Abstract
Effect of different level (60, 120 and 180 kg N/ha) of nitrogen (N) application on protein profiling, pasting and cooking quality characteristics of milled rice from different paddy cultivars was evaluated. N dose showed positive correlation with protein content and negative correlation with L*, whiteness and amylose content. N application significantly affected the protein profile, textural and pasting properties of different cultivars. All the cultivars expect PR120 and PAU201, showed an increase in the amount of accumulation of 60 kDa polypeptide with increase in N application. Accumulation of prolamines (16 and 14 kDa) and polypeptides of 38 and 35 kDa increased in all the cultivars. Size exclusion chromatography revealed decrease in low molecular weight subunits and increase in medium molecular weight subunits in all the cultivars upon N application. However, high molecular weight subunits increased in IET21214 and decreased in PR120 and PAU20 upon N application. N application resulted in increase in glutelins and decrease in peak and breakdown viscosity. PAU201 and PR120 showed lower AAC due to low accumulation of 60 kDa granule-bound starch synthase (GBSS), in response to N application. Gumminess and hardness of cooked rice increased with the increase in N dose and the increase was significant at 60 kg N/ha.
Keywords: Nitrogen, Rice, Protein profile, Gel chromatography, Pasting properties
Introduction
Rice cultivars differ in milling, cooking and textural properties as well as their suitability for different purpose. Genetic traits, environment and cultural practices are responsible for variation in rice quality Singh et al. (2005). Singh et al. (2014) showed that an increase in environmental temperature can affect grain color, protein, lipid and amylose content, and texture and pasting characteristics. The changes in pasting behaviour of rice have been associated with accumulation of Wx gene and the promoter of Wx gene is sensitive to temperature of the growing environment (Bao et al. 2004; Jiang et al. 2003). Nitrogen (N) application is important for high rice yields and also affects the processing and cooking quality of rice by changing the protein and amylose content in the rice grain (Prakash et al. 2002; Champagne et al. 2007). N effects both total protein and its subunits content. Protein is a major factor in determining the textural, pasting and sensory characteristics of milled rice (Ning et al. 2010). Majority of rice proteins are glutelins and prolamins however, glutelins are nutritionally more important than prolamins due to their higher lysine content and greater digestibility (Islam et al. 1996). N application is reported to have a favorable effect on milling quality by modifying protein distribution in rice grains (Leesawatwong et al. 2005). The apparent flexibility of rice farmers in adjusting the time and amount of fertilizer application offers potential to synchronize N application with the real-time demand of the rice crop (Witt et al. 2005). Rate and time of N application are critical in terms of their effects plant height, leaf size, panicle number, spikelet number, number of filled spikelets and yield (Shakouri et al. 2012). Improvement in protein quality by decrease in phytic acid content and increase in protein fractions and total protein with N application has also been reported (Ning et al. 2009). Various studies on effect of N on yield and few quality attributes have been conducted; however, the effect of application of variable doses N on protein profiling, pasting and textural properties of different rice cultivars is scanty. The present study was undertaken to evaluate the effects of varying doses of N application on physicochemical, protein profile and quality characteristics of different rice cultivars.
Materials and methods
Materials
The experiment was carried out at the research farm of the Punjab Agricultural University, Ludhiana, (30°56/N, 75°52/E) in northwest India during the wet seasons (June–October) of 2010. The regional climate is sub-tropical with a hot, wet summer with monsoon (July–September) and a cool, dry winter (November–February). Average annual rainfall at the research farm is 734 mm, 85 % of which falls during the monsoon period. The soil at the experimental site is Fatehpur loamy sand (Entisol, Typic Ustipsament,) with pH of 7.4, total N 0.04 %, P (Olsen P) 6.3 mg kg−1, and NH4OAc-exchangable K 45 mg kg−1. The depth to the groundwater at the site was below 25 m. The site had been under rice-wheat cropping system for 8 years prior to start of the experiment. The experiment, replicated thrice, was laid out in a split-plot design with four N levels (0, 60, 120 and 180 kg N ha−1) as main plots and six cultivars viz. IR64, IET21205, IET21208, IET21214, PR120 and PAU201, as subplots. Cultivars were selected on the basis of their superior performance (grain yield) in breeding trials on DSR. IR 64 was selected as a check. The field was disked twice, followed by planking after a pre-sowing irrigation. Seeds were sown on dry soil in the third week of June with a hand-held furrow opener at 20-cm row spacing and a seed rate of 30 kg ha−1 (Mahajan and Chauhan 2016). After sowing, two irrigations of 50 mm depth were given for proper germination and establishment (one immediately after sowing and a second 5 days after sowing). The subsequent irrigation, each of 50 mm depth, was applied with a Parshall flume when soil moisture potential, measured by a tube tensiometer installed at 15 cm depth, reached 15 kPa. The potentials were measured every morning at 9 A.M. with a Soil-Spec vacuum gauge to determine the need for irrigation on that day. N (urea) was applied in each plot with respective doses in four equal splits: 15, 30, 45, 60 days after sowing (DAS) (Mahajan et al. 2011). Weeds were controlled by applying a pre-emergence herbicide (pendimethalin 0.75 kg ha−1) 3 DAS, and a post-emergence herbicide (bispyribac 25 g ha−1) 20 DAS. Weeds that escaped these herbicides were removed manually. All plots were sprayed twice at weekly interval with 1 % ferrous sulfate solution (250 l ha−1) at 25 DAS to alleviate the iron deficiency. The crop was harvested at 15 %–18 % grain moisture. The paddy samples were dehusked on a McGill sample sheller (Rapsco, TX, USA). The brown rice samples obtained were polished in a McGill mill No. 2 (Rapsco, Brookshire, TX, USA) to obtain a 6 % degree of milling (Singh et al. 2006). Milled whole rice kernels were separated from broken rice for the evaluation of physicochemical, cooking and textural properties.
Methods
Colour characteristics
Color parameters (L*, a*, b*) of milled rice kernels from different cultivars were carried out in triplicates, using a ultra scan VIS Hunter Lab (Hunter Associates Laboratory Inc., Reston, VA, U.S.A). The L* value indicates the lightness, a* value gives the degree of red-green color, with a higher positive a* value indicating more red. The b* value indicating the degree of the yellow-blue color, with a higher positive b* indicating more yellow. Whiteness was determined using:
Length-breadth ratio of milled rice
Length and breadth arrangement of milled rice was done and their cumulative measurements (in mm) were taken. The value of L/B was determined by dividing length by breadth. A mean of 10 replications was reported.
Cooking characteristics
Head rice (2 g) samples were taken in test tube from each cultivar and cooked in 20 ml distilled water in a boiling water bath. The cooking time was determined by removing a few kernels at different time intervals during cooking and pressing them between two glass plates until no white core was left (Singh et al. 2005).
Length-breadth ratio (L/B)
Length and breadth of the cooked rice was determined as described above.
Gruel solid loss (GSS)
Head rice samples (2 g) in 20 ml distilled water, for each cultivar, were cooked for minimum cooking time in a boiling water bath. The gruel was transferred to 50 ml beakers with several washings and volume was made up with distilled water. The aliquot having leached solids was evaporated at 110 °C in an oven until completely dry. The solids were weighed and percent gruel solids were reported.
Textural properties
Textural profile analysis (TPA) of the cooked rice was performed using a texture analyzer (TA-XT2, Texture technologies corp. UK) with a 5 kg load cell using a two-cycle compression (Park et al. 2001; Juliano et al. 1984; Singh et al. 2014).
Flour characteristics
Milled rice was ground to a uniform size using 60 mesh sieve to obtain flour. Moisture, protein and ash content of rice were determined by AACC method (1995).
Apparent amylose content
Apparent amylose content (AAC) was determined using method of Williams et al. (1970).
Pasting properties
The pasting properties of rice bean starch gels were evaluated using Rheoplus (Anton Parr, Molecular Compact Rheometer, Model MCR302) from different legume varieties. Starch (3 g sample in 25 ml distill water) was weighed directly in the aluminium RVA sample canister and distilled water was added to a total constant sample weight 28 g. A programmed heating and cooling was used where samples were held at 50 °C for 1 min, heated to 95 °C in 7.5 min, held at 95 °C for 5 min, cooling from 95 °C to 50 °C in 7.5 mins and holding at 50 °C for 2 mins. Parameters recorded were pasting temperature, peak viscosity (PV), breakdown viscosity (BV), setback viscosity (SBV) and final viscosity (FV) (Kaur et al. 2015).
Gel filtration chromatography
Supernatant used earlier for SDS-PAGE was used for FPLC analysis. 1 ml supernatant was centrifuged at 14,000 rpm for 10 min and filtered through 0.22ηm syringe filter. Gel filtration was carried out by using an AKTA purifier system equipped with a Superose 12 HR 10/30 column (GE Healthcare, Barcelona, Spain). The 50 mM Tris-HCl pH 7.8 buffer at a flow of 0.4 ml /min, was used for elution (Ghumman et al. 2016; Vioque et al. 2012). The eluate was monitored at 280 nm. Apparent molecular masses were determined using blue dextran 2000 (2000 kDa), b-amylase (200 kDa), bovine serum albumin (67 kDa), and ribonuclease A (13.7 kDa) as molecular weight standards (Pharmacia) and calculating molecular weight using UNICORN software.
Protein profiling
Total seed storage proteins were extracted according to the method of Kawakatsu et al. (2008). SDS-PAGE analysis of seed storage proteins was carried out according to modified method of Laemmli (1970) as described by Singh et al. (2014).
Statistical analysis
The data were subjected to two way analysis of variance (ANOVA) and Duncan’s test (p ≤ 0.05) using Minitab Statistical Software (MINITABR v 14.12.0, State College, PA). Pearson correlation coefficients (r) were calculated for determining the relationship between different parameters.
Results and discussion
Milled rice characteristics
TGW of milled rice from different rice cultivars ranged from 17.39 to 24.18 g. TGW was observed to be the highest for IET21208 and the lowest for PR120 (Table 1). F value shows TGW vary significantly with cultivars and N application (Tables 2 and 3). TGW of milled rice from paddy grown with N doses was significantly higher than that of milled rice from paddy grown without N. TGW of milled rice showed positive correlation with L* value (r = 0.426, p ≤ 0.05) (Table 4). Both cultivars and N dose affected colour parameters of milled rice significantly. The L* value for milled rice from paddy grown without N dose ranged from 63.3 (PAU201) to 75 (IET21208). L* value was the highest for IET21208 indicating that the grains were the lightest amongst the cultivars studied and the lowest for PAU201 and PR120 milled rice. The results revealed that L* value of milled rice decreased with N dose. Minimum L* value of 60.60 was observed for PAU201 at 180 kg N dose. PAU201 and PR120 also showed minimum whiteness values. Whiteness of milled rice ranged between 58 and 71.9. Whiteness of milled rice without N dose was higher than paddy grown with N dose. This may be due to increase in protein with increase in N dose. The a* value of milled rice from paddy grown without N was significantly lower as compared with milled rice from paddy grown with different N doses. The a* value of milled rice increased progressively with N dose but b* value increased only upto 120 kg N application. Light coloured milled rice grains were found to have lower protein content (r = −0.591, p ≤ 0.005). L* value was correlated to packing of granules by Lanning et al. (2012). This indicated that the N dose affected the packing of granules in grain. Higher L* and lower b* value of milled rice has been attributed to higher chalkiness (Singh et al. 2014). A positive relationship between whiteness and chalkiness was observed earlier (Lanning and Siebenmorgen 2013). It was reported that whiteness generally increased with increase in chalkiness, whereas, yellowness decreased as chalkiness increased (Ashida et al. 2009).
Table 1.
Effect of different doses of nitrogen application on physicochemical properties of milled rice from different paddy cutivars
| Variety | Nitrogen application (Kg/ha) | TGW (g) | L* | a* | b* | Whiteness | Ash Content (%) | Protein Content | Amylose Content (%) |
|---|---|---|---|---|---|---|---|---|---|
| IR64 | 0 | 19.01 ± 0.13 | 68.87 ± 0.76 | 0.84 ± 0.03 | 13.24 ± 0.26 | 66.2 ± 0.12 | 0.32 ± 0.02 | 7.14 ± 0.15 | 15.78 ± 0.11 |
| 60 | 20.66 ± 0.12 | 65.22 ± 0.32 | 1.13 ± 0.04 | 13.27 ± 0.15 | 62.8 ± 0.19 | 0.37 ± 0.02 | 7.56 ± 0.04 | 15.50 ± 0.09 | |
| 120 | 20.82 ± 0.28 | 67.77 ± 0.33 | 1.12 ± 0.05 | 13.53 ± 0.39 | 65.0 ± 0.11 | 0.53 ± 0.02 | 7.70 ± 0.15 | 15.45 ± 0.05 | |
| 180 | 20.37 ± 0.01 | 66.45 ± 0.43 | 1.20 ± 0.05 | 13.25 ± 0.35 | 63.9 ± 0.13 | 0.34 ± 0.04 | 7.90 ± 0.01 | 15.11 ± 0.10 | |
| IET21205 | 0 | 17.89 ± 0.10 | 70.23 ± 0.24 | 0.62 ± 0.03 | 13.59 ± 0.12 | 67.3 ± 0.17 | 0.52 ± 0.20 | 6.87 ± 0.10 | 19.80 ± 0.11 |
| 60 | 21.11 ± 0.19 | 69.22 ± 0.31 | 1.72 ± 0.09 | 15.25 ± 0.25 | 65.6 ± 0.16 | 0.68 ± 0.01 | 7.12 ± 0.04 | 18.50 ± 0.13 | |
| 120 | 19.10 ± 0.21 | 69.63 ± 0.43 | 1.75 ± 0.03 | 14.14 ± 0.17 | 66.4 ± 0.18 | 0.63 ± 0.02 | 7.50 ± 0.04 | 17.68 ± 0.02 | |
| 180 | 18.65 ± 0.16 | 64.88 ± 0.12 | 1.78 ± 0.10 | 13.35 ± 0.37 | 62.4 ± 0.15 | 0.73 ± 0.02 | 7.80 ± 0.10 | 17.60 ± 0.02 | |
| IET21208 | 0 | 21.47 ± 0.17 | 75.00 ± 0.33 | 0.44 ± 0.04 | 12.83 ± 0.03 | 71.9 ± 0.14 | 0.41 ± 0.01 | 6.28 ± 0.08 | 19.15 ± 0.06 |
| 60 | 24.18 ± 0.09 | 74.00 ± 0.57 | 0.47 ± 0.03 | 12.85 ± 0.05 | 71.0 ± 0.13 | 0.46 ± 0.01 | 6.73 ± 0.07 | 18.80 ± 0.02 | |
| 120 | 23.06 ± 0.37 | 73.07 ± 0.27 | 0.75 ± 0.05 | 14.29 ± 0.25 | 69.5 ± 0.11 | 0.63 ± 0.02 | 6.76 ± 0.06 | 18.42 ± 0.09 | |
| 180 | 23.50 ± 0.05 | 72.5 ± 0.28 | 0.93 ± 0.03 | 13.43 ± 0.26 | 69.4 ± 0.11 | 0.47 ± 0.02 | 6.99 ± 0.08 | 18.05 ± 0.01 | |
| IET21214 | 0 | 18.06 ± 0.23 | 72.68 ± 0.33 | 0.61 ± 0.06 | 14.38 ± 0.31 | 69.1 ± 0.12 | 0.68 ± 0.02 | 7.05 ± 0.14 | 13.22 ± 0.05 |
| 60 | 18.77 ± 0.18 | 66.07 ± 0.11 | 0.66 ± 0.03 | 15.06 ± 0.16 | 62.9 ± 0.18 | 0.57 ± 0.03 | 7.41 ± 0.08 | 12.82 ± 0.07 | |
| 120 | 18.85 ± 0.21 | 68.78 ± 0.11 | 0.74 ± 0.04 | 15.26 ± 0.33 | 65.2 ± 0.15 | 0.47 ± 0.02 | 7.80 ± 0.10 | 12.30 ± 0.03 | |
| 180 | 17.95 ± 0.16 | 67.36 ± 0.46 | 0.76 ± 0.03 | 14.62 ± 0.30 | 64.2 ± 0.14 | 0.76 ± 0.03 | 7.95 ± 0.11 | 11.14 ± 0.05 | |
| PR120 | 0 | 17.39 ± 0.26 | 65.98 ± 0.18 | 2.13 ± 0.05 | 14.81 ± 0.09 | 62.8 ± 0.13 | 0.43 ± 0.03 | 7.47 ± 0.27 | 4.84 ± 0.04 |
| 60 | 20.25 ± 0.22 | 62.09 ± 0.29 | 2.32 ± 0.12 | 14.91 ± 0.12 | 59.2 ± 0.13 | 0.49 ± 0.01 | 7.36 ± 0.03 | 4.70 ± 0.08 | |
| 120 | 19.81 ± 0.23 | 62.63 ± 0.19 | 3.08 ± 0.02 | 15.74 ± 0.25 | 59.3 ± 0.13 | 0.58 ± 0.02 | 8.00 ± 0.10 | 4.11 ± 0.10 | |
| 180 | 19.16 ± 0.05 | 63.40 ± 0.09 | 3.16 ± 0.02 | 14.96 ± 0.03 | 60.3 ± 0.12 | 0.57 ± 0.02 | 8.10 ± 0.09 | 3.91 ± 0.07 | |
| PAU201 | 0 | 19.47 ± 0.24 | 63.33 ± 0.52 | 1.23 ± 0.05 | 15.18 ± 0.10 | 60.3 ± 0.12 | 0.58 ± 0.02 | 6.50 ± 0.18 | 2.90 ± 0.02 |
| 60 | 19.49 ± 0.06 | 63.13 ± 0.35 | 1.33 ± 0.14 | 14.88 ± 0.01 | 60.2 ± 0.11 | 0.47 ± 0.02 | 7.50 ± 0.14 | 2.82 ± 0.01 | |
| 120 | 21.89 ± 0.17 | 65.63 ± 0.10 | 1.56 ± 0.08 | 16.09 ± 0.21 | 62.0 ± 0.15 | 0.35 ± 0.05 | 7.20 ± 0.10 | 2.71 ± 0.01 | |
| 180 | 19.45 ± 0.34 | 60.60 ± 0.48 | 2.06 ± 0.11 | 14.36 ± 0.35 | 58.0 ± 0.09 | 0.43 ± 0.02 | 7.54 ± 0.13 | 2.67 ± 0.02 |
Values ± Standard deviation
Table 2.
F values obtained from two-way ANOVA of physicochemical properties of milled rice
| Source | TGW | L* | a* | b* | L/B ratio | Ash content | Protein content | AAC |
|---|---|---|---|---|---|---|---|---|
| Nitrogen | 346.64** | 358.12** | 39.54** | 27.5** | 54.41** | 6.48** | 14.87** | 59.51** |
| Cultivar | 851.38** | 1551.46** | 1395.29** | 79.15** | 242.7** | 47.45** | 11.4** | 3528.45** |
| Interaction | 50.54** | 58.84** | 92.41** | 6.95** | 38.41** | 12.16** | 0.62 | 12.7** |
*Indicates p < 0.05.**Indicates p < 0.005
Table 3.
F values obtained from two-way ANOVA of cooking, textural and pasting properties of milled rice
| Source | Cooking time | L/B ratio | Gruel Solid loss | Coh. | Gum. | Har. | PV | FV | SV | BDV |
|---|---|---|---|---|---|---|---|---|---|---|
| Nitrogen | 30.66** | 32.03** | 67.91** | 0.93 | 7.4** | 15.3** | 560.61** | 998.71** | 483.32** | 791.92** |
| Cultivar | 38.01** | 25.72** | 165.52** | 0.87 | 42.19** | 75.14** | 501.05** | 138.11** | 41.75** | 224.03** |
| Interaction | 37.88** | 17.76** | 46.73** | 0.96 | 12.75** | 32.22** | 346.72** | 33.63** | 10.89** | 141.6** |
TKW Thousand kernel weight, AAC apparent amylose content, Coh. Cohesiveness, Gum. Gumminess, Har. Hardness, PV Peak viscosity, FV Final viscosity, SV Setback viscosity, BDV Breakdown viscosity, *Indicates p < 0.05.**Indicates p < 0.005
Table 4.
Pearson correlation coefficients between different milled rice characteristics
| TGW | L* | a* | b* | L/B | ash | Protein | AAC | PV | BV | FV | SV | GSS | Coh. | Gum. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | 0.426* | ||||||||||||||
| a* | – | −0.757** | |||||||||||||
| b* | – | −0.491* | 0.585** | ||||||||||||
| L/B | – | – | – | – | |||||||||||
| Ash | – | – | – | – | −0.511* | ||||||||||
| Protein | −0.441* | −0.591** | – | – | – | – | |||||||||
| Amylose | – | 0.764** | −0.597** | −0.719** | – | – | −0.421* | ||||||||
| PV | 0.554** | – | – | – | – | – | – | ||||||||
| BV | – | −0.567** | 0.553** | 0.484* | – | – | – | −0.672** | 0.737** | ||||||
| FV | – | 0.735** | −0.697** | – | −0.497* | – | – | 0.749** | – | −0.478* | |||||
| SV | – | 0.600** | −0.725** | −0.417* | −0.419* | 0.479* | – | – | – | −0.656** | – | ||||
| L/B | 0.492* | – | – | – | – | – | – | – | – | – | – | – | |||
| GSS | – | 0.425* | – | −0.416* | – | – | – | 0.449* | – | – | – | – | |||
| Coh. | 0.514* | 0.705** | −0.503* | – | – | – | −0.654** | 0.486* | – | – | 0.551** | – | 0.450* | ||
| Gum. | 0.538** | 0.538** | −0.515* | – | – | – | −0.401* | 0.525** | – | – | 0.538* | – | – | 0.453* | |
| Hard. | 0.449* | 0.423* | −0.453* | – | – | – | – | 0.460* | – | – | 0.451* | – | – | – | 0.973** |
*Indicates p < 0.05, **Indicates p < 0.005
Proximate composition
Milled rice from paddy grown with different doses of N had ash content between 0.32 % and 0.76 % and protein content between 6.28 % and 8.1 % (Table 1). Both ash content and protein content significantly varied with cultivar and N dose (Table 1). IET21214 milled rice from paddy grown with different doses of N had higher ash content as compared to milled rice from other cultivars. Protein content of the milled rice was significantly increased with N application (Table 1). PR120 milled rice with or without N dose showed maximum protein content. N dose, cultivar and their interaction effect on AAC was significant; however the effect of cultivar was more prominent (Table 2). AAC of different cultivars ranged between 2.67 and 19.8 % (Table 1). AAC decreased with increase in N dose. Higher reduction in AAC was observed for IET21205 and the lowest for IET21208. A significant negative correlation between AAC and N application was reported earlier by Champagne et al. (2009). PAU201 and PR120 showed significantly lower AAC as compared to other cultivars. Prakash et al. (2002), observed a similar decrease in AAC and increase in protein content with increase in nitrogen dose. Grain protein is derived mainly from mobilization of endogenous sources of nitrogen (Perez et al. 1973). Lower AAC may be attributed to higher amino acid associated protein metabolism which may reduce starch metabolism for balancing energy consumption.
Protein profile
SDS-PAGE analysis showed presence of 20 major polypeptides ranged from 12.0–120.0 kDa (± 2.0 kDa) (Figs. 1 and 2a). Kawakatsu et al. (2008), classified these as waxy of 60 kDa, glutelin acidic subunits (GAS) of 38 and 35 kDa, globulins of 25 kDa, glutelin basic subunits (GBS) of 22 and 21 kDa and prolamins of 12, 14, 16 and 18 kDa. Polypeptides of 60, 38, 35, 16, 14 and 12 kDa appeared to be modulated by different N dose and were subjected to densitometry scanning to quantitatively analyze the effect of N dose on the accumulation of these proteins in different cultivars (Fig. 2b). The banding pattern of all the major polypeptides was highly conserved among all the cultivars except 60 kDa polypeptide, which was absent in PR120 and PAU201 (Fig. 2a). PR120 and PAU201 did not show any change in accumulation of 60 kDa polypeptide in response to N dose. As compared to control (0 kg ha−1 N), IET21208 and IET21214 showed significantly increased accumulation of 60 kDa polypeptide in response N application. On the contrary, IR64 and IET21205 showed increased accumulation of 60 kDa polypeptide in milled rice in response to 60 and 120 kg ha−1 N-application followed by a decline in response to 180 kg ha−1 of N application. Polypeptide of 60 kDa has been reported to be the granule-bound starch synthase (GBSS) protein. Higher level of accumulation of 60 kDa protein was reported to be a remarkable feature in Indica rice (Kawakatsu et al. 2008). GBSS is responsible for starch biosynthesis and PR120 and PAU201 showed very less quantity of AAC, therefore, it was likely that the absence of GBSS in both these cultivars may have resulted in reduced accumulation of amylose in these cultivars. On the contrary, AAC in IET21205, IET21214, PR120 and PAU201 showed negative correlation with N application and was dependent upon the quantity of N applied (Table 1). These cultivars showed decline in AAC when subjected to 60 and 120 kg ha−1 N-application followed by incline in storage of AAC at 180 kg ha−1 of N application. IET21208 and IR64, on the contrary, showed higher storage of AAC in response to 60, 120 and 180 kg ha−1 N application, respectively, which was further validated by the accumulation of GBSS in same cultivars (Fig. 2b). Accumulation of GBSS in these cultivars was also increased in response to N application (Fig. 2b).
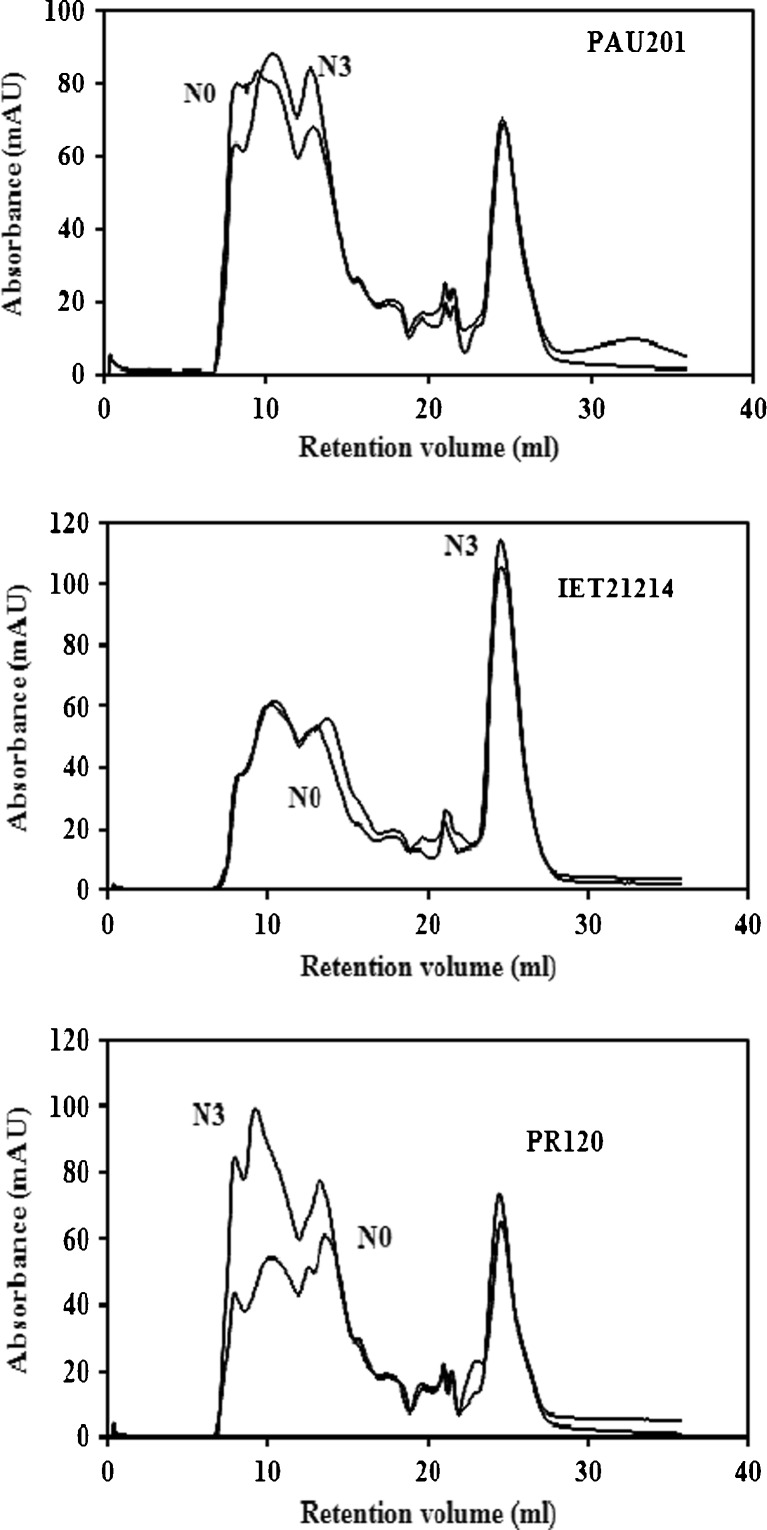
Fig. 1.
Effect of Nitrogen application on FPLC chromatograms of PAU201, IET21214, PR120, N0 and N3 represent 0 and 180 kg Nha−1, respectively
Fig. 2.
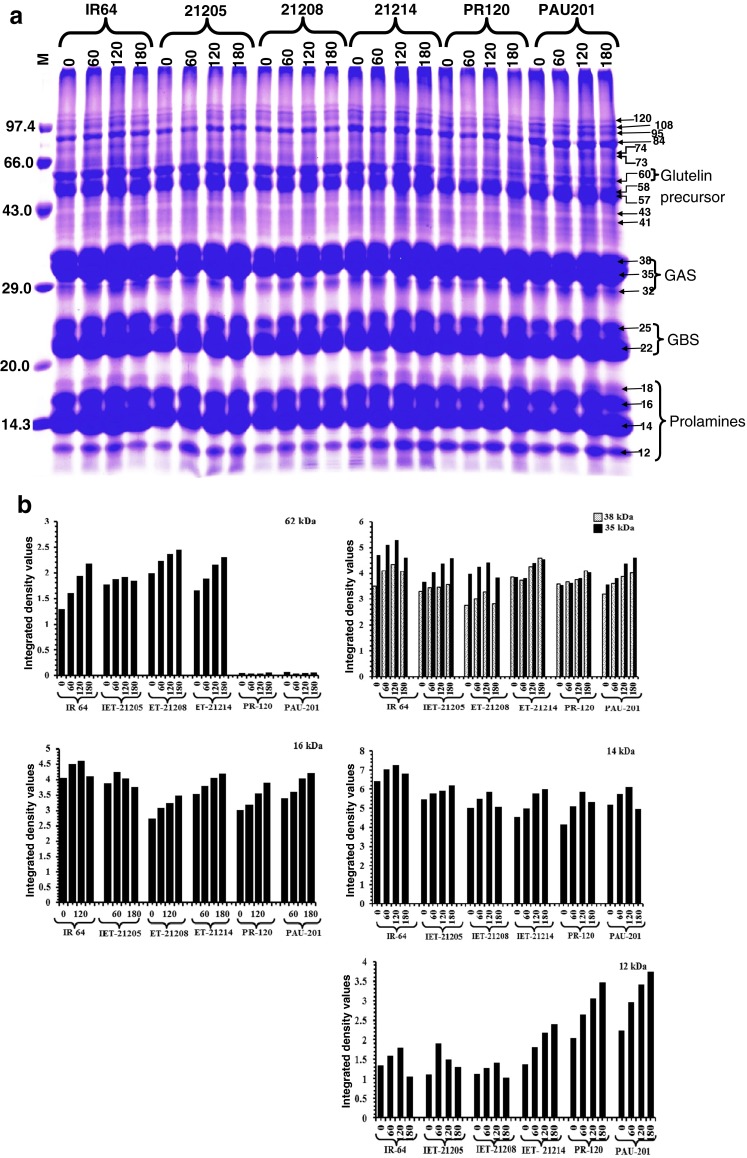
a SDS-PAGE analysis of rice seed storage proteins showing effect of amount (0, 60, 120 and 180 kg N ha−1) of nitrogen application on protein profiling. b Densitometric scanning of 62, 38, 35, 16, 14 and 12 kDa polypeptides using AlphaEaseFCv6.0.0 software
The storage of polypeptides of 38 kDa and 35 kDa (GAS) was also modulated by exogenous N application and was cultivar dependent. Comparing to the respective controls, IET21205, IET21214, PR120 and PAU201 showed higher storage of 38 kDa and 35 kDa polypeptides in response to N dose. On the contrary, IR64 and IET21208 showed higher accumulation of GAS polypeptides (38 kDa and 35 kDa) at 60 and 120 kg ha−1 N followed by reduction in storage of same polypeptides at 180 kg/ha of N-application (Fig. 2b). Leesawatwong et al. (2005), reported increase in storage protein but lysine rich glutelin fraction, the main storage protein of rice was mostly affected by N fertilization. Rice prolamines influences textural, cooking and physico-chemical properties of rice flour. Application of N at different quantities differentially modulates the accumulation of rice prolamines (12 kDa, 14 kDa, 16 kDa and 18 kDa) (Baxter et al. 2006). Accumulation of 16 kDa and 14 kDa polypeptides increased in response to 60 and 120 kg ha−1 N application in all the cultivars, except IET21205 which showed reduced storage of same polypeptides. IR64 and IET21205 showed reduced accumulation of 16 kDa polypeptide in response to 180 kg ha−1 N application. On the contrary, IET21208, IET21214, PR120 and PAU201 showed higher accumulation of same polypeptide in response to 180 kg ha−1 N application. Storage of 14 kDa polypeptide in milled rice of IET21205, IET21214 was higher upon 180 kg ha−1 Ndose, whereas, it was reduced in IR64, IET21208, PR120 and PAU201. Contrary to 14 kDa prolamin, 12 kDa polypeptide showed the identical accumulation pattern with 16 kDa polypeptide. However, the accumulation of polypeptide of 12 kDa was much higher as compared to 16 kDa polypeptide (Fig. 2b). Earlier, Pechanek et al. (1997) demonstrated that exogenous application of N significantly modulated the storage of wheat gluten proteins and it was cultivar dependent, however, the accumulation of albumins and globulins was not affected in different cultivars of wheat. Contrary to this, the ratio of low molecular weight and high molecular weight wheat glutenins was decreased consistently in all the cultivars studied. Leesawatwong et al. (2005) also reported that exogenous N fertilization can improve nutritional quality of rice by increasing the proportion of glutelin, which was rich in lysine. In lieu of this, studies carried out by Ning et al. (2010) demonstrated that rice prolamin and glutelin were largely modulated by N application, whereas, the accumulation of albumin and globulin was not affected by N fertilization but was mainly controlled by genotype (Ning et al. 2010). It was also demonstrated that exogenous application of N influences accumulation of amino acid composition in rice (Ning et al. 2010).
Gel chromatography
Protein fractions obtained from milled rice were categorized into high molecular weight (HMW), medium molecular weight (MMW) and low molecular weight (LMW) subunits Fig. 1. HMW subunits were obtained in 8 ml elution volume and ranged from 1397 to 1518 kDa. MMW and LMW subunits ranged from 331 to 576 kDa and 0.19 to 8 kDa, respectively. MMW and LMW subunits, respectively, were obtained in 10 to 12 ml and 15–25 ml elution volume. HMW, MMW and LMW-subunits proportion in rice without N application ranged from 8 to 15 %, 37 to 43 % and 10 to 58 %, respectively (Table 5). IET21214 milled rice had the highest proportion of HMW subunits and the lowest proportion of MMW and HMW. PAU201 milled rice had the highest MMW and PR120 had the lowest LMW subunits and the highest HMW subunits among different cultivars evaluated. The proportion of LMW subunits decreased from 59 % to 7 % in IET21214 and 16 % to 10 % in PAU201 upon N application. While the proportion of MMW subunits increased in IET21214 and PAU 201 from 34 to 42 % and 43 to 67 %, respectively. The proportion of HMW subunits in IET21214 increased from 8 to 51 % while in PAU201 and PR120 the proportion of HMW subunits decreased from 40 to 22 % and 51 to 34 %, respectively. The protein subunits having MW of 33 kDa and 64 to 500 kDa were designated as glutelin subunits by Zarins and Chrastil (1992).
Table 5.
Effect of nitrogen application on FPLC peak proportions obtained at different retention volumes
| Retention volume (ml) | Molecular weight (kDa) | Proportion (%) | |||||
|---|---|---|---|---|---|---|---|
| IET21214 | PR120 | PAU201 | |||||
| 0 kg/ha | 180 kg/ha | 0 kg/ha | 180 kg/ha | 0 kg/ha | 180 kg/ha | ||
| 7.92–8.29 | 1540–1184 | 8.05b | 6.68a | 10.5c | 15.6d | 16.3d | 10.1c |
| 9.15–10.39 | 644–267 | 27.9a | 36.7c | 30.6b | 35.6c | 28.6a | 40.1d |
| 12.41–13.31 | 63–40 | 5.69a | 5.17a | 7.73b | 14.4c | 14.9c | 27.5d |
| 17.6–17.8 | 1.6–1.4 | 0.04a | 1.45b | ND | ND | 0.01a | 1.51b |
| 19.4–19.65 | 0.45–0.38 | 0.04a | 0.02a | ND | ND | 0.34b | 0.37b |
| 21.03–21.52 | 0.14–0.1 | 1.64c | 1.3b | 1.9d | 0.72a | 1.45b | 1.15a |
| 24.48–24.64 | 0.01 | 55.8e | 48.6d | 44.3d | 33.5b | 38.3c | 19.3a |
Mean values with similar subscripts in a row do not vary significantly
ND not detected
Pasting properties
N application, cultivar and their interaction showed significant affect on pasting properties of rice flour. Effect of N application on pasting properties was more prominent than cultivars and their interaction (Table 3). PV and BDV of the milled rice from different cultivars ranged from 1990 to3681 cP and 489 to 1818 cP, respectively. IET21205 showed the lowest mean PV and BDV while IET21208 and PAU201 showed higher PV as compared to other cultivars (Table 6). FV and SB of different cultivars ranged from 2534 to 5882 cP and 1290 to 3296 cP, respectively. N application showed variable effects on the pasting properties of the flour from various rice cultivars. N application, particularly, at higher doses decreased PV as well as BDV. Majority of cultivars showed a decrease in FV also with N application at higher level of N application. The decreases in PV and FV with increasing N application may be due to a combination of increase in protein content as well surface lipids content as previously reported (Martin and Fitzgerald 2002; Perdon et al. 2001). BDV and SBV showed positive correlation with AAC (Table 4). Similar significant correlations were reported earlier by Champagne et al. (1999). Prolamines and glutelin were reported as major components of storage proteins in rice and increase in prolamin content caused by application of N at higher doses may have lowered PV and FV and increased breakdown (Baxter et al. 2006). Variation in changes in PV and BDV at different doses of N application amongst different cultivars may be related to the accumulation of prolamines to variable amount that was consistent with the earlier studies (Baxter et al. 2006). Pasting temperature was not significantly affected by exogenous application of N.
Table 6.
Effect of different doses of nitrogen application on cooking, textural and pasting properties of milled rice from different paddy cutivars
| Variety | N (Kg/ha) | Cooking time (mins) | L/B ratio | Water uptake | Gruel Solid loss (%) | Cohesiveness | Gumminess | Hardness | Peak viscosity | Breakdown viscosity | Final viscosity | Setback viscosity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N) | (cP) | (cP) | (cP) | (cP) | ||||||||
| IR64 | 0 | 16.5 ± 0.50 | 3.02 ± 0.09 | 4.94 ± 0.06 | 7.8 ± 0.11 | 0.234 ± 0.004 | 0.812 ± 0.04 | 3.47 ± 0.082 | 3657 ± 17 | 1002 ± 16 | 5481 ± 11 | 2826 ± 12 |
| 60 | 19.7 ± 0.42 | 3.29 ± 0.10 | 4.44 ± 0.08 | 7.1 ± 0.21 | 0.241 ± 0.005 | 0.807 ± 0.03 | 3.35 ± 0.014 | 2181 ± 10 | 775 ± 18 | 4419 ± 13 | 3013 ± 13 | |
| 120 | 19.5 ± 0.57 | 3.46 ± 0.06 | 3.79 ± 0.07 | 7.9 ± 0.18 | 0.210 ± 0.010 | 1.113 ± 0.04 | 5.30 ± 0.1 | 3374 ± 19 | 1062 ± 10 | 5604 ± 11 | 3292 ± 18 | |
| 180 | 18.6 ± 0.30 | 3.52 ± 0.08 | 3.63 ± 0.08 | 5.9 ± 0.11 | 0.224 ± 0.007 | 1.064 ± 0.07 | 4.75 ± 0.022 | 3453 ± 15 | 874 ± 18 | 5882 ± 21 | 3303 ± 11 | |
| IET21205 | 0 | 18.9 ± 0.40 | 2.89 ± 0.08 | 4.19 ± 0.08 | 9.6 ± 0.29 | 0.234 ± 0.025 | 1.11 ± 0.11 | 4.74 ± 0.034 | 3358 ± 15 | 1520 ± 16 | 4516 ± 28 | 2695 ± 13 |
| 60 | 18.2 ± 0.25 | 2.75 ± 0.09 | 4.02 ± 0.01 | 5.8 ± 0.27 | 0.243 ± 0.022 | 1.13 ± 0.120 | 4.65 ± 0.019 | 3174 ± 24 | 1337 ± 18 | 4388 ± 18 | 2551 ± 11 | |
| 120 | 16.5 ± 0.50 | 3.49 ± 0.08 | 4.64 ± 0.07 | 8.4 ± 0.20 | 0.247 ± 0.003 | 0.759 ± 0.01 | 3.07 ± 0.026 | 3067 ± 22 | 1222 ± 22 | 4785 ± 10 | 2940 ± 22 | |
| 180 | 19.3 ± 0.30 | 3.12 ± 0.09 | 4.35 ± 0.09 | 8.8 ± 0.16 | 0.225 ± 0.015 | 0.580 ± 0.03 | 2.58 ± 0.092 | 3035 ± 20 | 1360 ± 12 | 4426 ± 19 | 2551 ± 11 | |
| IET21208 | 0 | 17.6 ± 0.35 | 3.57 ± 0.04 | 4.51 ± 0.18 | 11.0 ± 0.15 | 0.301 ± 0.019 | 1.017 ± 0.05 | 3.39 ± 0.045 | 2525 ± 23 | 868 ± 16 | 4627 ± 27 | 2990 ± 15 |
| 60 | 17.2 ± 0.27 | 3.92 ± 0.06 | 4.68 ± 0.04 | 9.3 ± 0.10 | 0.279 ± 0.029 | 1.152 ± 0.14 | 4.13 ± 0.032 | 3647 ± 30 | 1007 ± 10 | 5768 ± 22 | 3128 ± 24 | |
| 120 | 19.6 ± 0.35 | 3.18 ± 0.10 | 4.20 ± 0.09 | 7.8 ± 0.20 | 0.251 ± 0.007 | 1.835 ± 0.07 | 7.31 ± 0.029 | 2507 ± 19 | 663 ± 11 | 5160 ± 18 | 3296 ± 11 | |
| 180 | 19.7 ± 0.30 | 3.29 ± 0.11 | 4.60 ± 0.03 | 9.1 ± 0.30 | 0.302 ± 0.032 | 1.307 ± 0.10 | 4.36 ± 0.075 | 1990 ± 11 | 489 ± 10 | 4782 ± 22 | 3281 ± 25 | |
| IET21214 | 0 | 19.2 ± 0.20 | 2.85 ± 0.11 | 4.27 ± 0.11 | 8.6 ± 0.35 | 0.261 ± 0.034 | 0.700 ± 0.09 | 2.68 ± 0.026 | 2955 ± 13 | 1238 ± 28 | 4148 ± 26 | 2431 ± 30 |
| 60 | 18.5 ± 0.35 | 3.25 ± 0.12 | 4.75 ± 0.07 | 8.1 ± 0.25 | 0.250 ± 0.019 | 0.737 ± 0.06 | 2.95 ± 0.15 | 2708 ± 32 | 1177 ± 19 | 4267 ± 27 | 2590 ± 22 | |
| 120 | 20.5 ± 0.50 | 3.31 ± 0.12 | 5.47 ± 0.18 | 7.7 ± 0.30 | 0.240 ± 0.026 | 0.436 ± 0.07 | 1.82 ± 0.12 | 2801 ± 23 | 1113 ± 16 | 4298 ± 18 | 2610 ± 16 | |
| 180 | 19.5 ± 0.32 | 3.42 ± 0.08 | 4.36 ± 0.07 | 7.0 ± 0.33 | 0.232 ± 0.010 | 0.858 ± 0.03 | 3.70 ± 0.15 | 2736 ± 28 | 1030 ± 10 | 3936 ± 22 | 2377 ± 19 | |
| PR120 | 0 | 20.7 ± 0.72 | 2.90 ± 0.11 | 4.63 ± 0.12 | 8.6 ± 0.34 | 0.210 ± 0.070 | 0.411 ± 0.02 | 1.96 ± 0.043 | 3208 ± 31 | 1073 ± 23 | 4807 ± 17 | 3164 ± 24 |
| 60 | 21.6 ± 0.37 | 3.40 ± 0.11 | 4.20 ± 0.10 | 8.3 ± 0.31 | 0.223 ± 0.007 | 0.41 ± 0.01 | 1.84 ± 0.061 | 3216 ± 26 | 1292 ± 12 | 5088 ± 22 | 3052 ± 21 | |
| 120 | 17.2 ± 0.25 | 2.99 ± 0.10 | 4.53 ± 0.14 | 8.1 ± 0.10 | 0.234 ± 0.030 | 0.33 ± 0.045 | 1.41 ± 0.041 | 2939 ± 20 | 1125 ± 15 | 4511 ± 21 | 2697 ± 17 | |
| 180 | 20.5 ± 0.50 | 3.25 ± 0.12 | 4.46 ± 0.09 | 8.4 ± 0.17 | 0.215 ± 0.008 | 0.467 ± 0.03 | 2.22 ± 0.032 | 2464 ± 19 | 878 ± 17 | 4638 ± 32 | 2672 ± 20 | |
| PAU201 | 0 | 18.5 ± 0.50 | 2.99 ± 0.10 | 3.88 ± 0.05 | 7.2 ± 0.30 | 0.250 ± 0.013 | 0.591 ± 0.05 | 2.55 ± 0.07 | 3841 ± 14 | 2010 ± 20 | 3652 ± 23 | 1290 ± 10 |
| 60 | 17.7 ± 0.25 | 3.41 ± 0.09 | 3.79 ± 0.08 | 6.5 ± .0.35 | 0.250 ± 0.011 | 1.66 ± 0.018 | 6.59 ± 0.09 | 3681 ± 18 | 1818 ± 10 | 3557 ± 27 | 1782 ± 12 | |
| 120 | 17.5 ± 0.50 | 3.35 ± 0.15 | 3.62 ± 0.02 | 6.4 ± 0.30 | 0.220 ± 0.004 | 0.754 ± 0.02 | 3.46 ± 0.11 | 3427 ± 27 | 1710 ± 12 | 3499 ± 25 | 1694 ± 14 | |
| 180 | 20.1 ± 0.33 | 3.08 ± 0.01 | 4.63 ± 0.05 | 6.6 ± 0.12 | 0.207 ± 0.004 | 0.368 ± 0.04 | 1.78 ± 0.04 | 2105 ± 15 | 990 ± 11 | 2534 ± 21 | 1821 ± 11 |
Data ± Standard deviation
Cooking and textural properties
The cultivar effect on cooking time and L/B ratio was more significant as comapred to interactive effect of N application and cultivar and N application alone (Table 2). The cooking characteristics of milled rice from various cultivars grown with and without N application are shown in Table 6. Cooking time of milled rice from the different cultivars varied from 16.5 to 20.7 min. PR120 milled rice from paddy grown both with and without N application took the longest time to cook. IR64 followed by IET21205 milled rice from paddy grown with and without N application, respectively had the lowest cooking time. N application resulted in significant increase in cooking time of milled rice at the highest dose (180 kg ha−1). Cooking time of less than 20 min for milled rice with low gelatinization temperature and more than 20 min for those with intermediate gelatinization temperature have been reported earlier. A negative correlation between cooking time and amylose content of milled rice has been reported earlier (Singh et al. 2003). The negative correlation between TGW and cooking time indicates that the rice with higher TGW i.e., compact structure showed a slower water uptake, resulting in longer cooking time. A disorganized cellular structure offers the opportunity for increased water absorption during cooking and, thus, a softer cooked grain (Lisle et al. 2000).
F value shows N application affected L/B value significantly (Table 2). L/B value of cooked rice varied from 2.75 to 3.57 for different cultivars. L/B value increased significantly with N application. L/B value of milled rice was observed to be the highest at 60 kg/ha of N application. GSS decreased significantly with N application. GSS has been reported to be influenced by L/B ratio and amylose content (Singh et al. 2003). Cultivars with higher L/B ratios offer large surface to contact with water hence caused more loss of gruels. Amylose was known to leach out during cooking and the higher amylose content was liable to leach more into the cooking water (Juliano et al. 1987).
N application and cultivars significantly affected all the textural properties except cohesiveness. Amongst cultivars and N application and their combined effect, cultivar had the highest significant effect on textural properties followed by interaction between cultivars and N application (Table 4). The textural properties of cooked rice from different rice cultivars grown under different doses of N application are shown in Table 6. Cohesiveness of cooked rice increased with increase in N application; however, the improvement was maximum when 120 kg N ha−1 was applied. Gumminess and hardness of cooked rice increased with the increase in N application and the increase was significant when 60 kg N ha−1 was applied. Among the various cultivars, IET21208 cooked rice grains showed the highest mean hardness and gumminess values. PR120 cooked rice showed the least value for all the textural parameters. The difference in textural properties between cultivars may be attributed to differences in the AAC, ratio of long/short chain amylopectin chains and granular structure. The higher value of hardness may be due to differences in their granular structure. A highest hardness value has been reported for rice cultivars having smallest size starch granules (Singh et al. 2003). A positive significant correlation between hardness and AAC was observed (Table 4). Similar correlations were reported earlier by many authors (Bao et al. 2006). Formation of film on the surface of the grain as result of leaching of amylose from starch granules during cooking contributes to the increased hardness of the grains (Yu et al. 2009). All the textural properties showed positive correlation with TGW and AAC. Cohesiveness was negatively dependent on the protein content (r = −0.496, p ≤ 0.005) and cooking time (r = −0.654, p ≤ 0.005) (Table 4). Grains with higher protein content and cooking time had higher cohesiveness.
Conclusion
N application affected milled rice characteristics significantly. TGW, total protein assimilation and SBV increased in response to increased N dose whereas AAC, L*, whiteness, husk content and GSS decreased with increased N dose. Grains with higher lightness value had higher TGW but lower protein content. N application resulted in increase in glutelins and decrease in AAC. PAU201 and PR120 showed lower AAC due to low accumulation of 60 kDa GBSS, in response to N application. The decrease in PV and FV at higher dose of N application was related to the higher protein content of rice cultivars.
Acknowledgment
The financial support to NS from Department of Science and Technology, Govt. of India, in the form of J.C. Bose National fellowship grant is gratefully acknowledged.
References
- AACC, American Association of Cereal Chemists . Approved methods of the AACC. 9. St Paul: The Association; 1995. [Google Scholar]
- Ashida K, Iida S, Yasui T. Morphological, physical, and chemical properties of grain and flour from chalky rice mutants. Cereal Chem. 2009;86:225–231. doi: 10.1094/CCHEM-86-2-0225. [DOI] [Google Scholar]
- Bao J, Kong X, Xie J, Xu L. Analysis of genotypic and environmental effects on rice starch, apparent amylose content, pasting viscosity, and gel texture. J Agric Food Chem. 2004;52:6010–6016. doi: 10.1021/jf049234i. [DOI] [PubMed] [Google Scholar]
- Bao J, Shen S, Sun M, Corke H. Analysis of genotypic diversity in the starch physicochemical properties of nonwaxy rice: apparent amylose content, pasting viscosity and gel texture. Starch-Starke. 2006;58:259–267. doi: 10.1002/star.200500469. [DOI] [Google Scholar]
- Baxter GJ, Blythe RA, Croft W, McKane AJ. Utterance selection model of language change. Phys Rev. 2006;73:46–118. doi: 10.1103/PhysRevE.73.046118. [DOI] [PubMed] [Google Scholar]
- Champagne ET, Bett KL, Vinyard BT, McClung AM, Barton FE, II, Moldenhauer K, Linscombe S, McKenzie K. Correlation between cooked rice texture and rapid visco analyser measurements. Cereal Chem. 1999;76:764–771. doi: 10.1094/CCHEM.1999.76.5.764. [DOI] [Google Scholar]
- Champagne ET, Bett-Garber KL, Grimm CC, McClung AM. Effects of organic fertility management on physicochemical properties and sensory quality of diverse rice cultivars. Cereal Chem. 2007;84:320–327. doi: 10.1094/CCHEM-84-4-0320. [DOI] [Google Scholar]
- Champagne ET, Bett-Garber L, Thomson JL, Fitzgerald MA. Unraveling the impact of nitrogen nutrition on cooked rice flavor and texture. Cereal Chem. 2009;86:274–280. doi: 10.1094/CCHEM-86-3-0274. [DOI] [Google Scholar]
- Ghumman A, Kaur A, Singh N. Impact of germination on flour, protein and starch characteristics of lentil (Lens culinaris) and horsegram (Macrotyloma uniflorum L.) lines. LWT Food Sci Technol. 2016;65:137–144. doi: 10.1016/j.lwt.2015.07.075. [DOI] [Google Scholar]
- Islam N, Inanaga S, Chishaki N, Horiguchii T. Effect of N top-dressing on protein content in japonica and indica rice grains. Cereal Chem. 1996;73:571–573. [Google Scholar]
- Jiang H, Dian W, Wu P. Effect of high temperature on fine structure of amylopectin in rice endosperm by reducing the activity of the starch branching enzyme. Phytochemistry. 2003;63:53–59. doi: 10.1016/S0031-9422(03)00005-0. [DOI] [PubMed] [Google Scholar]
- Juliano BO, Perez CM, Alyoshin EP, Romanov VB, Blakeney AB, Welsh LA, Choudhury NH, Delgado LL, Iwasaki T, Shibuya N, Mossman AP, Siwi B, Damardjati DS, Suzuki H, Kimura H. International cooperative test on texture of cooked rice. J Texture Stud. 1984;15:357–376. doi: 10.1111/j.1745-4603.1984.tb00392.x. [DOI] [Google Scholar]
- Juliano BO, Villareal RM, Perez CM, Villareal CP, Takeda Y, Jlizukuri Varietal differences in properties among high amylose rice starches. Starch. 1987;39:390. doi: 10.1002/star.19870391106. [DOI] [Google Scholar]
- Kaur A, Shevkani K, Singh N, Sharma P, Kaur P. Effect of guar gum and xanthan gum on pasting and noodle-making properties of potato, corn and mung bean starches. J Food Sci Technol. 2015;52(12):8113–8121. doi: 10.1007/s13197-015-1954-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakatsu T, Yamamoto MP, Hirose S, Yano M, Takaiwa F. Characterization of a new rice glutelin gene GluD-1 expressed in the starchy endosperm. J Exp Bot. 2008;59:4233–4245. doi: 10.1093/jxb/ern265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:681–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanning SB, Siebenmorgen TJ. Effects of pre-harvest nighttime air temperatures on whiteness of head rice. Cereal Chem. 2013;90:218–222. doi: 10.1094/CCHEM-07-12-0082-R. [DOI] [Google Scholar]
- Lanning SB, Siebenmorgen TJ, Ambardekar A, Counce PA, Bryant RJ. Effects of nighttime air temperature during kernel development of field-grown rice on physicochemical and functional properties. Cereal Chem. 2012;89:168–175. doi: 10.1094/CCHEM-12-11-0146. [DOI] [Google Scholar]
- Leesawatwong M, Jamjod S, Kuo J, Dell B, Rerkasem B. Nitrogen fertilizer increases seed protein and milling quality of rice. Cereal Chem. 2005;82:588–593. doi: 10.1094/CC-82-0588. [DOI] [Google Scholar]
- Lisle AJ, Martin M, Fitzgerald MA. Chalky and translucent rice grains differ in starch composition and structure, and cooking properties. Cereal Chem. 2000;77:627–632. doi: 10.1094/CCHEM.2000.77.5.627. [DOI] [Google Scholar]
- Mahajan G, Chauhan BS. Performance of dry direct-seeded rice in response to genotype and seeding rate. Agro Soils Env Qua. 2016;107:257–265. [Google Scholar]
- Mahajan G, Chauhan BS, Gill MS. Optimal nitrogen fertilization timing and rate in dry-seeded rice in Northwest India. Soil Fert Crop Nut. 2011;103:1676–1682. [Google Scholar]
- Martin M, Fitzgerald MA. Proteins in rice grains influence cooking properties. J Cereal Sci. 2002;36(3):285–294. doi: 10.1006/jcrs.2001.0465. [DOI] [Google Scholar]
- Ning H, Liu Z, Wang Q, Lin Z, Chen S, Li G, Wan S, Ding Y. Effect of nitrogen fertilizer application on grain phytic acid and protein concentrations in japonica rice and its variations with genotypes. J Cereal Sci. 2009;50:49–55. doi: 10.1016/j.jcs.2009.02.005. [DOI] [Google Scholar]
- Ning J, Li X, Hicks LM, Xiong L. A raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol. 2010;152:876–890. doi: 10.1104/pp.109.149856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JK, Kim SS, Kim KO. Effects of milling ratio on sensory properties of cooked rice and on physiochemical properties of milled and cooked rice. Cereal Chem. 2001;78:151–156. doi: 10.1094/CCHEM.2001.78.2.151. [DOI] [Google Scholar]
- Pechanek U, Karger A, Groger S, Charvat B, Schoggl G, Lelley T. Effect of nitrogen fertilization on quality of our protein components, dough properties, and breadmaking quality of wheat. Cereal Chem. 1997;74:800–805. doi: 10.1094/CCHEM.1997.74.6.800. [DOI] [Google Scholar]
- Perdon AA, Siebenmorgen TJ, Mauromoustakos A, Griffin VK, Johnson ER. Degree of milling effects on rice pasting properties. Cereal Chem. 2001;78(2):205–209. doi: 10.1094/CCHEM.2001.78.2.205. [DOI] [Google Scholar]
- Perez CM, Cagampang GB, Esmama BV, Monserrate RU, Juliano BO. Protein metabolism in leaves and developing grains of rices differing in grain protein content. Plant Physiol. 1973;51:537–542. doi: 10.1104/pp.51.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Bhadoria PBS, Amitava R, Rakshit A. Relative efficacy of organic manure in improving milling and cooking quality of rice. Int Rice Res. 2002;27:43–44. [Google Scholar]
- Shakouri MJ, Vajargah AV, Gavabar MG, Mafakheri S, Zargar M. Rice vegetative response to different biological and chemical fertilizer. Adv Environ Biol. 2012;6:859–863. [Google Scholar]
- Singh N, Sodhi NS, Kaur M, Saxena SK. Physicochemical, morphological, thermal, cooking and textural properties of chalky and translucent rice kernels. Food Chem. 2003;82:433–439. doi: 10.1016/S0308-8146(03)00007-4. [DOI] [Google Scholar]
- Singh N, Kaur L, Sodhi NS, Sekhon KS. Physicochemical, cooking and textural properties of milled rice from different Indian rice cultivars. Food Chem. 2005;89:253–259. doi: 10.1016/j.foodchem.2004.02.032. [DOI] [Google Scholar]
- Singh N, Kaur L, Sandhu KS, Kaur J, Nishinari K. Relationships between physicochemical, morphological, thermal, rheological properties of rice starches. Food Hydrocoll. 2006;20:532–542. doi: 10.1016/j.foodhyd.2005.05.003. [DOI] [Google Scholar]
- Singh N, Paul P, Virdi AS, Kaur P, Mahajan G. Influence of early and delayed transplantation of paddy on physico-chemical, pasting, cooking, textural and protein characteristics of milled rice. Cereal Chem. 2014;91:389–397. doi: 10.1094/CCHEM-09-13-0193-R. [DOI] [Google Scholar]
- Vioque J, Alaiz M, Girón-Calle J. Nutritional and functional properties of Vicia faba protein isolates and related fractions. Food Chem. 2012;132:67–72. doi: 10.1016/j.foodchem.2011.10.033. [DOI] [PubMed] [Google Scholar]
- Williams PC, Kuzina FD, Hlynka I. A rapid calorimetric procedure for estimating the amylose content of starches and flours. Cereal Chem. 1970;47:411–420. [Google Scholar]
- Witt C, Pasuquin JMCA, Mutters R, Buresh RJ. New leaf color chart for effective nitrogen management in rice. Better Crops. 2005;89:36–39. [Google Scholar]
- Yu S, Ma YY, Sun DW. Impact of amylose content on starch retrogradation and texture of cooked milled rice during storage. J Cereal Sci. 2009;50:139–144. doi: 10.1016/j.jcs.2009.04.003. [DOI] [Google Scholar]
- Zarins Z, Chrastil J. Separation and purification of rice oryzenin subunits by anion- exchange and gel permeation chromatography. J Agric Food Chem. 1992;40:1500–1601. doi: 10.1021/jf00021a024. [DOI] [Google Scholar]