Abstract
Gorham-Stout (GS) disease is a rare bone disorder of unknown etiology that is characterized by local proliferation of small vascular or lymphatic channels, resulting in progressive osteolysis and bone resorption. The diagnosis of GS disease is one of exclusion, with radiography and histopathology playing key roles. We describe a 9-year-old girl who presented to us with dyspnea and bone pain. She was found to have a cystic mass of the upper extremity, multiple cystic bone lesions, multiple fractures of different ages, and pleural effusions. We review the radiologic images that helped establish the diagnosis of GS disease.
Introduction
Gorham-Stout (GS) disease is a rare bone disorder of unknown etiology that is characterized by local proliferation of small vascular or lymphatic channels resulting in progressive osteolysis and bone resorption. The first case of GS was described by Jackson in 1838 and later described as a specific pathologic entity by Gorham and Stout in 1955 (1).
While GS disease is capable of affecting any part of the skeleton, a predilection exists for bones that develop by intramembranous ossification, such as the skull, shoulder, and pelvic girdles (2, 3, 4). Clinically, the disorder can present itself in a number of ways. Patients can present with an indolent onset of symptoms (including dull pain and weakness) or with a relatively abrupt onset of pain and swelling (likely secondary to pathologic fracture) (5, 6). Most cases of GS involve a single bone, but involvement of multiple bones is not uncommon (7).
The diagnosis of GS disease is one of exclusion and takes into account clinical, radiological, and histopathological features. Before the diagnosis of GS disease, other pathologies that are capable of causing a similar osteolytic picture, such as malignancy, infection, and hyperparathyroidism, must be excluded (6).
Radiologic evaluation with radiographs, computed tomography (CT), and magnetic resonance imaging (MRI) plays an important role in both diagnosis and evaluation of the extent of disease in patients with suspected or known GS disease. This article describes a case of GS disease that presented to our institution and reviews the radiologic findings.
Case report
A 9-year-old girl presented to Harbor-UCLA Medical Center complaining of shortness of breath and left-lower-extremity pain. A chest radiograph revealed a right-sided pleural effusion, a right clavicle lytic lesion, and several old left rib fractures (Fig. 1). Left-lower-extremity radiographs revealed multiple focal expansile lytic lesions involving the cortex of the tibia and fibula, with a pathologic fracture through the middle tibia (Fig. 2). A CT of the chest, abdomen, and pelvis was then obtained; it revealed extensive and diffuse involvement of the bones with lytic lesions. In addition, a large right-sided pleural effusion and multiple hypodense splenic lesions compatible with lymphangiomatosis were also present (Fig. 3).
Fig. 1.
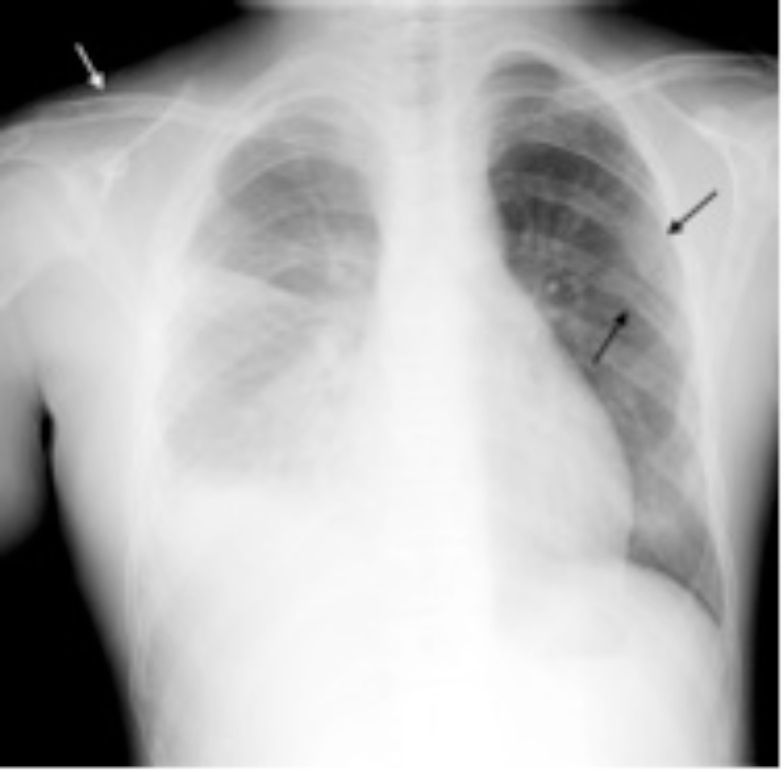
Chest radiograph demonstrates a right-sided pleural effusion, a right clavicle lytic lesion (white arrow). and several old left rib fractures (black arrows).
Fig. 2.
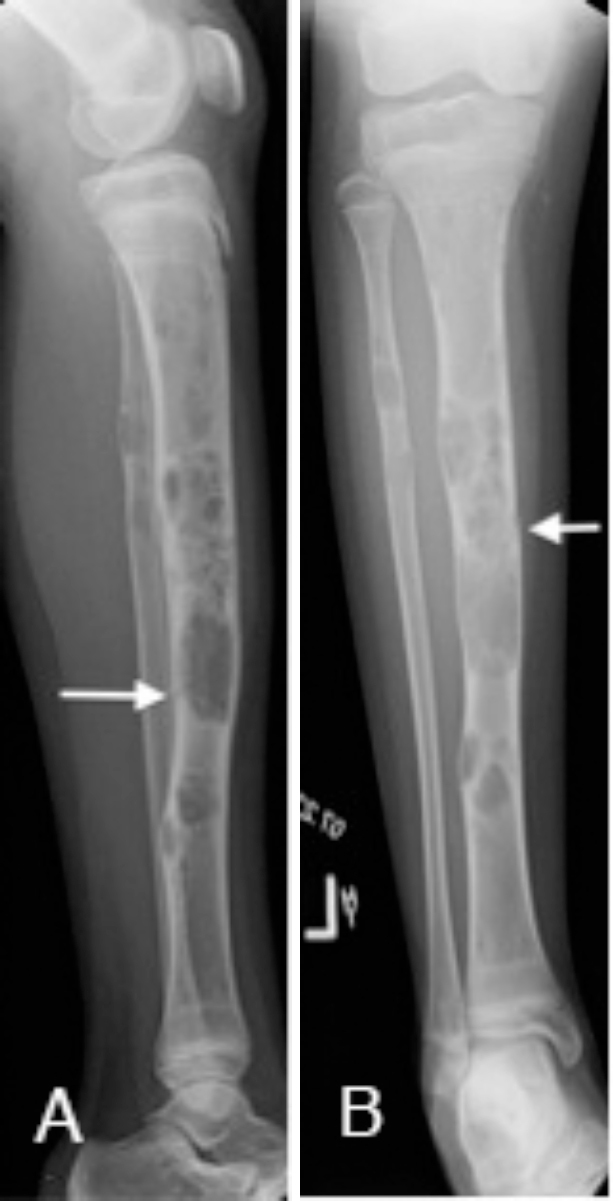
Left-lower-extremity radiographs show multiple focal expansile lytic lesions involving the cortex of the tibia and fibula, with a pathologic fracture through the middle tibia (white arrows).
Fig. 3.
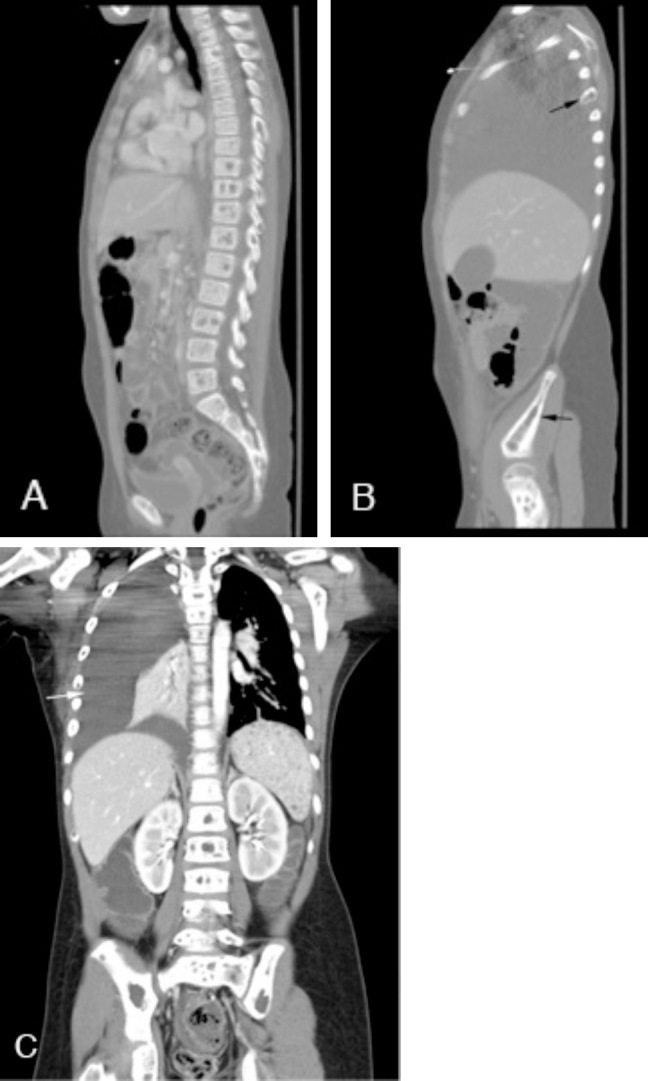
Contrast-enhanced CT of the chest, abdomen, and pelvis. Sagittal imaging (A and B) demonstrates multiple lytic lesions involving various vertebral bodies, ribs, and pelvis (black arrows). Coronal imaging (C) shows innumerable hypodense splenic lesions representing lymphangiomatosis and an extensive right-sided pleural effusion (white arrow).
A review of the patient's past medical history revealed the surgical removal of a large cystic mass from the right anterior chest wall four years before (Fig. 4). The histopathology associated with the lesion was compatible with a lymphangioma. In addition, the patient had a history of prior fractures and pleural effusions treated at an outside hospital. Given her history, a nuclear medicine lymphoscintigraphy study was performed and demonstrated injury to the lymphatic drainage system leading to chylothorax (Fig. 5). An MRI of the chest was then obtained for further evaluation of the patient's chest wall and the right humeral lesions in anticipation of biopsy (Fig. 6). A lesion within the right humerus was biopsied, and histopathology showed thin-walled vessels with flattened endothelium and marrow fibrosis.
Fig. 4.
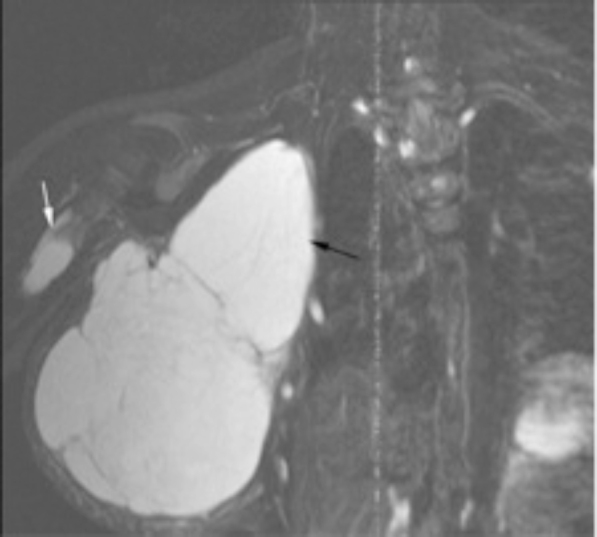
Coronal T2-weighted Fast Inversion Recovery image shows a large cystic mass extending from the right anterior thorax to the axilla (black arrow), and a cystic lesion in the proximal right humerus (white arrow). This right chest mass was surgically excised four years before presentation, and the histopathology revealed thin-walled, dilated lymphatic vessels consistent with a lymphangioma.
Fig. 5.
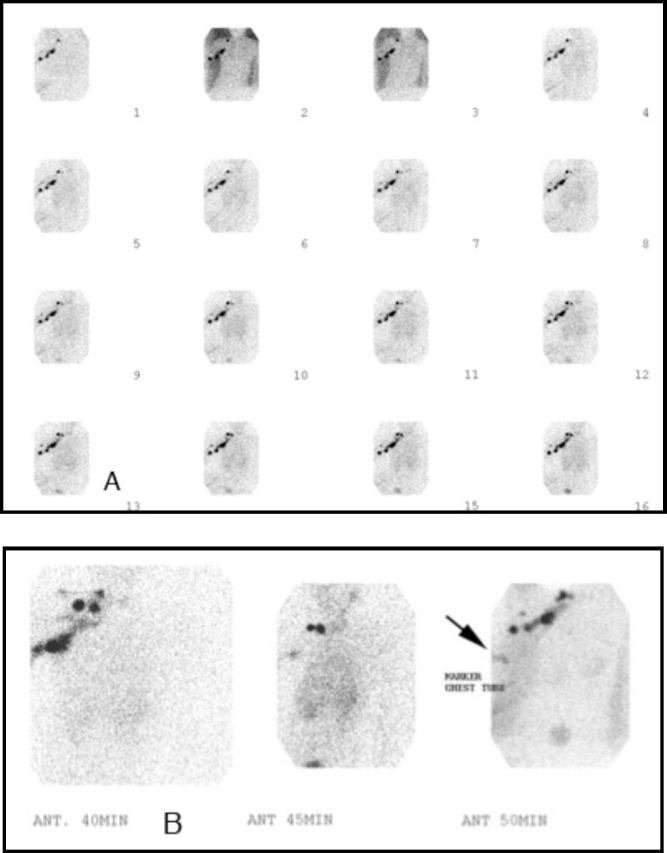
Nuclear medicine lymphoscintigraphy study performed with Tc-99m filtered sulfur colloid injected into the interdigital webs of the patient's right hand. Sequential imaging obtained at 2 minutes/frame. Normal lymphatic drainage was seen within the right upper chest. Faint early activity is seen within the right chest tube (A). On delayed imaging (B), significant activity is seen within the right chest tube (arrow) and drainage container. This study confirmed injury to the lymphatic drainage system leading to recurrent chylothorax.
Fig. 6.
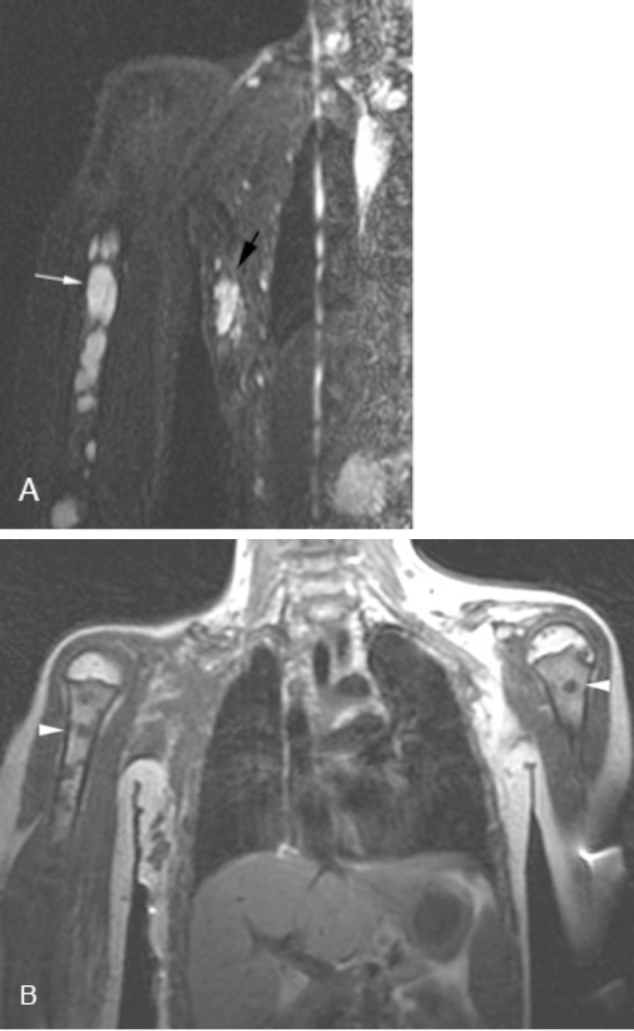
Coronal Short Tau Inversion Recovery (STIR) image (A) shows multiple hyperintense lesions in the right humerus (white arrow). Also seen is a remnant or recurrence of the previously excised lymphangioma (black arrow). Coronal T1-weighted imaging (B) shows multiple bilateral hypointense humerus lesions (white arrowheads).
Discussion
Gorham-Stout disease is a rare disease whose etiology and pathogenesis remain unknown. This nonfamilial disease affects men and women equally and can affect any bone in the body. Several potential mechanisms for osteolysis in GS have been hypothesized. Some authors implicate osteoclasts in bone resorption, while others believe that angiomatosis is responsible. Post-traumatic hyperaemia, local hypoxia, and an acidic environment have also been suggested (8, 9).
GS disease is a diagnosis of exclusion and is based on clinical, radiological, and histopathological findings. Other diagnoses that most often need to be excluded include essential osteolysis with nephropathy, hereditary multicentric osteolysis, angiosarcoma, angioma, metastasis, and osteomylelitis. Clinically, patients are typically asymptomatic until a pathologic fracture occurs, causing pain and swelling. In addition, involvement of the ribs can lead to pleural effusions (as was the case for our patient) or chylothorax, resulting in the associated symptoms.
Multiple imaging modalities are helpful in diagnosing and evaluating GS disease. Radiographs and CT are valuable for assessing the extent of bone destruction and spread of disease. MRI, with its superior soft-tissue resolution, is the preferred imaging modality for GS (7). Considerable variability exists in the MRI findings for GS; however, the most commonly reported findings are increased signal intensity on T1-weighted images and higher signal intensity on T2-weighted sequences. However, Yoo et al. reported MRI findings of low signal intensity on T1-weighted images and high signal intensity on T2-weighted images in a 20-year-old female with GS (10). The findings from our case support the findings of Yoo et al, although it is known that variability in the MR findings exists and that it may be due to a spectrum of neovascular progression and fibrosis (7).
Treatment options for GS disease currently include surgery and radiation therapy as the mainstays. Surgery with bone grafting provides the best chance for cure but is limited by frequent absorption of the graft (8). Radiation therapy has been used with good clinical outcomes in producing arrest of osteolysis and (in some cases) recalcification. Furthermore, many other medical treatments have been used with little evidence of benefit, including vitamin D, calcium, sodium fluoride, bisphosphonates, calcitonin, and androgens.
References
- 1.Sekharappa V, Arockiaraj J, Amritanand R, Krishnan V, David KS, David SG. Gorham's disease of spine. Asian Spine J. Sep 2013;7(3):242–247. doi: 10.4184/asj.2013.7.3.242. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gowin W, Rahmanzadeh R. Radiologic diagnosis of massive idiopathic osteolysis (Gorham-Stout Syndrome) Rontgenpraxis. 1985;38:128–134. [PubMed] [PubMed] [Google Scholar]
- 3.Horst M, Zsernaviczky J, Delling G. A rare case of so-called idiopathic osteolysis associated with a lymphangioma of the fibula. Z Orthop. 1979;117:88–95. [PubMed] [PubMed] [Google Scholar]
- 4.Gondivkar S, Gadbail A. Gorham-Stout syndrome: a rare clinical entity and review of the literature. Oral Radiol Endod. 2010;109:e41–e48. doi: 10.1016/j.tripleo.2009.08.043. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Bruch-Gerharz D, Gerharz CD, Stege H. Cutaneous vascular malformations in disappearing bone (Gorham-Stout) disease. JAMA. 2003;289(12):1479–1480. doi: 10.1001/jama.289.12.1479. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Rubel IF, Carrer A, Cohen G. Progressive Gorham disease of the forearm. Ortho. 2008;31(3):1–3. doi: 10.3928/01477447-20080301-33. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Kai B, Ryan A, Munk PL, Dunlop P. Gorham disease of the bone: three cases and review of radiological features. Clin Radiol. 2006;61:1058–1064. doi: 10.1016/j.crad.2006.04.014. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Shalilesh GM, Amol RG. Gorham-Stout syndrome: a rare clinical entity and review of the literature. Oral Surg Oral med Oral Pathol Oral Radiol Endod. 2010;109:e41–e48. doi: 10.1016/j.tripleo.2009.08.043. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Chung C, Yu JS, Resnick D. Gorham syndrome of the thorax and cervical spine: CT and MRI findings. Skeletal Radiol. 1997;26:55–59. doi: 10.1007/s002560050192. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Yoo SY, Hong SH, Chung HY. MRI of Gorham's disease: findings in two cases. Skeletal Radiol. 2002;31:301–306. doi: 10.1007/s00256-002-0487-y. [PubMed] [DOI] [PubMed] [Google Scholar]


