Abstract
Coccidioidomycosis is a pulmonary infection caused by the dimorphic fungi Coccidioides immitis and Coccidioidomycosis posadasii. This disease is endemic to the southwestern United States and has a predilection for immunocompromised patients. Diabetes mellitus has been shown to be a strong risk factor for acquiring this infection in these states. Most cases are asymptomatic or present with mild pulmonary symptoms. However, untreated pulmonary mycosis can lead to disseminated infection, most often involving meningitis, osteomyelitis, or skin and soft-tissue infections. When there is arthritis, the knee is the most common site of infection. We present a case of a 23-year-old male with longstanding, uncontrolled Type 1 diabetes mellitus who was found to have pulmonary coccidioidomycosis following diagnosis of coccidioidomycosis osteomyelitis of the knee.
Case report
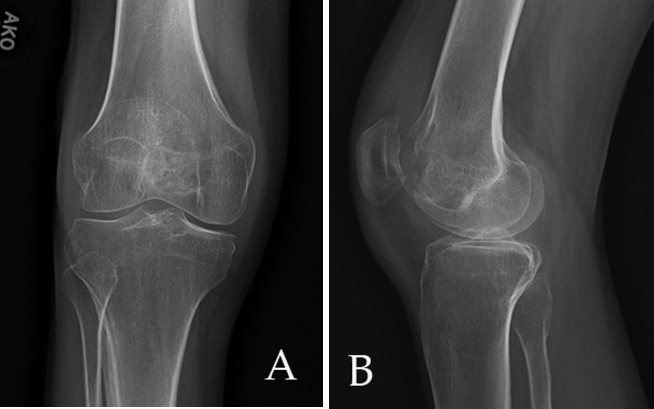
Our patient presented to the emergency department at 21 years of age with right knee pain for six months. He stated that he injured the knee in a mixed martial arts workout and had progressive swelling and pain upon flexion and weight bearing. The patient had no other complaints. He had a 10-year history of Type 1 diabetes mellitus that had progressed to neuropathy and gastroparesis at the time of presentation. Physical exam of the knee was normal except for medial joint line tenderness and pain on weight bearing and external rotation. AP and lateral views of the knee showed no fractures or evidence of arthritis, but a small suprapatellar effusion was present (Fig. 1).
Fig. 1.
AP (A) and lateral radiographs (B) of the knee demonstrate joint space narrowing and suprapatellar joint effusion.
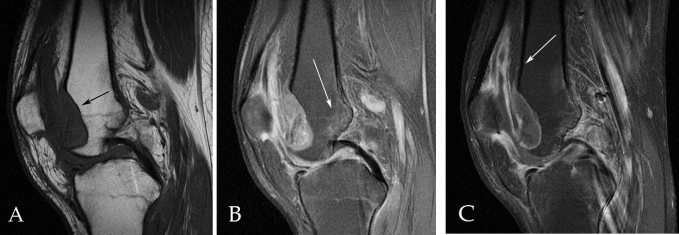
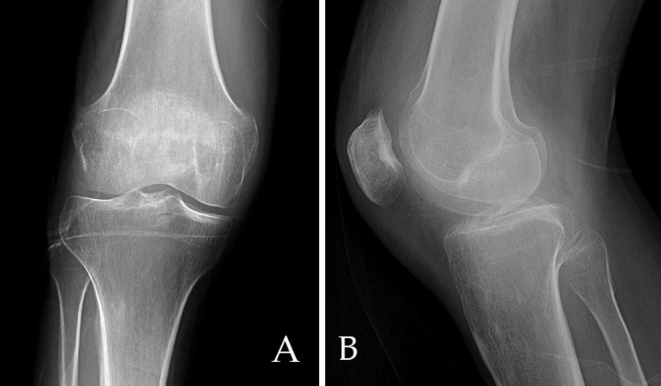
The patient was referred to the orthopedics clinic from the ED but did not follow up. He returned to the orthopedic clinic with similar complaints 16 months after his original presentation of knee pain in the ED. AP and lateral views of the knee, when compared to earlier radiographs, showed decreased bone density and a large erosion of the medial trochlear facet, with a small effusion of the suprapatellar bursa (Fig. 2). MRI revealed a 3-cm, sharply demarcated erosion of the anterior aspect of the trochlear notch and medial facet, with synovial thickening and enhancement and patchy edema of the femoral and tibial metaphyses (Fig. 3). The study was reported as a chronic synovitis, possibly related to a granulomatous infection, and synovial fluid aspiration for cytology and culture was recommended. Aspiration of the knee was performed, including removing a pus-like fluid. Orthopedics recommended surgical intervention with incision and debridement but the patient refused and was, again, lost to followup.
Fig. 2.
(A) AP and (B) Lateral radiograph of the knee demonstrates increased osteopenia, joint effusion, and erosion of the medial trochlear surface (arrow).
Fig. 3.
Sagittal (A) T1, (B) fat-saturated proton density, and (C) fat-saturated T1 postcontrast images demonstrate a large, well-defined erosion (black arrow in A), patchy bone marrow edema (long white arrow in B), and synovial thickening and enhancement (white arrow in C).
The patient returned again one month later with increased swelling of the knee and very limited range of motion. Incision and debridement performed at this time revealed a 3-cm × 3-cm × 2-cm cavitary lesion of the distal femur with surrounding granulation tissue and hypertrophied synovium.
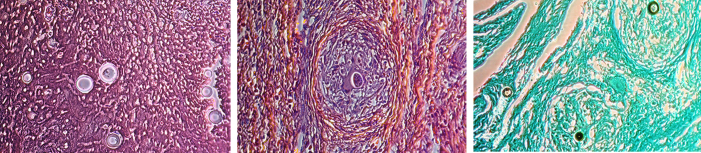
The original pathologic evaluation of surgical samples described a necrotizing granulomatous inflammation. Further pathologic testing showed that specimens stained negative for acid-fast bacteria but stained positive on Grocott's methenamine silver stain for fungus. Findings were described as most consistent with a subspecies of Coccidioides being the causal organism (Fig. 4).
Fig. 4.
A. H&E stain. Characteristic spherules of coccidioides from knee debridement. Various sizes and stages of spherules are typical. Fig 4B. H&E stain. Granuloma formation with histiocytes and giant cells in a background of chronic lymphocytic inflammation. Granuloma surrounds a coccidioidal spherule filled with endospores that could disseminate if ruptured. Fig 4c. GMS stain. Coccidioidal spherules within granulomas display variable uptake of silver stain.
Though the patient had experienced no pulmonary symptoms, a screening chest x-ray was performed. Radiographs demonstrated opacity in the medial right lung apex, with a central curviliniar lucency (Fig. 5). A CT scan of the chest demonstrated two cavitary lesions measuring 1.5 and 3 cm in the right upper lobe. A soft-tissue mass was present within the larger cavity, likely representing a mycetoma. There was adjacent bronchiectasis, parenchymal scarring, and multiple, small centrilobular nodules in the right upper lobe (Fig. 6).
Fig. 5.
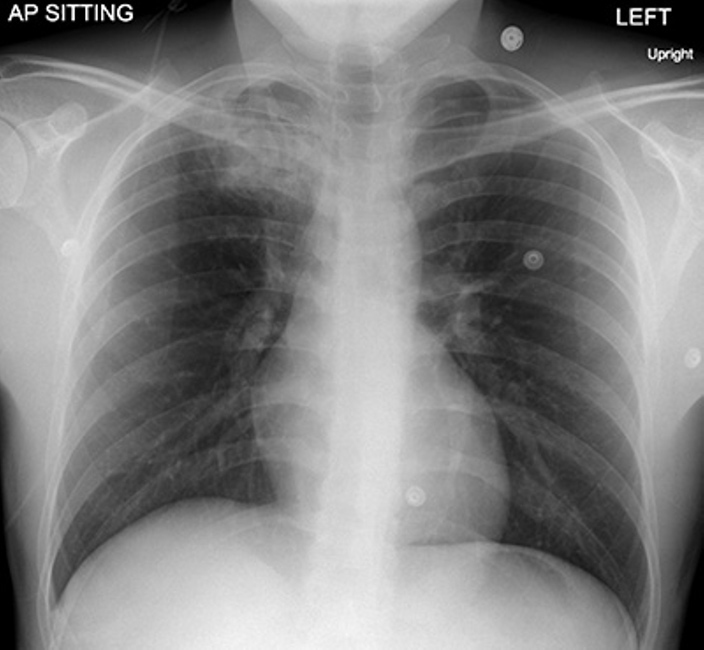
AP radiograph of the chest demonstrates a wedge-shaped area of air space consolidation in the right upper lobe containing a crescentic lucency (arrow). There is mild retraction of the right hilum likely related to volume loss and scarring.
Fig. 6.
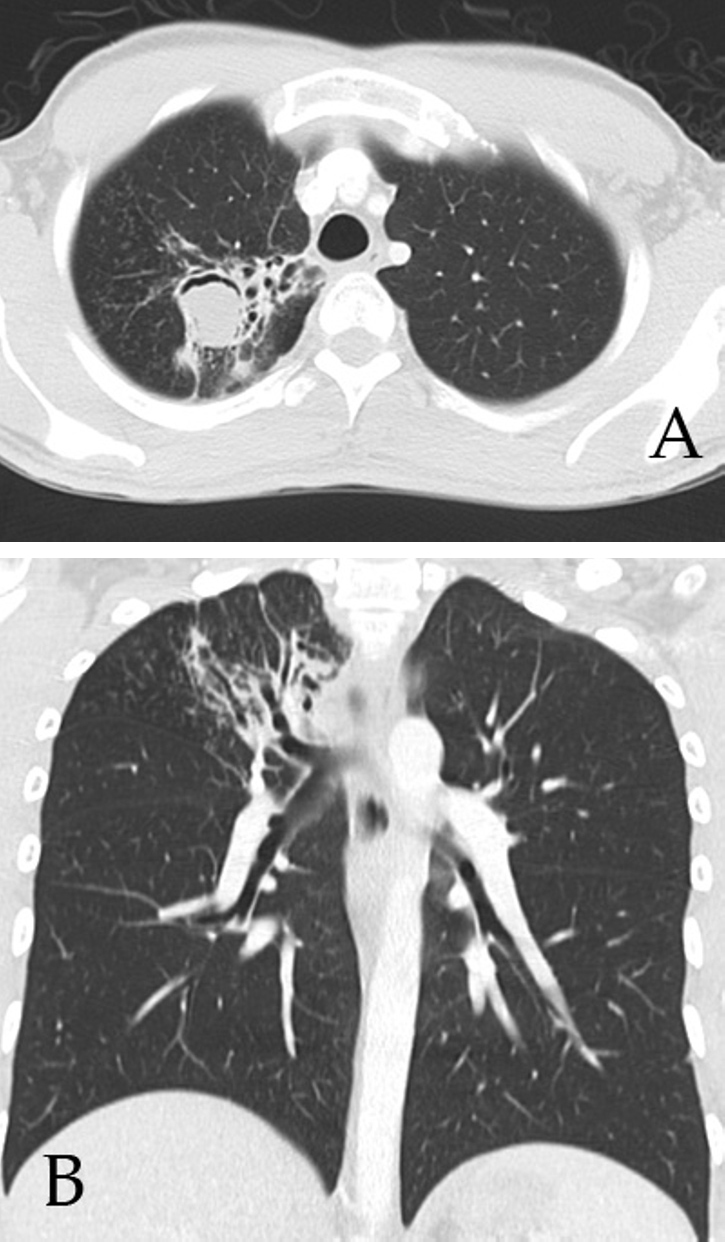
(A) Axial CT of the chest demonstrate a 3-cm cavitary lesion in the right upper lobe containing soft-tissue density with a crescentic lucency in the nondependent portion. (B) Coronal reformatted CT image demonstrates right-upper-lobe bronchiectasis, scarring, and multiple centrilobular nodules.
The patient has been treated almost three years with oral fluconazole, 600mg daily, for disseminated infection and followed up in a clinic checking coccidioides antibody titers. There have been heightened measures to improve his followup, and control of his diabetes and coccidioidomycosis infection, since surgery. In followup, his hemoglobin A1c has remained elevated around 14 to 15%, and he has shown signs of early glaucoma and renal disease. To date, his coccidioides antibody titers have remained elevated, and he has been unable to discontinue his antifungal therapy.
Discussion
Coccidioidomycosis, also known as “San Joaquin Valley Fever,” was first reported by Posadas and Wernicke in Argentina in 1892, followed shortly by cases in the San Joaquin Valley of California (1, 2, 3). Heightened focus was brought to this disease when the US army placed training sites within the San Joaquin Valley before WWII. This resulted in a drastic rise in hospitalizations for coccidioidomycosis in the region, prompting further research (1). These studies, and later research related to prisoner-of-war camps, dust storms, earthquakes, and soil displacement, have provided a larger understanding of the epidemiology and pathogenicity of this disease (1, 2, 3, 4).
Coccidioidomycosis is a disease of regional endemicity, highly concentrated within focal areas of the Western Hemisphere that have warm and arid to semi-arid climates (4, 5, 6). Endemic regions have been described along the US-Mexico border and in areas of Central and South America (4, 5, 6, 7, 8). It is thought that many other areas of Central and South America could also be considered endemic, but this is difficult to establish due to varying reporting measures and poor specificity of skin reaction tests to differentiate mycoses found in these regions (7).
Inhalation of spores from the soil-dwelling, dimorphic fungi Coccidioides immitis and Coccidioides posadasii causes pulmonary coccidioidomycosis (9). Within immunocompetent patients, a delayed-type cell-mediated response limits infections to being asymptomatic 60% of the time, or only causing mild flu-like symptoms 15% of the time (1, 5, 10). The remaining patients, considered to have clinically significant disease, can have a range of nonspecific symptoms including fever, night sweats, and chest pain. Patients with primary pulmonary coccidioidomycosis often demonstrate consolidation or hilar lymphadenopathy on chest x-ray. However, even with clinically apparent symptoms, chest x-rays can be normal (1, 3).
While most primary pulmonary coccidioidomycoses resolve spontaneously, a small proportion of patients' symptoms endure as persistent coccidioidal pneumonia. Persistent coccidioidal pneumonia frequently involves single or multiple nodules, with upper-lobe predilection. The nodules measure 1.5 cm on average and can resolve completely, remain stable, or rupture and disseminate. Persistent disease can also manifest as cavitations, including thick-walled and thin-walled lesions. Although rare, complications such as fungal mycetoma formation in the cavitary lesions have been reported. There are often adjacent areas of bronchiolar and bronchial wall thickening. Bronchiectasis and scarring may result as the end product of pulmonary cocccidioidal infection. However, areas of scarring and otherwise stable nodules may contain residual foci of coccidiomycosis and can lead to disseminated infection, especially in cases of immunosuppression (3).
Disseminated coccidioidomycosis occurs rarely, accounting for only 1% to 5% of all cases. African-American and Filipino patients have a much higher risk of developing disseminated disease compared to Caucasians (1, 4, 11, 12). Other risk factors include male gender, pregnancy, diabetes, and immunosuppression (4, 5, 7, 13). Patients with disseminated disease can present with meningitis, osteomyelitis, arthritis, and skin and soft-tissue infections (1, 6, 7). In cases of musculoskeletal involvement, osteomyelitis is the most common finding, multiple sites can be affected, and there is a strong predilection for the axial skeleton (3). The next most common finding was joint involvement, with the knee being most commonly involved (1, 3, 14, 15).
The most common radiographic findings with bony involvement are osteolytic lesions, either with punched-out, well-circumscribed borders or demonstrating a permeative (or moth-eaten) appearance. CT and MRI have been useful in evaluating the extent of bony erosion, soft-tissue damage, and bone marrow signal. In cases of arthritis, advanced imaging techniques can also be useful to assess synovial thickening, cartilage destruction, and the extent of subarticular bone loss (2, 14, 15). Followup radiographs can also be useful for evaluating the management and monitoring treatment of skeletal involvement. Radionucleotide scanning has proved useful for surveying dissemination (14).
Persistent pulmonary coccidioidomycosis and disseminated disease are treated with a number of antifungal therapies. Though amphotericin B was once the mainstay, its poor renal profile has caused drugs like fluconazole, itraconazole, and others of their class to become more commonly used. Patients may remain on antifungal therapy for months or even the rest of their lives to prevent recurrence of disseminated disease, especially if they are immunocompromised (6, 13). We present this case of a young male with poorly controlled diabetes to illustrate the radiographic findings of disseminated coccidiomycosis with pulmonary and joint involvement. This case also serves to illustrate the dangers of an unchecked granulomatous disease within an immunocompromised host and the importance of good followup with similar patients.
Footnotes
Published: February 5, 2015
References
- 1.Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394–1402. doi: 10.2105/ajph.36.12.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirschmann JV. The early history of coccidioidomycosis: 1892-1945. Clin Infect Dis. 2007;44(9):1202–1207. doi: 10.1086/513202. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.McGahan JP, Graves DS, Palmer PES, Stadalnik RC, Dublin AB. Classic and contemporary imaging of coccidioidomycosis. AJR Am J Roentgenol. 1981 Feb;136(2):393–404. doi: 10.2214/ajr.136.2.393. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Kirkland TN, Fierer J. Coccidioidomycosis: a reemerging infectious disease. Emerg Infect Dis. 1996 Jul-Sep;2(3):192–199. doi: 10.3201/eid0203.960305. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laniado-Laborin R. Expanding understanding of epidemiology of coccidioidomycosis in the Western hemisphere. Ann N Y Acad Sci. 2007 Sep;1111:19–34. doi: 10.1196/annals.1406.004. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Galgiani JN, Ampel NM, Blair JE. Coccidioidomycosis. Clin Infect Dis. 2005;14:1217–1223. doi: 10.1086/496991. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Brown J, Benedict K, Park BJ, Thompson GR., 3rd. Coccidioidomycosis: epidemiology. Clin Epidemiol. 2013 Jun 25;5:185–197. doi: 10.2147/CLEP.S34434. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winthrop KL, Chiller T. Preventing and treating biologic-associated opportunistic infections. Nat Rev Rheumatol. 2009 Jul;5(7):405–410. doi: 10.1038/nrrheum.2009.105. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Fisher MC, Koenig GL, White TJ, Taylor JW. Molecular and phenotypic description of Coccidioides posadasii sp nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia. 2002;94:73–84. [PubMed] [PubMed] [Google Scholar]
- 10.Ampel NM. Measurement of cellular immunity in human coccidioidomycosis. Mycopathologia. 2003;156(4):247–262. doi: 10.1023/b:myco.0000003580.93839.71. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Drutz DJ, Catanzara A. Coccidioidomycosis. Part I. Am Rev Respir Dis. 1978;117(3):559–585. doi: 10.1164/arrd.1978.117.3.559. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Drutz DJ, Catanzara A. Coccidioidomycosis. Part II. Am Rev Respir Dis. 1978;117(4):727–771. doi: 10.1164/arrd.1978.117.4.727. [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Blair JE. State-of-the-art treatment of coccidioidomycosis skeletal infections. Ann N Y Acad Sci. 2007 Sep;1111:422–433. doi: 10.1196/annals.1406.000. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Zeppa MA1, Laorr A, Greenspan A, McGahan JP, Steinbach LS. Skeletal coccidioidomycosis: imaging findings in 19 patients. Skeletal Radiol. 1996 May;25(4):337–343. doi: 10.1007/s002560050092. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Lund PJ1, Chan KM, Unger EC, Galgiani TN, Pitt MJ. Magnetic resonance imaging in coccidioidal arthritis. Skeletal Radiol. 1996 Oct;25(7):661–665. doi: 10.1007/s002560050154. [PubMed] [DOI] [PubMed] [Google Scholar]