Abstract
The pivotal role of epithelial cells is to secrete and absorb ions and water in order to allow the formation of a luminal fluid compartment that is fundamental for the epithelial function as a barrier against environmental factors. Importantly, epithelial cells also take part in the innate immune system. As a first line of defense they detect pathogens and react by secreting and responding to chemokines and cytokines, thus aggravating immune responses or resolving inflammatory states. Loss of epithelial anion transport is well documented in a variety of diseases including cystic fibrosis, chronic obstructive pulmonary disease, asthma, pancreatitis, and cholestatic liver disease. Here we review the effect of aberrant anion secretion with focus on the release of inflammatory mediators by epithelial cells and discuss putative mechanisms linking these transport defects to the augmented epithelial release of chemokines and cytokines. These mechanisms may contribute to the excessive and persistent inflammation in many respiratory and gastrointestinal diseases.
1. Introduction
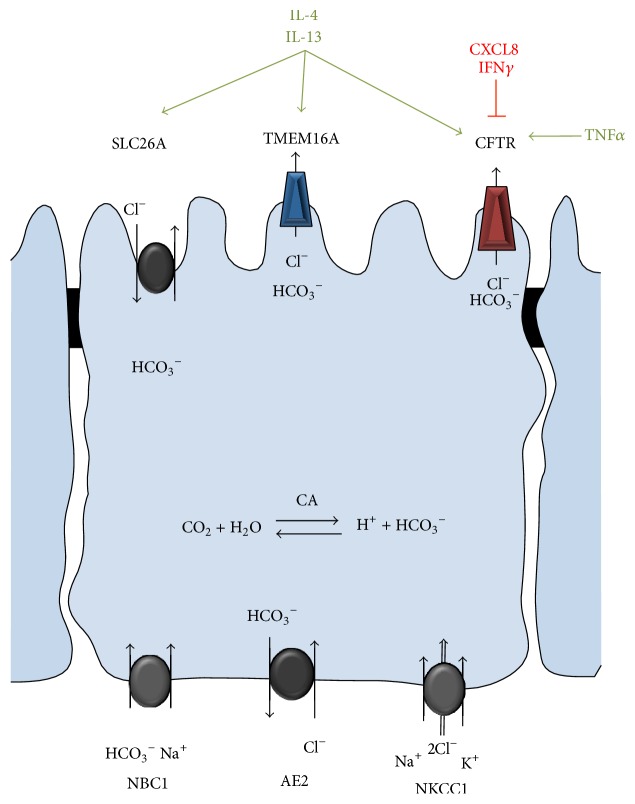
The ion and water homeostasis, mediated by channels and transporters at the apical and basolateral membranes of secretory epithelia that maintain a fluid compartment at the luminal surface, is essential for the function of different organ systems such as the airways, intestines, and pancreas [1, 2]. The most abundant anions, chloride and bicarbonate, are secreted through the apical cystic fibrosis transmembrane conductance regulator (CFTR) and calcium-activated chloride channel TMEM16A and anion exchangers of the SLC26A family. At the basolateral plasma membrane (PM) chloride and bicarbonate uptake are mediated by the sodium-potassium-chloride cotransporter (NKCC1) and sodium-bicarbonate cotransporters (NBC1), respectively [1] (Figure 1). Alternatively, basolateral chloride entry into the cell is conducted by the basolateral chloride-bicarbonate exchanger AE2 [3].
Figure 1.
Schematic depiction of the anion transport pathways in secretory epithelia. Apical chloride (Cl−) and bicarbonate (HCO3 −) efflux is mediated by CFTR and TMEM16A and probably members of the SLC26A family. Basolateral chloride and bicarbonate entry is conducted by cation cotransporters NKCC1 and NBC1, respectively. Alternatively chloride entry at the basolateral membrane is conducted by chloride-bicarbonate exchange via AE2. Carbonic anhydrase (CA) catalyzes the de novo formation of bicarbonate. Examples for the cytokine-mediated regulation of channel function or expression are indicated.
Secretory epithelial cells also play a pivotal role as part of the innate immune system. Besides providing a physical barrier by forming tight cell-cell contacts and a chemical barrier by secreting antimicrobial peptides and mucins, epithelial cells express a variety of pattern-recognition receptors that, when triggered, activate the expression and release of a number of chemokines and cytokines. These inflammatory mediators in concert with the production of reactive oxygen species (ROS) allow the epithelium to contribute to the host defense and to recruit specialized immune cells [4–7]. It is well established that the expression and function of ion channels and transporters, including the anion channel and transporters discussed in this review, are modulated by chemokines and cytokines [8–12]. Examples for these mechanisms include the inhibition and downregulation of the epithelial sodium channel (ENaC) by IL-4 and IL-13 [10, 13, 14], the IL-4 and IL-13-mediated increase in function and expression of CFTR [13], and TMEM16A [15] as well as pendrin (SLC26A4) [12, 16] and SLC26A9 [17]. The CFTR expression and activity are increased by TNFα [18] and decreased by IFNγ [19, 20] and its function is attenuated by CXCL8 via β2-adrenergic receptor (β2-AR) dependent mechanism [21] (Figure 1).
In contrast, little is known about the regulatory role of anion transport on cytokine, in particular chemokine, synthesis, and secretion of epithelial cell that serves as amplifier and regulator of inflammation in conditions like cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), asthma, cholestasis, and acute pancreatitis. The effects of aberrant anion transport activities in these diseases and putative mechanisms linking them to the augmented epithelial release of chemokines and cytokines are discussed in this review.
2. Diseases Exhibiting Defective Epithelial Anion Transport
2.1. Cystic Fibrosis
Cystic fibrosis (CF), the most common lethal hereditary disease in the Caucasian population, is caused by mutations in CFTR that lead to impaired anion conductance at the apical membrane of secretory epithelia [22–25]. More than 2,000 mutations in CFTR have been described that confer a range of molecular, cellular, and functional phenotypes [26, 27] and can be classified according to these complex phenotypes as recently reviewed [28]. Interestingly, mutants that are expressed at the apical membrane in densities comparable to the wild-type (WT) protein but are nonfunctional (e.g., G551D-CFTR) cause a similar CF disease severity as mutants with strongly reduced protein at the PM (e.g., ΔF508-CFTR) [27, 29], suggesting that the loss of CFTR function rather than its physical absence is required for the initiation of the disease.
CF is characterized by a multiorgan pathology mainly affecting the upper and lower airway, gastrointestinal and reproductive tract, and endocrine system [23, 25]. Pancreatic disease in CF has a high penetrance and the pancreas is the earliest intrauterine affected organ [30, 31]. Most CF patients (80–90%) suffer from pancreatic insufficiency (PI), a condition characterized by acinar atrophy, ductal irregularity, fibrosis, deficiency of exocrine pancreatic enzymes, and beta cell damage at late stages. Importantly, the level of pancreatic damage in CF is linked to the genotype [32, 33]. Patients that retain sufficient exocrine pancreatic function carry a small risk to develop pancreatitis [34, 35]. In the lung, imbalanced epithelial ion secretion and mucus thickening lead to decreased airway clearance and reoccurring bronchopulmonary infections that cumulate in tissue damage, the leading cause for morbidity and mortality in CF [36–39].
It is widely accepted that CF lung disease is associated with an excessive inflammatory response that is characterized by massive neutrophil recruitment into the lumen driven by augmented release of chemokines including CXCL8 by lung epithelial tissue. Once recruited and activated the neutrophils release antimicrobial peptides, neutrophil extracellular traps (NETs), reactive oxygen species, and proteases [40–42]. The sheer amount of neutrophil released serine proteases (neutrophil elastase (NE), proteinase 3, and cathepsin G) overwhelms the antiprotease production resulting in protease-antiprotease imbalance [43, 44]. Excessive amounts of NE in the airway-surface liquid in turn induce CXCL8 expression [45, 46], upregulate mucin production [47, 48], cleave antimicrobial peptides [49], and promote the degradation of CFTR [50] further perpetuating the inflammatory response that cumulates in progressive tissue damage. Despite their excessive infiltration into the CF lung, CF neutrophils fail to eradicate bacterial infections [41, 51]. A recent study shows that CF neutrophils exhibit abnormal degranulation responses and consequently impaired bacterial killing. Treatment of G551D-CFTR patient with the potentiator ivacaftor normalized the intracellular homeostasis and degranulation of their neutrophils, thus improved bacterial killing [52]. These findings and studies reporting reduced phagolysosomal chlorination in CF neutrophils [53, 54] suggest an intrinsic neutrophil function defect in CF. However, although hyperinflammation in CF is acknowledged, it is under debate whether the immunological aberrancy is directly caused by loss-of-CFTR-function or is rather the consequence of chronic bacterial infection (this controversy is reviewed in [42, 55–57]).
2.1.1. Animal Models of CF
Animal models allow investigating the early pathogenesis of CF and examining how the lack of CFTR is linked to the host-defense defect of the lung. Several CF mouse models exist, including null [58, 59] and mutant form of CFTR [60–62] expressing animals. However, none of these models develops spontaneous severe lung disease, characteristic of human CF. Yet CF mice do secrete dramatically higher concentrations of inflammatory mediators upon bacterial challenge, including the chemokines keratinocyte chemoattractant (KC/CXCL1, the functional CXCL8 analog in mice) and the macrophage inflammatory protein 2 (mip-2/CCL3) that can be measured in the bronchoalveolar lavage (BAL) fluid [63, 64]. Concordantly, inhibition of CFTR in WT mice using the inhibitor-172 exacerbates the acute inflammatory response in an induced peritonitis model, including increased polymorphonuclear leukocyte infiltration and cytokine release [65]. The specificity of the inhibitor-172 however is questioned by other studies [66–68]. Transgenic mice that overexpress the β-subunit of the epithelial sodium channel (ENaC) in the airway, mimicking the increased sodium reabsorption found in human airway epithelia by many researchers [36, 37, 69], exhibit mucus obstruction, goblet cell metaplasia, increased BAL levels of the chemokines CXCL1 and CXCL2, neutrophilic inflammation, and poor bacterial clearance [70].
Since mice do not recapitulate all of the hallmarks of human CF, considerable efforts were made to create other animal models. CFTR −/− rats were recently created using DNA editing and the resulting animals show a reduced airway-surface liquid (ASL) height and submucosal gland hypoplasia [71]. CFTR −/− and CFTR ΔF508/ΔF508 pigs as well as CFTR −/− ferrets were generated by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer [72, 73]. CF pigs and ferrets develop spontaneous lung disease characterized by airway inflammation, airway remodeling, impaired mucosal clearance, and bacterial infection [74–76]. The clearance upon bacterial challenge in the lung of both animal models is impaired likely as a result of the decreased pH and therefore attenuated antibacterial properties of the ASL [77, 78]. The onset of pulmonary inflammation however differs between CF pigs and ferrets. The CXCL8 and TNFα levels were increased in the CF ferret BAL at birth, suggesting an inherent innate immunity defect [77]. In contrast neutrophil counts and CXCL8 levels were not augmented in the neonatal CF pig BAL [74, 76]. Intriguingly, the pancreas of newborn CF pig shows increased proportion of innate immune cells and inflammatory response without apparent infection [79]. Similarly, zebrafish that lacks functional CFTR shows progressive destruction of the exocrine pancreas accompanied by a significant increase in neutrophil infiltration [80]. Thus, upregulation of chemokine secretion that promotes neutrophil recruitment likely contributes to the early onset of inflammation in these animals.
2.1.2. Human Studies
An early onset of excessive proinflammatory chemokine and cytokine release into the BAL of CF infants has been reported, but whether this precedes bacterial infection remains controversial [81–85]. CXCL8, neutrophils counts, and the concentration of free elastase were prominently increased in the BAL of CF patients in comparison to healthy controls [82–84, 86]. Other inflammatory mediators that are augmented in the CF lung include IL-6, IL-1β, and TNF-α [87], GM-CSF and G-CSF [88], IL-33 [89], HMGB1 [90], and the chemokine CCL18 [91]. Interestingly, a number of studies reported a decrease of the anti-inflammatory cytokine IL-10 in CF BAL [87, 92, 93]. By binding to the IL-10 receptor, IL-10 inhibits signaling pathways including the mitogen-activated kinase p38 and NF-κB pathways that are associated with proinflammatory chemokine and cytokine expression.
Using tracheal or bronchiolar grafts from CF and non-CF human foetuses implanted into severe combined immunodeficient (SCID) mice Tirouvanziam and colleagues investigated the inflammatory state prior to any infection [94, 95]. Both upper and lower airway CF grafts showed increased CXCL8 expression and leukocyte infiltration, but the inflammation was more severe in the bronchiolar grafts leading to progressive tissue destruction. These results provide evidence that CF lung inflammation could arise solely from CFTR mutations in sterile environment.
2.1.3. Cell Models
Increased CXCL8 expression in CF cell lines and CF primary human bronchial epithelial cells has been reported, both constitutively and following stimulation with Pseudomonas aeruginosa or TNF-α, implying CFTR-dependent alteration in the innate immune response [96–98]. However, elevated CXCL8 levels were not observed in other cellular models [99–101], while in some studies CFTR appeared to have a permissive role in CXCL8 secretion [102, 103]. The interpretation of these results is difficult since many derive from the comparison between clonally isolated CF and non-CF cell lines. Isolation of individual clones from heterologous cell populations, however, may favor unrepresentative phenotypes as clonal variations can exceed the underlying differences in the regulated CXCL8 secretion [67]. The genetic heterogeneity and possible epigenetic modification due to the repeated infection and inflammation in CF [104] restrict the comparability of primary human cells from CF patients and healthy individuals. However, a number of studies elegantly circumvented these limitations. Inhibition of CFTR function by continuous treatment with the inhibitor-172 in differentiated primary airway epithelial cells resulted in increased constitutive CXCL8 secretion that was further augmented by Pseudomonas aeruginosa infection [105]. CFTR inhibition by gene knockdown in the human intestinal epithelial cell lines Caco-2/15 and HT-29 [106] as well as in human the human airway epithelial cell line Calu-3 [107] similarly increased CXCL8 secretion. In another approach human CF respiratory epithelial models with the inducible expression of either CFTR or TMEM16A were generated. Induced expression of WT-CFTR or TMEM16A attenuated the proinflammatory chemokine CXCL8, CXCL1, and CXCL2 and cytokine IL-6 expression under air-liquid, but not liquid-liquid interface culture [67]. Likewise, transient lentiviral transduction that conferred the expression of WT-CFTR reduced CXCL8 secretion of primary human CF bronchial epithelia [67]. Interestingly, the expression of the nonfunctional G551D-CFTR or WT-CFTR in combination with channel inhibition failed to attenuate CXCL8 release, suggesting that CFTR-mediated anion transport activity at the apical PM is required to reduce the proinflammatory chemokine and cytokine secretion [67].
2.2. Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD), encompassing two clinical phenotypes, chronic bronchitis, and emphysema, is attributed to the exposure to cigarette smoke and other environmental toxins [108, 109]. Genetic factors such as α1-antitrypsin gene mutations are also associated with disease [110]. COPD affects 64 million people and currently is the 4th leading cause of mortality worldwide [111]. COPD is characterized by persistent airflow limitation, chronic inflammatory responses, excessive mucus production, and recurrent cough, concordant to the CF lung phenotype. COPD shares several other clinical, immunological, and biochemical features with CF. Both are characterized by reduced mucociliary clearance, goblet cell metaplasia, chronic bacterial infections, excessive innate immune responses, and persistent neutrophilic inflammation. In comparison with CF, however, the inflammatory response in COPD includes more pronounced involvement of the adaptive immune system. An inverse correlation between the number of CD8+ T cells and the CD4+/CD8+ ratio to the lung function has been observed [112–114]. The CD8+ T cells release perforins and cause cytolysis and apoptosis of alveolar epithelial cells that is correlated to emphysema [115, 116].
The phenotypic similarity between CF and COPD raises the possibility that CFTR inactivation and/or downregulation may play an important role in the pathogenesis of COPD. Indeed, a number of studies support this hypothesis. (i) COPD disease severity is inversely correlated with CFTR protein expression [117]. (ii) CFTR function and expression are markedly attenuated in the respiratory epithelia of smokers and patients suffering from COPD [118, 119]. (iii) CFTR downregulation was observed upon cigarette smoke or cigarette smoke extract exposure of respiratory and intestinal epithelial cultures [120–122]. (iv) Cigarette smoke decreases the level of CFTR in lipid rafts in mice leading to defective autophagy [123]. Overall, these studies suggest that an acquired loss of CFTR function might contribute to the pathogenesis of COPD.
The inflammation characteristics of COPD also resemble that seen in CF. Cigarette smoke extract and acrolein, a smoke component, have been shown to increase the CXCL8 mRNA stability by activating the p38 MAPK/MK2 signaling pathway [124]. Gene expression levels of CXCL8, IL-6, CCL2, and CCL8 were elevated in both smokers and COPD patients [125]. This is consistent with the increased levels of CXCL1, CXCL8, and CCL2 in induced sputum and/or BAL fluid [109] and the augmented levels of CCL18 in the serum of COPD patients [126].
2.3. Asthma
Asthma affects approximately 300 million people worldwide. Between 5 and 15% are classified as severe asthmatics, specified by a state of heightened morbidity that is refractory to normal pharmacological treatment. Asthma is characterized by airway hyperresponsiveness (AHR), tissue remodeling as indicated by an increased proportion of airway smooth muscle in the airway wall [127], and chronic airway inflammation that together results in reversible airflow obstruction [128]. In severe asthmatics' sputa, besides hallmarks of allergic inflammation, there is increased neutrophil presence that is correlated with lowest lung function and worst asthma control [129]. The increase in smooth muscle mass leads to more airway constriction and contributes to the inflammatory milieu, including synthesis of the neutrophil chemoattractant CXCL8 in severe asthmatics [130]. Recent studies in the CFTR knockout pig [131] and using TMEM16A inhibitors in mouse and human airway smooth muscle [132, 133] support a role of these anion channels in smooth muscle hyperresponsiveness. Moreover, as described in the previous sections, links between defects in anion transport in epithelial cells and increased CXCL8 production may have interesting parallels in asthmatic airway smooth muscle to regulate the inflammatory milieu, particularly in severe asthmatics. The intricate connection between inflammation and anion transport in airway smooth muscle is reviewed elsewhere [134].
Current understanding suggests that beyond the smooth muscle phenotype, the airway epithelium is a key player in the pathology of asthma [135]. In addition to goblet cell hyper- or metaplasia and mucus hypersecretion [136–138], the asthmatic airway epithelium exhibits barrier defects [139, 140] and increased release of the chemokines and cytokines CXCL8, IL-6, GM-CSF, TGF-β1, IL-13, and IL-1β [141, 142] that are capable of triggering both allergic (Th2-mediated [143]) and neutrophilic (Th17-mediated [144]) inflammation. Moreover, the ratio of CXCL8 to the anti-inflammatory lipid mediator lipoxin A4 is increased in severe asthmatics, favoring proinflammatory environment that promotes neutrophil recruitment [145]. Eicosanoids synthesis occurs via the cellular cooperation between structural cells (such as epithelial cells) and immune cells (like neutrophils). Changes in substrate availability (arachidonic acid (AA) versus docosahexaenoic acid (DHA)) can skew the inflammatory response, with allergic individual showing lower levels of DHA [146]. In a murine model of asthma, the proinflammatory balance was reversed using the vitamin A-derivative fenretinide to favor DHA over AA [147].
Some of these epithelial defects are reminiscent of the changes observed in cystic fibrosis. For example, in CF imbalance increasing the AA/DHA ratio has also been observed [148] that can be ameliorated with fenretinide in a CF mouse model [149]. Indeed at least 50% of CF patients show signs of airway hyperresponsiveness and bronchodilator treatment-reversible airway obstruction [150–153], a condition referred to as “CF asthma” [154, 155]. Concordantly, studies found an increased percentage of asthma patients to be carriers of mutations in CFTR [156–158] and the nasal potential difference values of some of these patients scored in the CF range [157]. In addition to CFTR, the involvement of anion transport in the pathophysiology of asthma is suggested by aberrant expression of other epithelial anion transporters. (i) The expression of the anion exchanger pendrin (SLC26A4) that functions to import chloride from the ASL in exchange to bicarbonate and thiocyanate [159] is upregulated in the asthmatic airway epithelium likely as a consequence of Th2 cytokine (IL-4 and IL-13) signaling [160, 161]. Interestingly, pendrin overexpression alone is sufficient to trigger mucus hypersecretion, hyperresponsiveness, and elevated secretion of the chemokines CXCL1 and CXLC2 that in turn lead to neutrophil infiltration into the airway [162]. (ii) A polymorphism in the 3′ UTR of SLC26A9 that functions as CFTR-regulated chloride-bicarbonate exchanger or chloride channel [163–165] is associated with reduced protein expression and an increased risk for asthma [17]. (iii) The calcium-activated chloride channel TMEM16A expression in airway secretory cells is increased in asthma and correlates with mucus hypersecretion [132].
Taken together these evidences show an important role of the airway epithelium to regulate the inflammatory balance in asthma in addition to mucus hypersecretion. Thus, anion transport can significantly influence neutrophil recruitment via increased chemokine synthesis, which is particularly important for the more severe form of the disease.
2.4. Pancreatitis
Acute pancreatitis (AP) is a sudden inflammatory disorder of the pancreas. AP is one of the most common disorders requiring acute hospitalization among the GI diseases [166]. In its chronic form, pancreatitis (CP) can lead to decreased quality of life with continuous pain, diarrhoea, maldigestion, and consequently weight loss. Moreover in some cases, on the base of chronic inflammation, pancreatic ductal adenocarcinoma can develop. The human exocrine pancreas consists of two main secretory cell types: ductal and acinar cells. The two cell types, as a functional unit, interact closely to mediate the secretion of the pancreatic juice and have a conjoint role in the pathogenesis of pancreatitis [167]. Acinar cells synthetize, store, and secrete digestive zymogens that under physiological conditions are carried along the ductal system into the duodenum. AP originates from the premature activation of pancreatic zymogens, leading to the autodigestion of the pancreas that causes the recruitment of inflammatory cells. In addition, acinar cells produce a large amount of protons which are secreted with the pancreatic zymogens resulting in acidic fluid [168]. Ductal cells secrete a bicarbonate-rich, alkaline fluid that is required to neutralize the acinar acid load and carry the zymogens into the duodenum and that acts as a protective mechanism to prevent premature zymogen activation [169]. The ductal secretion contains up to 140 mM HCO3 − that is achieved by the concerted activity of anion transporters. Basolateral Na+-HCO3 − cotransporters, Na+/H+ exchangers, and H+-ATPases mediate HCO3 − uptake into the epithelial cells. On the luminal side CFTR in concert with SCL26A family Cl−/HCO3 − exchangers, specifically SLC26A6 (PAT1) and SLC26A3 (DRA), mediate the bicarbonate secretion. DRA serves as 2Cl−/1HCO3 − exchanger, whereas PAT1 functions with 1 : 2 Cl− : HCO3 − stoichiometry that enables reaching the high bicarbonate concentration characteristic for the pancreatic juice [170, 171]. A direct molecular interaction between CFTR and SLC26A6 and A3 has been observed that results in reciprocal activation of both CFTR and the Cl−/HCO3 − exchangers [172, 173]. However, until now the exact role of SLC26A6 and SLC26A3 in the pancreatic ductal bicarbonate secretion remains controversial [174].
Etiological factors for AP mainly include biliary disease and alcohol consumption. Interestingly, these factors have been shown to impair the ductal bicarbonate transport. (i) Ethanol and its nonoxidative metabolites decrease the CFTR activity, mRNA expression, and its stability at the PM [175, 176]. (ii) At high concentration nonconjugated bile acids impair the ductal bicarbonate and fluid secretion by inhibiting ion transporters (Na+/H+ exchangers, Na+-HCO3 − cotransporters, and Cl−/HCO3 − exchangers) and by causing intracellular calcium overload that leads to mitochondrial damage and ATP depletion and could also affect CFTR [177–180]. (iii) Recent studies suggest that smoking increases the risk for pancreatitis [181–183]. Acrolein, a component of cigarette smoke, has a systematic inhibitory effect on CFTR activity [184] and therefore likely contributes to the CFTR inhibition in pancreatic ducts. (iv) Intra-acinar [185] or intraductal [186, 187] premature activation of trypsinogen to trypsin is a key event in the initiation of pancreatitis. Trypsin inhibits CFTR-mediated ion transport by a PAR-2 dependent pathway, thus leading to a decreased luminal pH that further accelerates the trypsinogen autoactivation [169]. (v) Lastly, heterozygous carriers of CFTR mutations have increased risk to develop pancreatitis [188–197]. The consequences of CFTR mutations on pancreatic function include high protein content and low-flow secretion that frequently results in obstruction and the destruction of the gland. Most CF patients with pancreatic insufficiency (PI) carry “severe” mutations on both alleles that result in the loss of exocrine function. In case “severe” CFTR mutation on one allele is paired with “mild” CFTR mutation on the second allele, most patients retain sufficient exocrine function for digestion, referred to as pancreas sufficient phenotype (PS) [198–200]. Because the exocrine function is necessary for the initiation of pancreatitis, the risk for pancreatitis is higher for those mutations manifesting in PS CF phenotype [201]. Interestingly, the CFTR variant R75Q, which has selective bicarbonate transport defect, is associated with pancreatitis but not classical CF. Overall, the aforementioned studies indicate that the alteration of pancreatic ductal anion transport plays a role in the development of pancreatitis, suggesting that CFTR could be a possible therapeutic target for AP [32].
AP results in local and systematic overproduction of inflammatory mediators [202]. Characteristic hallmark of the disease is the increased serum levels of CXCL8 and IL-6 as well as often IL-1 and TNF-α. CXLC8 and IL-6 serum levels correlate with disease severity [203, 204]. Similar to the CF airway epithelia, it is likely that the pancreatic epithelium itself is at least partly accountable for the augmented chemokine and cytokine production and therefore plays a role in the exacerbation of the pancreatic inflammation. This is supported by the expression of IL-33, IL-6, and CXCL8 in stressed acinar cells [205–208] as well as CXCL8 expression in pancreatic duct cells [209]. Furthermore, pancreatic ductal adenocarcinoma cell lines (CAPAN-1 and CAPAN-2) exhibit basal and LPS induced secretion of CXCL8 and IL-6 [210]. The results of these studies raise the intriguing possibility that impaired anion transport in the pancreatic ductal system could increase chemokine and cytokine secretion and therefore contribute to the progression of pancreatitis.
2.5. Cholestatic Liver Disease
Cholangiopathies, chronic progressive liver diseases (e.g., primary sclerosing cholangitis or primary biliary cirrhosis) characterized by chronic cholestasis, are frequently caused by drugs and antibiotics and are common in CF increasing the risk for mortality [211]. Cholestasis is bile flow stagnation that could result from either the failure of the bile formation in the liver cells (intrahepatic) or the blockade of the ductal secretory mechanism (extrahepatic), responsible for washing biliary secretion away from the hepatocytes. CFTR is expressed at the apical membranes of cholangiocytes and the gallbladder epithelium [212], where it acts as chloride channel. CFTR promotes chloride efflux to generate a luminal chloride gradient, the driving force for bicarbonate secretion via AE2 mediated chloride/bicarbonate exchange resulting in bicarbonate-rich choleresis [213, 214]. CFTR also regulates cholangiocyte secretion by stimulating ATP release into the ductal lumen. Luminal ATP triggers apical P2Y receptors resulting in calcium mobilization, which in turn activates TMEM16A-mediated bicarbonate secretion [215]. Accordingly, dysfunction of CFTR results in low ductal pH, thickened bile, impaired bile flow, and biliary obstruction [216, 217]. This condition leads to accumulation of hydrophobic bile acids and results in periductal inflammation [218, 219].
Similar to the lung epithelium, human biliary epithelial cells participate in the innate immunity. They express various Toll-like receptors and secrete chemokines and cytokines as well as antimicrobial factors [220]. Therefore these cells play a crucial role in the pathogenesis of different cholangiopathies, such as primary biliary cholangitis and biliary atresia [221–223]. Cultured biliary ductal epithelial cells secrete CXCL8 and CCL2 [224, 225] that drive neutrophil infiltration [226]. In addition several other chemokines, including CXCL1, CXCL5, CXCL6, CCL3, CCL4, CCL5, and CXCL10, are expressed by human biliary epithelial cells under both basal and stimulated conditions [227].
One study showed that a combination of proinflammatory cytokines inhibits bile ductular secretion through attenuating AE2 and CFTR function that is impeded by the inhibition of cAMP formation in isolated single biliary ductal units [228]. Other studies however highlighted the role of CFTR function in the innate immune response of the biliary epithelium. Dextran sodium sulphate induced colitis results in an induced inflammatory cholangiopathy in CF but not WT mice [229, 230]. Indeed, downregulation of CFTR in progressive familiar intrahepatic cholestasis and reduced AE2 in primary biliary cirrhosis patients have been reported [231, 232]. Additionally, non-CF-causing CFTR mutations have been associated with primary sclerosing cholangitis in some patients [233, 234], further supporting the role of CFTR in these diseases.
3. Putative Mechanisms Linking Aberrant Anion Transport to the Dysregulated Epithelial Chemokine Release
The inherited (CF) or acquired (COPD, asthma, pancreatitis, and cholestasis) loss of epithelial anion transport is correlated to the increased inflammation driven by the release of chemokines and subsequent immune cell infiltration of the respective organs (Table 1). While in some of the discussed diseases the causality of these events is incompletely established, there is little doubt that loss of epithelial CFTR function is the primary cause for CF. This raises the question how the lack of anion transport leads to the described disease pathogeneses or more specifically which chemical or physical alterations, caused by the lack of chloride, bicarbonate, or other anion transport, trigger the disease mechanisms.
Table 1.
Anion transport activity changes and proinflammatory chemokine and cytokine secretion in different diseases.
| Disease | Transporter/channel function | Augmented chemokines/cytokines | |
|---|---|---|---|
| Airway system | CF | CFTR ↓ | CXCL8, CXCL1, CXCL2, CCL3, CCL18, IL-1β, IL-6, IL-33, TNFα, GM-CSF, G-CSF, HMGB1 |
| COPD | CFTR ↓ | CXCL8, CXCL1, CCL2, CCL8, CCL18, IL-6 | |
| Asthma | CFTR∗
SLC26A4 ↑ TMEM16A ↑ SLC26A9 ↓ |
CXCL8, IL-1β, IL-6, IL-13, TGF-β1, GM-CSF | |
|
| |||
| Gastrointestinal system | Pancreatitis | CFTR∗
CFTR ↓ SLC26A3/A6 ↓ |
CXCL8, IL-1, IL-6, TNF-α |
| Cholestatic liver diseases | CFTR∗
CFTR ↓ AE2 ↓ |
CXCL8, CXCL1, CXCL5, CXCL6, CXCL10, CCL2, CCL3, CCL4, CCL5, | |
CFTR∗: carriers of CFTR mutations have an increased risk to develop disease.
3.1. Chloride Transport
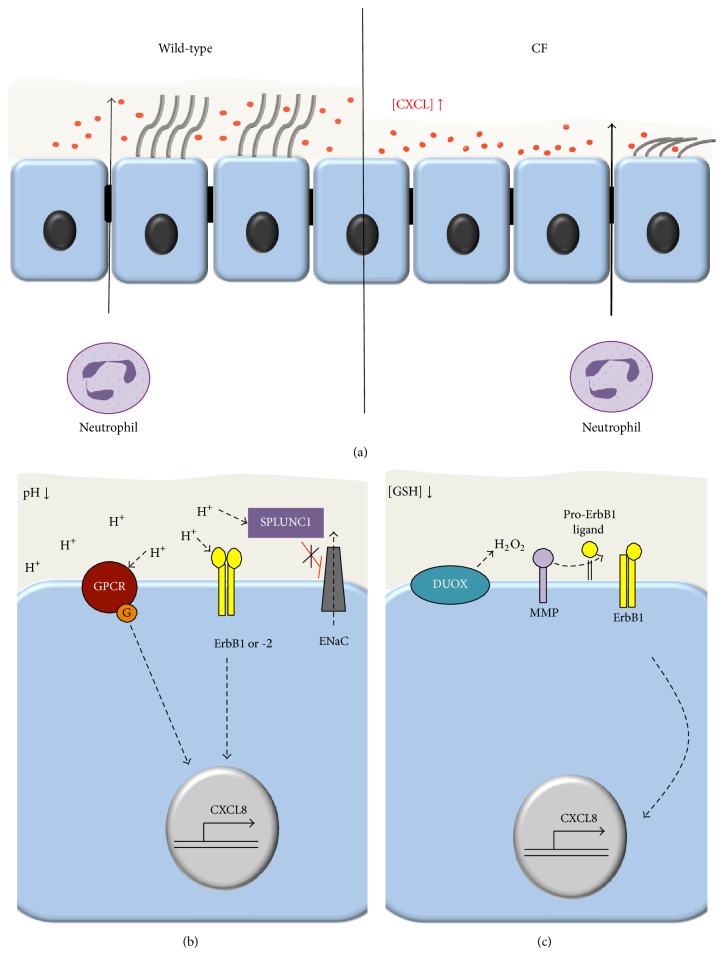
The loss of CFTR-mediated chloride transport resulting in a decreased ASL height was proposed as primary causes of impaired mucociliary clearance, recurrent bacterial infection, and chronic neutrophilic lung infiltration in CF [36, 235, 236]. Reduced ASL height was observed in primary human CF airway epithelial cultures [236], human CF airway biopsies [237], CF ferret tracheas [77], and CF rat tracheas [71]. However, it is under debate whether CFTR inhibits the ENaC channel to prevent sodium hyperabsorption, which if this inhibition is lost contributes to the decreased ASL height [235, 238–240]. To counteract the reduced ASL height by rehydrating the mucus in CF, administration of nebulized hypertonic saline (HTS) was proposed. Administration of 7% HTS increased the mucociliary clearance and lung function and decreased the frequency of exacerbations [241, 242] (Table 2). In cell models hyperosmolarity increases the CXCL8 production by activating the p38 mitogen-activated protein kinase pathway [243, 244]. In contrast, in the sputum of CF patients HTS had either no effect [245, 246] or decreased CXCL8 levels and reduced the neutrophil chemotactic efficiency [247]. The latter observation is consistent with the HTS-mediated release of glycosaminoglycan bound CXCL8 that promotes its proteolytic cleavage and reduces the half-life and function of CXCL8 as neutrophil chemoattractant [247–249]. This leads to a reduced number of neutrophils in the CF sputum after HTS treatment [246]. A direct role of reduced ASL height in the augmented neutrophil chemotaxis in CF was suggested by a study of WT and ΔF508-CFTR tracheal epithelial cells [250]. Here, Pseudomonas aeruginosa infection increased the overall apical and basolateral release of the chemokines CXCL1, CXCL2, CXCL5, and CXCL10 to a similar extent in CF and WT epithelial cells. However, when the apical chemokine concentration was adjusted to the reduced ASL volume in CF, a significantly increased concentration gradient across the epithelial monolayer became apparent that explains the doubled transmigration of neutrophils across the CF monolayers [250] (Figure 2(a)).
Table 2.
Clinical trials for CF therapy indirectly targeting the lack of CFTR-mediated anion transport in the lung by ion-replacement or activation of alternative anion channels.
| Therapeutic approach | Compound | Type of administration | Result | References |
|---|---|---|---|---|
| Mucus rehydration | Hypertonic saline | Nebulized | Modest increase in lung function | [241, 242] |
|
| ||||
| Antioxidant treatment | GSH | Nebulized | Small or no effect on lung function | [288, 289, 312] |
| N-acetylcysteine | Oral | Modest increase in lung function | [291, 294] | |
|
| ||||
| TMEM16A function | Denufosol | Inhaled | Small or no effect on lung function | [305, 313] |
Figure 2.
Putative mechanisms linking impaired anion transport to augmented epithelial chemokine signaling in secretory epithelia. (a) Low ASL in CF results in elevated concentrations of CXCL chemokines due to the reduced liquid volume in the luminal compartment. The increased concentration gradient enhances the neutrophil chemotaxis [250]. (b) Low ASL pH may directly activate chemokine expression by activating acid-sensing signaling pathways via proton-sensing GPCRs, ErbB1, and ErbB2 or the soluble protein SPLUNC1. (c) The decreased ROS buffering capacity of the ASL due to the reduced GSH concentration may promote hydrogen peroxide (H2O2) mediated metalloproteinase (MMP) activation. These in turn cleave pro-erbB1 ligands resulting in erbB1 activation, thus initiating signaling pathways that lead to the upregulation of chemokine expression [285].
3.2. Bicarbonate Transport
Lack of bicarbonate transport at the apical membrane of epithelia results in reduced pH of the epithelial lining fluid as indicated by measurements of the ASL, bile, or pancreatic fluid in CF [77, 78, 216, 251–254]. This pH difference might be attributed to the lack of CFTR-mediated bicarbonate transport [255, 256] or to the absence of CFTR-mediated regulation of the chloride/bicarbonate exchanger pendrin or other members of the SLC26 family [159, 163, 257]. The pH difference is augmented by the nongastric H+/K+ adenosine triphosphatase ATP12A activity that in human and pig mediates H+ transport into the ASL [258].
Reduced ASL bicarbonate and pH could provoke CF and CF-like phenotypes by multiple mechanisms. (i) Mucins packaging in goblet cell granules requires the presence of high intragranular calcium concentrations and acidic pH. Upon exocytosis, calcium chelation by bicarbonate and normal ASL pH facilitate mucin unfolding, prerequisite for their proteolytic cleavage and release from the cells surface [259, 260]. Impaired mucus detachment in CF pig submucosal glands and attenuated mucus release in CF mouse intestine highlight the importance of this mechanism [261–263]. In addition, the increased viscosity of mucins at acidic pH could contribute to the mucociliary clearance defect in CF [264]. (ii) Reduced ASL pH attenuates the bactericidal effect of antimicrobial peptides, key components of the innate immune response of the lung. pH-dependent activity was shown for lysozyme, lactoferrin, β-defensin-3, and LL-37 and a deleterious effect on their synergistic bactericidal properties upon pH-reduction was observed [78, 265]. (iii) The reduced ASL pH may also directly activate chemokine and cytokine expression and release by activating one or more of the following acid-sensing signaling pathways (Figure 2(b)). (a) Proton-sensing G-protein-coupled receptors, including GPR4, GPR65 (TDAG8), GPR68 (OGR1), and GPR132 (G2A), regulate the inflammatory response among other functions [266]. Upon activation by acidic pH GPR4 predominantly signals via the GS pathway resulting in activation of cAMP formation [267, 268] and increased expression of inflammatory genes including chemokines (CXCL2, CXCL8, and CCL20), cytokines, adhesion molecules, NF-κB pathway genes, and stress response genes [269]. GPR68, which is inactive at pH 7.8 and fully activated at pH 6.8, mainly acts through Gq [268]. It is expressed in airway epithelia and stimulates the phospholipase C activity as well as intracellular Ca2+ signaling and has been implicated in the acid-induced mucin5AC and IL-6 secretion [270, 271]. (b) The activity of the receptor tyrosine kinases ErbB1 and ErbB2 is increased by low extracellular pH [272], which can contribute to the increase in CXCL8 production [273]. (c) The short palate lung and nasal epithelial clone 1 (SPLUNC1) is a pH-sensitive regulator of ENaC and is unable to inhibit ENaC in the acidic CF airway environment [252]. Thus, the ensuing increased ENaC activity may result in decreased ALS volume [274] that can trigger augmented chemokine signaling as discussed above.
Aberrant mucin release and reduced bactericidal properties of the lining fluid could promote chronic bacterial infection that in concert with augmented chemokine and cytokine secretion would lead to the hyperinflammatory state seen in CF and other diseases. However, CFTR mutants like R75Q which have normal chloride but selective disruption of bicarbonate conductance do not cause classical CF [195] but are associated with recurrent acute and chronic pancreatitis [198].
3.3. Glutathione and Thiocyanate Transport
Another potential mechanism to perpetuate the imbalance in chemokine production in CF and perhaps other inflammatory disorders is oxidative stress arising from oxidant-antioxidant imbalance. Besides transporting chloride and bicarbonate, CFTR may serve as an efflux channel for reduced glutathione (GSH) [275, 276] and thiocyanate [277, 278]. The ASL concentration of GSH was estimated in the 275–430 μM range in healthy individuals [279, 280]. In contrast in CF patients total GSH concentrations are reduced to 92 μM but the concentration of the oxidised form (GSSG) was not affected by the absence of CFTR activity, thus shifting the redox potential from −175 mV to −128 mV [280]. This shift in redox potential may decrease the redox buffering capacity of the ASL and therefore affect the oxidation state of membrane proteins that could induce downstream proinflammatory signaling [281, 282]. However, it is not well understood how differences in extracellular GSH could result in aberrant gene expression. Increased or insufficiently buffered hydrogen peroxide production at the apical membrane of CF epithelia by NADPH oxidases, especially by the dual oxidase 1 (DUOX1) [283, 284], may increase metalloproteinase-mediated release of ligands that activate the ErbB1 signaling pathway resulting in augmented CXCL8 production [273, 285] (Figure 2(c)). This is consistent with the protective role of extracellular GSH supplementation upon infection with Pseudomonas aeruginosa in CF cells that reduced the release of CXCL8 and IL-6 [97, 286].
Direct GSH supplementation was explored as a therapeutic approach for CF as early as 1985. In this study the authors demonstrated that aerosol administered GSH led to an increase in BAL GSH and GSSG levels and decrease in reactive oxygen species [287]. While this and other initial studies were promising, showing an increase in BAL GSH level and improved lung function [288], the improvement could not be confirmed in a recent phase 2b trial [289]. Antioxidant treatment with N-acetylcysteine (NAC) that has a weak antioxidant character but predominantly acts through the replenishment of GSH in deficient cells [290] was explored as alternative to GSH. Initial short-term studies (≤3 month) did not report a significant improvement in CF lung function [291, 292]. Interestingly, administering high doses of NAC three times daily reduced NE, neutrophil counts, and CXCL8 in the sputum of CF patients [293]. A recent phase 2 trial reports a significant relative increase in FEV1 by 4.4% after 24-week treatment, which is predominantly due to stabilization of the lung function in the treatment group while it declined in the control group [294] (Table 2).
Thiocyanate acts both as an antioxidant [295] and precursor for the bactericidal hypothiocyanite [296]. Reduced level of thiocyanate in the epithelial lining fluid has been observed in CF cells, CF mice [297], and CF patients [298]. Restoring the thiocyanate levels in β-ENaC overexpressing mice, a model for CF [70], using nebulized thiocyanate decreased the bacterial burden after Pseudomonas aeruginosa infection and attenuated neutrophil infiltration and reduced the levels of the chemokine CXCL1 and cytokines IL-6 and TNF-α in the BAL [299].
4. Conclusion
The discussed mechanisms, linking attenuated anion transport to the inflammatory state in CF and other diseases, are not mutually exclusive. Therefore it is likely that symptomatic correction of any single one of these transport defects will result in only minor benefit. This is evident by the results of the clinical trials using HTS to rehydrate the mucus in CF that resulted in only a modest improvement in lung function [241, 242] and using inhaled GSH to increase the ASL antioxidant level that was not beneficial [289] (Table 2). Recent advances to correct the underlying defect in CF by folding correctors and gating potentiators of CFTR are promising, and drugs targeting a subset of CF-causing mutations are approved [25, 28]. These compounds might be beneficial for other diseases with an acquired loss of CFTR function phenotype like, for example, COPD. Treatment with the gating potentiator ivacaftor increased the WT-CFTR activity in cigarette smoke exposed primary human bronchial epithelia and partially reverted the smoke-induced mucociliary clearance defect [300]. The University of Alabama recently announced a phase I clinical trial with ivacaftor in COPD patients [301]. Considering the limited efficacy of this potentiator to increase the open probability of WT-CFTR [302] and the documented downregulation of CFTR expression in COPD [117, 122], stabilizing the protein with a corrector drug would be another promising strategy.
Alternative ion channels like TMEM16A or SLC26A9 may compensate for CFTR dysfunction [303]. For TMEM16A this was attempted using the stable UTP analog denufosol that stimulates P2Y-receptor-induced cytoplasmic calcium signaling and thus channel activity [304] (Table 2). This approach was unsuccessful [305], likely due to the rapid desensitization and internalization of the P2Y-receptor [67, 306–308]. These limitations could be overcome by the activator F that probably activates TMEM16A allosterically without triggering cytosolic calcium signaling [309] and that was shown to reduce CXCL8 secretion in a CF cell model [67]. TMEM16A is widely expressed [132], including in the airway smooth muscle where it is implicated in the hyperresponsiveness in asthma [133, 310]. To target this channel for therapy therefore requires the identification and exploitation of an epithelial cell specific splice isoform [311] or regulatory mechanism.
Acknowledgments
This work was supported by Cystic Fibrosis Canada, National Institutes of Health (NIH) DK075302, and Canadian Institutes of Health Research (CIHR) to Gergely L. Lukacs as well as the Hungarian Scientific Research Fund K116634 and the Momentum Grant of the Hungarian Academy of Sciences LP2014-10/2014 to Péter Hegyi. Andrea Schnúr was supported in part by postdoctoral fellowship from Cystic Fibrosis Canada and by postdoctoral fellowship of the CIHR-Funded Chemical Biology Training Program. Simon Rousseau acknowledges a salary award from the FRQ-S. Gergely L. Lukacs is recipient of a Canada Research Chair.
Competing Interests
The authors declare that there are no competing interests regarding the publication of this paper.
References
- 1.Frizzell R. A., Hanrahan J. W. Physiology of epithelial chloride and fluid secretion. Cold Spring Harbor Perspectives in Medicine. 2012;2(6) doi: 10.1101/cshperspect.a009563.a009563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollenhorst M. I., Richter K., Fronius M. Ion transport by pulmonary epithelia. Journal of Biomedicine and Biotechnology. 2011;2011:16. doi: 10.1155/2011/174306.174306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shan J., Huang J., Liao J., Robert R., Hanrahan J. W. Anion secretion by a model epithelium: more lessons from Calu-3. Acta Physiologica. 2011;202(3):523–531. doi: 10.1111/j.1748-1716.2011.02253.x. [DOI] [PubMed] [Google Scholar]
- 4.Hiemstra P. S., McCray P. B., Jr., Bals R. The innate immune function of airway epithelial cells in inflammatory lung disease. European Respiratory Journal. 2015;45(4):1150–1162. doi: 10.1183/09031936.00141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies D. E. Epithelial barrier function and immunity in asthma. Annals of the American Thoracic Society. 2014;11:S244–S255. doi: 10.1513/AnnalsATS.201407-304AW. [DOI] [PubMed] [Google Scholar]
- 6.Hammad H., Lambrecht B. N. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43(1):29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Parker D., Prince A. Innate immunity in the respiratory epithelium. American Journal of Respiratory Cell and Molecular Biology. 2011;45(2):189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanke F. The contribution of the airway epithelial cell to host defense. Mediators of Inflammation. 2015;2015:7. doi: 10.1155/2015/463016.463016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenhut M., Wallace H. Ion channels in inflammation. Pflugers Archiv—European Journal of Physiology. 2011;461(4):401–421. doi: 10.1007/s00424-010-0917-y. [DOI] [PubMed] [Google Scholar]
- 10.Galietta L. J. V., Folli C., Caci E., et al. Effect of inflammatory stimuli on airway ion transport. Proceedings of the American Thoracic Society. 2004;1(1):62–65. doi: 10.1513/pats.2306017. [DOI] [PubMed] [Google Scholar]
- 11.McKay D. M., Baird A. W. Cytokine regulation of epithelial permeability and ion transport. Gut. 1999;44(2):283–289. doi: 10.1136/gut.44.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nofziger C., Dossena S., Suzuki S., Izuhara K., Paulmichl M. Pendrin function in airway epithelia. Cellular Physiology and Biochemistry. 2011;28(3):571–578. doi: 10.1159/000335115. [DOI] [PubMed] [Google Scholar]
- 13.Galietta L. J. V., Pagesy P., Folli C., et al. IL-4 is a potent modulator of ion transport in the human bronchial epithelium in vitro. The Journal of Immunology. 2002;168(2):839–845. doi: 10.4049/jimmunol.168.2.839. [DOI] [PubMed] [Google Scholar]
- 14.Danahay H., Atherton H., Jones G., Bridges R. J., Poll C. T. Interleukin-13 induces a hypersecretory ion transport phenotype in human bronchial epithelial cells. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2002;282(2):L226–L236. doi: 10.1152/ajplung.00311.2001. [DOI] [PubMed] [Google Scholar]
- 15.Caputo A., Caci E., Ferrera L., et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322(5901):590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 16.Scudieri P., Caci E., Bruno S., et al. Association of TMEM16A chloride channel overexpression with airway goblet cell metaplasia. Journal of Physiology. 2012;590(23):6141–6155. doi: 10.1113/jphysiol.2012.240838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anagnostopoulou P., Riederer B., Duerr J., et al. SLC26A9-mediated chloride secretion prevents mucus obstruction in airway inflammation. Journal of Clinical Investigation. 2012;122(10):3629–3634. doi: 10.1172/JCI60429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bitam S., Pranke I., Hollenhorst M., et al. An unexpected effect of TNF-α on F508del-CFTR maturation and function. F1000Research. 2015;4, article 218 doi: 10.12688/f1000research.6683.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galietta L. J. V., Folli C., Marchetti C., et al. Modification of transepithelial ion transport in human cultured bronchial epithelial cells by interferon-γ . American Journal of Physiology—Lung Cellular and Molecular Physiology. 2000;278(6):L1186–L1194. doi: 10.1152/ajplung.2000.278.6.L1186. [DOI] [PubMed] [Google Scholar]
- 20.Besancon F., Przewlocki G., Baro I., Hongre A. S., Escande D., Edelman A. Interferon-gamma downregulates CFTR gene expression in epithelial cells. American Journal of Physiology. 1994;267(5):C1398–C1404. doi: 10.1152/ajpcell.1994.267.5.C1398. [DOI] [PubMed] [Google Scholar]
- 21.Roux J., McNicholas C. M., Carles M., et al. IL-8 inhibits cAMP-stimulated alveolar epithelial fluid transport via a GRK2/PI3K-dependent mechanism. The FASEB Journal. 2013;27(3):1095–1106. doi: 10.1096/fj.12-219295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins F. S. Cystic fibrosis: molecular biology and therapeutic implications. Science. 1992;256(5058):774–779. doi: 10.1126/science.1375392. [DOI] [PubMed] [Google Scholar]
- 23.Rowe S. M., Miller S., Sorscher E. J. Cystic fibrosis. The New England Journal of Medicine. 2005;352(19):1992–2001. doi: 10.1056/nejmra043184. [DOI] [PubMed] [Google Scholar]
- 24.Riordan J. R., Rommens J. M., Kerem B.-S., et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 25.Cutting G. R. Cystic fibrosis genetics: from molecular understanding to clinical application. Nature Reviews Genetics. 2015;16(1):45–56. doi: 10.1038/nrg3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cystic Fibrosis Mutation Database, http://www.genet.sickkids.on.ca/app.
- 27.Sosnay P. R., Siklosi K. R., Van Goor F., et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nature Genetics. 2013;45(10):1160–1167. doi: 10.1038/ng.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veit G., Avramescu R. G., Chiang A. N., et al. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Molecular Biology of the Cell. 2016;27(3):424–433. doi: 10.1091/mbc.e14-04-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The Clinical and Functional Translation of CFTR, http://www.cftr2.org/
- 30.Wilschanski M., Novak I. The cystic fibrosis of exocrine pancreas. Cold Spring Harbor Perspectives in Medicine. 2013;3(5) doi: 10.1101/cshperspect.a009746.a009746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zielenski J. Genotype and phenotype in cystic fibrosis. Respiration. 2000;67(2):117–133. doi: 10.1159/000029497. [DOI] [PubMed] [Google Scholar]
- 32.Hegyi P., Wilschanski M., Muallem S., et al. Reviews of Physiology, Biochemistry and Pharmacology. Vol. 170. Springer; 2016. CFTR: a new horizon in the pathomechanism and treatment of pancreatitis; pp. 37–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kristidis P., Bozon D., Corey M., et al. Genetic determination of exocrine pancreatic function in cystic fibrosis. American Journal of Human Genetics. 1992;50(6):1178–1184. [PMC free article] [PubMed] [Google Scholar]
- 34.Shwachman H., Lebenthal E., Khaw K. T. Recurrent acute pancreatitis in patients with cystic fibrosis with normal pancreatic enzymes. Pediatrics. 1975;55(1):86–95. [PubMed] [Google Scholar]
- 35.De Boeck K., Weren M., Proesmans M., Kerem E. Pancreatitis among patients with cystic fibrosis: correlation with pancreatic status and genotype. Pediatrics. 2005;115(4):e463–e469. doi: 10.1542/peds.2004-1764. [DOI] [PubMed] [Google Scholar]
- 36.Matsui H., Grubb B. R., Tarran R., et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95(7):1005–1015. doi: 10.1016/S0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 37.Tarran R., Grubb B. R., Parsons D., et al. The CF salt controversy: in vivo observations and therapeutic approaches. Molecular Cell. 2001;8(1):149–158. doi: 10.1016/s1097-2765(01)00286-6. [DOI] [PubMed] [Google Scholar]
- 38.Derichs N., Jin B.-J., Song Y., Finkbeiner W. E., Verkman A. S. Hyperviscous airway periciliary and mucous liquid layers in cystic fibrosis measured by confocal fluorescence photobleaching. FASEB Journal. 2011;25(7):2325–2332. doi: 10.1096/fj.10-179549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoltz D. A., Meyerholz D. K., Welsh M. J. Origins of cystic fibrosis lung disease. The New England journal of medicine. 2015;372(4):351–362. doi: 10.1056/nejmra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Downey D. G., Bell S. C., Elborn J. S. Neutrophils in cystic fibrosis. Thorax. 2009;64(1):81–88. doi: 10.1136/thx.2007.082388. [DOI] [PubMed] [Google Scholar]
- 41.Laval J., Ralhan A., Hartl D. Neutrophils in cystic fibrosis. Biological Chemistry. 2016;397(6) doi: 10.1515/hsz-2015-0271. [DOI] [PubMed] [Google Scholar]
- 42.Nichols D. P., Chmiel J. F. Inflammation and its genesis in cystic fibrosis. Pediatric Pulmonology. 2015;50(supplement 40):S39–S56. doi: 10.1002/ppul.23242. [DOI] [PubMed] [Google Scholar]
- 43.Twigg M. S., Brockbank S., Lowry P., Fitzgerald S. P., Taggart C., Weldon S. The role of serine proteases and antiproteases in the cystic fibrosis lung. Mediators of Inflammation. 2015;2015:10. doi: 10.1155/2015/293053.293053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Birrer P., Mcelvaney N. G., Rüdeberg A., et al. Protease-antiprotease imbalance in the lungs of children with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine. 1994;150(1):207–213. doi: 10.1164/ajrccm.150.1.7912987. [DOI] [PubMed] [Google Scholar]
- 45.Devaney J. M., Greene C. M., Taggart C. C., Carroll T. P., O'Neill S. J., McElvaney N. G. Neutrophil elastase up-regulates interleukin-8 via toll-like receptor 4. FEBS Letters. 2003;544(1–3):129–132. doi: 10.1016/s0014-5793(03)00482-4. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura H., Yoshimura K., McElvaney N. G., Crystal R. G. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. Journal of Clinical Investigation. 1992;89(5):1478–1484. doi: 10.1172/JCI115738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao M. X. G., Nadel J. A. Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-α-converting enzyme. Journal of Immunology. 2005;175(6):4009–4016. doi: 10.4049/jimmunol.175.6.4009. [DOI] [PubMed] [Google Scholar]
- 48.Park J.-A., He F., Martin L. D., Li Y., Chorley B. N., Adler K. B. Human neutrophil elastase induces hypersecretion of mucin from well-differentiated human bronchial epithelial cells in vitro via a protein kinase Cδ-mediated mechanism. American Journal of Pathology. 2005;167(3):651–661. doi: 10.1016/S0002-9440(10)62040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weldon S., McNally P., McElvaney N. G., et al. Decreased levels of secretory leucoprotease inhibitor in the Pseudomonas-infected cystic fibrosis lung are due to neutrophil elastase degradation. The Journal of Immunology. 2009;183(12):8148–8156. doi: 10.4049/jimmunol.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Gars M., Descamps D., Roussel D., et al. Neutrophil elastase degrades cystic fibrosis transmembrane conductance regulator via calpains and disables channel function in vitro and in vivo. American Journal of Respiratory and Critical Care Medicine. 2013;187(2):170–179. doi: 10.1164/rccm.201205-0875OC. [DOI] [PubMed] [Google Scholar]
- 51.Hayes E., Pohl K., McElvaney N. G., Reeves E. P. The cystic fibrosis neutrophil: a specialized yet potentially defective cell. Archivum Immunologiae et Therapiae Experimentalis. 2011;59(2):97–112. doi: 10.1007/s00005-011-0113-6. [DOI] [PubMed] [Google Scholar]
- 52.Pohl K., Hayes E., Keenan J., et al. A neutrophil intrinsic impairment affecting Rab27a and degranulation in cystic fibrosis is corrected by CFTR potentiator therapy. Blood. 2014;124(7):999–1009. doi: 10.1182/blood-2014-02-555268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Painter R. G., Valentine V. G., Lanson N. A., Jr., et al. CFTR expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry. 2006;45(34):10260–10269. doi: 10.1021/bi060490t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Painter R. G., Marrero L., Lombard G. A., Valentine V. G., Nauseef W. M., Wang G. CFTR-mediated halide transport in phagosomes of human neutrophils. Journal of Leukocyte Biology. 2010;87(5):933–942. doi: 10.1189/jlb.1009655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Machen T. E. Innate immune response in CF airway epithelia: hyperinflammatory? American Journal of Physiology—Cell Physiology. 2006;291(2):C218–C230. doi: 10.1152/ajpcell.00605.2005. [DOI] [PubMed] [Google Scholar]
- 56.Elizur A., Cannon C. L., Ferkol T. W. Airway inflammation in cystic fibrosis. Chest. 2008;133(2):489–495. doi: 10.1378/chest.07-1631. [DOI] [PubMed] [Google Scholar]
- 57.Cohen-Cymberknoh M., Kerem E., Ferkol T., Elizur A. Airway inflammation in cystic fibrosis: molecular mechanisms and clinical implications. Thorax. 2013;68(12):1157–1162. doi: 10.1136/thoraxjnl-2013-203204. [DOI] [PubMed] [Google Scholar]
- 58.Ratcliff R., Evans M. J., Cuthbert A. W., et al. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nature Genetics. 1993;4(1):35–41. doi: 10.1038/ng0593-35. [DOI] [PubMed] [Google Scholar]
- 59.Snouwaert J. N., Brigman K. K., Latour A. M., et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257(5073):1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 60.Delaney S. J., Alton E. W. F. W., Smith S. N., et al. Cystic fibrosis mice carrying the missense mutation G551D replicate human genotype-phenotype correlations. The EMBO Journal. 1996;15(5):955–963. [PMC free article] [PubMed] [Google Scholar]
- 61.van Doorninck J. H., French P. J., Verbeek E., et al. A mouse model for the cystic fibrosis ΔF508 mutation. The EMBO Journal. 1995;14(18):4403–4411. doi: 10.1002/j.1460-2075.1995.tb00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colledge W. H., Abella B. S., Southern K. W., et al. Generation and characterization of a ΔF508 cystic fibrosis mouse model. Nature Genetics. 1995;10(4):445–452. doi: 10.1038/ng0895-445. [DOI] [PubMed] [Google Scholar]
- 63.Gavilanes X., Huaux F., Meyer M., et al. Azithromycin fails to reduce increased expression of neutrophil-related cytokines in primary-cultured epithelial cells from cystic fibrosis mice. Journal of Cystic Fibrosis. 2009;8(3):203–210. doi: 10.1016/j.jcf.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Van Heeckeren A., Walenga R., Konstan M. W., Bonfield T., Davis P. B., Ferkol T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa . Journal of Clinical Investigation. 1997;100(11):2810–2815. doi: 10.1172/jci119828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dalli J., Rosignoli G., Hayhoe R. P. G., Edelman A., Perretti M. CFTR inhibition provokes an inflammatory response associated with an imbalance of the annexin A1 pathway. American Journal of Pathology. 2010;177(1):176–186. doi: 10.2353/ajpath.2010.091149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelly M., Trudel S., Brouillard F., et al. Cystic fibrosis transmembrane regulator inhibitors CFTRinh-172 and GlyH-101 target mitochondrial functions, independently of chloride channel inhibition. Journal of Pharmacology and Experimental Therapeutics. 2010;333(1):60–69. doi: 10.1124/jpet.109.162032. [DOI] [PubMed] [Google Scholar]
- 67.Veit G., Bossard F., Goepp J., et al. Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia. Molecular Biology of the Cell. 2012;23(21):4188–4202. doi: 10.1091/mbc.E12-06-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Melis N., Tauc M., Cougnon M., et al. Revisiting CFTR inhibition: a comparative study of CFTRinh-172 and GlyH-101 inhibitors. British Journal of Pharmacology. 2014;171(15):3716–3727. doi: 10.1111/bph.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stutts M. J., Canessa C. M., Olsen J. C., et al. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269(5225):847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 70.Mall M., Grubb B. R., Harkema J. R., O'Neal W. K., Boucher R. C. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nature Medicine. 2004;10(5):487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 71.Tuggle K. L., Birket S. E., Cui X., et al. Characterization of defects in ion transport and tissue development in Cystic Fibrosis Transmembrane Conductance Regulator (CFTR)-knockout rats. PLoS ONE. 2014;9(3) doi: 10.1371/journal.pone.0091253.e91253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rogers C. S., Hao Y., Rokhlina T., et al. Production of CFTR-null and CFTR-ΔF508 heterozygous pigs by adeno-associated virus—mediated gene targeting and somatic cell nuclear transfer. The Journal of Clinical Investigation. 2008;118(4):1571–1577. doi: 10.1172/jci34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun X., Yan Z., Yi Y., et al. Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. Journal of Clinical Investigation. 2008;118(4):1578–1583. doi: 10.1172/jci34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rogers C. S., Stoltz D. A., Meyerholz D. K., et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321(5897):1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun X., Sui H., Fisher J. T., et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. Journal of Clinical Investigation. 2010;120(9):3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ostedgaard L. S., Meyerholz D. K., Chen J., et al. The ΔF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Science Translational Medicine. 2011;3(74) doi: 10.1126/scitranslmed.3001868.74ra24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keiser N. W., Birket S. E., Evans I. A., et al. Defective innate immunity and hyperinflammation in newborn cystic fibrosis transmembrane conductance regulator-knockout ferret lungs. American Journal of Respiratory Cell and Molecular Biology. 2015;52(6):683–694. doi: 10.1165/rcmb.2014-0250oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pezzulo A. A., Tang X. X., Hoegger M. J., et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;486(7405):109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abu-El-Haija M., Sinkora M., Meyerholz D. K., et al. An activated immune and inflammatory response targets the pancreas of newborn pigs with cystic fibrosis. Pancreatology. 2011;11(5):506–515. doi: 10.1159/000332582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Navis A., Bagnat M. Loss of cftr function leads to pancreatic destruction in larval zebrafish. Developmental Biology. 2015;399(2):237–248. doi: 10.1016/j.ydbio.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khan T. Z., Wagener J. S., Bost T., Martinez J., Accurso F. J., Riches D. W. H. Early pulmonary inflammation in infants with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine. 1995;151(4):1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 82.Noah T. L., Black H. R., Cheng P.-W., Wood R. E., Leigh M. W. Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. Journal of Infectious Diseases. 1997;175(3):638–647. doi: 10.1093/infdis/175.3.638. [DOI] [PubMed] [Google Scholar]
- 83.Muhlebach M. S., Stewart P. W., Leigh M. W., Noah T. L. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. American Journal of Respiratory and Critical Care Medicine. 1999;160(1):186–191. doi: 10.1164/ajrccm.160.1.9808096. [DOI] [PubMed] [Google Scholar]
- 84.Armstrong D. S., Hook S. M., Jamsen K. M., et al. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatric Pulmonology. 2005;40(6):500–510. doi: 10.1002/ppul.20294. [DOI] [PubMed] [Google Scholar]
- 85.Balough K., McCubbin M., Weinberger M., Smits W., Ahrens R., Fick R. The relationship between infection and inflammation in the early stages of lung disease from cystic fibrosis. Pediatric Pulmonology. 1995;20(2):63–70. doi: 10.1002/ppul.1950200203. [DOI] [PubMed] [Google Scholar]
- 86.Koller D. Y., Nething I., Otto J., Urbanek R., Eichler I. Cytokine concentrations in sputum from patients with cystic fibrosis and their relation to eosinophil activity. American Journal of Respiratory and Critical Care Medicine. 1997;155(3):1050–1054. doi: 10.1164/ajrccm.155.3.9116985. [DOI] [PubMed] [Google Scholar]
- 87.Bonfield T. L., Panuska J. R., Konstan M. W., et al. Inflammatory cytokines in cystic fibrosis lungs. American Journal of Respiratory and Critical Care Medicine. 1995;152(6, part 1):2111–2118. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 88.Moser C., Jensen P. Ø., Pressler T., et al. Serum concentrations of GM-CSF and G-CSF correlate with the Th1/Th2 cytokine response in cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. APMIS. 2005;113(6):400–409. doi: 10.1111/j.1600-0463.2005.apm_142.x. [DOI] [PubMed] [Google Scholar]
- 89.Roussel L., Farias R., Rousseau S. IL-33 is expressed in epithelia from patients with cystic fibrosis and potentiates neutrophil recruitment. Journal of Allergy and Clinical Immunology. 2013;131(3):913–916. doi: 10.1016/j.jaci.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 90.Tiringer K., Treis A., Kanolzer S., et al. Differential expression of IL-33 and HMGB1 in the lungs of stable cystic fibrosis patients. European Respiratory Journal. 2014;44(3):802–805. doi: 10.1183/09031936.00046614. [DOI] [PubMed] [Google Scholar]
- 91.Hector A., Kröner C., Carevic M., et al. The chemokine CCL18 characterises Pseudomonas infections in cystic fibrosis lung disease. European Respiratory Journal. 2014;44(6):1608–1615. doi: 10.1183/09031936.00070014. [DOI] [PubMed] [Google Scholar]
- 92.Dosanjh A. K., Elashoff D., Robbins R. C. The bronchoalveolar lavage fluid of cystic fibrosis lung transplant recipients demonstrates increased interleukin-8 and elastase and decreased IL-10. Journal of Interferon and Cytokine Research. 1998;18(10):851–854. doi: 10.1089/jir.1998.18.851. [DOI] [PubMed] [Google Scholar]
- 93.Bonfield T. L., Konstan M. W., Burfeind P., Panuska J. R., Hilliard J. B., Berger M. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. American Journal of Respiratory Cell and Molecular Biology. 1995;13(3):257–261. doi: 10.1165/ajrcmb.13.3.7544594. [DOI] [PubMed] [Google Scholar]
- 94.Tirouvanziam R., De Bentzmann S., Hubeau C., et al. Inflammation and infection in naive human cystic fibrosis airway grafts. American Journal of Respiratory Cell and Molecular Biology. 2000;23(2):121–127. doi: 10.1165/ajrcmb.23.2.4214. [DOI] [PubMed] [Google Scholar]
- 95.Tirouvanziam R., Khazaal I., Péault B. Primary inflammation in human cystic fibrosis small airways. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2002;283(2):L445–L451. doi: 10.1152/ajplung.00419.2001. [DOI] [PubMed] [Google Scholar]
- 96.Ribeiro C. M. P., Paradiso A. M., Schwab U., et al. Chronic airway infection/inflammation induces a Ca2+ i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. The Journal of Biological Chemistry. 2005;280(18):17798–17806. doi: 10.1074/jbc.m410618200. [DOI] [PubMed] [Google Scholar]
- 97.Roussel L., Martel G., Bérubé J., Rousseau S. P. aeruginosa drives CXCL8 synthesis via redundant toll-like receptors and NADPH oxidase in CFTRΔF508 airway epithelial cells. Journal of Cystic Fibrosis. 2011;10(2):107–113. doi: 10.1016/j.jcf.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 98.Vandivier R. W., Richens T. R., Horstmann S. A., et al. Dysfunctional cystic fibrosis transmembrane conductance regulator inhibits phagocytosis of apoptotic cells with proinflammatory consequences. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2009;297(4):L677–L686. doi: 10.1152/ajplung.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fulcher M. L., Gabriel S. E., Olsen J. C., et al. Novel human bronchial epithelial cell lines for cystic fibrosis research. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2009;296(1):L82–L91. doi: 10.1152/ajplung.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hybiske K., Fu Z., Schwarzer C., et al. Effects of cystic fibrosis transmembrane conductance regulator and ΔF508CFTR on inflammatory response, ER stress, and Ca2+ of airway epithelia. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2007;293(5):L1250–L1260. doi: 10.1152/ajplung.00231.2007. [DOI] [PubMed] [Google Scholar]
- 101.Pizurki L., Morris M. A., Chanson M., et al. Cystic fibrosis transmembrane conductance regulator does not affect neutrophil migration across cystic fibrosis airway epithelial monolayers. The American Journal of Pathology. 2000;156(4):1407–1416. doi: 10.1016/s0002-9440(10)65009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.John G., Chillappagari S., Rubin B. K., Gruenert D. C., Henke M. O. Reduced surface toll-like receptor-4 expression and absent interferon-γ-inducible protein-10 induction in cystic fibrosis airway cells. Experimental Lung Research. 2011;37(6):319–326. doi: 10.3109/01902148.2011.569968. [DOI] [PubMed] [Google Scholar]
- 103.John G., Yildirim A. Ö., Rubin B. K., Gruenert D. C., Henke M. O. TLR-4-mediated innate immunity is reduced in cystic fibrosis airway cells. American Journal of Respiratory Cell and Molecular Biology. 2010;42(4):424–431. doi: 10.1165/rcmb.2008-0408OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brigati C., Banelli B., di Vinci A., et al. Inflammation,HIF-1,and the epigenetics that follows. Mediators of Inflammation. 2010;2010:5. doi: 10.1155/2010/263914.263914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perez A., Issler A. C., Cotton C. U., Kelley T. J., Verkman A. S., Davis P. B. CFTR inhibition mimics the cystic fibrosis inflammatory profile. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2007;292(2):L383–L395. doi: 10.1152/ajplung.00403.2005. [DOI] [PubMed] [Google Scholar]
- 106.Crites K. S., Morin G., Orlando V., et al. CFTR Knockdown induces proinflammatory changes in intestinal epithelial cells. Journal of Inflammation. 2015;12, article 62 doi: 10.1186/s12950-015-0107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bellec J., Bacchetta M., Losa D., Anegon I., Chanson M., Nguyen T. H. CFTR inactivation by lentiviral vector-mediated RNA interference and CRISPR-Cas9 genome editing in human airway epithelial cells. Current Gene Therapy. 2015;15(5):447–459. doi: 10.2174/1566523215666150812115939. [DOI] [PubMed] [Google Scholar]
- 108.Vestbo J., Hurd S. S., Agustí A. G., et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American Journal of Respiratory and Critical Care Medicine. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596pp. [DOI] [PubMed] [Google Scholar]
- 109.Barnes P. J. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clinics in Chest Medicine. 2014;35(1):71–86. doi: 10.1016/j.ccm.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 110.Brantly M. L., Paul L. D., Miller B. H., Falk R. T., Wu M., Crystal R. G. Clinical features and history of the destructive lung disease associated with alpha-1-antitrypsin deficiency of adults with pulmonary symptoms. American Review of Respiratory Disease. 1988;138(2):327–336. doi: 10.1164/ajrccm/138.2.327. [DOI] [PubMed] [Google Scholar]
- 111. http://www.who.int/respiratory/copd/en/
- 112.Glader P., von Wachenfeldt K., Löfdahl C.-G. Systemic CD4+ T-cell activation is correlated with FEV1 in smokers. Respiratory Medicine. 2006;100(6):1088–1093. doi: 10.1016/j.rmed.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 113.O'Shaughnessy T. C., Ansari T. W., Barnes N. C., Jeffery P. K. Inflammation in bronchial biopsies of subjects with chronic bronchitis: Inverse relationship of CD8+ T lymphocytes with FEV1. American Journal of Respiratory and Critical Care Medicine. 1997;155(3):852–857. doi: 10.1164/ajrccm.155.3.9117016. [DOI] [PubMed] [Google Scholar]
- 114.Saetta M., Baraldo S., Corbino L., et al. CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1999;160(2):711–717. doi: 10.1164/ajrccm.160.2.9812020. [DOI] [PubMed] [Google Scholar]
- 115.Chrysofakis G., Tzanakis N., Kyriakoy D., et al. Perforin expression and cytotoxic activity of sputum CD8+ lymphocytes in patients with COPD. Chest. 2004;125(1):71–76. doi: 10.1378/chest.125.1.71. [DOI] [PubMed] [Google Scholar]
- 116.Majo J., Ghezzo H., Cosio M. G. Lymphocyte population and apoptosis in the lungs of smokers and their relation to emphysema. European Respiratory Journal. 2001;17(5):946–953. doi: 10.1183/09031936.01.17509460. [DOI] [PubMed] [Google Scholar]
- 117.Bodas M., Min T., Mazur S., Vij N. Critical modifier role of membrane-cystic fibrosis transmembrane conductance regulator-dependent ceramide signaling in lung injury and emphysema. Journal of Immunology. 2011;186(1):602–613. doi: 10.4049/jimmunol.1002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dransfield M. T., Wilhelm A. M., Flanagan B., et al. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest. 2013;144(2):498–506. doi: 10.1378/chest.13-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cantin A. M., Hanrahan J. W., Bilodeau G., et al. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. American Journal of Respiratory and Critical Care Medicine. 2006;173(10):1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 120.Rab A., Rowe S. M., Raju S. V., Bebok Z., Matalon S., Collawn J. F. Cigarette smoke and CFTR: implications in the pathogenesis of COPD. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2013;305(8):L530–L541. doi: 10.1152/ajplung.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rasmussen J. E., Sheridan J. T., Polk W., Davies C. M., Tarran R. Cigarette smoke-induced Ca2+ release leads to cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction. Journal of Biological Chemistry. 2014;289(11):7671–7681. doi: 10.1074/jbc.m113.545137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Clunes L. A., Davies C. M., Coakley R. D., et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. The FASEB Journal. 2012;26(2):533–545. doi: 10.1096/fj.11-192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bodas M., Min T., Vij N. Critical role of CFTR-dependent lipid rafts in cigarette smoke-induced lung epithelial injury. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2011;300(6):L811–L820. doi: 10.1152/ajplung.00408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moretto N., Bertolini S., Iadicicco C., et al. Cigarette smoke and its component acrolein augment IL-8/CXCL8 mRNA stability via p38 MAPK/MK2 signaling in human pulmonary cells. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2012;303(10):L929–L938. doi: 10.1152/ajplung.00046.2012. [DOI] [PubMed] [Google Scholar]
- 125.Llinàs L., Peinado V. I., Ramon Goñi J., et al. Similar gene expression profiles in smokers and patients with moderate COPD. Pulmonary Pharmacology & Therapeutics. 2011;24(1):32–41. doi: 10.1016/j.pupt.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 126.Sin D. D., Miller B. E., Duvoix A., et al. Serum PARC/CCL-18 concentrations and health outcomes in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2011;183(9):1187–1192. doi: 10.1164/rccm.201008-1220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pepe C., Foley S., Shannon J., et al. Differences in airway remodeling between subjects with severe and moderate asthma. Journal of Allergy and Clinical Immunology. 2005;116(3):544–549. doi: 10.1016/j.jaci.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 128.Fanta C. H. Asthma. The New England Journal of Medicine. 2009;360(10):1002–1014. doi: 10.1056/nejmra0804579. [DOI] [PubMed] [Google Scholar]
- 129.Hastie A. T., Moore W. C., Meyers D. A., et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. Journal of Allergy and Clinical Immunology. 2010;125(5):1028–1036.e13. doi: 10.1016/j.jaci.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Robins S., Roussel L., Schachter A., et al. Steroid-insensitive ERK1/2 activity drives CXCL8 synthesis and neutrophilia by airway smooth muscle. American Journal of Respiratory Cell and Molecular Biology. 2011;45(5):984–990. doi: 10.1165/rcmb.2010-0450OC. [DOI] [PubMed] [Google Scholar]
- 131.Cook D. P., Rector M. V., Bouzek D. C., et al. Cystic Fibrosis Transmembrane Conductance Regulator in sarcoplasmic reticulum of airway smooth muscle. Implications for airway contractility. American Journal of Respiratory and Critical Care Medicine. 2016;193(4):417–426. doi: 10.1164/rccm.201508-1562oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huang F., Rock J. R., Harfe B. D., et al. Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(50):21413–21418. doi: 10.1073/pnas.0911935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang C.-H., Li Y., Zhao W., et al. The transmembrane protein 16A Ca2+-activated Cl− channel in airway smooth muscle contributes to airway hyperresponsiveness. American Journal of Respiratory and Critical Care Medicine. 2013;187(4):374–381. doi: 10.1164/rccm.201207-1303oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McCuaig S., Martin J. G. How the airway smooth muscle in cystic fibrosis reacts in proinflammatory conditions: Implications for airway hyper-responsiveness and asthma in cystic fibrosis. The Lancet Respiratory Medicine. 2013;1(2):137–147. doi: 10.1016/S2213-2600(12)70058-9. [DOI] [PubMed] [Google Scholar]
- 135.Loxham M., Davies D. E., Blume C. Epithelial function and dysfunction in asthma. Clinical and Experimental Allergy. 2014;44(11):1299–1313. doi: 10.1111/cea.12309. [DOI] [PubMed] [Google Scholar]
- 136.Aikawa T., Shimura S., Sasaki H., Ebina M., Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992;101(4):916–921. doi: 10.1378/chest.101.4.916. [DOI] [PubMed] [Google Scholar]
- 137.Kuyper L. M., Paré P. D., Hogg J. C., et al. Characterization of airway plugging in fatal asthma. American Journal of Medicine. 2003;115(1):6–11. doi: 10.1016/S0002-9343(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 138.Rubin B. K., Priftis K. N., Schmidt H. J., Henke M. O. Secretory hyperresponsiveness and pulmonary mucus hypersecretion. Chest. 2014;146(2):496–507. doi: 10.1378/chest.13-2609. [DOI] [PubMed] [Google Scholar]
- 139.Hardyman M. A., Wilkinson E., Martin E., et al. TNF-α-mediated bronchial barrier disruption and regulation by src-family kinase activation. Journal of Allergy and Clinical Immunology. 2013;132(3):665.e8–675.e8. doi: 10.1016/j.jaci.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 140.Xiao C., Puddicombe S. M., Field S., et al. Defective epithelial barrier function in asthma. The Journal of Allergy and Clinical Immunology. 2011;128(3):549–556.e1-12. doi: 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 141.Freishtat R. J., Watson A. M., Benton A. S., et al. Asthmatic airway epithelium is intrinsically inflammatory and mitotically dyssynchronous. American Journal of Respiratory Cell and Molecular Biology. 2011;44(6):863–869. doi: 10.1165/rcmb.2010-0029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hackett T.-L., Singhera G. K., Shaheen F., et al. Intrinsic phenotypic differences of asthmatic epithelium and its inflammatory responses to respiratory syncytial virus and air pollution. American Journal of Respiratory Cell and Molecular Biology. 2011;45(5):1090–1100. doi: 10.1165/rcmb.2011-0031OC. [DOI] [PubMed] [Google Scholar]
- 143.Robinson D. S., Hamid Q., Ying S., et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. The New England Journal of Medicine. 1992;326(5):298–304. doi: 10.1056/nejm199201303260504. [DOI] [PubMed] [Google Scholar]
- 144.Bullens D. M. A., Truyen E., Coteur L., et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respiratory Research. 2006;7, article 135 doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vachier I., Bonnans C., Chavis C., et al. Severe asthma is associated with a loss of LX4, an endogenous anti-inflammatory compound. Journal of Allergy and Clinical Immunology. 2005;115(1):55–60. doi: 10.1016/j.jaci.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 146.Yu G., Björkstén B. Polyunsaturated fatty acids in school children in relation to allergy and serum IgE levels. Pediatric Allergy and Immunology. 1998;9(3):133–138. doi: 10.1111/j.1399-3038.1998.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 147.Kanagaratham C., Kalivodová A., Najdekr L., et al. Fenretinide prevents inflammation and airway hyperresponsiveness in a mouse model of allergic asthma. American Journal of Respiratory Cell and Molecular Biology. 2014;51(6):783–792. doi: 10.1165/rcmb.2014-0121OC. [DOI] [PubMed] [Google Scholar]
- 148.Freedman S. D., Blanco P. G., Zaman M. M., et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. The New England Journal of Medicine. 2004;350(6):560–569. doi: 10.1056/nejmoa021218. [DOI] [PubMed] [Google Scholar]
- 149.Guilbault C., De Sanctis J. B., Wojewodka G., et al. Fenretinide corrects newly found ceramide deficiency in cystic fibrosis. American Journal of Respiratory Cell and Molecular Biology. 2008;38(1):47–56. doi: 10.1165/rcmb.2007-0036OC. [DOI] [PubMed] [Google Scholar]
- 150.Colombo J. L. Long-acting bronchodilators in cystic fibrosis. Current Opinion in Pulmonary Medicine. 2003;9(6):504–508. doi: 10.1097/00063198-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 151.Sanchez I., Powell R. E., Pasterkamp H. Wheezing and airflow obstruction during methacholine challenge in children with cystic fibrosis and in normal children. American Review of Respiratory Disease. 1993;147(3):705–709. doi: 10.1164/ajrccm/147.3.705. [DOI] [PubMed] [Google Scholar]
- 152.van Haren E. H. J., Lammers J.-W. J., Festen J., Van Herwaarden C. L. A. Bronchial vagal tone and responsiveness to histamine, exercise and bronchodilators in adult patients with cystic fibrosis. European Respiratory Journal. 1992;5(9):1083–1088. [PubMed] [Google Scholar]
- 153.Weinberger M. Airways reactivity in patients with CF. Clinical Reviews in Allergy and Immunology. 2002;23(1):77–85. doi: 10.1385/CRIAI:23:1:077. [DOI] [PubMed] [Google Scholar]
- 154.Balfour-Lynn I. M., Elborn J. S. ‘CF asthma’: what is it and what do we do about it? Thorax. 2002;57(8):742–748. doi: 10.1136/thorax.57.8.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kent B. D., Lane S. J., van Beek E. J., Dodd J. D., Costello R. W., Tiddens H. A. W. M. Asthma and cystic fibrosis: a tangled web. Pediatric Pulmonology. 2014;49(3):205–213. doi: 10.1002/ppul.22934. [DOI] [PubMed] [Google Scholar]
- 156.Dahl M., Tybjærg-Hansen A., Lange P., Nordestgaard B. G. ΔF508 heterozygosity in cystic fibrosis and susceptibility to asthma. The Lancet. 1998;351(9120):1911–1913. doi: 10.1016/s0140-6736(97)11419-2. [DOI] [PubMed] [Google Scholar]
- 157.Schulz A., Tümmler B. Non-allergic asthma as a CFTR-related disorder. Journal of Cystic Fibrosis. 2015 doi: 10.1016/j.jcf.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 158.Tzetis M., Efthymiadou A., Strofalis S., et al. CFTR gene mutations—including three novel nucleotide substitutions- and haplotype background in patients with asthma, disseminated bronchiectasis and chronic obstructive pulmonary disease. Human Genetics. 2001;108(3):216–221. doi: 10.1007/s004390100467. [DOI] [PubMed] [Google Scholar]
- 159.Garnett J. P., Hickman E., Burrows R., et al. Novel role for pendrin in orchestrating bicarbonate secretion in cystic fibrosis transmembrane conductance regulator (CFTR)-expressing airway serous cells. The Journal of Biological Chemistry. 2011;286(47):41069–41082. doi: 10.1074/jbc.m111.266734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kuperman D. A., Lewis C. C., Woodruff P. G., et al. Dissecting asthma using focused transgenic modeling and functional genomics. Journal of Allergy and Clinical Immunology. 2005;116(2):305–311. doi: 10.1016/j.jaci.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 161.Pedemonte N., Caci E., Sondo E., et al. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels. Journal of Immunology. 2007;178(8):5144–5153. doi: 10.4049/jimmunol.178.8.5144. [DOI] [PubMed] [Google Scholar]
- 162.Nakao I., Kanaji S., Ohta S., et al. Identification of pendrin as a common mediator for mucus production in bronchial asthma and chronic obstructive pulmonary disease. Journal of Immunology. 2008;180(9):6262–6269. doi: 10.4049/jimmunol.180.9.6262. [DOI] [PubMed] [Google Scholar]
- 163.Bertrand C. A., Zhang R., Pilewski J. M., Frizzell R. A. SLC26A9 is a constitutively active, CFTR-regulated anion conductance in human bronchial epithelia. Journal of General Physiology. 2009;133(4):421–438. doi: 10.1085/jgp.200810097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chang M.-H., Plata C., Zandi-Nejad K., et al. Slc26a9—anion exchanger, channel and Na+ transporter. Journal of Membrane Biology. 2009;228(3):125–140. doi: 10.1007/s00232-009-9165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dorwart M. R., Shcheynikov N., Wang Y., Stippec S., Muallem S. SLC26A9 is a Cl- channel regulated by the WNK kinases. Journal of Physiology. 2007;584(1):333–345. doi: 10.1113/jphysiol.2007.135855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Peery A. F., Dellon E. S., Lund J., et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179–1187.e3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hegyi P., Pandol S., Venglovecz V., Rakonczay Z., Jr. The acinar-ductal tango in the pathogenesis of acute pancreatitis. Gut. 2011;60(4):544–552. doi: 10.1136/gut.2010.218461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hegyi P., Maléth J., Venglovecz V., Rakonczay Z., Jr. Pancreatic ductal bicarbonate secretion: challenge of the acinar acid load. Frontiers in Physiology. 2011;2, article 36 doi: 10.3389/fphys.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pallagi P., Venglovecz V., Rakonczay Z., et al. Trypsin reduces pancreatic ductal bicarbonate secretion by inhibiting CFTR Cl− channels and luminal anion exchangers. Gastroenterology. 2011;141(6):2228.e6–2239.e6. doi: 10.1053/j.gastro.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Steward M. C., Ishiguro H. Molecular and cellular regulation of pancreatic duct cell function. Current Opinion in Gastroenterology. 2009;25(5):447–453. doi: 10.1097/MOG.0b013e32832e06ce. [DOI] [PubMed] [Google Scholar]
- 171.Steward M. C., Ishiguro H., Case R. M. Mechanisms of bicarbonate secretion in the pancreatic duct. Annual Review of Physiology. 2005;67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- 172.Ko S. B. H., Zeng W., Dorwart M. R., et al. Gating of CFTR by the STAS domain of SLC26 transporters. Nature Cell Biology. 2004;6(4):343–350. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gray M. A. Bicarbonate secretion: it takes two to tango. Nature Cell Biology. 2004;6(4):292–294. doi: 10.1038/ncb0404-292. [DOI] [PubMed] [Google Scholar]
- 174.Hegyi P., Rakonczay Z., Farkas K., et al. Controversies in the role of SLC26 anion exchangers in pancreatic ductal bicarbonate secretion. Pancreas. 2008;37(2):232–234. doi: 10.1097/MPA.0b013e318164f8ff. [DOI] [PubMed] [Google Scholar]
- 175.Maléth J., Balázs A., Pallagi P., et al. Alcohol disrupts levels and function of the cystic fibrosis transmembrane conductance regulator to promote development of pancreatitis. Gastroenterology. 2015;148(2):427–439.e16. doi: 10.1053/j.gastro.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Judák L., Hegyi P., Rakonczay Z., Jr., Maléth J., Gray M. A., Venglovecz V. Ethanol and its non-oxidative metabolites profoundly inhibit CFTR function in pancreatic epithelial cells which is prevented by ATP supplementation. Pflugers Archiv European Journal of Physiology. 2014;466(3):549–562. doi: 10.1007/s00424-013-1333-x. [DOI] [PubMed] [Google Scholar]
- 177.Venglovecz V., Rakonczay Z., Jr., Ózsvári B., et al. Effects of bile acids on pancreatic ductal bicarbonate secretion in guinea pig. Gut. 2008;57(8):1102–1112. doi: 10.1136/gut.2007.134361. [DOI] [PubMed] [Google Scholar]
- 178.Maléth J., Venglovecz V., Rázga Z., Tiszlavicz L., Rakonczay Z., Jr., Hegyi P. Non-conjugated chenodeoxycholate induces severe mitochondrial damage and inhibits bicarbonate transport in pancreatic duct cells. Gut. 2011;60(1):136–138. doi: 10.1136/gut.2009.192153. [DOI] [PubMed] [Google Scholar]
- 179.Maléth J., Hegyi P., Rakonczay Z., Venglovecz V. Breakdown of bioenergetics evoked by mitochondrial damage in acute pancreatitis: mechanisms and consequences. Pancreatology. 2015;15, supplement 4:S18–S22. doi: 10.1016/j.pan.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 180.Maléth J., Hegyi P. Calcium signaling in pancreatic ductal epithelial cells: an old friend and a nasty enemy. Cell Calcium. 2014;55(6):337–345. doi: 10.1016/j.ceca.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 181.Ye X., Lu G., Huai J., Ding J. Impact of smoking on the risk of pancreatitis: a systematic review and meta-analysis. PLoS ONE. 2015;10(4) doi: 10.1371/journal.pone.0124075.e0124075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Majumder S., Gierisch J. M., Bastian L. A. The association of smoking and acute pancreatitis: a systematic review and meta-analysis. Pancreas. 2015;44(4):540–546. doi: 10.1097/mpa.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 183.Yuhara H., Ogawa M., Kawaguchi Y., Igarashi M., Mine T. Smoking and risk for acute pancreatitis a systematic review and meta-analysis. Pancreas. 2014;43(8):1201–1207. doi: 10.1097/MPA.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 184.Raju S. V., Jackson P. L., Courville C. A., et al. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. American Journal of Respiratory and Critical Care Medicine. 2013;188(11):1321–1330. doi: 10.10.1164/rccm.201304-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lerch M. M., Gorelick F. S. Early trypsinogen activation in acute pancreatitis. Medical Clinics of North America. 2000;84(3):549–563. doi: 10.1016/S0025-7125(05)70239-X. [DOI] [PubMed] [Google Scholar]
- 186.Renner I. G., Rinderknecht H., Douglas A. P. Profiles of pure pancreatic secretions in patients with acute pancreatitis: the possible role of proteolytic enzymes in pathogenesis. Gastroenterology. 1978;75(6):1090–1098. [PubMed] [Google Scholar]
- 187.Geokas M. C., Rinderknecht H. Free proteolytic enzymes in pancreatic juice of patients with acute pancreatitis. The American Journal of Digestive Diseases. 1974;19(7):591–598. doi: 10.1007/BF01073012. [DOI] [PubMed] [Google Scholar]
- 188.Sharer N., Schwarz M., Malone G., et al. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. The New England Journal of Medicine. 1998;339(10):645–652. doi: 10.1056/nejm199809033391001. [DOI] [PubMed] [Google Scholar]
- 189.Castellani C., Bonizzato A., Rolfini R., Frulloni L., Cavallini G. C., Mastella G. Increased prevalence of mutations of the cystic fibrosis gene in idiopathic chronic and recurrent pancreatitis. The American Journal of Gastroenterology. 1999;94(7):1993–1995. doi: 10.1016/s0002-9270(99)00232-4. [DOI] [PubMed] [Google Scholar]
- 190.Cohn J. A., Friedman K. J., Noone P. G., Knowles M. R., Silverman L. M., Jowell P. S. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. The New England Journal of Medicine. 1998;339(10):653–658. doi: 10.1056/nejm199809033391002. [DOI] [PubMed] [Google Scholar]
- 191.Cohn J. A., Neoptolemos J. P., Feng J., et al. Increased risk of idiopathic chronic pancreatitis in cystic fibrosis carriers. Human Mutation. 2005;26(4):303–307. doi: 10.1002/humu.20232. [DOI] [PubMed] [Google Scholar]
- 192.Noone P. G., Zhou Z., Silverman L. M., Jowell P. S., Knowles M. R., Cohn J. A. Cystic fibrosis gene mutations and pancreatitis risk: relation to epithelial ion transport and trypsin inhibitor gene mutations. Gastroenterology. 2001;121(6):1310–1319. doi: 10.1053/gast.2001.29673. [DOI] [PubMed] [Google Scholar]
- 193.Ockenga J., Stuhrmann M., Ballmann M., et al. Mutations of the cystic fibrosis gene, but not cationic trypsinogen gene, are associated with recurrent or chronic idiopathic pancreatitis. American Journal of Gastroenterology. 2000;95(8):2061–2067. doi: 10.1016/S0002-9270(00)01020-0. [DOI] [PubMed] [Google Scholar]
- 194.Truninger K., Malik N., Ammann R. W., et al. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. The American Journal of Gastroenterology. 2001;96(9):2657–2661. doi: 10.1016/s0002-9270(01)02610-7. [DOI] [PubMed] [Google Scholar]
- 195.LaRusch J., Jung J., General I. J., et al. Mechanisms of CFTR functional variants that impair regulated bicarbonate permeation and increase risk for pancreatitis but not for cystic fibrosis. PLoS Genetics. 2014;10(7) doi: 10.1371/journal.pgen.1004376.e1004376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Masson E., Chen J.-M., Audrézet M.-P., Cooper D. N., Férec C. A conservative assessment of the major genetic causes of idiopathic chronic pancreatitis: data from a comprehensive analysis of PRSS1, SPINK1, CTRC and CFTR Genes in 253 young french patients. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0073522.e73522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rosendahl J., Landt O., Bernadova J., et al. CFTR, SPINK1, CTRC and PRSS1 variants in chronic pancreatitis: Is the role of mutated CFTR overestimated? Gut. 2013;62(4):582–592. doi: 10.1136/gutjnl-2011-300645. [DOI] [PubMed] [Google Scholar]
- 198.Ooi C. Y., Durie P. R. Cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations in pancreatitis. Journal of Cystic Fibrosis. 2012;11(5):355–362. doi: 10.1016/j.jcf.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 199.Ravi Kanth V., Nageshwar Reddy D. Genetics of acute and chronic pancreatitis: an update. World Journal of Gastrointestinal Pathophysiology. 2014;5(4):427–437. doi: 10.4291/wjgp.v5.i4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hegyi P., Rakonczay Z. Insufficiency of electrolyte and fluid secretion by pancreatic ductal cells leads to increased patient risk for pancreatitis. American Journal of Gastroenterology. 2010;105(9):2119–2120. doi: 10.1038/ajg.2010.191. [DOI] [PubMed] [Google Scholar]
- 201.Ooi C. Y., Dorfman R., Cipolli M., et al. Type of CFTR mutation determines risk of pancreatitis in patients with cystic fibrosis. Gastroenterology. 2011;140(1):153–161. doi: 10.1053/j.gastro.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 202.Makhija R., Kingsnorth A. N. Cytokine storm in acute pancreatitis. Journal of Hepato-Biliary-Pancreatic Surgery. 2002;9(4):401–410. doi: 10.1007/s005340200049. [DOI] [PubMed] [Google Scholar]
- 203.Pezzilli R., Billi P., Miniero R., et al. Serum interleukin-6, interleukin-8, and β2-microglobulin in early assessment of severity of acute pancreatitis comparison with serum C-reactive protein. Digestive Diseases and Sciences. 1995;40(11):2341–2348. doi: 10.1007/bf02063235. [DOI] [PubMed] [Google Scholar]
- 204.Gross V., Andreesen R., Leser H.-G., et al. Interleukin-8 and neutrophil activation in acute pancreatitis. European Journal of Clinical Investigation. 1992;22(3):200–203. doi: 10.1111/j.1365-2362.1992.tb01826.x. [DOI] [PubMed] [Google Scholar]
- 205.Kempuraj D., Twait E. C., Williard D. E., Yuan Z., Meyerholz D. K., Samuel I. The novel cytokine interleukin-33 activates acinar cell proinflammatory pathways and induces acute pancreatic inflammation in mice. PLoS ONE. 2013;8(2, article e56866) doi: 10.1371/journal.pone.0056866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Motoo Y., Xie M. J., Mouri H., Sawabu N. Expression of interleukin-8 in human obstructive pancreatitis. Journal of the Pancreas. 2004;5(3):138–144. [PubMed] [Google Scholar]
- 207.Blinman T. A., Gukovsky I., Mouria M., et al. Activation of pancreatic acinar cells on isolation from tissue: cytokine upregulation via p38 MAP kinase. American Journal of Physiology—Cell Physiology. 2000;279(6):C1993–C2003. doi: 10.1152/ajpcell.2000.279.6.C1993. [DOI] [PubMed] [Google Scholar]
- 208.Xie M.-J., Motoo Y., Su S.-B., Mouri H., Sawabu N. Induction of chemokines in rat pancreatic acinar cell injury. Pancreas. 2002;24(2):198–204. doi: 10.1097/00006676-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 209.Movahedi B., Gysemans C., Jacobs-Tulleneers-Thevissen D., Mathieu C., Pipeleers D. Pancreatic duct cells in human islet cell preparations are a source of angiogenic cytokines interleukin-8 and vascular endothelial growth factor. Diabetes. 2008;57(8):2128–2136. doi: 10.2337/db07-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Blanchard J. A., II, Barve S., Joshi-Barve S., Talwalker R., Gates L. K., Jr. Cytokine production by CAPAN-1 and CAPAN-2 cell lines. Digestive Diseases and Sciences. 2000;45(5):927–932. doi: 10.1023/A:1005573024448. [DOI] [PubMed] [Google Scholar]
- 211.Rowland M., Gallagher C., Gallagher C. G., et al. Outcome in patients with cystic fibrosis liver disease. Journal of Cystic Fibrosis. 2015;14(1):120–126. doi: 10.1016/j.jcf.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 212.Cohn J. A., Strong T. V., Picciotto M. R., Nairn A. C., Collins F. S., Fitz J. G. Localization of the cystic fibrosis transmembrane conductance regulator in human bile duct epithelial cells. Gastroenterology. 1993;105(6):1857–1864. doi: 10.1016/0016-5085(93)91085-v. [DOI] [PubMed] [Google Scholar]
- 213.Banales J. M., Arenas F., Rodríguez-Ortigosa C. M., et al. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology. 2006;43(2):266–275. doi: 10.1002/hep.21042. [DOI] [PubMed] [Google Scholar]
- 214.Banales J. M., Prieto J., Medina J. F. Cholangiocyte anion exchange and biliary bicarbonate excretion. World Journal of Gastroenterology. 2006;12(22):3496–3511. doi: 10.3748/wjg.v12.i22.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Minagawa N., Nagata J., Shibao K., et al. Cyclic AMP regulates bicarbonate secretion in cholangiocytes through release of ATP into bile. Gastroenterology. 2007;133(5):1592–1602. doi: 10.1053/j.gastro.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Uc A., Giriyappa R., Meyerholz D. K., et al. Pancreatic and biliary secretion are both altered in cystic fibrosis pigs. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2012;303(8):G961–G968. doi: 10.1152/ajpgi.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nagel R. A., Javaid A., Meire H. B., et al. Liver disease and bile duct abnormalities in adults with cystic fibrosis. The Lancet. 1989;334(8677):1422–1425. doi: 10.1016/s0140-6736(89)92035-7. [DOI] [PubMed] [Google Scholar]
- 218.Herrmann U., Dockter G., Lammert F. Cystic fibrosis-associated liver disease. Best Practice and Research: Clinical Gastroenterology. 2010;24(5):585–592. doi: 10.1016/j.bpg.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 219.Kelly T., Buxbaum J. Gastrointestinal manifestations of cystic fibrosis. Digestive Diseases and Sciences. 2015;60(7):1903–1913. doi: 10.1007/s10620-015-3546-7. [DOI] [PubMed] [Google Scholar]
- 220.Harada K., Nakanuma Y. Biliary innate immunity: function and modulation. Mediators of Inflammation. 2010;2010:9. doi: 10.1155/2010/373878.373878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Harada K., Nakanuma Y. Innate immunity in the pathogenesis of cholangiopathy: a recent update. Inflammation and Allergy—Drug Targets. 2012;11(6):478–483. doi: 10.2174/187152812803589976. [DOI] [PubMed] [Google Scholar]
- 222.Dong R., Zheng S. Interleukin-8: a critical chemokine in biliary atresia. Journal of Gastroenterology and Hepatology. 2015;30(6):970–976. doi: 10.1111/jgh.12900. [DOI] [PubMed] [Google Scholar]
- 223.Choi J., Selmi C., Leung P. S., Kenny T. P., Roskams T., Gershwin M. E. Chemokine and chemokine receptors in autoimmunity: the case of primary biliary cholangitis. Expert Review of Clinical Immunology. 2016;12(6):661–672. doi: 10.1586/1744666x.2016.1147956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Morland C. M., Fear J., McNab G., Joplin R., Adams D. H. Promotion of leukocyte transendothelial cell migration by chemokines derived from human biliary epithelial cells in vitro. Proceedings of the Association of American Physicians. 1997;109(4):372–382. [PubMed] [Google Scholar]
- 225.Kruglov E. A., Nathanson R. A., Nguyen T., Dranoff J. A. Secretion of MCP-1/CCL2 by bile duct epithelia induces myofibroblastic transdifferentiation of portal fibroblasts. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2006;290(4):G765–G771. doi: 10.1152/ajpgi.00308.2005. [DOI] [PubMed] [Google Scholar]
- 226.Isse K., Harada K., Nakanuma Y. IL-8 expression by biliary epithelial cells is associated with neutrophilic infiltration and reactive bile ductules. Liver International. 2007;27(5):672–680. doi: 10.1111/j.1478-3231.2007.01465.x. [DOI] [PubMed] [Google Scholar]
- 227.Shimoda S., Harada K., Niiro H., et al. Biliary epithelial cells and primary biliary cirrhosis: the role of liver-infiltrating mononuclear cells. Hepatology. 2008;47(3):958–965. doi: 10.1002/hep.22102. [DOI] [PubMed] [Google Scholar]
- 228.Spiral C., Nathanson M. H., Fiorotto R., et al. Proinflammatory cytokines inhibit secretion in rat bile duct epithelium. Gastroenterology. 2001;121(1):156–169. doi: 10.1053/gast.2001.25516. [DOI] [PubMed] [Google Scholar]
- 229.Fiorotto R., Scirpo R., Trauner M., et al. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4NF-κB-mediated inflammatory response in mice. Gastroenterology. 2011;141(4):1498–1508. doi: 10.1053/j.gastro.2011.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Blanco P. G., Zaman M. M., Junaidi O., et al. Induction of colitis in cftr−/− mice results in bile duct injury. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2004;287(2):G491–G496. doi: 10.1152/ajpgi.00452.2003. [DOI] [PubMed] [Google Scholar]
- 231.Medina J. F., Martínez-Ansó E., Vázquez J. J., Prieto J. Decreased anion exchanger 2 immunoreactivity in the liver of patients with primary biliary cirrhosis. Hepatology. 1997;25(1):12–17. doi: 10.1002/hep.510250104. [DOI] [PubMed] [Google Scholar]
- 232.Demeilliers C., Jacquemin E., Barbu V., et al. Altered hepatobiliary gene expressions in PFIC1: ATP8B1 gene defect is associated with CFTR downregulation. Hepatology. 2006;43(5):1125–1134. doi: 10.1002/hep.21160. [DOI] [PubMed] [Google Scholar]
- 233.Pall H., Zielenski J., Jonas M. M., et al. Primary sclerosing cholangitis in childhood is associated with abnormalities in cystic fibrosis-mediated chloride channel function. Journal of Pediatrics. 2007;151(3):255–259. doi: 10.1016/j.jpeds.2007.03.062. [DOI] [PubMed] [Google Scholar]
- 234.Henckaerts L., Jaspers M., Van Steenbergen W., et al. Cystic fibrosis transmembrane conductance regulator gene polymorphisms in patients with primary sclerosing cholangitis. Journal of Hepatology. 2009;50(1):150–157. doi: 10.1016/j.jhep.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 235.Tarran R., Trout L., Donaldson S. H., Boucher R. C. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. Journal of General Physiology. 2006;127(5):591–604. doi: 10.1085/jgp.200509468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tarran R., Grubb B. R., Gatzy J. T., Davis C. W., Boucher R. C. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. Journal of General Physiology. 2001;118(2):223–236. doi: 10.1085/jgp.118.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Griesenbach U., Soussi S., Larsen M. B., et al. Quantification of periciliary fluid height in human airway biopsies is feasible, but not suitable as a biomarker. American Journal of Respiratory Cell and Molecular Biology. 2011;44(3):309–315. doi: 10.1165/rcmb.2009-0265OC. [DOI] [PubMed] [Google Scholar]
- 238.Hobbs C. A., Da Tan C., Tarran R. Does epithelial sodium channel hyperactivity contribute to cystic fibrosis lung disease? Journal of Physiology. 2013;591(18):4377–4387. doi: 10.1113/jphysiol.2012.240861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Itani O. A., Chen J.-H., Karp P. H., et al. Human cystic fibrosis airway epithelia have reduced Cl− conductance but not increased Na+ conductance. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(25):10260–10265. doi: 10.1073/pnas.1106695108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chen J.-H., Stoltz D. A., Karp P. H., et al. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell. 2010;143(6):911–923. doi: 10.1016/j.cell.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Elkins M. R., Robinson M., Rose B. R., et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. The New England Journal of Medicine. 2006;354(3):229–240. doi: 10.1056/nejmoa043900. [DOI] [PubMed] [Google Scholar]
- 242.Donaldson S. H., Bennett W. D., Zeman K. L., Knowles M. R., Tarran R., Boucher R. C. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. The New England Journal of Medicine. 2006;354(3):241–250. doi: 10.1056/nejmoa043891. [DOI] [PubMed] [Google Scholar]
- 243.Chan M. M., Chmura K., Chan E. D. Increased NaCl-induced interleukin-8 production by human bronchial epithelial cells is enhanced by the ΔF508/W1282X mutation of the cystic fibrosis transmembrane conductance regulator gene. Cytokine. 2006;33(6):309–316. doi: 10.1016/j.cyto.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 244.Hashimoto S., Matsumoto K., Gon Y., Nakayama T., Takeshita I., Horie T. Hyperosmolarity-induced interleukin-8 expression in human bronchial epithelial ceils through p38 mitogen-activated protein kinase. American Journal of Respiratory and Critical Care Medicine. 1999;159(2):634–640. doi: 10.1164/ajrccm.159.2.9712090. [DOI] [PubMed] [Google Scholar]
- 245.Suri R., Marshall L. J., Wallis C., Metcalfe C., Bush A., Shute J. K. Effects of recombinant human DNase and hypertonic saline on airway inflammation in children with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine. 2002;166(3):352–355. doi: 10.1164/rccm.2110015. [DOI] [PubMed] [Google Scholar]
- 246.Aitken M. L., Greene K. E., Tonelli M. R., et al. Analysis of sequential aliquots of hypertonic saline solution-induced sputum from clinically stable patients with cystic fibrosis. Chest. 2003;123(3):792–799. doi: 10.1378/chest.123.3.792. [DOI] [PubMed] [Google Scholar]
- 247.Reeves E. P., Williamson M., O'Neill S. J., Greally P., McElvaney N. G. Nebulized hypertonic saline decreases IL-8 in sputum of patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine. 2011;183(11):1517–1523. doi: 10.1164/rccm.201101-0072OC. [DOI] [PubMed] [Google Scholar]
- 248.Goger B., Halden Y., Rek A., et al. Different affinities of glycosaminoglycan oligosaccharides for monomeric and dimeric interleukin-8: a model for chemokine regulation at inflammatory sites. Biochemistry. 2002;41(5):1640–1646. doi: 10.1021/bi011944j. [DOI] [PubMed] [Google Scholar]
- 249.Marshall L. J., Ramdin L. S. P., Brooks T., DPhil P. C., Shute J. K. Plasminogen activator inhibitor-1 supports IL-8-mediated neutrophil transendothelial migration by inhibition of the constitutive shedding of endothelial IL-8/heparan sulfate/syndecan-1 complexes. Journal of Immunology. 2003;171(4):2057–2065. doi: 10.4049/jimmunol.171.4.2057. [DOI] [PubMed] [Google Scholar]
- 250.Farberman M. M., Ibricevic A., Joseph T. D., et al. Effect of polarized release of CXC-chemokines from wild-type and cystic fibrosis murine airway epithelial cells. American Journal of Respiratory Cell and Molecular Biology. 2011;45(2):221–228. doi: 10.1165/rcmb.2009-0249OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Freedman S. D., Kern H. F., Scheele G. A. Pancreatic acinar cell dysfunction in CFTR−/− mice is associated with impairments in luminal pH and endocytosis. Gastroenterology. 2001;121(4):950–957. doi: 10.1053/gast.2001.27992. [DOI] [PubMed] [Google Scholar]
- 252.Garland A. L., Walton W. G., Coakley R. D., et al. Molecular basis for pH-dependent mucosal dehydration in cystic fibrosis airways. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(40):15973–15978. doi: 10.1073/pnas.1311999110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Coakley R. D., Grubb B. R., Paradiso A. M., et al. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):16083–16088. doi: 10.1073/pnas.2634339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Song Y., Salinas D., Nielson D. W., Verkman A. S. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. American Journal of Physiology—Cell Physiology. 2006;290(3):C741–C749. doi: 10.1152/ajpcell.00379.2005. [DOI] [PubMed] [Google Scholar]
- 255.Poulsen J. H., Fischer H., Illek B., Machen T. E. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(12):5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Smith J. J., Welsh M. J. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. Journal of Clinical Investigation. 1992;89(4):1148–1153. doi: 10.1172/JCI115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ko S. B. H., Shcheynikov N., Choi J. Y., et al. A molecular mechanism for aberrant CFTR-dependent CO3 − transport in cystic fibrosis. The EMBO Journal. 2002;21(21):5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Shah V. S., Meyerholz D. K., Tang X. X., et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351(6272):503–507. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Schütte A., Ermund A., Becker-Pauly C., et al. Microbial-induced meprin β cleavage in MUC2 mucin and a functional CFTR channel are required to release anchored small intestinal mucus. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(34):12396–12401. doi: 10.1073/pnas.1407597111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yang N., Garcia M. A. S., Quinton P. M. Normal mucus formation requires cAMP-dependent HCO3—secretion and Ca2+-mediated mucin exocytosis. Journal of Physiology. 2013;591(18):4581–4593. doi: 10.1113/jphysiol.2013.257436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gustafsson J. K., Ermund A., Ambort D., et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. The Journal of Experimental Medicine. 2012;209(7):1263–1272. doi: 10.1084/jem.20120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hoegger M. J., Fischer A. J., McMenimen J. D., et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345(6198):818–822. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Garcia M. A. S., Yang N., Quinton P. M. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. Journal of Clinical Investigation. 2009;119(9):2613–2622. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bhaskar K. R., Gong D., Bansil R., et al. Profound increase in viscosity and aggregation of pig gastric mucin at low pH. American Journal of Physiology—Gastrointestinal and Liver Physiology. 1991;261(5, part 1):G827–G832. doi: 10.1152/ajpgi.1991.261.5.G827. [DOI] [PubMed] [Google Scholar]
- 265.Abou Alaiwa M. H., Reznikov L. R., Gansemer N. D., et al. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(52):18703–18708. doi: 10.1073/pnas.1422091112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Justus C. R., Dong L., Yang L. V. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Frontiers in Physiology. 2013;4, article 354 doi: 10.3389/fphys.2013.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Chen A., Dong L., Leffler N. R., Asch A. S., Witte O. N., Yang L. V. Activation of GPR4 by acidosis increases endothelial cell adhesion through the cAMP/Epac pathway. PLoS ONE. 2011;6(11) doi: 10.1371/journal.pone.0027586.e27586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ludwig M.-G., Vanek M., Guerini D., et al. Proton-sensing G-protein-coupled receptors. Nature. 2003;425(6953):93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- 269.Dong L., Li Z., Leffler N. R., Asch A. S., Chi J.-T., Yang L. V. Acidosis activation of the proton-sensing GPR4 receptor stimulates vascular endothelial cell inflammatory responses revealed by transcriptome analysis. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0061991.e61991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Liu C., Li Q., Zhou X., Kolosov V. P., Perelman J. M. Regulator of G-protein signaling 2 inhibits acid-induced mucin5AC hypersecretion in human airway epithelial cells. Respiratory Physiology and Neurobiology. 2013;185(2):265–271. doi: 10.1016/j.resp.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 271.Ichimonji I., Tomura H., Mogi C., et al. Extracellular acidification stimulates IL-6 production and Ca2+ mobilization through proton-sensing OGR1 receptors in human airway smooth muscle cells. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2010;299(4):L567–L577. doi: 10.1152/ajplung.00415.2009. [DOI] [PubMed] [Google Scholar]
- 272.Skelton L. A., Boron W. F. Effect of acute acid-base disturbances on ErbB1/2 tyrosine phosphorylation in rabbit renal proximal tubules. American Journal of Physiology—Renal Physiology. 2013;305(12):F1747–F1764. doi: 10.1152/ajprenal.00307.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kim S., Beyer B. A., Lewis C., Nadel J. A. Normal CFTR inhibits epidermal growth factor receptor-dependent pro-inflammatory chemokine production in human airway epithelial cells. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0072981.e72981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Garcia-Caballero A., Rasmussen J. E., Gaillard E., et al. SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(27):11412–11417. doi: 10.1073/pnas.0903609106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Linsdell P., Hanrahan J. W. Glutathione permeability of CFTR. American Journal of Physiology—Cell Physiology. 1998;275(1):C323–C326. doi: 10.1152/ajpcell.1998.275.1.C323. [DOI] [PubMed] [Google Scholar]
- 276.Gao L., Kim K. J., Yankaskas J. R., Forman H. J. Abnormal glutathione transport in cystic fibrosis airway epithelia. American Journal of Physiology—Lung Cellular and Molecular Physiology. 1999;277(1):L113–L118. doi: 10.1152/ajplung.1999.277.1.L113. [DOI] [PubMed] [Google Scholar]
- 277.Linsdell P. Thiocyanate as a probe of the cystic fibrosis transmembrane conductance regulator chloride channel pore. Canadian Journal of Physiology and Pharmacology. 2001;79(7):573–579. doi: 10.1139/cjpp-79-7-573. [DOI] [PubMed] [Google Scholar]
- 278.Fragoso M. A., Fernandez V., Forteza R., Randell S. H., Salathe M., Conner G. E. Transcellular thiocyanate transport by human airway epithelia. Journal of Physiology. 2004;561(1):183–194. doi: 10.1113/jphysiol.2004.071548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Cantin A. M., North S. L., Hubbard R. C., Crystal R. G. Normal alveolar epithelial lining fluid contains high levels of glutathione. Journal of Applied Physiology. 1987;63(1):152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 280.Roum J. H., Buhl R., McElvaney N. G., Borok Z., Crystal R. G. Systemic deficiency of glutathione in cystic fibrosis. Journal of Applied Physiology. 1993;75(6):2419–2424. doi: 10.1152/jappl.1993.75.6.2419. [DOI] [PubMed] [Google Scholar]
- 281.Galli F., Battistoni A., Gambari R., et al. Oxidative stress and antioxidant therapy in cystic fibrosis. Biochimica et Biophysica Acta (BBA)—Molecular Basis of Disease. 2012;1822(5):690–713. doi: 10.1016/j.bbadis.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 282.Ziady A. G., Hansen J. Redox balance in cystic fibrosis. International Journal of Biochemistry and Cell Biology. 2014;52:113–123. doi: 10.1016/j.biocel.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Fischer H. Mechanisms and function of DUOX in epithelia of the lung. Antioxidants & Redox Signaling. 2009;11(10):2453–2465. doi: 10.1089/ars.2009.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Geiszt M., Witta J., Baffi J., Lekstrom K., Leto T. L. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. The FASEB Journal. 2003;17(11):1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 285.Burgel P.-R., Nadel J. A. Epidermal growth factor receptor-mediated innate immune responses and their roles in airway diseases. European Respiratory Journal. 2008;32(4):1068–1081. doi: 10.1183/09031936.00172007. [DOI] [PubMed] [Google Scholar]
- 286.Bérubé J., Roussel L., Nattagh L., Rousseau S. Loss of cystic fibrosis transmembrane conductance regulator function enhances activation of p38 and erk mapks, increasing interleukin-6 synthesis in airway epithelial cells exposed to Pseudomonas aeruginosa. Journal of Biological Chemistry. 2010;285(29):22299–22307. doi: 10.1074/jbc.M109.098566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Roum J. H., Borok Z., McElvaney N. G., et al. Glutathione aerosol suppresses lung epithelial surface inflammatory cell-derived oxidants in cystic fibrosis. Journal of Applied Physiology. 1999;87(1):438–443. doi: 10.1152/jappl.1999.87.1.438. [DOI] [PubMed] [Google Scholar]
- 288.Griese M., Ramakers J., Krasselt A., et al. Improvement of alveolar glutathione and lung function but not oxidative state in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine. 2004;169(7):822–828. doi: 10.1164/rccm.200308-1104OC. [DOI] [PubMed] [Google Scholar]
- 289.Griese M., Kappler M., Eismann C., et al. Inhalation treatment with glutathione in patients with cystic fibrosis. A randomized clinical trial. American Journal of Respiratory and Critical Care Medicine. 2013;188(1):83–89. doi: 10.1164/rccm.201303-0427oc. [DOI] [PubMed] [Google Scholar]
- 290.Rushworth G. F., Megson I. L. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacology and Therapeutics. 2014;141(2):150–159. doi: 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 291.Duijvestijn Y. C. M., Brand P. L. P. Systematic review of N-acetylcysteine in cystic fibrosis. Acta Paediatrica. 1999;88(1):38–41. doi: 10.1080/08035259950170574. [DOI] [PubMed] [Google Scholar]
- 292.Skov M., Pressler T., Lykkesfeldt J., et al. The effect of short-term, high-dose oral N-acetylcysteine treatment on oxidative stress markers in cystic fibrosis patients with chronic P. aeruginosa infection—a pilot study. Journal of Cystic Fibrosis. 2015;14(2):211–218. doi: 10.1016/j.jcf.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 293.Tirouvanziam R., Conrad C. K., Bottiglieri T., Herzenberg L. A., Moss R. B., Herzenberg L. A. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(12):4628–4633. doi: 10.1073/pnas.0511304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Conrad C., Lymp J., Thompson V., et al. Long-term treatment with oral N-acetylcysteine: affects lung function but not sputum inflammation in cystic fibrosis subjects. A phase II randomized placebo-controlled trial. Journal of Cystic Fibrosis. 2015;14(2):219–227. doi: 10.1016/j.jcf.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 295.Xu Y., Szép S., Lu Z. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(48):20515–20519. doi: 10.1073/pnas.0911412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Moskwa P., Lorentzen D., Excoffon K. J. D. A., et al. A novel host defense system of airways is defective in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine. 2007;175(2):174–183. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Gould N. S., Gauthier S., Kariya C. T., Min E., Huang J., Day B. J. Hypertonic saline increases lung epithelial lining fluid glutathione and thiocyanate: two protective CFTR-dependent thiols against oxidative injury. Respiratory Research. 2010;11, article 119 doi: 10.1186/1465-9921-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Minarowski Ł., Sands D., Minarowska A., et al. Thiocyanate concentration in saliva of cystic fibrosis patients. Folia Histochemica et Cytobiologica. 2008;46(2):245–246. doi: 10.2478/v10042-008-0037-0. [DOI] [PubMed] [Google Scholar]
- 299.Chandler J. D., Min E., Huang J., et al. Antiinflammatory and antimicrobial effects of thiocyanate in a cystic fibrosis mouse model. American Journal of Respiratory Cell and Molecular Biology. 2015;53(2):193–205. doi: 10.1165/rcmb.2014-0208OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Sloane P. A., Shastry S., Wilhelm A., et al. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS ONE. 2012;7(6, article e39809) doi: 10.1371/journal.pone.0039809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.ClinicalTrials.gov. TOPIC trial for COPD. ClinicalTrials.gov NCT02135432, 2014.
- 302.Van Goor F., Hadida S., Grootenhuis P. D. J., et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(44):18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Mall M. A., Galietta L. J. V. Targeting ion channels in cystic fibrosis. Journal of Cystic Fibrosis. 2015;14(5, article 1230):561–570. doi: 10.1016/j.jcf.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 304.Yerxa B. R., Sabater J. R., Davis C. W., et al. Pharmacology of INS37217 [P1(uridine 5′)-P4 -(2′-deoxycytidine 5′)tetraphosphate, tetrasodium salt], a next-generation P2Y2 receptor agonist for the treatment of cystic fibrosis. Journal of Pharmacology and Experimental Therapeutics. 2002;302(3):871–880. doi: 10.1124/jpet.102.035485. [DOI] [PubMed] [Google Scholar]
- 305.Accurso F. J., Moss R. B., Wilmott R. W., et al. Denufosol tetrasodium in patients with cystic fibrosis and normal to mildly impaired lung function. American Journal of Respiratory and Critical Care Medicine. 2011;183(5):627–634. doi: 10.1164/rccm.201008-1267oc. [DOI] [PubMed] [Google Scholar]
- 306.Flores R. V., Hernández-Pérez M. G., Aquino E., Garrad R. C., Weisman G. A., Gonzalez F. A. Agonist-induced phosphorylation and desensitization of the P2Y2 nucleotide receptor. Molecular and Cellular Biochemistry. 2005;280(1-2):35–45. doi: 10.1007/s11010-005-8050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Hoffmann C., Ziegler N., Reiner S., Krasel C., Lohse M. J. Agonist-selective, receptor-specific interaction of human P2Y receptors with β-arrestin-1 and -2. Journal of Biological Chemistry. 2008;283(45):30933–30941. doi: 10.1074/jbc.M801472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Morris G. E., Nelson C. P., Everitt D., et al. G protein-coupled receptor kinase 2 and arrestin2 regulate arterial smooth muscle P2Y-purinoceptor signalling. Cardiovascular Research. 2011;89(1):193–203. doi: 10.1093/cvr/cvq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Namkung W., Yao Z., Finkbeiner W. E., Verkman A. S. Small-molecule activators of TMEM16A, a calcium-activated chloride channel, stimulate epithelial chloride secretion and intestinal contraction. The FASEB Journal. 2011;25(11):4048–4062. doi: 10.1096/fj.11-191627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Huang F., Zhang H., Wu M., et al. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(40):16354–16359. doi: 10.1073/pnas.1214596109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Ferrera L., Caputo A., Ubby I., et al. Regulation of TMEM16A chloride channel properties by alternative splicing. The Journal of Biological Chemistry. 2009;284(48):33360–33368. doi: 10.1074/jbc.m109.046607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Calabrese C., Tosco A., Abete P., et al. Randomized, single blind, controlled trial of inhaled glutathione vs placebo in patients with cystic fibrosis. Journal of Cystic Fibrosis. 2015;14(2):203–210. doi: 10.1016/j.jcf.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 313.Ratjen F., Durham T., Navratil T., et al. Long term effects of denufosol tetrasodium in patients with cystic fibrosis. Journal of Cystic Fibrosis. 2012;11(6):539–549. doi: 10.1016/j.jcf.2012.05.003. [DOI] [PubMed] [Google Scholar]