Abstract
A 63-year-old man with right hemiparesis was found (on MRI) to have an expansive intramedullary tumorous lesion at the C2-C3 level. After complete neurosurgical tumor resection, the tumor was histologically categorized as an intermediate grade of intramedullary melanocytoma, an uncommon neoplasm. Based on this peculiar case and review of the literature, radical surgical resection appears to be the therapy of choice for intramedullary melanocytomas. However, their high recurrence rate and aggressive behavior suggest the need for close followup with serial MRI.
Introduction
Primary melanocytic neoplasms of the meninges (meningeal melanocytomas) are uncommon neoplasms of the central nervous system; only 23 cases have been published up to now (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14; Table 1). These neoplasms may occur in a diffuse or localized form (15, 16). Contrary to metastases from malignant skin melanoma, primary melanocytic lesions are very rare, with an incidence of 1 per 10 million for melanocytomas and 0.5 cases per 10 million for primary malignant melanomas (17). They usually have an intracranial localization but also involve the spinal column, where they are most often detected intradurally and extramedullary (5, 18, 19, 20, 21, 22, 23, 24).
This tumor entity was first described as meningeal melanocytoma by Limas and Tio in 1972 (25). Diffuse entities such as melanocytosis and the highly malignant melanomatosis generally occur in the setting of dermatologic syndromes—for example, neurocutaneous melanosis and nevus of Ota (26, 27, 28). Localized tumors present as leptomeningeal masses and range from well-differentiated melanocytomas to lesions of intermediate malignancy and overtly malignant melanomas (29). According to the World Health Organisation (WHO) classification of primary brain tumors, meningeal melanocytomas belong to the subgroup of primary melanocytic lesions (15, 30). Based on a proposition by Brat et al. (29), the WHO classification assigns an intermediate grade to melanocytomas with increased mitotic activity and infiltrative growth that fail to meet all characteristics of malignant melanoma (15).
Melanocytomas are derived from scattered melanocytes that are present in the leptomeninges, primarily at the base of the brain, in the posterior fossa, and around the upper cervical spinal cord. In cases with an intraparenchymal localization, the melanocytes most probably originate from the Virchow-Robin spaces (2). Melanocytomas are commonly solitary, low-grade neoplasms that do not invade surrounding structures (15). They are usually characterized by a benign clinical course, but local recurrence may occur (7, 31).
Intermediate-grade lesions show histological features suggestive of aggressive behavior such as increased mitotic activity and/or invasion of the CNS, but lack the overt cytological atypia of melanomas (15, 29).
Although the terminologies introduced in the literature suggest a clear dichotomy between low-grade melanocytoma and high-grade melanoma, their differentiation is neither absolute nor clearly defined in published cases. Appropriate descriptions and the nosology of lesions along that spectrum are still not transparent, especially for intermediate tumors between the two extremes (intermediate-grade melanocytomas).
| Authors, year (rei) | Age | Sex | Location | Resection (macroscopic) | Followup (months) | Recurrence | Radiotherapy | Comment |
|---|---|---|---|---|---|---|---|---|
| Barth et al. 1993 (1) | 49 | F | T10-T12 | subtotal | 48 | yes | no | patient died after CSF seeding |
| Glick et al. 1997 [2] | 69 | M | C1-C2 | total | 60 | no | no | |
| 39 | F | T8-T9 | subtotal | 12 | no | yes | ||
| 27 | F | T1-T6 | total | 24 | yes | no | ||
| 56 | F | T12 | total | 48 | no | no | ||
| 74 | F | T11-T12 | total | 12 | no | no | re-resection | |
| 70 | M | C1 | total | no followup | patient died 10 days after surgery | |||
| 24 | F | T12-L1 | total | 24 | no | no | re-resection | |
| Delhaye et al. 2001 [3] | 38 | F | T6-T9 | subtotal | 48 | yes | no | patient died after CSF seeding |
| Iida et al. 2002 [4] | 42 | M | T10 | not described | 4 | no | no | patient died 4 months after surgery: urinary infection |
| Turhan et al. 2004 [5] | 19 | F | T8 | total | 36 | no | no | |
| Van Paesschen et al. 2004 [6] | 51 | M | C1-C2 | total | no followup | |||
| Horn et al. 2008 [7] | 37 | F | C1-C3 | total | 38 | yes | no | |
| 37 | F | T9-T10 | total | 16 | yes | no | ||
| 48 | M | T12 | total | 185 | yes | no | ||
| Chacko et al. 2008 [8] | 22 | M | T6-T11 | total | 96 | no | no | |
| Karikari et al. 2009 [9] | 32 | F | T10 | total | 3 | no | no | |
| 20 | M | T12 | total | 2 | no | no | ||
| Caruso et al. 2009 [10] | 62 | M | T11-T12 | total | 24 | no | no | |
| Perrini et al. 2010 [11] | 79 | F | T10-T11 | subtotal | 30 | yes | no | re-resection; malignant transformation |
| Eskandari et al. 2010 [12] | 45 | M | T11 | subtotal | 36 | yes | yes | |
| Muthappan et al. 2012 [13] | 61 | F | C3-C4 | total | 36 | no | nos | |
| Kahilogullari 2012 [14] | 28 | F | T | total | no followup | |||
| Present report 2014 | 63 | M | C2-C3 | total | 18 | yes | yes | |
The differential diagnosis of melanocytomas includes primary or metastatic malignant melanoma (32, 33), melanotic schwannoma (34), melanotic meningioma (1, 2, 25, 34, 35, 36), and melanoblastosis (37).
Here we report a rare case of a cervical intramedullary melanocytoma of intermediate grade. Our diagnosis was based on imaging, histomorphological criteria as described by Brat et al. (15, 29), and immunohistochemical staining.
Case report
History and clinical presentation
A 63-year-old male patient was referred to our emergency department with slight progressive weakness of the right side of his body for over one year and concomitant paraesthesia of his right hand. On initial examination, the patient was in good general condition. We noticed a global weakness of the muscles of the right-side upper and lower limbs (strength grade 3/5 according to the Medical Research Council scale). Tendon reflexes were preserved. Particularly striking was a bilateral positive Babinski sign. His neurological status was otherwise unremarkable. Secondary diagnoses comprised pulmonary fibrosis with respiratory partial insufficiency, pulmonary hypertension, and diabetes mellitus. Pulmonary embolism was noted in the patient's medical history seven years ago.
Due to the overt muscle weakness, MR imaging of the entire spinal canal was performed under suspicion for a pathology of the upper cervical spine—for example, disc herniation, spinal canal stenosis, or a cervical polyradiculopathy.
Preoperative imaging
Spinal MRI included precontrast sagittal and axial T1- and T2-weighted images, a sagittal T2-gradient echo sequence (GRE), sagittal diffusion-weighted imaging (DWI), and T2-weighted 3D space; after contrast application, sagittal and axial fat-suppressed T1-weighted images were acquired. The initial MRI examination indicated an intramedullary, paracentral right located, T1w hyper- and T2w-isointense, expansive tumorous lesion at the level of the second and third cervical vertebrae (Fig. 1, A-D). The solitary, solid, enhancing mass showed an axial expansion of 11 × 13 mm and measured 16 mm in craniocaudal extent. The neoplasm was not diffusion restricted, and there were no associated susceptibility artifacts in the T2w-GRE. Additionally, a T2w-hyperintense per focal tumoral edema was noticed, suggestive of myelopathy. No atrophy of the cervical myelon was observed.
Fig. 1.
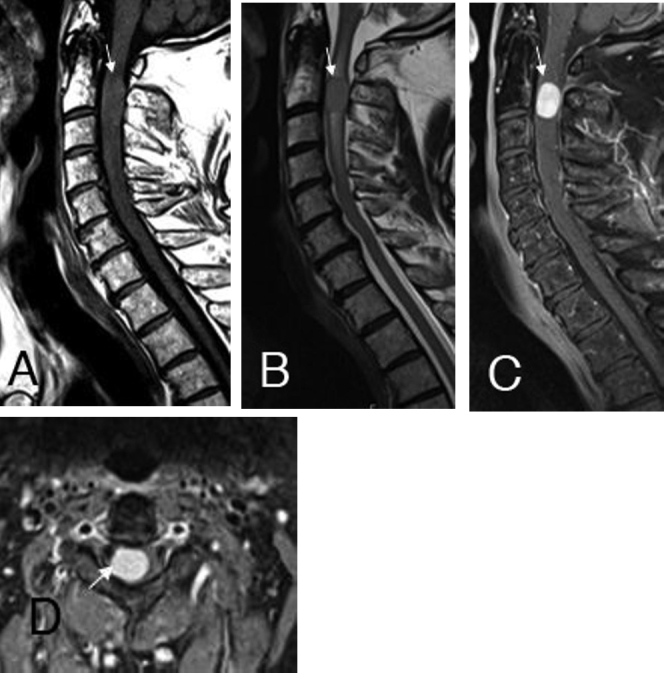
The sagittal T1- (A) and T2-weighted (B) images and the postcontrast sagittal (C) and axial (D) fat-suppressed T1-weighted images clearly show the intramedullary, paracentral right located, expansive melanocytoma at the C2 / C3 level with homogeneous contrast enhancement. Additionally, a T2w hyperintense (B) per focal tumoral edema was noticed, suggestive of myelopathy.
Differential diagnosis encompassed i) ependymoma, ii) astrocytoma, iii) melanotic meningioma, and iv) melanotic schwannoma.
Operation report
The patient underwent microsurgical operation with the purpose of complete tumor resection. Under sonographic and constant electrophysiologic intraoperative monitoring, a C2 and C3 hemi-laminectomy was performed, and the dura was opened longitudinally. A bulge was noted ventral to the C2 and the C3 dorsal nerve rootlets on the right side, with enlargement of the swollen spinal cord. Through a small tumor nodule protruding between the right dorsal nerves C2 and C3, the pia mater was prepared to enable access to the intramedullary tumor mass (Figs. 2, A-C). With the operating microscope under high magnification, it was possible to dissolve the soft parts of the tumor and to separate the tumor mass from the spinal cord. The tumor almost covered the entire circumference of the spinal cord and could be removed. Laterally, the tumor mass was firmly adherent to the two dorsal nerve roots C2 and C3. This part of the tumor mass was sharply dissected and removed. At the end of the operation, there was no macroscopic evidence of a residual tumor. Hemostasis was achieved, and a watertight dural closure was performed. During the entire operation, the potentials of the electrophysiological monitoring remained stable.
Fig. 2.
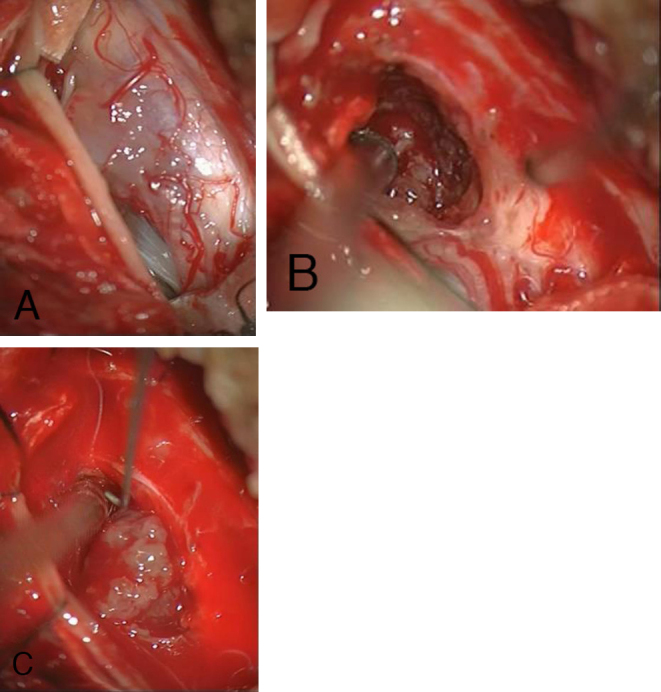
Intraoperative view of the spinal cord after opening the dura mater via dorsal laminectomy; the tumor shines brightly in (A). Melanocytoma with visible caudal tumor edge (B) and partially dissected melanocytoma (C).
Histopathology
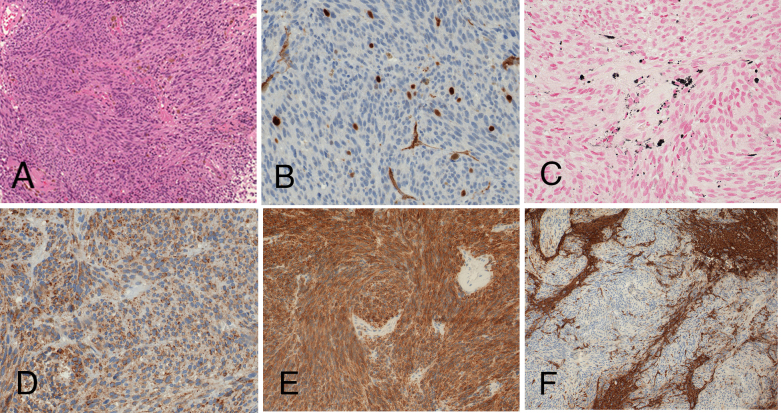
Histopathological examination revealed a spindle-cell neoplasm with melanin pigmentation, moderate mitotic activity (4 mitoses/10 high-power fields; MIB1 overall 5%, focally up to 8%), positivity for melanocytic markers by immunohistochemistry (S100 protein, Melan A, HMB-45), and infiltrative growth pattern (Figs. 3A-F). The tumor cells did not express GFAP, EMA, CD34, DOG1, CD117, broad-spectrum cytokeratins, desmin, smooth-muscle actin, CD99, synaptophysin, or chromogranin.
Fig. 3.
Histological examination revealed a melanocytic neoplasm with moderate mitotic activity and infiltrative growth pattern. Conventional hematoxilin and eosin staining (A; x100) shows lack of substantial cytologic atypia; a mitotic figure is depicted in the inset. Moderate MIB1 staining supports higher proliferative potential (B; x400). Melanocytic differentiation is disclosed by melanin pigmentation (C; Fontana-Masson staining, x400), and immunohistochemical positivity for the melanocytic markers HMB45 (D; x400) and Melan A (E; x200). Staining for glial fibrillary acidic protein (F; GFAP, x100) highlights the invasive tumor growth pattern, with unstained tumor cells and positively stained infiltrated central nervous tissue.
Whole body PET-CT, gastroscopy/colonoscopy, and a dermatologic consultation (including retina and tympanic membrane screening) excluded a peripherally located primary malignant melanoma or metastases. In summary, a primary intramedullary cervical melanocytoma was diagnosed. An intermediate grade was assigned due to the moderate mitotic activity and infiltrative growth pattern, without significant cytological atypia or necroses (15, 29).
Postoperative evaluation and followup
The patient quickly regained motor function in the muscles of the upper and lower limb (postoperative strength grade 4/5, according to the Medical Research Council scale). The concomitant paraesthesia of his right hand improved in the postoperative followup.
The immediate postoperative native and postcontrast MRI scans of the spine confirmed the total tumor resection; the signs of cervical myelopathy were still recognizable, although not as marked as before the operational intervention.
There was no evidence of a peripherally located primary malignant melanoma or metastases by whole body PET-CT, gastroscopy, colonoscopy, and dermatologic examination (including retina and tympanic membrane screening). For monitoring of the patient, clinical exams and serial MRIs were performed every 3 months. The first followup MRI revealed a solid enhancing tumor nodule at the right adherent lateral resection border (4 × 7 × 13 mm) following the same signal characteristics as the primary melanocytoma, in keeping with tumor recurrence (Figs. 4A-D). Percutaneous stereotactic radiotherapy with a fraction of 5 × 1.8 Gy per week and a cumulative dose of 45 (linear accelerator, 6 MV photon beam) was initiated. Nine months after radiotherapy, the recurrent tumor nodule was unchanged in size and configuration, in native and postcontrast MRI. Eighteen months after surgical resection and 15 months after radiation therapy, the patient remained neurologically stable with intermittent slight weakness (strength grade 4/5, according to the Medical Research Council scale) of the lower and upper right extremities.
Fig. 4.
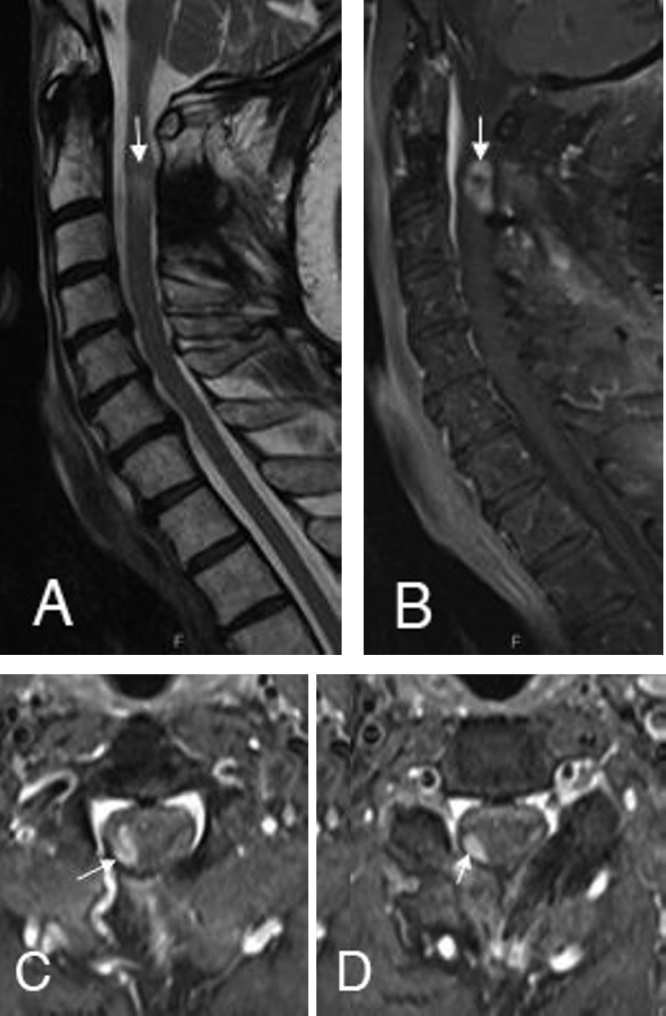
The followup MRI after 3 months indicates the solid enhancing tumor recurrence (B) at the right adherent lateral resection border at the C2 (C) and C3 level (D). The signs of cervical myelopathy were still recognizable on the sagittal T2-weighted (A) images.
Discussion
Primary melanocytic tumors of the spine are part of a spectrum of rare neoplasms derived from scattered melanocytes located in the leptomeninges. These melanocytes are derived from the neural crest during early embryonic development and are most frequently encountered in the recesses of the sulci at the base of the brain, and around the brain stem and upper part of the cervical spinal cord (38). Up to now, the genetic alterations associated with these neoplasms are unknown (39).
Melanocytomas consist of spindle-shaped cells with oval nuclei, small nucleoli, a nested growth pattern, and different amounts of melanin pigment. The existence of amelanotic subtypes is well established, rendering immunohistochemical analysis even more crucial in firmly establishing melanocytic differentiation and excluding other and far more frequent low-grade spindle-cell tumors (meningioma, schwannoma, and paraganglioma). Melanocytomas are immunohistochemically positive for S100 protein and the more specific melanocytic markers HMB-45 and Melan A (MART-1). According to the WHO criteria, low-grade melanocytomas are cytologically bland, solitary neoplasms with inconspicuous mitotic activity (<1 Mitosis/10 HPF, MIB1 <1–2%). At the other end of the spectrum, malignant melanomas are frankly malignant, widely infiltrating neoplasms with overt cytological atypia, brisk mitotic activity, and necrosis. The grading of tumors between those extremes is problematic, particularly due to their rarity. According to the WHO and based on a proposition by Brat et al., an intermediate-grade tumor comparable to our case is assigned to melanocytomas with increased mitotic activity that fail to meet all characteristics of malignant melanoma (15, 29, 35, 40). This categorization means that they show some histological features suggestive of aggressive behavior.
Intraoperatively, surgeons usually encounter a localized soft but solid pigmented mass with meningeal attachment (5, 7, 29). Contrary to the report of Turhan et al. (5), who described a capsule delimiting the tumor, we did not observe any delineating peritumoral capsule; this was confirmed by the infiltrative pattern in the histology examination. The absence of a characteristic tumor capsule thus means that even macroscopically apparent total extirpation cannot exclude the possibility of minimal residual tumor. Diagnosis at the time of surgery is often difficult and misleading, as distinctive cell types are not obvious until histopathological analysis is completed.
Twenty-three cases (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, Table 1) of spinal intramedullary melanocytomas have been reported in the literature since 1993; we add a further case description of this rare tumor entity. We would like to point out that Iida et al. (4) reported two cases with spinal melanocytoma—one intradural extramedullary located and the other intramedullary manifested—and we review only the one case with the intramedullary tumor.
Spinal melanocytic melanomas are more common in women, typically in the fourth decade (13), in keeping with in our review (14 female and 10 male). Our patient was male; his age was consistent with the patient population of 45.5 years (range, 19 to 79 years). Similar to our case, all patients described a slowly progressive evolution of clinical signs of myelopathy or radiculopathy. The length of the postoperative clinical and radiographic evaluation in 21 of these 24 (87.5%) patients varied from 2 months to 185 months (average, 38 months). In two (8.3%) of the reviewed cases, the postoperative course was not documented. One patient (4.2%) died 10 days after surgery, so no followup observation was possible.
On first MRI, the tumor mass was located central and intramedullary in the thoracic spine in 18 of the 24 (75%) analyzed cases. In the remaining 6 patients (25%), the location of the intramedullary melanocytoma was cervical.
Local recurrence occurred in five of the 21 (23.8%) cases with followup and documented macroscopic complete tumor resection (2, 7; current case), as well as in four patients (19%) with subtotal tumor extirpation (1, 3, 11, 12), probably due to the persistence of a very small (macroscopic or nonvisible) residue (2). We believe that there is another explanation for the spectrum of low-grade to intermediate-grade melanocytomas determined by histopathological features, as described for melanocytic neoplasms elsewhere in the central nervous system (29). Perrini et al. graded their case as an intermediate-grade melanocytoma (11). A clear histological grading or description to distinguish low-grade from intermediate-grade neoplasms is lacking in most of the published cases. Taking into account the high recurrence rates of the reviewed intramedullary melanocytomas, we conclude either that there is a higher rate of intermediate-grade melanocytomas in an intramedullary spinal localization compared to melanocytomas elsewhere in the central nervous system, or that histologically classified low-grade melanocytomas have a relevant potential for local recurrence.
Among the previously mentioned studies with total and subtotal tumor removal and recurrence in the followup, only two patients (9.5%) received adjuvant radiotherapy (12; current case). One patient (4.8%) with subtotal tumor surgery received adjuvant radiotherapy without local tumor recurrence (2). In summary, only 3 of 21 patients (14.3%) with intramedullary melanocytoma underwent adjuvant radiotherapy (2, 12; current case). However, overall, 10 of 21 patients (47.6%) had local tumor relapse, including our patient with local tumor recurrence in the 3-month followup MRI. In our current patient, the multidisciplinary tumor board committee decided to initiate percutaneous stereotactic radiotherapy.
Thus, there is uncertainty as to whether immediate postoperative radiotherapy would have prevented or delayed tumor recurrence, especially in patients with histologically verified intermediate-grade melanocytoma.
Our literature review aims to supports the view that intramedullary melanocytoma lacks characteristic imaging features. Typical MRI findings are that of an intramedullary lesion iso-to hyperintense on T1-weighted sequences, hypointense on T2 weighted sequences, and with homogeneous enhancement—in keeping with the MRI characteristics of our patient (Fig. 1A-D). These signal features are biased by variable degrees of tumor melanisation that affect the signal characteristics on MRI (9, 41).
Thus, nonspecific imaging characteristics, in addition to the rarity of tumors in the intramedullary spinal cord, lead to their misdiagnosis and frequent exclusion from the differential diagnosis. In the current patient, the axial MRI revealed a paracentral extension of the tumor, unlike the more centrally localized lesions associated with ependymoma or astrocytoma. Hence, meningeal melanocytoma should be included in the differential diagnosis of lesions that show a slight T1w hyperintensity and a paracentral component on MRI.
The aim of the treatment should be complete tumor resection with preservation of neurological function. In the past, local tumor control rates were four times higher if complete resection was achieved, and morbidity and mortality rates for incomplete resection now mandate use of adjuvant radiation therapy (7, 42, 43, 44, 45). The aggressive nature of intramedullary melanocytomas with frequent local recurrence has already been documented by Chacko and Rajshekhar (8), who observed what they referred to as “an infiltrative residue of the spinal cord substance during intraoperative inspection of the resection cavity of the tumor at the cranial and caudal poles.” The exact tissue type involved is still unknown, and although these authors advocated a radical excision, they did not report aggressive resection of the infiltrative portions from the spinal cord. Although their patient showed no radiographic recurrence at 8 years post-surgery, the infiltrative nature as well as aggressive and seemingly inconsistent behavior of this tumor type must— in our opinion—take into account intraoperative and followup imaging.
The risks of recurrence or residual tumor progression are important, and several reports focus on possible leptomeningeal seeding even years after surgical resection (46, 47, 48). Our review includes reports of two patients who died of subarachnoidial dissemination, both after a four-year course of disease (1, 9).
In the present case, we decided to defer initial radiotherapy. In the event of early local recurrence after 3-month followup, we resolved to pursue adjuvant radiation, because operative re-resection is often associated with significant morbidity (13). Rades et al. (43) achieved a 80% five-year local disease control after complete resection, compared to 100% with complete resection and radiotherapy. Interestingly, Rades et al. (43) showed 72% disease control with radiotherapy compared to 8% after incomplete resection only (p <0.001). Although these tumors are slow-growing, they have a propensity to recur early (3-month interval) and metastasize via the cerebrospinal fluid. Malignant transformation is described in the literature for some extramedullary cases (48, 49, 50, 51) and one intramedullary melanocytoma (11). Therefore, Rades and Schild (44) proposed the routine use of radiation in cases of subtotal tumor resection and even after complete surgical extirpation; however, long-term clinical data are limited. In consequence, we also emphasize the importance of very careful postoperative monitoring. Long-term monitoring is necessary to determine the outcomes of spinal intramedullary melanocytomas after surgical resection and radiotherapy, to improve our understanding of these tumors, and to enable improved treatment strategies with special regard to the treatment of local tumor recurrence.
The question still remains open whether more aggressive tumor treatment with early postoperative adjuvant therapy should be recommended for patients with locally aggressive tumors, especially in cases of histopathologically determined intermediate-grade melanocytoma.
Conclusions
Spinal melanocytomas are rare, especially when located in an intramedullary region, and are often not considered during differential diagnosis of intramedullary spinal cord tumors. The lack of distinctive imaging characteristics increases the challenge during preoperative diagnosis. The high recurrence rate of intermediate-grade intramedullary melanocytomas even after total tumor resection, and their histopathological features of aggressive behavior (for example, increased mitotic activity, infiltrative pattern) recommend close followup with serial MR imaging.
Footnotes
Published: March 7, 2015
References
- 1.Barth A, Pizzolato GP, Berney J. Intramedullary meningeal melanocytoma. Neurochirurgie. 1993;39:188–194. [PubMed] [PubMed] [Google Scholar]
- 2.Glick R, Baker C, Husain S, Hays A, Hibshoosh H. Primary melanocytomas of the spinal cord: a report of seven cases. Clin Neuropathol. 1997;16:127–132. [PubMed] [PubMed] [Google Scholar]
- 3.Delhaye M, Menei P, Rousselet MC, Diabira S, Mercier P. A case of intramedullary primary melanocytic tumor: meningeal melanocytoma or malignant melanoma? Neurochirurgie. 2001;47:133–136. [PubMed] [PubMed] [Google Scholar]
- 4.Iida M, Llena JF, Suarez MA. Two cases of spinal meningeal melanocytoma. Brain Tumor Pathol. 2002;19:41–45. doi: 10.1007/BF02482455. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Turhan T, Oner K, Yurtseven T, Akalin T, Ovul I. Spinal meningeal melanocytoma. Report of two cases and review of the literature. J Neurosurg. 2004;100:287–290. [PubMed] [PubMed] [Google Scholar]
- 6.Van Paescchen R, Van Calenbergh F, Demaerel P, Sciot T. Intramedullary melanocytoma associated with syringobulbia. Acta Neurologica Belgica. 2004;104:132–133. [Google Scholar]
- 7.Horn EM, Nakaji P, Coons SW, Dickman CA. Surgical treatment of intramedullary spinal cord melanocytomas. J Neurosurg Spine. 2008;9:48–54. doi: 10.3171/SPI/2008/9/7/048. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Chacko G, Rajshekhar V. Thoracic intramedullary melanocytoma with long term follow-up. J Neurosurg Spine. 2008;9:589–592. doi: 10.3171/SPI.2008.9.08323. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Karikari IO, Powers CJ, Bagley CA, Cummings TJ, Radhakrishnan S, Friedman AH. Primary intramedullary melanocytoma of the spinal cord: case report. Neurosurgery. 2009;64:E777–E778. doi: 10.1227/01.NEU.0000341516.22126.AA. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Caruso R, Marrocco L, Wierzbicki V, Salvati M. Intramedullary melanocytoma: case report and review of literature. Tumori. 2009;95:389–393. doi: 10.1177/030089160909500322. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Perrini P, Caniglia M, Pieroni M, Castagna M, Parenti GF. Malignant transformation of intramedullary melanocytoma: case report. Neurosurgery. 2010;67:E867–E869. doi: 10.1227/01.NEU.0000372919.96651.34. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Eskandari R, Schmidt MH. Intramedullary spinal melanocytoma. Rare Tumors. 2010;2:e24. doi: 10.4081/rt.2010.e24. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muthappen M, Muthu T, Hussain Z, Lamont D, Belakrishnan V. Cervical intramedullary melanocytoma: A case report and review of literature. J Clin Neurosci. 2012;19:1450–1453. doi: 10.1016/j.jocn.2011.09.040. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Kahilogullari G. Primary intramedullary melanocytoma: a case report. J Clinic Case Reports. 2012;2:6. [Google Scholar]
- 15.Brat DJ, Perry A. Melanocytic lesions. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumours of the central nervous system. 4th ed. IARC; Lyon: 2007. pp. 181–183. [Google Scholar]
- 16.Burger PC, Scheithauer BW, Vogel FS. Surgical pathology of the nervous system and its coverings. Churchill Livingstone; New York: 2009. [Google Scholar]
- 17.Liubinas SV, Maartens N, Drummond KJ. Primary melanocytic neoplasms of the central nervous system. J Clin Neurosci. 2010;17:1227–1232. doi: 10.1016/j.jocn.2010.01.017. [PubMed] [DOI] [PubMed] [Google Scholar]
- 18.Das A, Ratnagopal P, Puvanendran K, Teo JG. Spinal meningeal melanocytoma with hydrocephalus and intracranial superficial siderosis. Intern Med J. 2001;31:562–564. doi: 10.1046/j.1445-5994.2001.00119.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 19.Ibanez J, Weil B, Ayala A, Jimenez A, Acedo C, Rodrigo I. Meningeal melanocytoma: a case report and review of the literature. Histopathology. 1997;30:576–581. doi: 10.1046/j.1365-2559.1997.5660798.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto S, Kang Y, Sato S. Spinal meningeal melanocytoma presenting with superficial siderosis of central nervous system. Case report and review of literature. J Neurosurg. 1998;88:890–894. doi: 10.3171/jns.1998.88.5.0890. [DOI] [PubMed] [Google Scholar]
- 21.Painter TJ, Chaljub G, Sethi R, Singh H, Gelman B. Intracranial and intraspinal meningeal melanocytosis. AJNR Am J Neuroradiol. 2000;21:1349–1353. [PubMed] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen PV, Andersen C, Ulhoi BP. Intraspinal melanocytoma in a 16-year old girl. Ugeskr Leager. 2000;162:2052–2053. [PubMed] [Google Scholar]; Rasmussen PV, Andersen C, Ulhoi BP. Intraspinal melanocytoma in a 16-year old girl. Ugeskr Leager. 2000;162:2052–2053. [PubMed] [Google Scholar]
- 23.Shimoda H, Oka K, Naoi Y. Primary melanocytoma arising from the thoracic leptomeninges: case. Clin Neuropath. 1999;18:80–83. [PubMed] [PubMed] [Google Scholar]
- 24.Jellinger K, Bock F, Brenner H. Meningeal melanocytoma. Report of a case and review of literature. Acta Neurochir Wien. 1988;94:78–87. doi: 10.1007/BF01406621. [PubMed] [DOI] [PubMed] [Google Scholar]
- 25.Limas C, Tio FO. Meningeal melanocytoma (melanotic menigeoma). Its melanotic origin as revealed by electron microscopy. Cancer. 1972;30:1286–1294. doi: 10.1002/1097-0142(197211)30:5<1286::aid-cncr2820300522>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 26.Balmaceda CM, Fetell MR, O’Brien JL, Housepian EH. Nevus of Ota and leptomeningeal melanocytic lesions. Neurology. 1993;43:381–386. doi: 10.1212/wnl.43.2.381. [PubMed] [DOI] [PubMed] [Google Scholar]
- 27.Kadonaga JN, Frieden IJ. Neurocutaneous melanosis: definition and review of the literature. J Am Acad Dermatol. 1991;24:747–755. doi: 10.1016/0190-9622(91)70115-i. [PubMed] [DOI] [PubMed] [Google Scholar]
- 28.Livingstone E, Claviez A, Spengler D. Neurocutaneous melanosis: a fatal disease in early childhood. J Clin Oncol. 2009;27:2290–2291. doi: 10.1200/JCO.2008.20.4388. [PubMed] [DOI] [PubMed] [Google Scholar]
- 29.Brat DJ, Giannini C, Scheithauer BW, Burger PC. Primary melanocytic neoplasms of the central nervous systems. Am J Surg Pathol. 1999;23:745–754. doi: 10.1097/00000478-199907000-00001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 30.Jellinger K, Chou P, Paulus W. Melanocytic lesions. In: Kleihues P, Cavenee WK, editors. World Health Organisation Classification of Tumours: Pathology and Genetics of Tumours of the Nervous System. IARC Press; Lyon: 2000. pp. 193–195. [Google Scholar]
- 31.Rutten I, Bolle S, Kaschten B, Kaschten B, Stevenaert A, Deneufbourg JM, Deprez M. Recurrent intracranial melanocytoma associated with a nevus of Ota. Acta Neurochir. 2005;147:313–315. doi: 10.1007/s00701-004-0457-3. [PubMed] [DOI] [PubMed] [Google Scholar]
- 32.Lach B, Russel N, Benoit B, Atack D. Cellular blue nevus (“melanocytoma”) of the spinal meninges: electron microscopic and immunohistochemical features. Neurosurgery. 1988;22:773–780. doi: 10.1227/00006123-198804000-00030. [PubMed] [DOI] [PubMed] [Google Scholar]
- 33.Steinberg JM, Gillespie JJ, MacKay B. Meningeal melanocytoma with invasion of the thoracic spinal cord. Case report. J Neurorsurg. 1978;48:818–824. doi: 10.3171/jns.1978.48.5.0818. [PubMed] [DOI] [PubMed] [Google Scholar]
- 34.Larson TC, Houser OW, Onofrio BM. Primary spinal melanoma. J Neurosurg. 1987;66:47–49. doi: 10.3171/jns.1987.66.1.0047. [PubMed] [DOI] [PubMed] [Google Scholar]
- 35.Litofsky NS, Zee CS, Breeze RE. Meningeal melanocytoma: diagnostic criteria for a rare lesion. Neurosurgery. 1992;31:945–948. doi: 10.1227/00006123-199211000-00019. [PubMed] [DOI] [PubMed] [Google Scholar]
- 36.Winston KR, Setrel A, Schnitt STJ. Meningeal melanocytoma. Case report and review of the clinical and histological features. J Neurosurg. 1987;66:50–57. doi: 10.3171/jns.1987.66.1.0050. [PubMed] [DOI] [PubMed] [Google Scholar]
- 37.Czarnecki Ej, Silbergleit R, Guierrez JA. MR of the spinal meningeal melanocytoma. AJNR. Am J Neuroradiol. 1997;18:180–182. [PubMed] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldgeier MH, Klein LE, Klein-Angerer S, Moellma nn G, Nordlund JJ. The distribution of melanocytes in the leptomeninges of the human brain. J Invest Dermatol. 1984;82:235–238. doi: 10.1111/1523-1747.ep12260111. [PubMed] [DOI] [PubMed] [Google Scholar]
- 39.Küsters-Vandevelde HVN, Klaasen A, Küsters B. Activating mutations of the GNAQ gene: a frequent event in primary melanocytic neoplasms of the central nervous system. Acta Neuropathol. 2010;119:317–323. doi: 10.1007/s00401-009-0611-3. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O´Brien TF, Moran M, Miller HJ, Hensley SD. Meningeal melanocytoma: An uncommon diagnostic pitfall in surgical neuropathology. Arch Pathol Lab Med. 1995;119:542–546. [PubMed] [PubMed] [Google Scholar]
- 41.Demirci A, Kawamura Y, Sze G, Duncan C. MR of parenchymal neurocutaneous melanosis. Am J Neuroradiol. 1995;16:603–606. [PubMed] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahluwalia S, Ashkan K, Casey AT. Meningeal melanocytoma: clinical features and review of literature. Br J Neurosur. 2003;g17:347–351. doi: 10.1080/02688690310001601243. [PubMed] [DOI] [PubMed] [Google Scholar]
- 43.Rades D, Heidenreich F, Tatagiba M, Brandis A, Karstens JH. Therapeutic options for meningeal melanocytoma. Case report. J Neurosurg. 2001;9(5 (2 Suppl Spine)):225–231. doi: 10.3171/spi.2001.95.2.0225. [PubMed] [DOI] [PubMed] [Google Scholar]
- 44.Rades D, Schild SE. Dose-response relationship for fractionated irradiation in the treatment of spinal meningeal melanocytomas: a review of literature. J Neurooncol. 2006;77:311–314. doi: 10.1007/s11060-005-9048-2. [PubMed] [DOI] [PubMed] [Google Scholar]
- 45.Rades D, Schild SE, Tatagiba M, Molina HA, Alberti W. Therapy of meningeal melanocytomas. Cancer. 2004;100:2442–2447. doi: 10.1002/cncr.20296. [PubMed] [DOI] [PubMed] [Google Scholar]
- 46.Bydon A, Gutierrez JA, Mahmood A. Meningeal melanocytoma: an aggressive course for a benign tumor. J Neurooncol. 2003;64:259–263. doi: 10.1023/a:1025628802228. [PubMed] [DOI] [PubMed] [Google Scholar]
- 47.Ali Y, Rahme R, Moussa R, Abadjian G, Menassa-Moussa L, Samaha E. Multifocal meningeal melanocytoma: a new pathological entity or the result of leptomeningeal seeding? J Neurosurg. 2009;11:488–491. doi: 10.3171/2009.3.JNS081096. [PubMed] [DOI] [PubMed] [Google Scholar]
- 48.Uozumi Y, Kawano T, Kawaguchi T. Malignant transformation of meningeal melanocytoma: a case report. Brain Tumor Pathol. 2003;20:21–25. doi: 10.1007/BF02478943. [PubMed] [DOI] [PubMed] [Google Scholar]
- 49.Roser F, Nakamura M, Brandis A, Hans V, Vorkapic P, Samil M. Transition from meningeal melanocytoma to primary cerebral melanoma. Case report. J Neurosurg. 2004;101:528–531. doi: 10.3171/jns.2004.101.3.0528. [PubMed] [DOI] [PubMed] [Google Scholar]
- 50.Wang F, Li X, Chen L, Pu X. Malignant transformation of spinal meningeal melanocytoma. Case report and review of the literature. J Neurosurg. 2007;6:451–454. doi: 10.3171/spi.2007.6.5.451. [PubMed] [DOI] [PubMed] [Google Scholar]
- 51.Rades D, Tatagiba M, Brandis A, Dubben HH, Karstens JH. The value of radiotherapy in treatment of meningeal melanocytoma. Strahlenther Onkol. 2002;178:336–342. doi: 10.1007/s00066-002-0930-y. [PubMed] [DOI] [PubMed] [Google Scholar]