Abstract
Background
Abnormalities of the hypothalamic-pituitary-thyroid (HPT) axis have been reported in alcoholism, however, there is no definitive agreement on the specific thyroid abnormalities and their underlying mechanisms in alcohol dependence (AD). The biological activity of thyroid hormones or the availability of T3 is regulated by the three deiodinase enzymes D1, D2 and D3. In the context of alcohol use, functionally significant single nucleotide polymorphisms (SNP’s) of these deiodinase genes may play a role in HPT dysfunction.
Methods
The present study explored the effect of three functionally significant SNP’s (D1: rs2235544, D2: rs225014 and rs12885300) of deiodinase genes on drinking behavior and thyroid stimulating hormone (TSH) levels in alcohol dependent (N=521) and control subjects (N=228).
Results
Rs225014 was associated with significant differences in the amount of naturalistic alcohol drinking assessed by the Timeline Follow-Back (TLFB). Alcohol-dependent subjects had significantly higher thyroid stimulating hormone levels compared to controls; however, there was no effect of genotype on TSH levels for either group.
Conclusions
These findings extend previous studies on thyroid dysfunction in alcoholism and provide novel, albeit preliminary, information by linking functionally significant genetic polymorphisms of the deiodinase enzymes with alcohol drinking behavior.
Keywords: Thyroid hormone, deiodinases, alcohol dependence, genetics, single nucleotide polymorphisms, hypothalamic-pituitary-thyroid axis
Introduction
Abnormalities of the hypothalamic-pituitary-thyroid (HPT) axis have been reported in alcoholism [for review, see: (Hermann et al., 2002)] but are difficult to interpret as they have often focused on different stages of the disease, e.g. early, late abstinence or active drinking. Therefore, it is challenging to draw final conclusions on the specific thyroid abnormalities and their underlying mechanisms in alcoholism. A review of 33 studies assessing thyroid function in alcoholism reported that one third of alcoholic patients have a blunted thyroid stimulating hormone (TSH) response on the thyrotrophin-releasing hormone test (TRH-stim test) (Hermann et al., 2002). In addition in early abstinence, there is a reduction in total T4 as well as total and free T3 concentrations. Of note, excessive chronic alcohol use may have direct toxic effects to the thyroid gland. For example, Hegedus and colleagues (Hegedus et al., 1988) in an ultrasound study of the thyroid gland in alcohol-dependent patients, found a significant reduction in thyroid volume, with a dose-dependent effect of alcohol on thyroid fibrosis. This reduction was independent of the severity of liver damage. The reduced thyroid size was accompanied by reductions in T3 and free T3 levels and normal T4, free T4, and TSH values. In animal models of chronic alcohol exposure, these functional and structural abnormalities of thyroid function result in a dysregulation of feedback mechanisms regulating thyroid release [for review: (Hermann et al., 2002)]. Reduced thyroid hormone levels, chiefly T3, result in chronically elevated TRH, which causes a down regulation of TRH receptors in the pituitary gland and a blunted TSH response in the TRH stimulation test (Hermann et al., 2002). In a study that measured TRH release in acute cold exposure in rats that were chronically treated with alcohol, TRH mRNA in the paraventricular nucleus of hypothalamus was increased, demonstrating that, in chronic alcohol exposure, the thyroid gland can no longer adequately respond to TRH stimulation (Zoeller et al., 1996). In non-cirrhotic alcoholic patients, Garbutt and colleagues (Garbutt et al., 1992) found that suppressive doses of T3 are required to blunt TRH induced TSH response. The biological activity of thyroid hormone or the availability of T3 is regulated by the three deiodinase enzymes: D1, D2 and D3. D1 and D2 are activating enzymes, converting T4 to T3 by outer ring diodination, while D3 inactivates thyroid hormones by inner ring deiodination converting T3 to T2 and T4 to rT3 (Bianco et al., 2002). Genetic variation in deiodinase enzymes encoding genes influences thyroid hormone levels and ratios (de Jong et al., 2007; Panicker et al., 2008; Peeters et al., 2005a; Peeters et al., 2005b; Peeters et al., 2003). The C allele of the common single nucleotide polymorphism (SNP) of the Deiodinase 1 gene (DIo1; rs2235544) is associated with increased D1 function with resulting increase in free T3/T4 ratio and free T3 and decrease in free T4 and rT3 (Panicker et al., 2008). While common SNP’s of D2 and 3 have not been shown to alter peripheral thyroid hormone levels, the commonly occurring Thr92Ala D2 variant (rs225014) is associated with a decreased rate of acute TSH-stimulated T3 release consistent with a decrease in intrathyroidal deiodination (Butler et al., 2010). In addition, the G variant of another D2 SNP, K258A/G (rs12885300), associated with increased enzymatic activity, is associated with a decreased rate of acute TSH-stimulated FT4 secretion with a normal T3 release from the thyroid gland (Peltsverger et al., 2012).
In rats chronically exposed to ethanol, Baumgartner and colleagues (Baumgartner et al., 1994) found a reduction in D2 enzyme activity in cortico-limbic brain areas. Additionally, during alcohol withdrawal, D2 activity was reduced in prefrontal cortex, and striatum in alcohol dependent vs. naïve rats (Baumgartner et al., 1994). Given that nervous system tissues contain relatively high ratios of T3/T4 (Baumgartner et al., 1997), examining the effect of these genetic variants on thyroid hormone levels, i.e., TSH in the context of alcohol withdrawal might further elucidate the link between the HPT axis and alcoholism. The withdrawal state can be a stressor which is analogous to a TSH stimulation test, where the effect of these deiodinase SNPs on thyroid function are apparent. In this exploratory analysis we also examined whether functionally significant SNP’s of these deiodinase genes might affect drinking behavior. Therefore, in this exploratory study, we examined, a priori, the effect of the common functional SNP’s for D1 and D2 enzymes: D1; rs2235544, D2; rs225014; rs12885300 on alcohol drinking behavior and on serum TSH levels in alcohol-dependent vs. non-dependent subjects. As summarized above, the selection of these SNPs was based on the fact that these SNPs seem to be functional and influence thyroid hormone levels during provocative tests.
Materials and Methods
Participants and Assessments
809 individuals (521 patients with alcohol dependence [AD], 288 controls subjects without AD) participated in IRB-approved screening protocols for treatment-seeking alcoholic patients and control subjects at the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Subjects were recruited by advertisement and community outreach; data were collected over approximately the last 10 years. Exclusion criteria for the control subjects were alcohol dependence and any clinically significant medical illness or major psychiatric disorders such as Bipolar, Major Depressive or psychotic disorders. The exclusion criteria for the AD patients included clinically neuro-psychiatric disorders such as psychosis or dementia. The control population was not required to be abstinent from alcohol; rather, they were not alcohol dependent and in good health. All participants provided written consent before participating. Assessments upon intake included a medical history and physical examination, the Structured Clinical Interview for DSM-IV Disorders (SCID, DSM-IV) to diagnose AD and any other psychiatric diagnoses, and the alcohol Time-Line Follow Back (TLFB) to assess current drinking patterns over the last ninety days with the following: heavy drinking days, average number of drinks per drinking day, number of drinking days, days of abstinence, total number of drinks and calculated average number of drinks in past 30 days. For control subjects, serum TSH levels were measured during the one-day screening outpatient visit. For AD subjects, who were admitted to the inpatient unit for approximately four weeks, TSH levels were measured in early abstinence, i.e., one day following admission, therefore most of the AD patients were in early withdrawal at the time of the TSH draw. The average length of abstinence for the AD group was 2.4± 3.7 days and 4.1 ± 7.0 days for the control group. For AD and control subject characteristics, genotype frequencies and drinking behavior, see Table 1.
Table 1.
Patient Characteristics
| Alcohol Dependent (n = 521) | Controls (n = 288) | Test for Group Differences | |
|---|---|---|---|
| Demographics
| |||
| Age | 42.1 (9.9) | 31.3 (10.9) | t = 13.9, p < 0.0001 |
| Female | 159 (30.5%) | 111 (38.5%) | χ2 = 5.4, p = 0.02 |
| Caucasian1 | 308 (59.1%) | 186 (64.6%) | χ2 = 59.9, p = 0.007 |
| Body Mass Index | 26.6 (5.5) | 25.9 (4.9) | t = 2.03, p = 0.04 |
|
| |||
| Genotype
| |||
| rs22355442 | AA: 110 (21.2%) | AA: 60 (20.8%) | χ2 = 0.2, p = 0.92 |
| AC: 244 (46.9%) | AC: 132 (45.8%) | ||
| CC: 166 (31.9%) | CC: 96 (33.3%) | ||
| rs225015 | AA: 181 (34.7%) | AA: 104 (36.1%) | χ2 = 0.4, p = 0.84 |
| AG: 245 (47.0%) | AG: 136 (47.2%) | ||
| GG: 95 (18.2%) | GG: 48 (16.7%) | ||
| rs128853003 | AA/AG: 219 (42.4%) | AA/AG: 138 (47.9%) | χ2 = 2.3, p = 0.13 |
| GG: 298 (57.6%) | GG: 150 (52.1%) | ||
|
| |||
| Alcohol Use (90 days)
| |||
| Total Drinks | 1062.4 (721.0) | 158.5 (180.1) | t = 26.6, p < 0.0001 |
| Number of Drinking Days | 70.9 (22.0) | 36.1 (24.7) | t = 17.7, p < 0.0001 |
| Number of Non-Drinking Days | 18.8 (21.9) | 53.5 (24.8) | t = −17.6, p < 0.0001 |
| Average Drinks/day | 14.5 (8.0) | 3.7 (2.5) | t = 27.6, p < 0.0001 |
| Heavy Drinking Days | 65.6 (25.6) | 14.5 (20.3) | t = 28.3, p < 0.0001 |
| Number of Days Abstinent before TSH level | 2.4 (3.7) | 4.1 (7.0) | T = −3.27, p = 0.001 |
|
| |||
| Major Depression
| |||
| Current Diagnosis | 57 (10.9%) | 3 (1.1%) | χ2 = 25.5, p < 0.0001 |
A majority of the remaining subjects were Black/African American.
Missing genotype data for 1 subject
Missing genotype data for 4 subjects. AG and AA subjects were combined due to the small number of AA homozygotes
Genotyping
Genotyping was conducted at the NIAAA Laboratory of Neurogenetics. Genomic DNA was extracted from whole blood using standard protocols. DNA samples were genotyped using the Illumina OmniExpress BeadChip (Illumina Inc, San Diego, CA) for all participants. The number of homozygotes for the minor A allele for rs12885300 was very small, consequently the AA and AG subjects were combined for analysis (see Table 1 for genotype frequencies).
Data Analysis
Group differences in baseline characteristics between AD subjects and controls were determined using chi-square for categorical measures and independent t-tests for continuous measures. Differences in baseline characteristics between gene groups were determined using chi-square and analysis of variance (ANOVA). Baseline characteristics that were found significantly different between the two groups were included in the analyses as covariates. Hardy-Weinberg equilibrium was determined for the full sample using chi-square. In addition, the presence/absence of a current diagnosis of major depressive disorder (MDD) was used as a covariate given that thyroid axis abnormalities often occur in the context of MDD (Tichomirowa et al., 2005).
ANCOVA was used to analyze the effects of genotype, diagnosis and their interaction on TLFB-related drinking measures and TSH levels in a 3 x 2 ANCOVA model [genotype (3 levels) x group(2 levels)]. Ancestry informative marker scores were added to the ANCOVA to control for population stratification.
Since TSH levels particularly in the AD group could be affected by length of abstinence, the relationship between days of abstinence and TSH level was determined separately for AD and control groups first using simple linear regression. In addition, ANCOVA was used to test for significant gene group differences in the correlation between TSH levels and days of abstinence, as determined by an interaction between genotype and days of abstinence.
Results
Subject baseline characteristics
AD and control subjects differed significantly by age, gender, race, current diagnosis of major depressive disorder (MDD) and body mass index (BMI) (p’s < .05; Table 1). Specifically, AD subjects were older (average age = 42 years) and had a greater BMI (average = 26.6 kg/m2) as well as significantly more MDD compared to controls; furthermore, there were lower percentages of females and of Caucasians in the AD group. Therefore, MDD diagnosis, age, gender, and BMI were also included in the analyses of both groups as covariates. Ancestry informative marker scores were added to the ANCOVA to control for population stratification.
Comparison of the different genotype groups for each polymorphism of interest yielded significant differences in race attribution, both in the sample as a whole and in the AD sample (Table 2). There were no significant differences in genotype frequencies for the three SNP’s between the AD and control groups (Table 1). Each of the 3 SNP’s was found to be in Hardy-Weinberg equilibrium: rs2235544, p=0.10, rs225014, p=0.43, rs12885300, p=0.17. No subject exhibited evidence of clinical thyroid disease.
Table 2.
Genotype differences in patient characteristics
| Full Sample (N = 809) | Alcohol Dependent Patients (N = 521) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| SNP | Variable | ||||||||
| rs2235544 | AA | AC | CC | AA | AC | CC | |||
|
|
|
||||||||
| Age | 37.7 | 37.7 | 39.3 | F = 1.74 | 41.8 | 41.6 | 43.0 | F = 1.03 | |
| (11.6) | (11.5) | (11.3) | p = 0.18 | (10.0) | (10.0) | (9.7) | p = 0.36 | ||
| Female | 62 | 113 | 95 | χ2 = 3.6 | 38 | 66 | 55 | χ2 = 2.8 | |
| (36.5%) | (30.1%) | (36.3%) | p = 0.17 | (34.6%) | (27.1%) | (33.1%) | p = 0.25 | ||
| Caucasian1 | 126 | 250 | 118 | χ2 = 94.5 | 84 | 157 | 67 | χ2 = 61.1 | |
| (74.12%) | (66.5%) | (45.0%) | p < 0.0001 | (76.4%) | (64.3%) | (40.4%) | p < 0.0001 | ||
| Body Mass Index | 26.0 | 26.3 | 26.6 | F = 0.58 | 26.9 | 26.7 | 26.3 | F = 0.33 | |
| (4.9) | (5.7) | (4.8) | p = 0.56 | (5.2) | (6.0) | (4.7) | p = 0.72 | ||
| rs225014 | AA | AG | GG | AA | AG | GG | |||
|
|
|
||||||||
| Age | 38.5 | 38.3 | 37.7 | F = 0.23 | 42.6 | 41.9 | 41.4 | F = 0.56 | |
| (11.9) | (11.4) | (10.8) | p = 0.80 | (10.4) | (9.7) | (9.5) | p = 0.57 | ||
| Female | 90 | 132 | 48 | χ2 = 0.7 | 58 | 76 | 25 | χ2 = 1.0 | |
| (31.6%) | (34.7%) | (33.6%) | p = 0.71 | (32.0%) | (31.0%) | (26.3%) | p = 0.60 | ||
| Caucasian1 | 197 | 225 | 72 | χ2 = 15.4 | 122 | 138 | 48 | χ2 = 9.4 | |
| (69.1%) | (59.1%) | (50.4%) | p = 0.004 | (67.4%) | (56.3%) | (50.5%) | p = 0.05 | ||
| Body Mass Index | 26.4 | 26.3 | 26.5 | F = 0.07 | 26.6 | 26.8 | 26.5 | F = 0.11 | |
| (4.8) | (6.7) | (4.9) | p = 0.93 | (4.7) | (6.2) | (4.8) | p = 0.89 | ||
| rs12885300 | AA/AG | GG | AA/AG | GG | |||||
|
|
|
||||||||
| Age | 37.8 | 38.6 | F = 1.01 | 41.9 | 42.3 | F = 0.19 | |||
| (11.8) | (11.2) | p = 0.32 | (10.3) | (9.6) | p = 0.66 | ||||
| Female | 126 | 144 | χ2 = 0.9 | 75 | 84 | χ2 = 2.18 | |||
| (46.7%) | (53.3%) | p = 0.35 | (34.3%) | (28.2%) | p = 0.14 | ||||
| Caucasian1 | 278 | 213 | χ2 = 92.4 | 168 | 137 | χ2 = 59.6 | |||
| (77.9%) | (47.6%) | p < 0.0001 | (76.7%) | (46.0%) | p < 0.0001 | ||||
| Body Mass Index | 26.2 | 26.5 | F = 0.49 | 26.5 | 36.7 | F = 0.06 | |||
| (5.7) | (4.9) | p = 0.48 | (6.0) | (5.0) | p = 0.80 | ||||
A majority of the remaining subjects were Black/African American
Drinking behavior
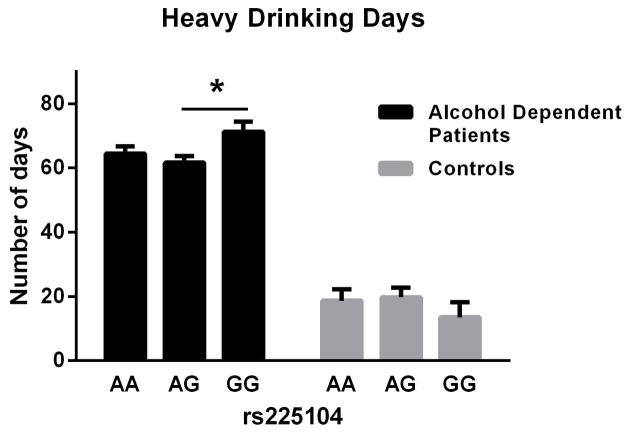
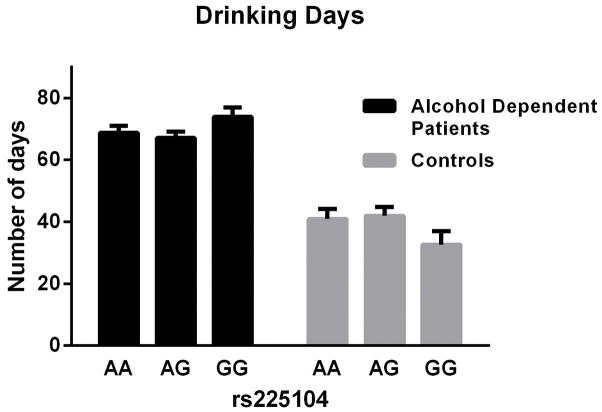
For the TLFB-related drinking measures, there was a significant main effect of rs225014 on heavy drinking days (i.e., ≥4 standard drinks per day for women and ≥5 drinks per day for men) in the AD patients [F(2, 688) = 3.82, p = 0.02; Fig. 1]. Post-hoc tests (Tukey tests) showed a significant difference between the AG and GG genotype in the AD patients only. Also in the AD patients, there was a main effect of the same SNP on number of drinking days [F(2,688)=4.59, p=0.01; Fig. 2]. No post-hoc tests were significant. There was no effect of this SNP in the control group. The effect size (Cohen’s d) for the difference between heavy drinking days was small (d = 0.32). There was no significant effect of either rs2235544 or rs12885300 in the AD or control groups.
Figure 1.
Effect of genotype (rs 225014) on heavy drinking days in alcohol dependent subjects, Mean (SEM).
Figure 2.
Effect of genotype (rs2235544) on average drinks per drinking day in control subjects, Mean (SEM).
TSH Levels
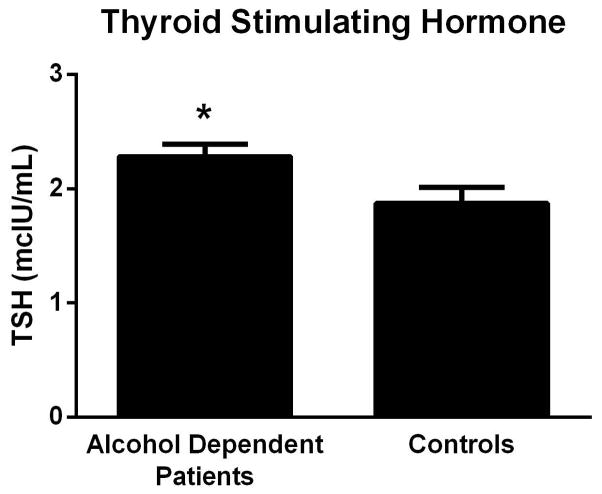
There was a significant main effect of diagnosis (AD vs. controls) on TSH levels (F[1, 759] = 13.67, p = 0.0002, Fig 3), but no main effect of genotype. There was also no significant genotype x diagnosis interaction for TSH levels. The effect size (Cohen’s d for the AD vs Control differences in TSH levels was small (d = 0.17).
Figure 3.
Thyroid Stimulating Hormone (TSH) levels in alcohol dependent patients and controls, Mean (SEM).
Days of abstinence did not affect TSH levels in either group. There was no interaction between gene group and days of abstinence for TSH levels. Omitting subjects with a diagnosis of MDD in analyses of drinking behavior and TSH did not change the results.
Discussion
We report here that a functionally significant D2 SNP (rs225014) is associated with drinking behavior in a sample of AD individuals, but not in controls. AD subjects with the variant genotype (GG), which is associated with reduced D2 enzymatic activity, resulting in less conversion of T4 to T3, reported significantly greater number of heavy drinking days. This finding extends previous studies on thyroid dysfunction in alcoholism by linking a functionally significant genetic polymorphism of the D2 enzyme with drinking behavior.
D2 catalyzes the intracellular conversion of T4 to T3 in several human tissues including brown fat, brain, pituitary, thyroid and skeletal muscle. T3 provides important regulatory signals for the control of motivated behaviors including appetite, satiety and reproductive function as well as the TRH/TSH feedback mechanism (Bianco et al., 2002; Fekete and Lechan, 2007; Fliers et al., 2006; Lechan and Fekete, 2005; Peeters et al., 2005a). In D2 knockout mice, TSH levels are two times higher compared to wild type (Bianco and Kim, 2006).
The D2 rs 225014 SNP is a common missense variant of the gene in which a threonine becomes an alanine at codon 92 (D2Thr92Ala; A92G) and is associated with decreased D2 enzyme velocity (Canani et al., 2005; Mentuccia et al., 2002). The rs 225014 variant also has been associated with delayed T3 secretion in a TRH stimulation test (Butler et al., 2010). In the setting of AD, where T3 feedback on hypothalamus and pituitary is deficient (Hermann et al., 2002), this genetic variant may act to amplify the dysregulated T3 feedback mechanisms that exist in AD. While in active drinking alcohol-dependent subjects, free T3 levels have been positively correlated with alcohol craving (Aoun et al., 2015; Leggio et al., 2008), it is known that thyroid abnormalities normalize for the most part with sustained abstinence and return with relapse. Presumably, exposure to alcohol entrains a cascade of HPT axis dysfunction but it is unclear how the latter modulates drinking behavior. The results of this study suggest that in the context of AD, genetic variation in D2 activity may impact drinking behavior, perhaps by worsening subclinical thyroid dysfunction.
Overall, TSH levels were significantly higher in AD patients compared to controls. Elevation of TSH may reflect a response, in early abstinence, when reduction in T3 and T4 is most pronounced (Hermann et al., 2002). In studies to date, an effect of genetic variants of deiodinase enzymes has not been observed on peripheral thyroid hormone levels, rather the effect on the HPT axis has been shown in provocative tests such as the TRH stimulation test (Butler et al., 2010; Peltsverger et al., 2012). In this study, while we hypothesized that early withdrawal, analogous to a TRH stimulation test, might render gene group differences in TSH levels apparent, we found no effect of genotype on levels of TSH for the AD group or for controls. The genetic variants studied here have been shown to affect outcome on this provocative test in control populations only. Therefore, future studies will need to investigate the effect of these genetic variants on the TRH stimulation test in AD subjects. Finally, it is worth noting that, the heterozygous gene group demonstrated the lowest drinking levels with no difference between homozygous gene groups. Small gene group sizes of the homozygous gene groups relative to the heterozygous group could be one reason for this result. In addition, a heterozygous effect has been noted in other studies of genetic polymorphisms in psychiatric populations(Gosso et al., 2008; Gratacos et al., 2007; Lee et al., 2002; Pooley et al., 2004; Retz et al., 2003).
Study strengths include the a priori approach of this analysis and that this is the first study investigating the effect of functionally significant SNP’s of deiodinase genes on alcohol drinking in an AD population. Limitations of the study include the lack of data from a TRH stimulation test to provide evidence of dynamic changes in thyroid function as hypothesized above. The non-dependent group had a mean alcohol consumption of 3.7 drinks per day, therefore, it is difficult to infer how consumption is affected by genotype in this group. Grouping the data into low/light drinkers (i.e., less than 2 drinks/day), yielded only 39 subjects which is too small to compare gene groups. Therefore, future studies are needed to investigate if the genetic variants here analyzed may also play a role in alcohol use in low/light drinkers. Also, this was an exploratory study, analyzing several parameters of drinking behavior, therefore, the results were not corrected for multiple comparisons; follow-up replication studies will be needed. Our approach is consistent with Bender and Lang (Bender and Lange, 2001) who suggested that exploratory analyses be done without multiplicity adjustment and that such results from these analyses be clearly labeled as exploratory and confirmed in follow up confirmatory studies.
In conclusion, the results of this a priori hypothesis-driven analysis link a functionally significant genetic variant of the deiodinase enzyme that controls T3 synthesis to drinking behavior. Further directions such as haplotype analyses and pathway analyses may elucidate other genetic influences over thyroid function that may account for the differences seen here in the D2 SNP, rs225014. These findings are very preliminary but nonetheless warrant further investigation as they may lead to a better understanding of the role that HTP axis plays in influencing motivated behaviors such as alcohol drinking.
Acknowledgments
Source of Support: This study was supported by NIH intramural funding ZIA-AA000218 (PI: Leggio) jointly supported by the Division of Intramural Clinical and Biological Research of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the Intramural Research Program of the National Institute on Drug Abuse (NIDA).
The authors gratefully acknowledge the NIAAA and Clinical Center clinical and research staff members involved in data collection support.
Footnotes
Contributors
MRL and LL thought of the rationale for this analysis; MRL managed the literature searches and analyses; JWB, AAD and ENO assisted with the literature searches and analyses; MRL and MLS conducted the statistical analyses; CAH and DG conducted the genetic analyses; MRL wrote the first draft of the manuscript; all authors contributed to and have approved the final manuscript.
Conflict of interest
All authors declare no conflict of interest.
References
- Aoun EG, Lee MR, Haass-Koffler CL, Swift RM, Addolorato G, Kenna GA, Leggio L. Relationship between the thyroid axis and alcohol craving. Alcohol and alcoholism. 2015;50:24–29. doi: 10.1093/alcalc/agu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner A, Eravci M, Pinna G, Hiedra L, Prengel H, Brodel O, Meinhold H. Thyroid hormone metabolism in the rat brain in an animal model of ‘behavioral dependence’ on ethanol. Neuroscience letters. 1997;227:25–28. doi: 10.1016/s0304-3940(97)00290-5. [DOI] [PubMed] [Google Scholar]
- Baumgartner A, Heyne A, Campos-Barros A, Kohler R, Muller F, Meinhold H, Rommelspacher H, Wolffgramm J. Hypothalamic-pituitary-thyroid axis in chronic alcoholism. II. Deiodinase activities and thyroid hormone concentrations in brain and peripheral tissues of rats chronically exposed to ethanol. Alcoholism, clinical and experimental research. 1994;18:295–304. doi: 10.1111/j.1530-0277.1994.tb00017.x. [DOI] [PubMed] [Google Scholar]
- Bender R, Lange S. Adjusting for multiple testing--when and how? Journal of clinical epidemiology. 2001;54:343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. The Journal of clinical investigation. 2006;116:2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocrine reviews. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- Butler PW, Smith SM, Linderman JD, Brychta RJ, Alberobello AT, Dubaz OM, Luzon JA, Skarulis MC, Cochran CS, Wesley RA, Pucino F, Celi FS. The Thr92Ala 5′ type 2 deiodinase gene polymorphism is associated with a delayed triiodothyronine secretion in response to the thyrotropin-releasing hormone-stimulation test: a pharmacogenomic study. Thyroid: official journal of the American Thyroid Association. 2010;20:1407–1412. doi: 10.1089/thy.2010.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canani LH, Capp C, Dora JM, Meyer EL, Wagner MS, Harney JW, Larsen PR, Gross JL, Bianco AC, Maia AL. The type 2 deiodinase A/G (Thr92Ala) polymorphism is associated with decreased enzyme velocity and increased insulin resistance in patients with type 2 diabetes mellitus. The Journal of clinical endocrinology and metabolism. 2005;90:3472–3478. doi: 10.1210/jc.2004-1977. [DOI] [PubMed] [Google Scholar]
- de Jong FJ, Peeters RP, den Heijer T, van der Deure WM, Hofman A, Uitterlinden AG, Visser TJ, Breteler MM. The association of polymorphisms in the type 1 and 2 deiodinase genes with circulating thyroid hormone parameters and atrophy of the medial temporal lobe. The Journal of clinical endocrinology and metabolism. 2007;92:636–640. doi: 10.1210/jc.2006-1331. [DOI] [PubMed] [Google Scholar]
- Fekete C, Lechan RM. Negative feedback regulation of hypophysiotropic thyrotropin-releasing hormone (TRH) synthesizing neurons: role of neuronal afferents and type 2 deiodinase. Frontiers in neuroendocrinology. 2007;28:97–114. doi: 10.1016/j.yfrne.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliers E, Alkemade A, Wiersinga WM, Swaab DF. Hypothalamic thyroid hormone feedback in health and disease. Progress in brain research. 2006;153:189–207. doi: 10.1016/S0079-6123(06)53011-0. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, McDavid J, Mason GA, Quade D, Loosen PT. Evidence for normal feedback inhibition of triiodothyronine on the thyrotropin (TSH) response to thyrotropin-releasing hormone (TRH) in abstinent male alcoholics. Alcoholism, clinical and experimental research. 1992;16:881–883. doi: 10.1111/j.1530-0277.1992.tb01886.x. [DOI] [PubMed] [Google Scholar]
- Gosso MF, de Geus EJ, Polderman TJ, Boomsma DI, Heutink P, Posthuma D. Catechol O-methyl transferase and dopamine D2 receptor gene polymorphisms: evidence of positive heterosis and gene-gene interaction on working memory functioning. European journal of human genetics: EJHG. 2008;16:1075–1082. doi: 10.1038/ejhg.2008.57. [DOI] [PubMed] [Google Scholar]
- Gratacos M, Gonzalez JR, Mercader JM, de Cid R, Urretavizcaya M, Estivill X. Brain-derived neurotrophic factor Val66Met and psychiatric disorders: meta-analysis of case-control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biological psychiatry. 2007;61:911–922. doi: 10.1016/j.biopsych.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Hegedus L, Rasmussen N, Ravn V, Kastrup J, Krogsgaard K, Aldershvile J. Independent effects of liver disease and chronic alcoholism on thyroid function and size: the possibility of a toxic effect of alcohol on the thyroid gland. Metabolism: clinical and experimental. 1988;37:229–233. doi: 10.1016/0026-0495(88)90100-x. [DOI] [PubMed] [Google Scholar]
- Hermann D, Heinz A, Mann K. Dysregulation of the hypothalamic-pituitary-thyroid axis in alcoholism. Addiction. 2002;97:1369–1381. doi: 10.1046/j.1360-0443.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- Lechan RM, Fekete C. Role of thyroid hormone deiodination in the hypothalamus. Thyroid: official journal of the American Thyroid Association. 2005;15:883–897. doi: 10.1089/thy.2005.15.883. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim SH, Lee HJ, Kim L, Lee SK, Jang DW, Lee MS, Son BG, Suh KY, Kim S. Gender-specific molecular heterosis of dopamine D2 receptor gene (DRD2) for smoking in schizophrenia. American journal of medical genetics. 2002;114:593–597. doi: 10.1002/ajmg.10641. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, Malandrino N, Mirijello A, D’Angelo C, Vonghia L, Miceli A, Capristo E, Kenna GA, Gasbarrini G, Swift RM, Addolorato G. Relationship between the hypothalamic-pituitary-thyroid axis and alcohol craving in alcohol-dependent patients: a longitudinal study. Alcoholism, clinical and experimental research. 2008;32:2047–2053. doi: 10.1111/j.1530-0277.2008.00792.x. [DOI] [PubMed] [Google Scholar]
- Mentuccia D, Proietti-Pannunzi L, Tanner K, Bacci V, Pollin TI, Poehlman ET, Shuldiner AR, Celi FS. Association between a novel variant of the human type 2 deiodinase gene Thr92Ala and insulin resistance: evidence of interaction with the Trp64Arg variant of the beta-3-adrenergic receptor. Diabetes. 2002;51:880–883. doi: 10.2337/diabetes.51.3.880. [DOI] [PubMed] [Google Scholar]
- Panicker V, Cluett C, Shields B, Murray A, Parnell KS, Perry JR, Weedon MN, Singleton A, Hernandez D, Evans J, Durant C, Ferrucci L, Melzer D, Saravanan P, Visser TJ, Ceresini G, Hattersley AT, Vaidya B, Dayan CM, Frayling TM. A common variation in deiodinase 1 gene DIO1 is associated with the relative levels of free thyroxine and triiodothyronine. The Journal of clinical endocrinology and metabolism. 2008;93:3075–3081. doi: 10.1210/jc.2008-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters RP, van den Beld AW, Attalki H, Toor H, de Rijke YB, Kuiper GG, Lamberts SW, Janssen JA, Uitterlinden AG, Visser TJ. A new polymorphism in the type II deiodinase gene is associated with circulating thyroid hormone parameters. American journal of physiology Endocrinology and metabolism. 2005a;289:E75–81. doi: 10.1152/ajpendo.00571.2004. [DOI] [PubMed] [Google Scholar]
- Peeters RP, van den Beld AW, van Toor H, Uitterlinden AG, Janssen JA, Lamberts SW, Visser TJ. A polymorphism in type I deiodinase is associated with circulating free insulin-like growth factor I levels and body composition in humans. The Journal of clinical endocrinology and metabolism. 2005b;90:256–263. doi: 10.1210/jc.2004-1301. [DOI] [PubMed] [Google Scholar]
- Peeters RP, van Toor H, Klootwijk W, de Rijke YB, Kuiper GG, Uitterlinden AG, Visser TJ. Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects. The Journal of clinical endocrinology and metabolism. 2003;88:2880–2888. doi: 10.1210/jc.2002-021592. [DOI] [PubMed] [Google Scholar]
- Peltsverger MY, Butler PW, Alberobello AT, Smith S, Guevara Y, Dubaz OM, Luzon JA, Linderman J, Celi FS. The -258A/G (SNP rs12885300) polymorphism of the human type 2 deiodinase gene is associated with a shift in the pattern of secretion of thyroid hormones following a TRH-induced acute rise in TSH. European journal of endocrinology/European Federation of Endocrine Societies. 2012;166:839–845. doi: 10.1530/EJE-11-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley EC, Fairburn CG, Cooper Z, Sodhi MS, Cowen PJ, Harrison PJ. A 5-HT2C receptor promoter polymorphism (HTR2C - 759C/T) is associated with obesity in women, and with resistance to weight loss in heterozygotes. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2004;126B:124–127. doi: 10.1002/ajmg.b.20143. [DOI] [PubMed] [Google Scholar]
- Retz W, Rosler M, Supprian T, Retz-Junginger P, Thome J. Dopamine D3 receptor gene polymorphism and violent behavior: relation to impulsiveness and ADHD-related psychopathology. Journal of neural transmission. 2003;110:561–572. doi: 10.1007/s00702-002-0805-5. [DOI] [PubMed] [Google Scholar]
- Tichomirowa MA, Keck ME, Schneider HJ, Paez-Pereda M, Renner U, Holsboer F, Stalla GK. Endocrine disturbances in depression. Journal of endocrinological investigation. 2005;28:89–99. doi: 10.1007/BF03345535. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Fletcher DL, Simonyl A, Rudeen PK. Chronic ethanol treatment reduces the responsiveness of the hypothalamic-pituitary-thyroid axis to central stimulation. Alcoholism, clinical and experimental research. 1996;20:954–960. doi: 10.1111/j.1530-0277.1996.tb05277.x. [DOI] [PubMed] [Google Scholar]