusted to acid pHs must be conserved under cold storage in order to avoid remarkable changes on hue and lightness, not exerting the temperature of storage a remarkable influence on possible colour changes of low-acidic foodstuffs. This study is an important step towards diversification of colorants in the direction of the displacement of synthetic colorants that provoke several inconveniences and value such as a low-exploited natural resource.
Acknowledgments
We are indebted to Consejería de Economía, Innovación y Ciencia, Junta de Andalucía, Spain (Project P11-AGR-7843) for financial support. We thank technical staff of Biology Service (SGI, Universidad de Sevilla) for technical assistance and University of Nariño, San Juan de Pasto, Colombia.
Contributor Information
María Jesús Cejudo-Bastante, Email: mjcejudo@us.es.
Nelson Hurtado, Email: nhurtado@unal.edu.co.
Angélica Delgado, Email: amadeju_18@hotmail.com.
Francisco J. Heredia, Phone: +34 954556495, Email: heredia@us.es
References
- Castellanos-Santiago E, Yahia EM. Identification and quantification of betalains from the fruits of 10 Mexican prickly pear cultivars by high-performance liquid chromatography and electrospray ionization mass spectrometry. J Agric Food Chem. 2008;56:5758–5764. doi: 10.1021/jf800362t. [DOI] [PubMed] [Google Scholar]
- Castellar MR, Obón JM, Alacid M, Fernández-López JA. Color properties and stability of betacyanins form Opuntia fruits. J Agric Food Chem. 2003;51:2772–2776. doi: 10.1021/jf021045h. [DOI] [PubMed] [Google Scholar]
- Cejudo-Bastante MJ, Chaalal M, Louaileche H, Parrado J, Heredia FJ. Betalain profile, phenolic content, and color characterization of different parts and varieties of Opuntia ficus-indica. J Agric Food Chem. 2014;62:8491–8499. doi: 10.1021/jf502465g. [DOI] [PubMed] [Google Scholar]
- Cejudo-Bastante MJ, Hurtado N, Mosquera N, Heredia FJ. Potential use of new Colombian sources of betalains. Color stability of ulluco (Ullucus tuberosus) extracts under different pH and thermal conditions. Food Res Int. 2014;64:465–471. doi: 10.1016/j.foodres.2014.07.036. [DOI] [PubMed] [Google Scholar]
- Chemah TC, Aminah A, Noriham A, Wan Aida WM. Determination of pitaya seeds as a natural antioxidant and source of essential fatty acids. Intern Food Res J. 2010;17:1003–1010. [Google Scholar]
- CIE . Technical Report Colorimetry; Commission Internationale de l'Eclairage Central Bureau. Vienna: Austria; 2004. [Google Scholar]
- De Azeredo HMC, Pereira AC, De Souza ACR, Gouveia ST, Mendes KCB. Study on efficiency of betacyanin extraction from red beetroots. Int J Food Sci Technol. 2009;44:2464–2469. doi: 10.1111/j.1365-2621.2009.02037.x. [DOI] [Google Scholar]
- Esquivel P, Stintzing FC, Carle R. Fruit characteristics during growth and ripening of different hylocereus genotypes. Eur J Hortic Sci. 2007;7:231–238. [Google Scholar]
- Esquivel P, Stintzing FC, Carle R. Phenolic compound profiles and their corresponding antioxidant capacity of purple pitaya (Hylocereus sp.) genotypes. Zeitschrift fur Naturforschung – Section C J Biosci. 2007;62:636–644. doi: 10.1515/znc-2007-9-1003. [DOI] [PubMed] [Google Scholar]
- Esquivel P, Stintzing FC, Carle R. Pigment pattern and expression of colour in fruits from different Hylocereus sp. genotypes. Innovative Food Sci Emerg Technol. 2007;8:451–457. doi: 10.1016/j.ifset.2007.03.022. [DOI] [Google Scholar]
- Fernández-López JA, Angosto JM, Giménez PJ, León G. Thermal Stability of Selected Natural Red Extracts Used as Food Colorants. Plant Foods Hum Nutr. 2013;68:11–17. doi: 10.1007/s11130-013-0337-1. [DOI] [PubMed] [Google Scholar]
- Fernández-Zurbano P, Ferreira V, Peña C, Escudero A, Serrano F, Cacho J. Prediction of oxidative browning in white wines as a function of their chemical composition. J Agric Food Chem. 1995;43:2813–2817. doi: 10.1021/jf00059a008. [DOI] [Google Scholar]
- Gordillo B, Rodríguez-Pulido FJ, Escudero-Gilete ML, González-Miret ML, Heredia FJ. Comprehensive colorimetric study of anthocyanic copigmentation in model solutions. Effects of pH and molar ratio J Agric Food Chem. 2012;60:2896–2905. doi: 10.1021/jf2046202. [DOI] [PubMed] [Google Scholar]
- Havlíková L, Miková K, Kyzlink V. Heat stability of betacyanins. Z Lebensm Unters Forsch. 1983;177:247–250. doi: 10.1007/BF01082487. [DOI] [Google Scholar]
- Herbach KM, Stintzing FC, Carle R. Impact of thermal treatment on color and pigment pattern of red beet (Beta vulgaris L.) preparations. J Food Sci. 2004;69:C491–C498. doi: 10.1111/j.1365-2621.2004.tb10994.x. [DOI] [Google Scholar]
- Herbach KM, Stintzing FC, Carle R. Betalain stability and degradation - Structural and chromatic aspects. J Food Sci. 2006;71:R41–R50. doi: 10.1111/j.1750-3841.2006.00022.x. [DOI] [Google Scholar]
- Herbach MK, Stintzing FC, Carle R. Thermal degradation of betacyanins in juices from purple pitaya [Hylocereus polyrhizus (Weber) Brittonv & Rose] monitored by high-performance liquid chromatography-tandem mass spectometric analyses. Eur Food Res Technol. 2004;219:377–385. doi: 10.1007/s00217-004-0948-8. [DOI] [Google Scholar]
- Heredia FJ, Álvarez C, González-Miret ML, Ramírez A (2004) CromaLab, análisis de color. In, vol. Registro General de la Propiedad Intelectual: SE-1052-04. Seville, Spain.
- Jiang YL, Lin TS, Lee CL, Yen CR, Yang WJ. Phenology, canopy composition, and fruit quality of yellow pitaya in Tropical Taiwan. HortSci. 2011;46:1497–1502. [Google Scholar]
- Kugler F, Graneis S, Schreiter PPY, Stintzing FC, Carle R. Determination of free amino compounds in betalainic fruits and vegetables by gas chromatography with flame ionization and mass spectrometric detection. J Agric Food Chem. 2006;54:4311–4318. doi: 10.1021/jf060245g. [DOI] [PubMed] [Google Scholar]
- Kugler F, Stintzing FC, Carle R. Evaluation of the antioxidant capacity of betalainic fruits and vegetables. J Appl Bot Food Qual. 2007;81:69–76. [Google Scholar]
- Lee S, Suh DH, Heo DY, Kim YS, Cho SK, Lee CH. Metabolite profiling of red and white pitayas (Hylocereus polyrhizus and Hylocereus undatus) for comparing betalain biosynthesis and antioxidant activity. J Agric Food Chem. 2014;62:8764–8771. doi: 10.1021/jf5020704. [DOI] [PubMed] [Google Scholar]
- Martínez JA, Melgosa M, Pérez MM, Hita E, Negueruela AI. Note. Visual and instrumental color evaluation in red wines. Food Sci TechnolIntern. 2001;7:439–444. [Google Scholar]
- Nemzer B, Pietrzkowski Z, Spórna A, Stalica P, Thresher W, Michałowski T, Wybraniec S. Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem. 2011;127:42–53. doi: 10.1016/j.foodchem.2010.12.081. [DOI] [Google Scholar]
- Nerd A, Mizrahi Y. Fruit development and ripening in yellow pitaya. J Am Soc Hortic Sci. 1998;123:560–562. [Google Scholar]
- Nerd A, Mizrahi Y. The effect of ripening stage on fruit quality after storage of yellow pitaya. Postharvest Biol Technol. 1999;15:99–105. doi: 10.1016/S0925-5214(98)00080-5. [DOI] [Google Scholar]
- Nerd A, Mizrahi Y. Reproductive biology of fruit cacti. Hortic Rev. 1997;18:322–346. [Google Scholar]
- Palazón MA, Pérez-Conesa D, Abellán P, Ros G, Romero F, Vidal ML. Determination of shelf-life of homogenized apple-based beikost storage at different temperatures using Weibull hazard model. LWT Food Sci Technol. 2009;42:319–326. doi: 10.1016/j.lwt.2008.03.011. [DOI] [Google Scholar]
- Pavokovi D, Krsnik-Rasol M. Complex biochemistry and biotechnological production of betalains. Food Technol Biotechnol. 2011;49:145–155. [Google Scholar]
- Schweiggert RM, Villalobos-Gutierrez MG, Esquivel P, Carle R. Development and optimization of low temperature enzyme-assisted liquefaction for the production of colouring foodstuff from purple pitaya (Hylocereus sp. [Weber] Britton & Rose) Eur Food Res Technol. 2009;230:269–280. doi: 10.1007/s00217-009-1167-0. [DOI] [Google Scholar]
- Statistica . StatSoft Inc. OK: Tulsa; 2007. [Google Scholar]
- Stintzing FC, Carle R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci Technol. 2004;15:19–38. doi: 10.1016/j.tifs.2003.07.004. [DOI] [Google Scholar]
- Stintzing FC, Conrad J, Klaiber I, Beifuss U, Carle R. Structural investigations on betacyanin pigments by LC NMR and 2D NMR spectroscopy. Phytochem. 2004;65:415–422. doi: 10.1016/j.phytochem.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Stintzing FC, Schieber A, Carle R (2002) Identification of betalains from yellow beet (Beta vulgaris L.) and cactus pear [Opuntia ficus-indica (L.) Mill.] by high-performance liquid chromatography-electrospray ionization mass spectrometry. J Agric Food Chem 502302–2307 [DOI] [PubMed]
- Vaillant F, Pérez A, Davila I, Dornier M, Reynes M. Colourant and antioxidant properties of red-purple pitaya (Hylocereus sp.) Fruits. 2005;60:1–10. doi: 10.1051/fruits:2005007. [DOI] [Google Scholar]
- Weiss J, Scheinvar L, Mizrahi Y. Selenicereus megalanthus (the yellow pitaya) a climbing cactus from Colombia and Peru. Cactus and Succulent J. 1995;67:280–283. [Google Scholar]
- Woo KK, Ngou FH, Ngo LS, Soong WK, Tang PY. Stability of betalain pigment from red dragon fruit (Hylocereus polyrhizus) Am J Food Technol. 2011;6:140–148. doi: 10.3923/ajft.2011.140.148. [DOI] [Google Scholar]
- Wybraniec S, Mizrahi Y. Fruit flesh betacyanin pigments in Hylocereus cacti. J Agric Food Chem. 2002;50:6086–6089. doi: 10.1021/jf020145k. [DOI] [PubMed] [Google Scholar]