Abstract
Granular-cell tumor is an uncommon cause of breast mass in premenopausal women that presents as a painless chronic lump. It mimics infiltrating carcinoma clinically and radiologically. Granular-cell tumor is usually benign, and the treatment is wide local excision. Definitive pre-operative diagnosis helps to avoid unnecessary mastectomy. We present clinical, mamographic, and sonographic characteristics of a benign granular-cell tumor of the breast in a 57-year-old woman.
Case report
A 57-year-old female patient was referred for a diagnostic mammogram. She presented with complaints of a lump in the right breast areolar region for the past 8 months. No discharge was present, either from the nipple or from the lump. There was no family or personal history of carcinoma of the breast.
On examination, there was a firm, immobile, subareolar lump at the 8 o'clock position. It pushed the nipple medially. The overlying skin was stretched and had hypopigmented areas. No axillary adenopathy was noted (Fig. 1).
Figure 1.
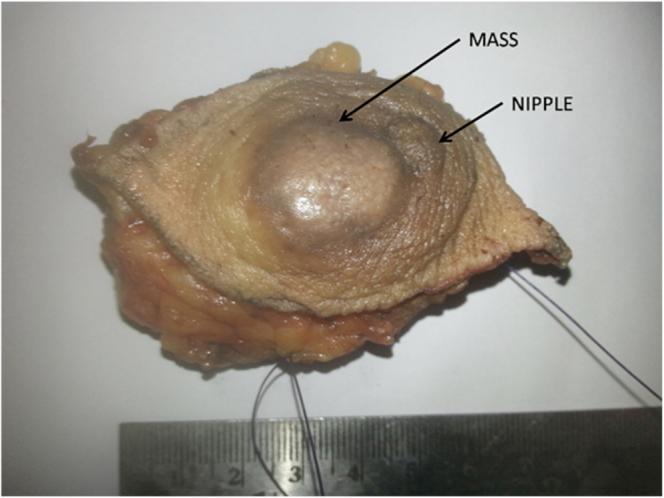
57-year-old female with benign granular-cell tumor in right breast. Wide local excision specimen shows lesion in subareolar region, outer quadrant, pushing the nipple medially, with hypopigmented overlying skin.
Diagnostic mammography (mediolateral oblique and craniocaudal views) demonstrated a dense, well-defined mass lesion in the upper and lower outer quadrant of the subareolar region (Figs. 2A, B). The lesion was seen predominantly in the subcutaneous plane, with thickening of the overlying skin. The adjacent duct appeared hyperdense and had speckled calcification.
Figure 2.
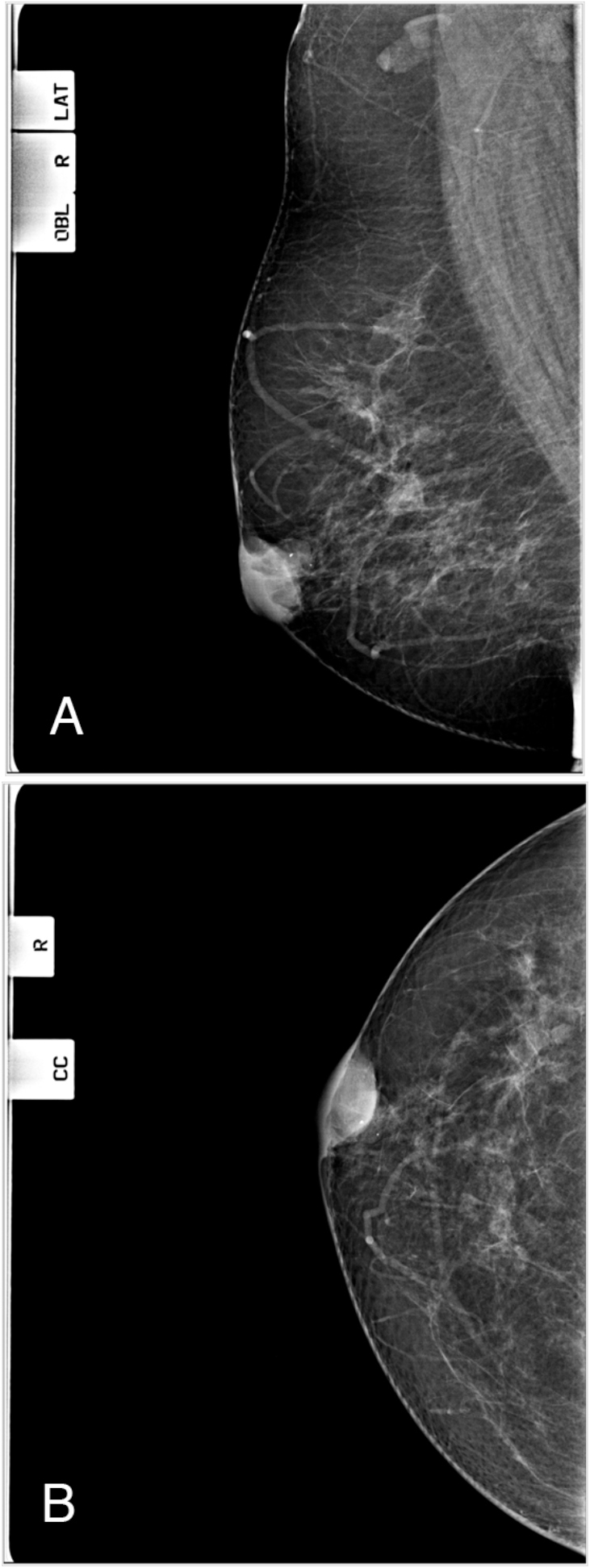
57-year-old female with benign granular-cell tumor in right breast.A. Computed radiography mammogram, right mediolateral oblique (MLO) view, Factors 28 kVp, 104 mAs. B. Right craniocaudal (CC) view, Factors 30 kVp,66 mAs. These show a dense, well-circumscribed subareolar mass with overlying skin thickening and adjacent prominent duct with calcification.
High-resolution sonography (HRS) over the lesion showed a hetero-echoic lesion with posterior acoustic enhancement measuring 2.4 × 1.6 cm (Fig. 3A). The epicenter of the lesion was seen in the subcutaneous plane. The margins were well defined, and no increased color flow was seen (Fig. 3B). The adjacent duct, in the inferomedial aspect, also showed an extension of the lesion, with similar echogenicity.
Figure 3.
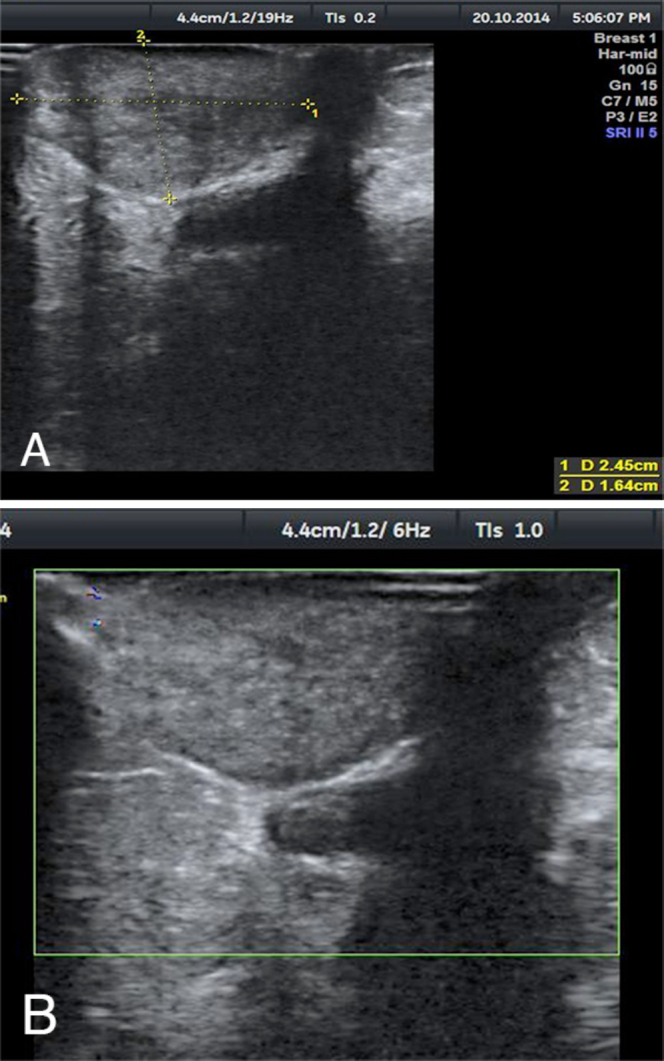
57-year-old female with benign granular-cell tumor in right breast.A. High-resolution sonography (HRS) over the lesion shows well-defined mixed echoic mass with posterior acoustic enhancement measuring 2.4 × 1.6 cm. B. HRS with color Doppler shows no increased color flow. The adjacent duct in the inferomedial aspect is enlarged and shows similar echogenic material within it. Technique: Linear 5–12 MHz transducer (frequency of 12 MHz) performed on a GE Voluson P8 ultrasound machine.
Given a well-defined, dense, subareolar mass with speckled calcification and skin thickening, a final report of BI-RADS category 4 was given, and a core biopsy was done. It revealed granular-cell tumor (GCT) of the breast.
Wide local excision of the lesion was done. A specimen cut section (Fig. 4) showed a grayish-white, circumscribed lesion arising below the skin. Histopathology showed a lesion in the subepithelium composed of nests and sheets of polygonal cells, with abundant eosinophilic, granular cytoplasm and a cuneiform, small, round nucleus with prominent nucleolus. The stroma showed many blood vessels and aggregates of lymphocytes and plasma cells. Mitotic activity was not seen. The cytoplasm showed periodic acid-Schiff stain (PAS)-positive inclusion bodies. A conclusion of benign GCT with margins negative for tumor was given. At the six-month postoperative followup, HRS showed no residual or recurrent lesion. The patient was advised to continue routine followup.
Figure 4.
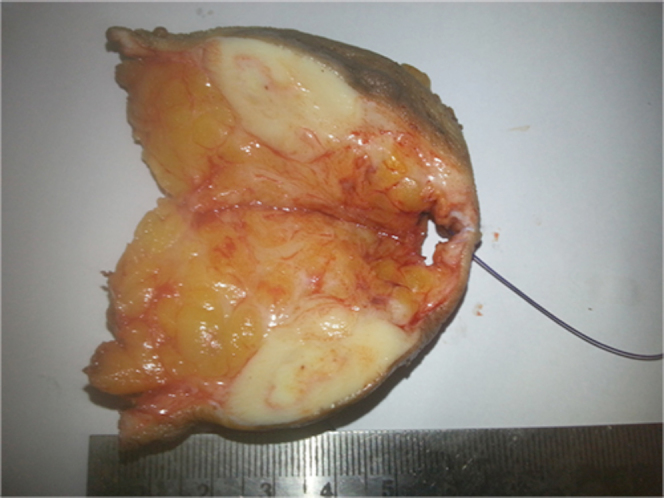
57-year-old female with benign granular-cell tumor in right breast. Cut section of the specimen shows circumscribed grayish-white mass beneath the skin.
Discussion
GCT is a rare, usually benign neoplasm occurring anywhere in visceral or cutaneous sites (1), predominantly in the head, neck, and chest-wall regions (2). It is commonly seen on the tongue (1). A literature review reveals that it occurs in subcutaneous, intradermal, and submucosal layers (3). It is thought to be derived from perineural cells (1, 4). It was first described by Abrikosoff as "myoblastic myoma" in 1926 (5).
Between 4 and 6% of GCTs are seen in the breast (6). Among breast cancers, GCTs are seen in 1 in 1,000 cases (2). GCT has also been reported in the male breast, with a 1:9 male:female ratio (3). A review of the literature reveals that the tumor is commonly located in the upper inner quadrant (1, 7), axillary region, and periareolar region. The distribution of supraclavicular nerve cutaneous sensory territory is said to be the cause for this predilection for the upper inner quadrant (1, 7). Most of the reported cases are seen in the right breast (6, 8). The youngest reported patient is 14 years (1). GCT is commonly seen in premenopausal women and African-American women (1). These tumors do not depend on the hormones estrogen and progesterone (1).
Clinically, GCT presents as a chronic, painless lump. Except in one case, where an axillary GCT lesion was reported in a screening mammogram (2), all other cases were reported via diagnostic mammogram or HRS.
Imaging diagnosis
GCT has a growth pattern similar to that of infiltrating carcinoma, and mimics this more sinister process clinically and on diagnostic imaging (4). Infiltration into superficial tissue is seen as skin dimpling, and underlying structures as a fixation to the pectoralis muscle (3).
As seen with HRS, GCT presents as an indeterminate-category hetero-echoic mass with an ill-defined margin, with or without increased vascularity (9). Depending upon the reactive fibrosis, it can show posterior acoustic shadowing (4) or enhancement. A surrounding halo can be present (3). Our patient had a well-defined hetero-echoic mass with posterior acoustic enhancement but without increased vascularity.
As seen with mammography, GCT is an isodense spiculated mass with an indistinct margin. No calcifications are described in the literature (2). Our patient had a well-defined, dense, subareolar mass in the subcutaneous plane with speckled calcification and skin thickening.
As seen with magnetic resonance imaging (MRI) mammography, GCT is a low-signal lesion in the T1 sequence, an iso- to slightly hyperintense lesion in T2, and a lobulated, variably enhancing mass with indistinct margin in contrast. Its hyperintensity in T2 is less than that of other masses and is considered as characteristic for GCT (3, 10).
As seen with computed tomography (CT), GCT is an enhancing oval or spiculated mass without lymphadenopathy (6).
Positron-emission tomography (PET) can differentiate a benign from malignant GCT. A benign GCT shows an uptake value of 1.8 in a series reported by Hoess et al, which is less than the cutoff value of 2.5 (11).
Radiological criteria for malignancy
-
1.
Tumor size greater than 5 cm.
-
2.
Presence of enlarged lymph nodes.
-
3.
Heterogeneity in MRI.
-
4.
Adjacent tissue infiltration (2).
Histopathology
The characteristic cytomorphological features of GCT are the presence of large granular cells with abundant granular cytoplasm, and stroma with thin-walled blood vessels (9). Cells positive for S-100 protein, CD68 (KP-1), neuron-specific enolase (NSE), and CEA support Schwann cell origin (4, 12). GCT shows a negative reaction against cytokeratin (4). It is negative for desmin and estrogen receptors (6). Reactivity for vimentin differentiates GCT from carcinoma (1).
In histopathology, when malignant lesions with granular cells are seen, apocrine carcinoma and alveolar soft-part carcinoma are considered as possible diagnoses (12).
Postmastectomy scar granular-cell traumatic neuroma (12), co-localized GCT, and infiltrative ductal carcinoma (8) are reported in the literature.
Core biopsy and histopathology give a definitive diagnosis. It is important to make definitive diagnosis pre-operatively to avoid extensive resection and axillary clearance for carcinoma. Wide local excision is the treatment of choice (1). Local recurrence is seen with incomplete resection (4). 1% of GCT cases are malignant (4). Metastasis to liver, lung, bone, and axillary lymph nodes are reported with malignant GCT (3).
Histopathologic criteria for malignancy
-
1.
Spindling.
-
2.
Necrosis.
-
3.
Vesicular nuclei with large nucleoli.
-
4.
High nuclear cytoplasm ratio.
-
5.
Nuclear pleomorphism.
-
6.
Increased mitotic activity.
2 out of 6 of above features are considered as atypical, and 3 out of 6 as malignant, by Le et al (13) and Adeniran et al (7).
Differential diagnosis
The differential diagnosis includes carcinoma, fibroadenoma, lymphoma, and nodular fasciitis.
-
•
Carcinoma is commonly seen in the upper outer quadrant of the breast. Carcinoma is typically a rapidly growing, spiculated mass with ill-defined margins, microcalcifications, and enlarged lymph nodes on mammography, and a hetero-echoic lesion with posterior acoustic shadowing and increased vascularity on HRS. It is nonreactive for vimentin (1) but may be positive for desmin and estrogen receptors (6).
-
•
Fibroadenoma may be seen as single or multiple lesions in one or both breasts in a younger age group. On a mammogram, it is seen as a well-defined dense lesion with or without popcorn calcification. On HRS, a round or oval lesion may be seen in fibroglandular parenchyma that is iso-, hyper-, or slightly hypo-echoic with posterior acoustic enhancement (14).
-
•
Lymphoma may present as unilateral or bilateral, single or multiple masses, or as diffuse involvement of the breast; core needle biopsy is confirmatory (15, 16, 17).
-
•
Nodular fasciitis is a self-limited condition seen as a rapidly growing mass. When present in the breast, it has nonspecific imaging findings similar to those of carcinoma. Followup shows reduction in the size of the lesion (14).
Teaching point
In imaging, GCT can be mistaken for carcinoma. So GCT has to be considered as part of the differential diagnosis in BI-RADS category 4 or 5 lesions. As it is said to occur in subcutaneous, intradermal, and submucosal layers, a breast lesion with its epicenter in the subcutaneous plane can be used as a clue to aid in the imaging diagnosis.
GCT is usually benign, and the treatment option is wide excision. Preoperative definitive diagnosis is possible only with image-guided biopsy, and this is mandatory, as it helps to avoid unnecessary mastectomy and axillary clearance, done in the case of carcinoma.
References
- 1.De Simone N, Aggon A, Christy C. Granular cell tumor of the breast: Clinical and pathologic characteristics of a rare case in a 14-year-old girl. Journal of Clinical Oncology. 2011 Aug;29(22):656–657. doi: 10.1200/JCO.2011.35.9448. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Gavriilidis P, Michalopoulou I, Baliaka A, Nikolaidou A. Granular cell breast tumour mimicking infiltrating carcinoma. BMJ Case Rep. 2013 Feb doi: 10.1136/bcr-2012-008178. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim EY, Kang DK, Kim TH, Jung YS, Kim KS, Yim H. Granular cell tumor of the male breast: Two case descriptions and brief review of the literature. J Ultrasound Med. 2011;30:1295–1301. doi: 10.7863/jum.2011.30.9.1295. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Shetty A and Radswiki et al. Granular cell tumour of the breast. radiopaedia.org.
- 5.Abrikosoff AI. (1926). Uber Myome, ausgehend von der quergestreiften willktirlichen Muskulature (in German) Virchows Arch Path Anat. 1926;260:215–233. [Google Scholar]
- 6.Vasselli F, Pediconi F, Luciani ML, Casali V, Telesca M, Miglio E, Catalano C. Breast localization of granular cell tumor (GCT) Euro Rad. 2011 doi: 10.1007/s11547-011-0630-8. [DOI] [PubMed] [Google Scholar]
- 7.Adeniran A, Al-Ahmadie H, Mahoney MC, Robinson-Smith TM. Granular cell tumor of the breast: a series of 17 cases and review of the literature. Breast J. 2004 Nov-Dec;10(6):528–531. doi: 10.1111/j.1075-122X.2004.21525.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Al-Ahmadie H, Hasselgren PO, Yassin R, Mutema G. Colocalized granular cell tumor and infiltrating ductal carcinoma of the breast. Arch Pathol Lab Med. 2002 Jun;126(6):731–733. doi: 10.5858/2002-126-0731-CGCTAI. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Yang WT, Edeiken-Monroe B, Sneige N, Fornage BD. Sonographic and mammographic appearances of granular cell tumors of the breast with pathological correlation. J Clin Ultrasound. 2006 May;34(4):153–160. doi: 10.1002/jcu.20227. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Scaranelo AM, Bukhanov K, Crystal P, Mulligan AM, O'Malley FP. Granular cell tumour of the breast: MRI findings and review of the literature. Br J Radiol. 2007;80:970–974. doi: 10.1259/bjr/95130566. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Hoess C, Freitag K, Kolben M. FDG PET evaluation of granular cell tumor of the breast. J Nucl Med. 1998;39:1398–1401. [PubMed] [PubMed] [Google Scholar]
- 12.Rosso RI, Scelsi M, Carnevali L. Granular cell traumatic neuroma: a lesion occurring in mastectomy scars. Arch Pathol Lab Med. 2000 May;124(5):709–711. doi: 10.5858/2000-124-0709-GCTN. [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Le BH, Boyer PJ, Lewis JE. Granular cell tumor, immunohistochemical assessment of inhibin-alpha, protein gene product 9,5, S100 protein, CD68 and ki-67 proliferase index with clinical correction. Arch Pathol Lab Med. 2004;128:771–775. doi: 10.5858/2004-128-771-GCTIAO. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Skaane P, Engedal K. Analysis of sonographic features in the differentiation of fibroadenoma and invasive ductal carcinoma. AJR. 1998 January;17O:110–114. doi: 10.2214/ajr.170.1.9423610. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Samardzic D, Chetlen A, Malysz J. Nodular fasciitis in the axillary tail of the breast. J Radiol Case Rep. 2014 May;8(5):16–26. doi: 10.3941/jrcr.v8i5.1903. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer J, Kopans D, Long J. Mammographic appearance of malignant lymphoma of the breast. Radiology. 1980;135:623–626. doi: 10.1148/radiology.135.3.7384445. [PubMed] [DOI] [PubMed] [Google Scholar]
- 17.Amador-Ortiz C, Chen L, Hassan A. Combined core needle biopsy and fine-needle aspiration with ancillary studies correlate highly with traditional techniques in the diagnosis of nodal-based lymphoma. American Journal of Clinical Pathology. 2011;135:516–524. doi: 10.1309/AJCP3WZ8ZDRJQDOU. [PubMed] [DOI] [PubMed] [Google Scholar]


