Abstract
Pomegranate (Punica granatum) peel possess higher antioxidant activity than the edible portion. Efforts were made to extract dry peel powder at room temperature (28–30 °C) using combination of water and ethanol (EtOH, a green combination) and standardize the factors that may affect the extraction efficiency. The storage stability of the extract has been studied for >100 days at room temperature (28–30 °C), 4, 0 and −80 °C. The extract quality was monitored by measuring the radical scavenging activity (RSA) by diphenyl picrylhydrazyl (DPPH) method, total polyphenol content and by estimating the contents of punicalagins and ellagic acid, the two major ellagitannins present in the peel. The standardized conditions for extraction were found to be; Water and EtOH in 1:1 (v/v) ratio, duration of 24–48 h, ratio of 1:10 for solid to solvent and particle size in the range of 100–400 μ. Consecutive extraction of the peel powder did neither improve the yield nor polyphenol content, hence single extraction was adopted. The extract stored at room temperature for 110 days resulted in 13.2 % loss of polyphenol content followed by 8.9, 2.8, 27.5 and 14.1 % loss in Punicalgin A, B, Ellagic acid and RSA content, respectively.
Keywords: Punica granatum, Polyphenols, RSA, Punicalagin, Ellagic acid
Introduction
Pomegranate (Punica granatum L., family Punicaceae) is used in folkloric medicine for the treatment of various diseases (Abdel Moneim et al. 2011; Ajaikumar et al. 2005). It has become popular owing to its health-promoting phytonutrients (Adams et al. 2006; Faria et al. 2007; Khan et al. 2007). Pomegranate consumption has been reported to reduce platelet aggregation, oxidative stress and protection of LDL against atherogenic modifications (Aviram et al. 2000). It also possesses anticancer, antibacterial and antiviral properties (Negi and Jayaprakasha 2003; Zhang et al. 1995). Pomegranate possesses strong antioxidant and anti-inflammatory properties and exhibits anti-cancer activity in several human cancers (Adhami and Mukhtar 2007; Qu et al. 2009). Pomegranate peels have been shown to possess higher antioxidant activity than edible portion (arils) owing to an abundance of flavonoids and tannins present (Yunfeng et al. 2006). However, attention towards value-added utilization of the peel, which gets generated in large quantities [73 % of the marc (combination of pomegranate peel and seed after the extraction of juice from the total fruit; Wang et al. 2011)] by the processing industries and is difficult to dispose of, as they have a high biological oxygen demand (BOD), is scanty. As a result, it is mostly used as cattle feeds with low value or directly disposed of in the field that could cause environmental problems.
In general, polarity of the solvent (Mashkor 2014), extractant, extraction temperature, solid–solvent ratio and particle size are influential parameters for extraction process (Qu et al. 2010). Various extractants may not dramatically affect the characteristics of antioxidants, but may affect the composition of the extracts (Kulkarni et al. 2004; Singh et al. 2002). The water – EtOH combination, which is a combination of non-toxic food grade solvents and approved by US Food and Drug Administration (USFDA, Tabaraki et al. 2012), has been used to increase the efficiency of the extractant for extracting grape seed polyphenols (Shi et al. 2003). Water (Wang et al. 2011; Çam and İçyer 2013) and EtOH (Ibrahium 2010) alone could be used as environment friendly solvents for the extraction of antioxidant rich fractions from pomegranate peel, but generally, the individual pure solvent exhibits poor extractability (Mashkor 2014). Pomegranate peel has also been extracted using ultrasound assisted extraction with 70 % aqueous EtOH (Tabaraki et al. 2012) at 60 °C for 30 min. Foss et al. (2014) employed 90 % hydroalcoholic solution at room temperature in dark for 5 days to extract pomegranate peel and studied its antifungal activity.
The objective of the present work was to find an appropriate combination of water and EtOH with minimum use of energy and to see the effect of various parameters that may affect the efficiency of extraction. The quality of extracts was monitored by their potential of scavenging 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical, contents of punicalagins and ellagic acid (Awika et al. 2003; Bondet et al. 1997) and total polyphenol content.
Materials and methods
Materials
Pomegranate fruits were procured from local market of Mysore. The peels were removed, washed with water, cut into small pieces and dried in hot air oven at 40 °C for 5–6 h (moisture content <1 %). The dried pieces of peel were coarsely powdered in a blender (coded as dried pomegranate peel powder, DPPP) and stored in 250 gauge polythene bags (water vapor transmission rate: 6 g/m2/day under 90 % RH gradient at 38 °C; oxygen transmission rate: 2500 cm3/m2/day/atm at 65 % RH/27 °C). DPPH, punicalagin and ellagic acid were procured from Sigma-Aldrich. All other chemicals and solvents used were of analytical / HPLC grade.
Extraction
The extraction of DPPP was carried out with water, EtOH and their various combinations. DPPP was soaked overnight (or for desired period) at room temperature (28–30 °C) for extraction with intermittent shaking. After extraction, it was centrifuged (2000 rpm × 5 min) and filtered through Whatman No. 2 filter paper and the effective volume was recorded.
Determination of radical scavenging activity (RSA)
Determination of RSA was carried out (Murthy et al. 2002) using DPPH as stable free radical and butylated hydroxyanisole (BHA) as standard. Different aliquots of extract and BHA were taken in 100 μl fixed volume. 0.1 mM methanolic solution of DPPH (100 μM, 5 ml) was added and vortexed. The tubes were kept at room temperature in dark for 20 min. MeOH was used for baseline correction. The changes in the absorbance were measured at 517 nm (Genesys-5, Milton Roy, USA) and RSA was expressed as percentage inhibition using the formula.
Determination of total phenolics
The concentration of phenolic compounds in the extracts was determined (Heinonen et al. 1998) and results were expressed as tannic acid equivalents. DPPP extract (0.2 ml) was mixed with of 10-fold diluted Folin–Ciocalteu reagent (1.0 ml) and of 7.5 % sodium carbonate solution (0.8 ml), the mixture was allowed to stand for 30 min at room temperature and the absorbance was measured at 765 nm.
The RSA profiling by DPPH method and total polyphenol content of the extract were used as criteria for evaluating the quality of extract and effectiveness of the extraction. The following parameters pertaining to standardize the extraction procedure of DPPP have been studied.
Effect of extractant composition
The effect of extractant was studied by soaking DPPP in EtOH and water alone and varying ratio of EtOH: water. The extracts were prepared, as described above and their RSA and polyphenol content were determined.
Effect of solid to solvent ratio
The effect of ratio of solid to solvent on the efficiency of extraction was studied by overnight extraction of DPPP with water-EtOH (1:1 v/v) with intermittent shaking. The ratio of powder to solvent was kept at 1:5, 1:10, 1:25, 1:50 and 1:100 and the RSA and polyphenols of various extracts were determined as described above.
Scanning Electron Microscopy (SEM) of DPPP particles after extraction
The surface topography of the DPPP after extraction was studied by SEM studies using scanning electron microscope (Model 435VP Leo Electronic Systems, Cambridge, UK). DPPP samples were dried in a hot air oven and separately placed on the sample holder and sputter coated with gold. The samples were observed at 15KV and vacuum of 9.75 × 10−5 torr. The 20,000X scanning electron micrographs of the DPPP particles extracted with water, water: EtOH (1:1 v/v) and EtOH are presented as Fig. 2a, b and c, respectively.
Fig. 2.
Scanning electron micrographs (magnification x20,000) of DPPP extracted with (a) water (b) water:EtOH (50:50 v/v) and (c) EtOH
Effect of duration of extraction
DPPP was separately extracted with Water, EtOH and water-EtOH (1:1 v/v), in 1:10 ratio of powder to solvent, for 0, 1, 2, 4, 8, 12 and 24 h with intermittent shaking to optimize the duration of extraction. After respective duration of extraction and centrifugation, RSA and polyphenols were determined. DPPP was separately extracted in EtOH:water (1:1 v/v) for 2, 5, 8 and 12 days at room temperature and RSA and polyphenol content of the extracts were determined.
Effect of number of extractions
In order to find the efficiency of extractions, DPPP was consecutively extracted with water-EtOH mixture (1:1 v/v) by soaking the residue left over after the first extraction with a fresh batch of solvent system (second extraction). The process was repeated again to get the third extraction and the RSA and polyphenol content of the extracts were determined.
Effect of particle size
To determine the effect of particle size on the extraction, DPPP was consecutively sieved through a series of sieves with decreasing particle size of 1610 to 85 μ. DPPP fractions obtained from different sieves were soaked separately with water EtOH (1:1 v/v) mixture in 1:10 solid-solvent ratio at room temperature, as described above. RSA and polyphenol content of the all the extracts were determined as above.
Effect of storage on the RSA profile of extract
The extracts from DPPP were stored in Teflon tubes at room temperature (28–30 °C), and 4, 0 and −80 °C, to study the profile of RSA and the contents of polyphenol, punicalagins and ellagic acid during storage, as described above.
Analyses of polyphenols, punicalagins and ellagic acid during storage
The contents of polyphenol, punicalagins and ellagic acid, were also determined in the extracts stored at different temperatures. The punicalagin content of the extracts was determined on analytical reverse phase HPLC (Waters Alliance e2695 model, Austria, integrated with Empower-3 software, Varian C-18 column 250 × 4.6 mm id, 5 μm pore size, 2998 model PDA detector 260 nm), as described by Baugh et al. (2014) using 1 % formic acid in Milli-Q water and acetonitrile as mobile phase. The gradient mode was; 0–18 min, 95 % formic acid, 18–20 min, 85 % formic acid, 20–25 min, 35 % formic acid, 25–30 min, 95 % formic acid, with a flow rate of 1 mL/min. The variable injection volumes were from 1 to 10 μl and the quantitation wavelength was set at 260 nm.
The ellagic acid content was determined on analytical reverse phase HPLC, as described by Panichayupakarananta et al. (2010) on Waters HPLC system, as for punicalagin analysis. The mobile phase consisted of methanol and 2 % aqueous acetic acid, gradient mode (0–15 min, 40–60 % v/v methanol and 15–20 min, 60%v/v methanol) with a flow rate of 1 mL/min. The variable injection volumes were from 1 to 10 μl. The quantitation wavelength was set at 254 nm.
Statistical analysis
All the experiments were carried out in triplicates and the results are expressed as Mean ± SD. Data analyzed by non-parametric one-way ANOVA followed by post hoc Tukey’s test (P < 0.05).
Results and discussion
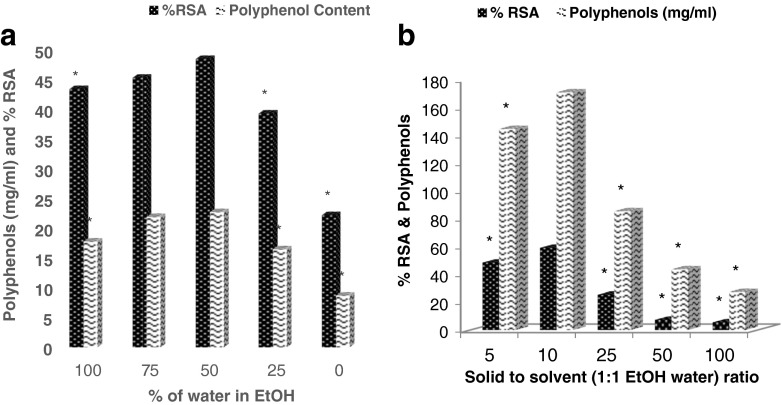
Effect of extractant composition and solid solvent ratio (Fig. 1a and b)
Fig. 1.
Effect of (a) extractant composition and (b) solid-solvent ratio on the extraction of DPPP (* p < 0.05 as compared to the values for 1:1 EtOH water)
From the data presented in Fig. 1a, it was obvious that although water alone could efficiently extract polyphenols from DPPP exhibiting good RSA, however, with increasing concentration of EtOH till 50 % concentration, an increase in both RSA and quantity of polyphenol was observed. Interestingly, beyond 50 % EtOH concentration, a significant decrease in RSA and polyphenol (p < 0.05) was noted. Hence 1:1 (v/v) ratio of water:EtOH could be chosen for extraction of DPPP. Figure 1b depicts the effect of solid to solvent ratio on the efficiency of extraction, RSA profile and yield of polyphenols. It was observed that solid to solvent ratio of 1:10 yielded significantly high RSA and polyphenol content than other combinations, hence, was considered for further studies. Our results are also in concurrence with the observations of Shi et al. (2003) who recommended the use of 50 % aqueous EtOH for the extraction of polyphenols from grape seeds and obtained maximum yield of polyphenols at solid solvent ratio of 1:7.5. The addition of water may increase the extraction efficiency of the solvent (Mashkor 2014). Qu et al. (2010) also mentioned the increase in the extract yield from pomegranate marc by increasing the solid-solvent ratio using water as extractant. Tabaraki et al. (2012) described the application of 70 % aqueous EtOH for extracting pomegranate peel at 60 °C temperature for 30 min. Foss et al. (2014) employed 90 % hydroalcoholic solution to extract pomegranate peel at room temperature for 5 days extraction. The use of EtOH in combination with water could probably bring about higher order of damage to the peel tissue than caused by individual components. This may also increase the bulging of the peel particles, thus increasing the surface area for more contact with solvent system (Mashkor 2014). This effect could be visualized in scanning electron micrographs of the particles after extraction. In addition, our method of extraction did not employ any extensive use of energy or very large duration, as described above while using EtOH-water combination.
Scanning Electron Microscopy of DPPP particles after extraction (Fig. 2a, b and c)
The scanning electron micrographs of the DPPP extracted with (A) water (B) water:EtOH (1:1 v/v) and (C) EtOH are presented in Fig. 2. The SEM topography of the surface of the DPPP depicts the smoothness of the surface of the water extracted particles, thus may allow the movement of the solvent by absorption / imbibition (Fig. 2a), while the surface of the EtOH extracted particles (Fig. 2c) presents a shrunken and dehydrated topography (shown by arrows), hence the free movement of solvent may be restricted as compared to particles extracted in water. On the other hand, the surface of particles extracted with 1:1 (v/v) combination of water and EtOH (Fig. 2b) appears to be extensively damaged with many pores and cervices and larger surface area generated, which may facilitate the movement of the solvent(s). This could be correlated with the effective surface sterilization by aqueous EtOH than with EtOH alone resulting in higher yields of polyphenols and high RSA of the extract (Ankita et al. 2012).
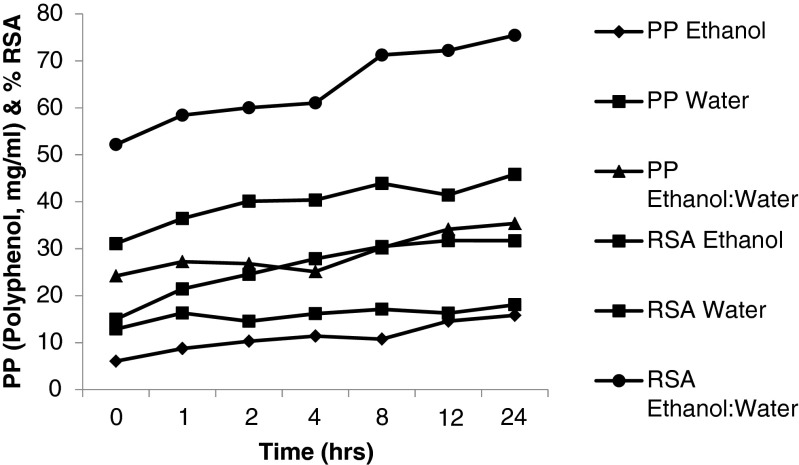
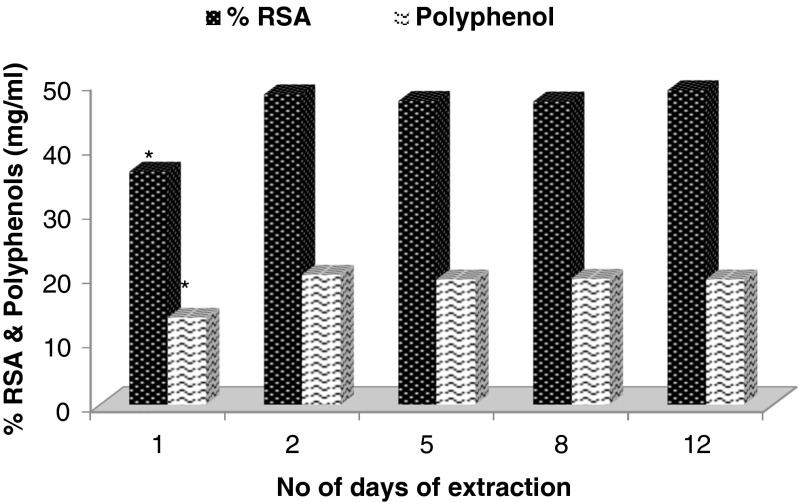
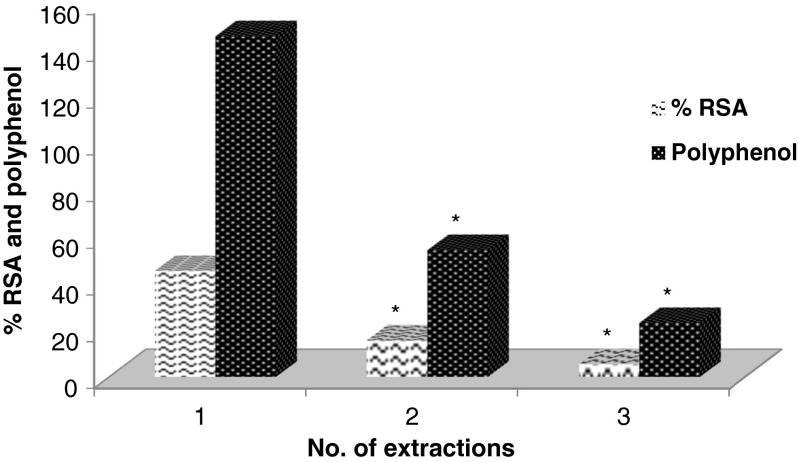
Effect of duration and number of extractions (Figs. 3, 4 and 5)
Fig. 3.
Effect of time and solvent on the extraction of DPPP
Fig. 4.
Effect of duration on the extraction of DPPP with 1:1 EtOH:water at room temperature (* p < 0.05 as compared to the values for 2 days extraction)
Fig. 5.
Effect of no. of extractions on the RSA and yield of polyphenol from DPPP (* p < 0.05 as compared to the values for first extraction)
Figure 3 shows a gradual increase in the RSA and polyphenol content of the extracts with increase in the duration of extraction. The yield of polyphenol and RSA was found to be higher for 1:1 (v/v) ratio of EtOH: water than either by water or EtOH alone. The effect of duration on the extraction was also studied by separately extracting DPPP for different durations and determining RSA and polyphenols in the respective extracts and the data are presented in Fig. 4. The RSA and polyphenol content have been shown to be increasing in the extracts prepared on day two than on day one. However, extraction beyond 2 days neither resulted in any significant increase in RSA profile nor in polyphenol content. Similar pattern of extraction was reported for grape seed meal by Shi et al. (2003) who used aqueous EtOH for extraction of polyphenols and showed that two consecutive extractions with aqueous EtOH may bring out >90 % of phenolics from the source. Hence, further extractions were carried out for 1 day duration only to standardize other parameters.
To determine the efficiency of extraction of DPPP, the effect of number of extractions was carried out. DPPP was consecutively extracted with EtOH-water (1:1 v/v) and the results of RSA and polyphenols of the extracts are exhibited in Fig. 5. It is evident that the first extraction removed the major content of polyphenols from DPPP and the extract exhibited pronounced RSA. Hence, further studies were carried out only with single extraction of DPPP, which could be a method of choice to minimize the use of solvent system. Similar pattern of extraction was reported for grape seed meal by Shi et al. (2003) who used of aqueous EtOH for extraction of polyphenols and showed that two consecutive extractions may bring out >90 % of phenolics from the source.
Effect of particle size (Table 1)
Table 1.
Effect of particle size of the DPPP on the efficiency of extraction (* p < 0.05 as compared to control)
| Sl. No. | Mesh size μ | %RSA | Polyphenol mg/ml |
|---|---|---|---|
| 1. | Native | 48.45 ± 0.08 | 22.74 ± 0.63 |
| 2. | 1610 | 42.02 ± 0.66* | 21.63 ± 0.35 |
| 3. | 1420 | 43.78 ± 0.66* | 22.81 ± 0.93 |
| 4. | 1110 | 46.31 ± 0.42 | 23.50 ± 0.47 |
| 5. | 400 | 47.48 ± 1.91 | 23.65 ± 0.13 |
| 6. | 150 | 47.72 ± 0.08 | 24.31 ± 0.13* |
| 7. | 132 | 47.81 ± 0.91 | 25.25 ± 0.68* |
| 8. | 118 | 48.13 ± 0.33 | 26.09 ± 0.29* |
| 9. | 85 | 50.41 ± 2.08* | 26.48 ± 0.33* |
| 10. | <85 | 54.70 ± 0.65* | 27.81 ± 0.71* |
Table 1 depicts %RSA and polyphenols of the extracts prepared with different particle sizes of DPPP which does not indicate any precise pattern of change in RSA and polyphenol with changing particle size and only minor changes in RSA and polyphenols have been noticed. Thus, the particle size of 100–400 μ could be recommended for the efficient extraction of DPPP.
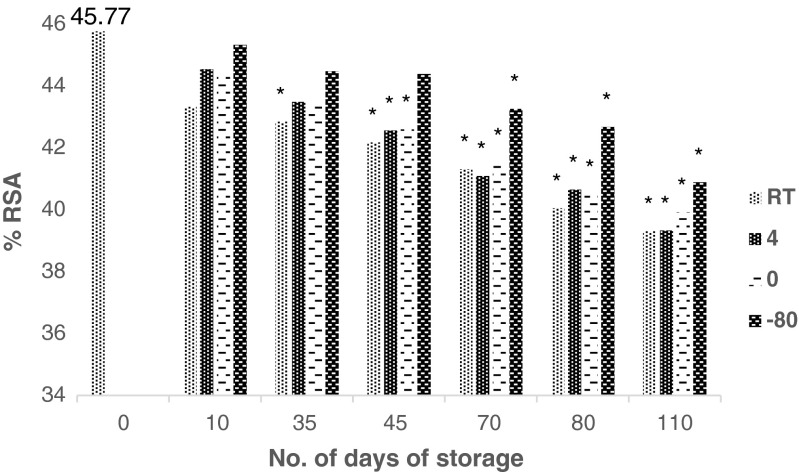
Effect of storage stability of extract (Fig. 6 and Table 2)
Fig. 6.
Effect of storage at different temperatures (room temperature 28–30, 4, 0 and −80 °C) on RSA profile of the extract (* p < 0.05, as compared to the values for zero day)
Table 2.
Contents of polyphenols, punicalagin and ellagic acid during storage (Ei : initial extract, ERT : extract at room temperature, E0 ; extract at 0 °C, E−80: extract at −80 °C (* p < 0.05 as compared to control)
| No of days | Sample | Polyphenols | Punicalagin (mg/g DPPP) | Ellagic acid (mg/g DPPP) | |
|---|---|---|---|---|---|
| mg/ml extract | B | A | |||
| 0 | Ei | 22.59 ± 0.41 | 43.71 ± 1.21 | 69.53 ± 1.91 | 2.98 ± 0.21 |
| 45 | ERT | 20.15 ± 0.31* | 42.89 ± 0.46 | 68.63 ± 2.74 | 2.92 ± 0.32 |
| E4 | 20.07 ± 0.37* | 38.89 ± 1.07* | 67.95 ± 1.89 | 2.84 ± 0.53 | |
| EO | 20.51 ± 0.45* | 38.72 ± 2.99* | 66.14 ± 2.14 | 2.87 ± 0.23 | |
| E−80 | 22.16 ± 0.24 | 40.79 ± 2.71* | 63.79 ± 0.87* | 2.85 ± 0.41 | |
| 80 | ERT | 20.45 ± 0.34* | 40.28 ± 2.44* | 68.06 ± 1.31 | 2.84 ± 0.49 |
| E4 | 20.69 ± 0.53* | 38.11 ± 2.95* | 66.30 ± 3.17 | 2.51 ± 0.48* | |
| EO | 20.45 ± 0.24* | 37.59 ± 1.76* | 65.34 ± 0.97* | 2.43 ± 0.32* | |
| E−80 | 21.60 ± 0.37* | 38.88 ± 1.12* | 63.19 ± 1.19* | 2.42 ± 0.38* | |
| 110 | ERT | 19.60 ± 0.46* | 39.79 ± 2.35* | 67.55 ± 0.35 | 2.16 ± 0.49* |
| E4 | 19.70 ± 0.49* | 38.47 ± 1.94* | 66.07 ± 0.76 | 1.96 ± 0.34* | |
| EO | 19.12 ± 0.30* | 37.29 ± 2.55* | 64.89 ± 2.47* | 1.91 ± 0.50* | |
| E−80 | 20.38 ± 0.06* | 38.18 ± 1.89* | 61.53 ± 1.68* | 1.98 ± 0.36* | |
The RSA pattern of the extracts stored at different temperatures are shown in Fig. 6. The polyphenols, punicalagins and ellagic acid contents were determined in the extracts at regular intervals and the results are expressed in Table 2. The RSA profile of the extracts stored at different temperatures did not change drastically (6–15 % for RT, 3–14 % for 4 °C, 3–13 % for 0 °C and 1–11 % for −80 °C) after 110 days of storage. In case of storage at room temperature, significant change in RSA profile was observed in contrast to storage at 4, 0 and −80 °C. The observations are in concurrence with the data on the contents of polyphenol, punicalagins and ellagic acid in the stored extracts. The polyphenol content was reduced by 14, 13, 16 and 10 %, respectively, during 110 days of storage at RT, 4, 0 and −80 °C. The levels of punicalagin A were reduced by 9, 12, 15 and 13 % and punicalagin B by 3, 5, 7 and 12 % during 110 days of storage at RT, 4, 0 and −80 °C. The content of ellagic acid was reduced to a larger extent than either polyphenol or punicalgin A or B (28, 34, 36 and 34 % change) during 110 days of storage at RT, 4, 0 and −80 °C, respectively.
Conclusions
Various reports indicate the superiority of the pomegranate peel in possessing health beneficial properties than arils, hence studies have been carried out for the extraction of DPPP using different solvent / solvent systems. Water alone is a good extractor of the polyphenols from pomegranate peel and stands only second to methanol. However, the use of combination of EtOH and water gives higher yield and RSA of the extract than from individual solvents, as EtOH improved the efficiency of extraction with water. The surface topography studies of extracted DPPP particles, as observed by SEM also supports the concept. This could also bear correlation with surface sterilization by aqueous EtOH which is more effective than EtOH alone, hence resulting in higher yield of extract with high RSA and polyphenol content in the extracts prepared with water-EtOH combination. The extraction of DPPP at room temperature with EtOH-water may be an ecofriendly approach in view of the concerns pertaining to the residual solvent and energy. In a nutshell, single extraction with a combination of EtOH and water (1:1 v/v) with solid to solvent ratio of 1:10 for the duration of 24–48 h at room temperature (energy efficient) for the particle size of 100–400 μ may be recommended for the efficient extraction of DPPP. The data obtained could be further amplified to scale up of the process at pilot level and the studies may be taken forward towards efficient and economical drying and characterization of the dried extract to establish the effectiveness of the extract for its various applications, including in food industries.
Acknowledgments
The authors thank Head, Department of Biochemistry and Nutrition and Director, CSIR-CFTRI, Mysore for their kind help and encouragement. Venkataramanamma D. and Aruna P. express their gratitude and sincere thanks to Department of Science & Technology (DST), New Delhi for awarding INSPIRE Fellowship to them.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Abdel Moneim AE, Mohamed AD, Al-Quraishy S. Studies on the effect of pomegranate (Punica granatum) juice and peel on liver and kidney in adult male rats. J Med Plants Res. 2011;5(20):5083–5088. [Google Scholar]
- Adams L, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate tannins and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006;54:980–985. doi: 10.1021/jf052005r. [DOI] [PubMed] [Google Scholar]
- Adhami VM, Mukhtar H. Anti-oxidants from green tea and pomegranate for chemoprevention of prostate cancer. J Mol Biotechnol. 2007;37:52–57. doi: 10.1007/s12033-007-0047-8. [DOI] [PubMed] [Google Scholar]
- Ajaikumar KB, Asheef M, Babu BH, Padikkala J. The inhibition of gastric mucosal injury by Punica granatum L (pomegranate) methonalic extract. J Ethanopharmacol. 2005;91:171–176. doi: 10.1016/j.jep.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Ankita, Preethi S, Singh RP, Prapulla SG (2012) Innovative methods of preparation of antioxidant rich fractions from non-edible portions of pomegranate, presented in 22nd Indian Convention of Food Scientists and Technologists, (ICFoST 2012) “SAFEST INNOVATIONS” organized by AFST (I)
- Aviram M, Dornfeld L, Rosenblat M, Volkova N, Kaplan M, Coleman R. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications of LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr. 2000;71:1062–1076. doi: 10.1093/ajcn/71.5.1062. [DOI] [PubMed] [Google Scholar]
- Awika JM, Rooney LW, Wu XL, Prior RL, Cisneros-Zevallos L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J Agric Food Chem. 2003;51(23):6657–6662. doi: 10.1021/jf034790i. [DOI] [PubMed] [Google Scholar]
- Bondet V, Brand-Williams W, Berset C. Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. J Food Sci Technol. 1997;30(6):609–615. [Google Scholar]
- Çam M, Icyer NC. Phenolics of pomegranate peels: extraction optimization by central composite design and alpha glucosidase inhibition potentials. J Food Sci Technol. 2013 doi: 10.1007/s13197-013-1148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria A, Monteiro R, Mateus N, Azevedo I, Calhau C. Effect of pomegranate (Punica granatum) juice intake on hepatic oxidative stress. Eur J Nutr. 2007;46:271–278. doi: 10.1007/s00394-007-0661-z. [DOI] [PubMed] [Google Scholar]
- Foss SR, Nakamura CV, Ueda-Nakamura T, Cortez DAG, Endo EH, Filho BPD. Antifungal activity of pomegranate peel extract and isolated compound punicalagin against dermatophytes. Ann Clin Microbiol Antimicrob. 2014;13:32–37. doi: 10.1186/s12941-014-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen IM, Meyer AS, Frankel EN. Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidation. J Agric Food Chem. 1998;46:4107–4112. doi: 10.1021/jf980181c. [DOI] [Google Scholar]
- Ibrahium MI. Efficiency of pomegranate peel extract as antimicrobial, antioxidant and protective agents. J Agric Sci. 2010;6(4):338–344. [Google Scholar]
- Khan N, Afaq F, Kweon MH, Kim K, Mukhtar H. Oral consumption of pomegranate fruit extract inhibits growth and progression of primary lung tumors in mice. Cancer Res. 2007;67:3475–3482. doi: 10.1158/0008-5472.CAN-06-3941. [DOI] [PubMed] [Google Scholar]
- Kulkarni AP, Aradhya SM, Divakar S. Isolation and identification of a radical scavenging antioxidant–punicalagin from pith and carpellary membrane of pomegranate fruit. J Food Chem. 2004;87:551–557. doi: 10.1016/j.foodchem.2004.01.006. [DOI] [Google Scholar]
- Mashkor IMAA. Total phenol, total flavonoids and antioxidant activity of pomegranate peel. Int J ChemTech Res. 2014;6(11):4656–4661. [Google Scholar]
- Murthy KNC, Jayaprakasha GK, Singh RP. Studies on the antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J Agric Food Chem. 2002;50(17):4791–4795. doi: 10.1021/jf0255735. [DOI] [PubMed] [Google Scholar]
- Negi PS, Jayaprakasha GK. Antioxidant and antibacterial activities of Punica granatum peel extracts. J Food Sci. 2003;68(4):1473–1477. doi: 10.1111/j.1365-2621.2003.tb09669.x. [DOI] [Google Scholar]
- Panichayupakarananta P, Issuriya A, Sirikatitham A, Wang W. Antioxidant assay-guided purification and LC determination of ellagic acid in pomegranate peel. J Chromatogr Sci. 2010;48:456–459. doi: 10.1093/chromsci/48.6.456. [DOI] [PubMed] [Google Scholar]
- Qu WJ, Pan ZL, Zhang RH, Ma HL, Chen XG, Zhu BN. Integrated extraction and anaerobic digestion process for recovery of nutraceuticals and biogas from pomegranate marc. Trans ASABE. 2009;52(6):1997–2006. doi: 10.13031/2013.29196. [DOI] [Google Scholar]
- Qu WJ, Pan ZL, Ma HL. Extraction modeling and activities of antioxidants from pomegranate marc. J Food Eng. 2010;99:16–23. doi: 10.1016/j.jfoodeng.2010.01.020. [DOI] [Google Scholar]
- Shi J, Yu J, Pohorly J, Young JC, Bryan M, Wu Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. Food Agric Environ. 2003;1(2):42–47. [Google Scholar]
- Singh RP, Murthy KNC, Jayaprakasha GK. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem. 2002;50(1):81–86. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- Tabaraki R, Heldarrizadi E, Benvidi A. Optimization of ultrasonic-assisted extraction of pomegranate (Puncia granatum L.) by response surface methodology. Sep Purif Technol. 2012;98:6–23. doi: 10.1016/j.seppur.2012.06.038. [DOI] [Google Scholar]
- Wang ZB, Pan ZL, Ma HL, Atungulu GG. Extract of phenolics from pomegranate peels. J Open Food Sci. 2011;5:17–25. doi: 10.2174/1874256401105010017. [DOI] [Google Scholar]
- Yunfeng L, Changjiang G, Jijun Y, Jingyu W, Jing X, Shuang C. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. J Food Chem. 2006;96:254–260. doi: 10.1016/j.foodchem.2005.02.033. [DOI] [Google Scholar]
- Zhang J, Zhan B, Yao X, Shong J. Antiviral activity of tannin from the pericarp of Punica granatum L. against genital herpes virus in vitro. Zhongguo Zhongyao Zazhi. 1995;20(9):556–558. [PubMed] [Google Scholar]
Web references
- Baugh S, Revell J, Eastman K (2014) Determination of the Punicalagins found in pomegranate by high performance liquid chromatography. http://www.dionex.com. Accessed 24.01.2014