Abstract
Detecting at-risk individuals within a healthy population is critical for preventing or delaying Alzheimer’s disease. The systems biology integration of brain and body metabolism enables peripheral metabolic biomarkers to serve as reporters of brain bioenergetic status. Using clinical metabolic data derived from healthy postmenopausal women in the ELITE trial, we conducted principal components and k-means clustering analyses of nine biomarkers to define metabolic phenotypes. Metabolic clusters were correlated with cognitive performance and analyzed for change over five years. Metabolic biomarkers at baseline generated three clusters, representing women with healthy, high blood pressure, and poor metabolic phenotypes. Compared to healthy women, poor metabolic women had lower verbal memory performance at baseline. Hormone therapy provided metabolic benefit to women in high blood pressure and poor metabolic phenotypes. This panel of well-established clinical peripheral biomarkers represents an initial step towards developing an affordable, rapidly deployable, and clinically relevant strategy to detect an atrisk phenotype of late-onset Alzheimer’s disease.
Keywords: Cognitive aging, Metabolism, Biomarker, Alzheimer’s disease, Menopause, Hormone therapy
1. INTRODUCTION
Effective prevention and delay of Alzheimer’s disease (AD) will require intervention during the preclinical phase (Carrillo et al., 2013, Jack et al., 2011, Sperling et al., 2011, Yao et al., 2011). Achievement of this goal entails accurate identification of at-risk individuals prior to clinically symptomatic disease. Successful screening of at-risk populations requires an accurate, rapidly deployable, clinically accessible, and economically feasible biomarker strategy. To achieve these criteria, biomarkers based on peripheral indicators that accurately predict early risk status of the brain would be advantageous. Interrogating the metabolic system through peripheral indicators provides one such strategy, as substantial evidence supports the hypothesis that midlife metabolism affects cognitive health in older age (Cheng et al., 2012, Gottesman et al., 2014, Kenna et al., 2013, Kivipelto et al., 2011, Norton et al., 2014, Rawlings et al., 2014, Roberts et al., 2014, Wharton et al., 2014, Whitmer et al., 2005).
One strategy to enrich an at-risk population for biomarker development is to focus on individuals with a greater lifetime risk of Alzheimer’s disease. Women have a two-fold greater lifetime risk of developing AD and thus constitute a target population for which biomarkers for early detection of risk could have substantial public health impact (Alzheimer’s Association, 2014). While the biological basis for gender differences in AD remains to be established, basic and clinical science indicate that the menopausal transition and decline in estrogen can adversely affect brain and whole-body metabolism (Brinton, 2015, Henderson and Brinton, 2010, Rettberg et al., 2014; Yin et al., 2015).
Based on basic and clinical science, we hypothesized that metabolically-based biomarkers would identify individuals at the tipping point for developing an at-risk for Alzheimer’s phenotype in a population of healthy postmenopausal women. To test this hypothesis, we conducted a clustering analysis using baseline data from the Early vs. Late Intervention Trial with Estradiol (ELITE) (Hodis et al., 2015) to identify metabolic phenotypes. We subsequently investigated the association of these phenotypes with cognitive performance, as well as the longitudinal change in both metabolic phenotypes and cognitive performance over five years. We further hypothesized that administration of hormone therapy (HT) would differentially impact both overall metabolism and cognitive performance within women of different metabolic phenotypes.
2. METHODS
2.1. The ELITE clinical trial
ELITE was a double-blinded, placebo-controlled clinical trial randomizing 643 postmenopausal women. It was designed to test the timing hypothesis of postmenopausal HT, such that HT benefits and risks depend on the temporal initiation of HT relative to time-since-menopause, which is in turn related to underlying tissue health (Henderson et al., 2013, Karim et al., 2015). Women were recruited into two cohorts: early menopause (n=271), defined as within 6 years of menopause, and late menopause (n=372), defined as 10 or more years postmenopause.
Eligible women were postmenopausal, defined as absence of menses for ≥6 months or surgical menopause and serum estradiol below 25 pg/mL. Of the women included, 14 were between 6 months and 1 year postmenopausal, and the remainder were all >1 year postmenopausal. Women were excluded if they had clinical signs, symptoms, or personal history of cardiovascular disease; diabetes mellitus (fasting serum glucose ≥140 mg/dL); uncontrolled hypertension (diastolic blood pressure ≥110 mmHg); untreated thyroid disease; plasma triglyceride levels >500 mg/dL; serum creatinine >2.0 mg/dL; cirrhosis or liver disease; a life threatening disease with prognosis less than 5 years; or inability to determine time-since-menopause. Women with a history of deep vein thrombosis, pulmonary embolism, or breast cancer were excluded. Within each postmenopause cohort, women were randomized to receive either HT (17β-estradiol, 1 mg daily) or placebo. Women who had not undergone a hysterectomy also used vaginal 4% progesterone (or placebo) gel for the last 10 days of each month.
The primary trial outcome was rate of change of distal common carotid artery far wall intima-media thickness (CIMT) (Hodis et al., 2015). A secondary outcome was change in cognitive function (Henderson et al., 2013). A comprehensive battery of neuropsychological tests was administered prior to randomization, at about 2.5 years, and at each participant’s final study visit, approximately 5 years after randomization. The battery included 14 neuropsychological tests that emphasized standardized tests sensitive to age-associated change in middle-aged and older adults (Henderson et al., 2013). ELITE was approved by the Institutional Review Board of the University of Southern California. All participants provided written informed consent.
For the longitudinal analysis, the full sample of 643 women was restricted to those completing cognitive testing at baseline and again at either 2.5 years, 5 years, or both (n=502). Of the 502 women, 216 were in the early menopause and 286 in the late menopause groups.
2.2. Clinical and laboratory measurements
At each 6-month clinic visit, 8-hour fasting blood was drawn and blood pressure was measured. Current medication use was recorded. Samples were prepared and stored at −70°C.
Fasting glucose, β-hydroxybutyrate, and insulin were measured in stored plasma using kits (glucose and β-hydroxybutyrate: Cayman Chemical, Ann Arbor, MI; insulin: Alpco Diagnostics, Salem, NH), according to each manufacturer’s protocol. Fasting total cholesterol, triglycerides, and HDL-cholesterol levels were measured in fresh plasma using an enzymatic method of the Standardization Program of the National Centers for Disease Control and Prevention as described previously (Hodis et al., 2015). LDL-cholesterol was computed using the Friedewald equation (Friedewald et al., 1972). Fasting HbA1c was measured in fresh whole blood using the Bio-Rad Hemoglobin A1c HPLC test.
2.3. Statistical analysis
The analysis included nine metabolic variables: glucose, the HOMA score (homeostatic model assessment; a measure of insulin resistance: [glucose mmol/L*insulin]/22.5), ketones (β-hydroxybutyrate), HDL-cholesterol, LDL-cholesterol, triglycerides, HbA1c, and systolic and diastolic blood pressure (SBP, DBP). These biomarkers were selected on the basis of their contribution to metabolic, cardiovascular, and neurological health. Insulin and total cholesterol were excluded as these were respectively highly correlated with the HOMA score (R2=0.98, p<0.0001) and LDL-cholesterol (R2=0.89, p<0.0001). All variables were standardized using baseline averages and standard deviations from the entire ELITE sample. A principal components analysis on the nine standardized variables identified the number of potential clusters that best explained the variance in the dataset. Specifying three clusters, a nonhierarchical K-means clustering algorithm was performed; the resulting three clusters were descriptively identified based on their means profile. The three clusters were compared on demographic factors and metabolic variables using analysis of variance (ANOVA) and covariance (ANCOVA) for continuous variables and chi-square tests for categorical variables.
Three cognitive composite scores (global cognition, executive functions, and verbal memory) were generated from the 14-item test battery. Composite scores were a linear sum of the standardized test scores within each domain, with each standard test score inversely weighted by its correlation with other contributing cognitive tests (Henderson et al., 2013). The verbal memory composite score was defined a priori by Word List Free Recall (a short version of the California Verbal Learning Test II) immediate and delayed recall, and Paragraph Recall (East Boston Memory Test) immediate and delayed recall (Henderson et al., 2013). Tests included in the executive functions composite score were Symbol Digit Modalities Test, Trail Making Test part B, Shipley Abstraction Scale, and category fluency (Animal Naming). These tests were determined by a principal components analysis of baseline scores (Henderson et al., 2013). The composite score for global cognition was similarly calculated as a weighted average, including all tests in the battery. ANCOVA was used to test for overall cross-sectional differences among metabolic clusters on each cognitive composite and test; covariates included postmenopause cohort (early/late), random intervention assignment, and education.
Measurements of longitudinal change in metabolic biomarkers used measures at three time points (baseline, 2.5 years, and end of study at approximately 5 years) to match with cognitive assessment times. Modeling each metabolic biomarker or cognitive composite separately as the longitudinal dependent variable, data were analyzed using mixed effects linear models, testing the effects of baseline metabolic cluster as well as menopause cohort on metabolic or cognitive change. The regression coefficient for time (years) since randomization estimated the slope of metabolic/cognitive change (in units/year). In the mixed model, random effects were specified to allow for subject-specific deviations around the average baseline (regression intercept) and slope of change. Interaction terms of metabolic cluster and treatment condition with time tested whether the slopes significantly differed by these variables. All metabolic analyses included menopause cohort and randomized treatment allocation as independent variables; all cognitive analyses additionally included years of education. All statistical tests used an overall 2-sided alpha of 0.05; post-hoc pairwise comparisons corrected for multiple comparisons using the Tukey-Kramer method. All associations were evaluated for differences by menopause strata; as no significant differences were found, the analyses for early and late menopause women were collapsed within each phenotype. 3.
RESULTS
3.1. Baseline metabolic phenotypes and demographics
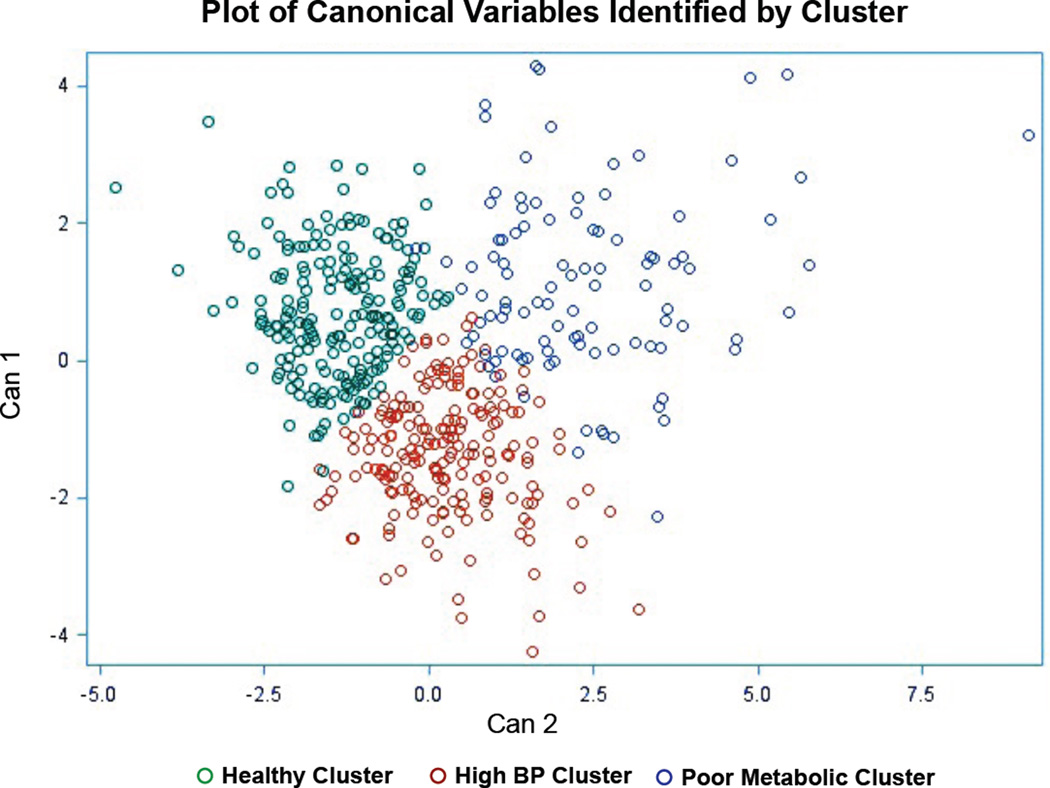
The three clusters (Figure 1) were descriptively identified based on their mean profile (Table 1): Healthy Metabolic (n=209, 41.6%), High Blood Pressure (n=191, 38.1%), and Poor Metabolic (n=102, 20.3%). Reflecting the clustering algorithm, the Healthy and Poor Metabolic phenotypes significantly differed on all metabolic biomarkers (Table 1). Healthy and High BP phenotypes significantly differed on all biomarkers except glucose and HbA1c; High BP and Poor Metabolic significantly differed on all biomarkers except ketones and LDL-cholesterol (Table 1). The majority of the metabolic biomarker means were within a normal range, consistent with recruitment of a healthy population of postmenopausal women. However, the metabolic biomarker means in the Poor Metabolic group were at the margins of clinically healthy values (Table 1).
Figure 1. Cluster development.
Women in the Healthy Metabolic cluster are shown in green. Women in the High Blood Pressure cluster are shown in red. Women in the Poor Metabolic cluster are shown in blue. Can1, the first canonical variable: the linear combination of the clustering variables that best explains cluster group differences (i.e., is most correlated with cluster group). Can2, the second canonical variable: the linear combination of clustering variables that is most correlated with the cluster groups but is uncorrelated with Can1.
Table 1.
Sample Characteristics by Cluster
| Comparisons Between Clusters | ||||||||
|---|---|---|---|---|---|---|---|---|
| HEALTHY METABOLIC |
HIGH BLOOD PRESSURE |
POOR METABOLIC |
Main Effect p-value |
|||||
| (n) | 209 (41.6%) | 191 (38.1%) | 102 (20.3%) | |||||
| Mean Age, years (SD) | 60.0 (7.3) | 60.9 (6.8) | 61.1 (6.5) | 0.39 | ||||
| Mean Time Since Menopause, years (SD) | 9.9 (7.4) | 10.4 (7.7) | 11.5 (8.4) | 0.23 | ||||
| Mean Education, years (SD) | 16.4 (2.2) | 16.2 (2.1) | 15.7 (2.3) * | 0.06 | ||||
| Menopause Cohort | 0.48 | |||||||
| Early Menopause, n (%) | 95 (45.5%) | 82 (42.9%) | 39 (38.2%) | |||||
| Late Menopause, n (%) | 114 (54.5%) | 109 (57.1%) | 63 (61.8%) | |||||
| Race or ethnicity |
n, % of cluster |
% of racial group |
n, % of cluster |
% of racial group |
n, % of cluster |
% of racial group |
0.0014 | |
| White, non-Hispanic | 157 (75.1%) | 44.2% | 140 (73.3%) | 39.5% | 58 (56.9%) | 16.3% | ||
| Black | 14 (6.7%) | 34.1% | 18 (9.4%) | 43.9% | 9 (8.8%) | 22.0% | ||
| Hispanic | 17 (8.1%) | 27.0% | 21 (11.0%) | 33.3% | 25 (24.5%) | 39.7% | ||
| Asian | 21 (10.1 %) | 48.8% | 12 (6.3%) | 27.9% | 10 (9.8%) | 23.3% | ||
| Biomarkers | ||||||||
| Glucose (mg/dL) | 80.60 (7.58) | 80.28 (7.46) | 91.55 (9.77) *‡ | <0.0001 | ||||
| Insulin Resistance (HOMA Score) | 0.98 (0.48) | 1.16 (0.46) *‡ | 2.62 (1.12) *‡ | <0.0001 | ||||
| Ketones (mM) | 0.12 (0.06) | 0.10 (0.03) * | 0.10 (0.04) * | 0.0002 | ||||
| HDL Cholesterol (mg/dL) | 74.96 (17.88) | 65.40 (15.67) * | 52.08 (10.77) *‡ | <0.0001 | ||||
| LDL Cholesterol (mg/dL) | 129.95 (29.64) | 137.11 (29.11) * | 144.96 (33.43) * | 0.0009 | ||||
| Triglycerides (mg/dL) | 80.41 (26.99) | 97.31 (33.36) * | 166.59 (65.59) *‡ | <0.0001 | ||||
| HbA1c (%) | 5.60 (0.38) | 5.52 (0.40) | 5.80 (0.45) *‡ | <0.0001 | ||||
| Systolic Blood Pressure (mmHg) | 105.83 (8.96) | 125.27 (10.26) * | 121.18 (10.74) *‡ | <0.0001 | ||||
| Diastolic Blood Pressure (mmHg) | 67.95 (5.52) | 80.86 (5.82) * | 76.31 (7.73) *‡ | <0.0001 | ||||
Demographics and biomarker values (average, standard deviation) for the three phenotypes.
Average value is significantly different from the Healthy phenotype (p < 0.05).
Average value is significantly different from the High BP phenotype (p < 0.05). Age, years since menopause, and education were compared using ANOVA. Differences between clusters on menopause cohort and race were assessed using Chi-square analysis. Biomarker results were compared using ANOVA, and adjusted for random intervention assignment and menopause cohort (early vs. late). The Tukey-Kramer method was used to adjust for multiple comparisons.
Cluster groups did not significantly differ on age, years since menopause, or postmenopause cohort (early/late) (Table 1). Women in the Healthy phenotype had on average 7 more months of education relative to the Poor Metabolic phenotype (p=0.045). The race/ethnic distribution significantly differed across clusters (p=0.0014); Caucasian (44%) and Asian (49%) women had a greater likelihood of membership in the Healthy Metabolic phenotype. African American women had greater likelihood of membership in the High BP phenotype (44%), whereas Hispanic women were most represented in the Poor Metabolic phenotype (40%).
3.2. Baseline cognitive performance
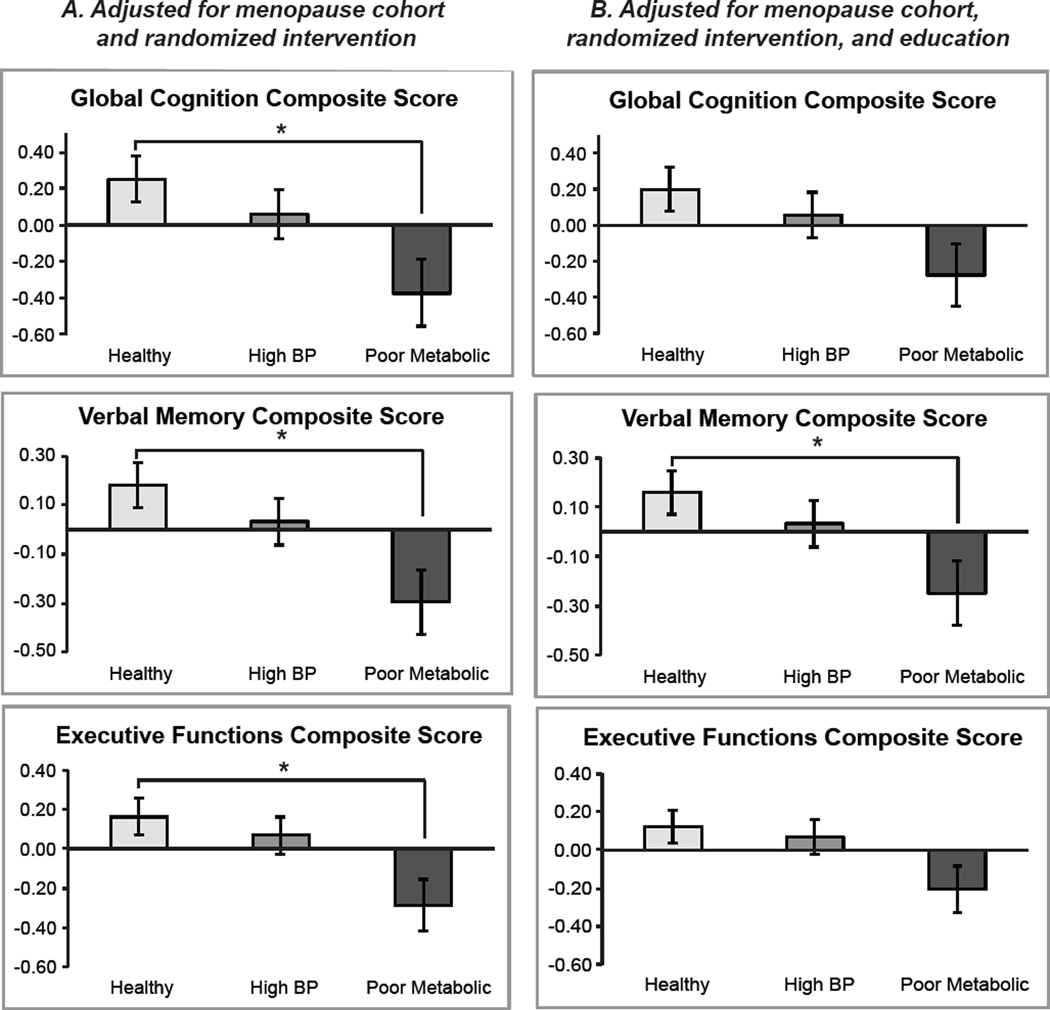
Cognitive test comparisons were initially adjusted for postmenopause cohort (early vs. late menopause) and random intervention assignment. Following these adjustments, the phenotypes significantly differed on global cognition (p=0.020), verbal memory (p=0.013), and executive functions (p=0.019). Women in the Healthy phenotype performed significantly better than women in the Poor Metabolic phenotype on global cognition (p=0.015), verbal memory (p=0.009), and executive functions (p=0.015) (Figure 2A). Women in the Healthy versus High BP phenotypes, and women in the High BP versus Poor Metabolic phenotypes, did not significantly differ on cognitive performance. With additional adjustment for education, cluster groups did not significantly differ on global cognition (p=0.085) and executive functions (p=0.089); group differences on verbal memory remained statistically significant (p=0.037), with verbal memory significantly different between women in the Healthy and Poor Metabolic phenotypes (p=0.028) (Figure 2B).
Figure 2. Comparison of cognitive composite scores between phenotypes at baseline.
Significant differences between phenotypes on the three cognitive composite scores: global cognition, verbal memory, and executive functions. A. Results after adjusting for menopause cohort and random intervention assignment. B. Results after adding an adjustment for education. *p < 0.05. Error bars represent SEM. The Tukey-Kramer method was used to adjust for multiple comparisons.
3.3. Effect of aging on metabolic biomarkers and cognition
We initially evaluated the impact of aging over the 5-year trial among the women randomized to placebo using mixed effects models. All women showed a significant amount of metabolic change regardless of metabolic phenotype (Table 2A, showing mean (SEM) estimates of annual change). Three metabolic biomarkers, HbA1c, HDL-cholesterol, and LDL-cholesterol, showed changes that can be considered predominantly age-related because the magnitude of change was similar regardless of metabolic phenotype. HbA1c values increased significantly in all women over 5 years, which is a known effect of age even in non-diabetic populations (Pani et al., 2008). Additionally, nearly all women had a significant longitudinal increase in HDL-cholesterol and decrease in LDL-cholesterol.
Table 2.
Longitudinal metabolic and cognitive change in women randomized to placebo
| Within-Cluster Changes and Pairwise Comparisons | |||||
|---|---|---|---|---|---|
| HEALTHY METABOLIC |
HIGH BLOOD PRESSURE |
POOR METABOLIC |
P-values between clusters |
||
| A. Metabolic Biomarkers | |||||
| Glucose (mg/dL) | 0.29 (0.30) | 0.42 (0.32) | −0.21 (0.43) | 0.48 | |
| HOMA Score | 0.018 (0.01) | 0.025 (0.01) *a | −0.025 (0.01)ab | 0.02 | |
| Ketones (mM) | −0.04 (0.01) * | −0.01 (0.01) | −0.02 (0.01) | 0.01 | |
| HDL Cholesterol (mg/dL) | 1.02 (0.25) * | 1.24 (0.27) * | 0.59 (0.37) | 0.37 | |
| LDL Cholesterol (mg/dL) | −2.75 (0.68) * | −2.17 (0.72) * | −4.82 (0.98) *b | 0.09 | |
| Triglycerides (mg/dL) | 0.01 (0.01) | −0.0001 (0.01) | −0.03 (0.01) *ab | 0.003 | |
| HbA1c (%) | 0.04 (0.01) * | 0.05 (0.01) * | 0.06 (0.01) * | 0.38 | |
| Systolic Blood Pressure (mmHg) | 0.79 (0.30) * | −1.68 (0.32) *a | −0.64 (0.43)a | <0.0001 | |
| Diastolic Blood Pressure (mmHg) | 0.42 (0.19) * | −1.31 (0.21) *a | −0.43 (0.28)ab | <0.0001 | |
| B. Cognitive Performance | |||||
| Global Cognition | 0.141 (0.03)* | 0.104 (0.03)* | 0.163 (0.04)* | 0.46 | |
| Verbal Memory | 0.134 (0.03)* | 0.133 (0.03)* | 0.138 (0.04)* | 0.99 | |
| Executive Functions | 0.002 (0.02) | −0.012 (0.02) | 0.006 (0.02) | 0.78 | |
A. Average (SEM) longitudinal change per year for each metabolic biomarker within each phenotype. Changes in HOMA, ketones, and triglycerides are expressed as log values due to a skewed distribution of values within the population. B. Average (SEM) longitudinal change per year for each cognitive composite score within each phenotype.
Significant longitudinal change from baseline (p < 0.05).
Longitudinal change is significantly different from the Healthy phenotype (p < 0.05).
Longitudinal change is significantly different from the High BP phenotype (p < 0.05). Data were analyzed using mixed effects linear models. The regression coefficient for time (years) since randomization estimated the slope of metabolic/cognitive change (in units/year). The Tukey-Kramer method was used to adjust for multiple comparisons.
Aside from the age-related changes that were apparent in all three clusters, the clusters significantly differed on changes in the HOMA score (p=0.02), ketones (p=0.01), triglycerides (p=0.003), SBP (p<0.0001), and DBP (p<0.0001). Women in the Healthy phenotype showed a slight but significant increase in SBP (p=0.009) and DBP (p=0.031). Women in the High BP phenotype had an increase in the HOMA score (p=0.025), but a significant decrease in both SBP (p<0.0001) and DBP (p<0.0001). Women in the Poor Metabolic phenotype had a significant decrease in their triglyceride levels (p=0.001).
With respect to the cognitive composite scores, women in the Healthy, High BP, and Poor Metabolic phenotypes had significant increases in global cognition and verbal memory (all p<0.05), with no significant change in executive functions (Table 2B). The increases in global cognition and verbal memory were likely a learning effect as they were seen in all groups. The magnitude of cognitive change did not significantly differ between metabolic phenotypes.
3.4. Longitudinal metabolic and cognitive changes among women randomized to HT
In mixed effects models, women in all three metabolic phenotypes randomized to HT showed a significant increase in HbA1c and HDL-cholesterol, and a significant decrease in LDL-cholesterol (Table 3A). Further, all phenotypes showed a significant decrease in ketones. The clusters significantly differed on changes in the HOMA score (p=0.02), triglycerides (p<0.0001), SBP (p<0.0001), and DBP (p<0.0001). Women in the Healthy phenotype showed a significant increase in triglycerides (p<0.05) (Authors, 1995). Women in the High BP phenotype had a significant decrease in both SBP (P<0.0001) and DBP (p<0.0001), similar to those randomized to placebo. Women in the Poor Metabolic phenotype had a significant longitudinal improvement in the HOMA score (p=0.004), triglycerides (p=0.005), and SBP (p=0.05).
Table 3.
Longitudinal metabolic and cognitive change in women randomized to hormone therapy
| Within-Cluster Changes and Pairwise Comparisons | |||||
|---|---|---|---|---|---|
| HEALTHY METABOLIC |
HIGH BLOOD PRESSURE |
POOR METABOLIC |
P-values between clusters |
||
| A. Metabolic Biomarkers | |||||
| Glucose (mg/dL) | 0.16 (0.21) | 0.18 (0.21) | 0.08 (0.30) | 0.96 | |
| HOMA Score | 0.01 (0.01) | −0.01 (0.01) | −0.05 (0.02) *a | 0.02 | |
| Ketones (mM) | −0.03 (0.01) * | −0.03 (0.01) * | −0.03 (0.01) * | 1.00 | |
| HDL Cholesterol (mg/dL) | 1.28 (0.25) * | 1.48 (0.26) * | 1.70 (0.37) * | 0.63 | |
| LDL Cholesterol (mg/dL) | −3.48 (0.67) * | −5.16 (0.70) * | −6.12 (0.99) *a | 0.06 | |
| Triglycerides (mg/dL) | 0.03 (0.01) * | 0.004 (0.01) a | −0.03 (0.01) *ab | <0.0001 | |
| HbA1c (%) | 0.03 (0.01) * | 0.03 (0.01) * | 0.03 (0.01) * | 0.90 | |
| Systolic Blood Pressure (mmHg) | 0.32 (0.28) | −1.72 (0.29) *a | −0.81 (0.41) *a | <0.0001 | |
| Diastolic Blood Pressure (mmHg) | 0.35 (0.20) | −1.56 (0.21) *a | −0.41 (0.29) ab | <0.0001 | |
| B. Cognitive Performance | |||||
| Global Cognition | 0.114 (0.03)* | 0.118 (0.03)* | 0.117 (0.04)* | 0.99 | |
| Verbal Memory | 0.080 (0.03)* | 0.078 (0.03)* | 0.071 (0.04) | 0.98 | |
| Executive Functions | 0.003 (0.02) | −0.013 (0.02) | 0.019 (0.03) | 0.58 | |
A. Average (SEM) longitudinal change per year for each metabolic biomarker within each phenotype. Changes in HOMA, ketones, and triglycerides are expressed as log values due to a skewed distribution of values within the population. B. Average (SEM) longitudinal change per year for each cognitive composite score within each phenotype.
Significant longitudinal change from baseline (p < 0.05).
Longitudinal change is significantly different from the Healthy phenotype (p < 0.05).
Longitudinal change is significantly different from the High BP phenotype (p < 0.05). Data were analyzed using mixed effects linear models. The regression coefficient for time (years) since randomization estimated the slope of metabolic/cognitive change (in units/year). The Tukey-Kramer method was used to adjust for multiple comparisons.
A general improvement in cognitive performance was also observed in women randomized to HT (Table 3B). Women in all three metabolic phenotypes showed significant increases in global cognition (all p<0.05), and women in the Healthy and High BP phenotypes had a significant increase in verbal memory (both p<0.05). Again, this can likely be attributed to a learning effect. There was no significant change in executive functions, and the magnitude of cognitive change did not significantly differ between metabolic phenotypes.
3.5. Treatment comparisons on longitudinal metabolic and cognitive changes by cluster groups
On mixed effects models, there were no significant cluster by treatment interactions on the longitudinal trajectory of any metabolic biomarker (Table 4B). A pairwise comparison of placebo and HT within the Healthy phenotype indicated that women on HT had a significantly greater increase in triglycerides than women on placebo (p=0.018) (Table 4A). Within the High BP phenotype, women on HT had a greater decline in the HOMA score (p=0.017) and LDL-cholesterol (p=0.004), indicating that High BP women randomized to HT experienced a metabolic benefit (Table 4A). Within the Poor Metabolic phenotype, women on HT had a greater increase in HDL-cholesterol (p=0.031) and a smaller increase in HbA1c (p=0.048), indicating that HT was also of metabolic benefit to these women (Table 4A).
Table 4.
Longitudinal cluster by treatment interaction effects on longitudinal change in metabolic biomarkers and cognitive composite scores
| A. Pairwise Comparisons | ||||||||
|---|---|---|---|---|---|---|---|---|
| HEALTHY METABOLIC: HT vs. Placebo |
HIGH BP: HT vs. Placebo |
POOR METABOLIC: HT vs. Placebo |
B. Cluster- Treatment Interaction p- value |
|||||
|
HT Effect (SEM) |
p-value |
HT Effect (SEM) |
p-value |
HT Effect (SEM) |
p-value | |||
| Metabolic Biomarkers | ||||||||
| Glucose (mg/dL) | −0.126 (0.36) | 0.73 | −0.239 (0.38) | 0.53 | 0.280 (0.53) | 0.60 | 0.73 | |
| HOMA Score | −0.010 (0.01) | 0.47 | −0.037 (0.02) | 0.017 | −0.021 (0.02) | 0.32 | 0.46 | |
| Ketones (mM) | 0.016 (0.01) | 0.21 | −0.022 (0.01) | 0.10 | −0.010 (0.02) | 0.58 | 0.11 | |
| HDL Cholesterol (mg/dL) | 0.275 (0.35) | 0.43 | 0.270 (0.37) | 0.46 | 1.098 (0.51) | 0.031 | 0.35 | |
| LDL Cholesterol (mg/dL) | −0.733 (0.96) | 0.44 | −2.962 (1.01) | 0.004 | −1.311 (1.40) | 0.35 | 0.26 | |
| Triglycerides (mg/dL) | 0.024 (0.01) | 0.019 | 0.005 (0.01) | 0.65 | 0.003 (0.01) | 0.84 | 0.33 | |
| HbA1c (%) | −0.010 (0.01) | 0.35 | −0.013 (0.01) | 0.26 | −0.031 (0.02) | 0.049 | 0.53 | |
| Systolic Blood Pressure (mmHg) | −0.460 (0.41) | 0.26 | −0.035 (0.43) | 0.94 | −0.178 (0.60) | 0.77 | 0.77 | |
| Diastolic Blood Pressure (mmHg) | −0.076 (0.28) | 0.78 | −0.265 (0.29) | 0.36 | 0.023 (0.40) | 0.95 | 0.82 | |
| Cognitive Performance | ||||||||
| Global Cognition | −0.028 (0.05) | 0.48 | 0.015 (0.04) | 0.71 | −0.046 (0.06) | 0.42 | 0.63 | |
| Verbal Memory | −0.053 (0.04) | 0.16 | −0.056 (0.04) | 0.16 | −0.065 (0.06) | 0.24 | 0.98 | |
| Executive Functions | 0.001 (0.03) | 0.96 | −0.0008 (0.02) | 0.97 | 0.012 (0.03) | 0.73 | 0.95 | |
A. Pairwise comparisons of the magnitude of longitudinal change between women randomized to HT and those randomized to placebo within each metabolic phenotype. Data are presented as estimate (SEM). B. Cluster by treatment interaction effect on the longitudinal trajectory of the nine metabolic biomarkers and three cognitive composite scores.
Pairwise comparisons revealed no significant differences in the magnitude of cognitive composite score change between women randomized to placebo and HT within the three phenotypes (Table 4A). There was no significant cluster by treatment interaction on the longitudinal trajectory of any of the cognitive composite scores (Table 4B).
3.6. Metabolic biomarkers and cognition at study end among women randomized to placebo
At the end of the study, significant differences remained between women in the Healthy, High BP, and Poor Metabolic phenotypes on glucose (p<0.0001), the HOMA score (p<0.0001), HDL-cholesterol (p<0.0001), triglycerides (p<0.0001), HbA1c (p<0.0001), SBP (p<0.0001), and DBP (p<0.0001) (Table 5A). Women in the High BP phenotype only remained significantly different from women in the Healthy phenotype on four biomarkers (HOMA score, HDL-cholesterol, SBP, and DBP, all p<0.05; Table 4A); women in the Poor Metabolic phenotype remained significantly different from women in the Healthy phenotype on seven biomarkers (glucose, HOMA score, HDL-cholesterol, triglycerides, HbA1c, SBP, and DBP, all p<0.05; Table 5A).
Table 5.
Study end metabolic and cognitive values in women randomized to placebo
| Pairwise Comparisons | |||||
|---|---|---|---|---|---|
| HEALTHY METABOLIC |
HIGH BLOOD PRESSURE |
POOR METABOLIC |
Main Effect of Cluster at Study End |
||
| A. Metabolic Biomarkers | |||||
| Glucose (mg/dL) | 81.23 (1.05) | 81.37 (1.14) | 91.53 (1.58) ab | <0.0001 | |
| HOMA Score | −0.04 (0.06) | 0.20 (0.06) a | 0.79 (0.09) ab | <0.0001 | |
| Ketones (mM) | −2.36 (0.04) | −2.40 (0.04) | −2.38 (0.06) | 0.80 | |
| HDL Cholesterol (mg/dL) | 80.14 (2.00) | 69.24 (2.18) a | 56.96 (3.01) ab | <0.0001 | |
| LDL Cholesterol (mg/dL) | 118.94 (3.53) | 122.27 (3.85) | 120.84 (5.32) | 0.81 | |
| Triglycerides (mg/dL) | 4.41 (0.04) | 4.53 (0.05) | 4.84 (0.06) ab | <0.0001 | |
| HbA1c (%) | 5.77 (0.04) | 5.64 (0.05) | 6.12 (0.07) ab | <0.0001 | |
| Systolic Blood Pressure (mmHg) | 108.22 (1.27) | 117.47 (1.38) a | 118.32 (1.91) a | <0.0001 | |
| Diastolic Blood Pressure (mmHg) | 69.23 (0.78) | 75.27 (0.85) a | 73.71 (1.17) a | <0.0001 | |
| B. Cognitive Performance | |||||
| Global Cognition | 0.71 (0.18) | 0.45 (0.20) | 0.57 (0.28) | 0.61 | |
| Verbal Memory | 0.51 (0.13) | 0.55 (0.14) | 0.38 (0.20) | 0.79 | |
| Executive Functions | 0.14 (0.14) | −0.17 (0.15) | −0.11 (0.21) | 0.29 | |
A. Average (SEM) value at study end for each metabolic biomarker within each phenotype. HOMA, ketones, and triglycerides are expressed as log values due to a skewed distribution of values within the population. B. Average (SEM) value at study end for each cognitive composite score within each phenotype.
Significant longitudinal change from baseline (p < 0.05).
Longitudinal change is significantly different from the Healthy phenotype (p < 0.05).
Longitudinal change is significantly different from the High BP phenotype (p < 0.05). ANCOVA was used to test for overall cross-sectional differences among metabolic clusters on each biomarker and cognitive composite score. The Tukey-Kramer method was used to adjust for multiple comparisons.
Cognitive composite scores did not differ between women in the three metabolic phenotypes for global cognition (p=0.61), verbal memory (p=0.79), and executive functions (p=0.29) at the end of the study (Table 5B).
3.7. Metabolic biomarkers and cognition at study end among women randomized to HT
At the end of the study, significant differences remained between women randomized to HT in the Healthy, High BP, and Poor Metabolic phenotypes on glucose (p<0.0001), the HOMA score (p<0.0001), HDL-cholesterol (p<0.0001), triglycerides (p<0.0001), HbA1c (p<0.0001), SBP (p<0.0001), and DBP (p=0.0004) (Table 6A). Women in the High BP phenotype only remained significantly different from women in the Healthy phenotype on SBP and DBP (both p<0.05, Table 6A), but women in the Poor Metabolic phenotype remained significantly different from women in the Healthy phenotype on seven biomarkers (glucose, HOMA score, HDL-cholesterol, triglycerides, HbA1c, SBP, and DBP, all p<0.05; Table A).
Table 6.
Study end metabolic and cognitive values in women randomized to HT
| Pairwise Comparisons | |||||
|---|---|---|---|---|---|
| HEALTHY METABOLIC |
HIGH BLOOD PRESSURE |
HEALTHY METABOLIC |
Main Effect of Cluster at Study End |
||
| A. Metabolic Biomarkers | |||||
| Glucose (mg/dL) | 81.42 (0.98) | 80.07 (1.05) | 93.66 (1.50) ab | <0.0001 | |
| HOMA Score | −0.12 (0.05) | 0.01 (0.06) | 0.75 (0.08) ab | <0.0001 | |
| Ketones (mM) | −2.35 (0.04) | −2.43 (0.04) | −2.46 (0.06) | 0.24 | |
| HDL Cholesterol (mg/dL) | 79.28 (1.86) | 74.19 (1.99) | 55.99 (2.82) ab | <0.0001 | |
| LDL Cholesterol (mg/dL) | 112.91 (3.37) | 120.38 (3.61) | 115.24 (5.13) | 0.31 | |
| Triglycerides (mg/dL) | 4.46 (0.04) | 4.54 (0.05) | 4.89 (0.07) ab | <0.0001 | |
| HbA1c (%) | 5.67 (0.03) | 5.65 (0.04) | 5.95 (0.05) ab | <0.0001 | |
| Systolic Blood Pressure (mmHg) | 108.23 (1.31) | 117.19(1.40) a | 116.87 (1.98) a | <0.0001 | |
| Diastolic Blood Pressure (mmHg) | 69.47 (0.80) | 73.46 (0.86) a | 74.30 (1.22) a | 0.0004 | |
| B. Cognitive Performance | |||||
| Global Cognition | 0.89 (0.18) | 0.87 (0.19) | 0.52 (0.27) | 0.50 | |
| Verbal Memory | 0.84 (0.15) | 0.47 (0.16) | 0.34 (0.22) | 0.10 | |
| Executive Functions | 0.23 (0.13) | 0.32 (0.14) | 0.07 (0.19) | 0.59 | |
A. Average (SEM) value at study end for each metabolic biomarker within each phenotype. HOMA, ketones, and triglycerides are expressed as log values due to a skewed distribution of values within the population. B. Average (SEM) value at study end for each cognitive composite score within each phenotype.
Significant longitudinal change from baseline (p < 0.05).
Longitudinal change is significantly different from the Healthy phenotype (p < 0.05).
Longitudinal change is significantly different from the High BP phenotype (p < 0.05). ANCOVA was used to test for overall cross-sectional differences among metabolic clusters on each biomarker and cognitive composite score. The Tukey-Kramer method was used to adjust for multiple comparisons.
Cognitive composite scores did not differ between women in the three metabolic phenotypes for global cognition (p=0.50), verbal memory (p=0.10), and executive functions (p=0.59) at the end of the study (Table 6B). A pairwise comparison revealed that verbal memory was slightly but non-significantly higher in Healthy compared to Poor Metabolic women randomized to HT at study end (p=0.067).
3.8. Treatment comparisons on metabolic biomarkers and cognitive composite scores at study end
There were no significant cluster by treatment interactions for any metabolic biomarkers at study end (Table 7). There were also no significant cluster by treatment interactions on the cognitive composite scores at study end (Table 7). An analysis of the individual cognitive tests revealed an interaction effect of treatment by cluster on the Trails-B test (p=0.041). In the Healthy phenotype, women randomized to HT performed the test an average of 6 seconds slower than women randomized to placebo. This was significantly different from High BP (p=0.037), where women randomized to HT performed the test an average of 9 seconds faster than women randomized to placebo, and Poor Metabolic, (p=0.035), where women randomized to HT performed the test an average of 15 seconds faster than women randomized to placebo (data not shown).
Table 7.
Study end cluster by treatment interaction effects on metabolic biomarkers and cognitive composite scores
| A. Pairwise Comparisons | ||||||||
|---|---|---|---|---|---|---|---|---|
| HEALTHY METABOLIC: HT vs. Placebo |
HIGH BP: HT vs. Placebo |
POOR METABOLIC: HT vs. Placebo |
B. Cluster- Treatment Interaction p-value |
|||||
|
HT Effect (SEM) |
p-value |
HT Effect (SEM) |
p-value |
HT Effect (SEM) |
p-value | |||
| Metabolic Biomarkers | ||||||||
| Glucose (mg/dL) | 0.034 (−4.11, 4.17) | 1.00 | −1.419 (−5.86, 3.02) | 0.94 | 2.148 (−4.07, 8.36) | 0.92 | 0.41 | |
| HOMA Score | −0.082 (−0.31, 0.14) | 0.90 | −0.196 (−0.44, 0.05) | 0.20 | −0.042 (−0.38, 0.30) | 1.00 | 0.49 | |
| Ketones (mM) | 0.016 (−0.14, 0.18) | 1.00 | −0.020 (−0.19, 0.15) | 1.00 | −0.078 (−0.32, 0.16) | 0.94 | 0.65 | |
| HDL Cholesterol (mg/dL) | −0.707 (−8.57, 7.15) | 1.00 | 5.157 (−3.28, 13.59) | 0.50 | −0.469 (−12.27, 11.33) | 1.00 | 0.30 | |
| LDL Cholesterol (mg/dL) | −6.59 (−20.64, 7.45) | 0.76 | −2.44 (−17.52,12.63) | 1.00 | −6.092 (−27.19, 15.00) | 0.96 | 0.83 | |
| Triglycerides (mg/dL) | 0.044 (−0.13, 0.22) | 0.98 | 0.010 (−0.17, 0.19) | 1.00 | 0.058 (−0.20, 0.31) | 0.99 | 0.89 | |
| HbA1c (%) | −0.105 (−0.27, 0.06) | 0.44 | 0.015 (−0.16, 0.19) | 1.00 | −0.172 (−0.42, 0.07) | 0.34 | 0.16 | |
| Systolic Blood Pressure (mmHg) | 0.174 (−5.05, 5.40) | 1.00 | −0.101 (−5.71, 5.51) | 1.00 | −1.222 (−9.07, 6.63) | 1.00 | 0.91 | |
| Diastolic Blood Pressure (mmHg) | 0.126 (−3.09, 3.34) | 1.00 | −1.907 (−5.36, 1.54) | 0.61 | 0.565 (−4.26, 5.39) | 1.00 | 0.36 | |
| Cognitive Performance | ||||||||
| Global Cognition | 0.022 (−0.71, 0.76) | 1.00 | 0.263 (−0.53, 1.05) | 0.93 | −0.214 (−1.33, 0.90) | 0.99 | 0.59 | |
| Verbal Memory | 0.222 (−0.35, 0.79) | 0.87 | −0.168 (−0.78, 0.44) | 0.97 | −0.106 (−0.97, 0.75) | 1.00 | 0.37 | |
| Executive Functions | −0.057 (−0.59, 0.48) | 1.00 | 0.340 (−0.23, 0.91) | 0.53 | 0.052 (−0.76, 0.86) | 1.00 | 0.34 | |
A. Pairwise comparisons of values at study end between women randomized to HT and those randomized to placebo within each metabolic phenotype. Data are presented as estimate (95% confidence interval). B. Cluster by treatment interaction effect on the study end values of the nine metabolic biomarkers and three cognitive composite scores.
4. DISCUSSION
Using a set of nine clinically accessible biomarkers, we identified metabolically distinct groups of women within a population characterized as healthy (specifically excluding individuals with cardiovascular disease or diabetes). The data indicate wide variability on each biomarker within this study population that was selected for positive health. However, the variability within this population indicated multiple phenotypes: one met criteria for healthy metabolism, whereas two were at the margins of healthy. Evaluating only one indicator of metabolic health would not have provided such a robust identification of phenotypes of risk, because even the individuals that fell within the Poor Metabolic cluster had mean values at the margin of normal. Thus, in the preclinical transition to disease, multiple indicators may have a higher probability of identifying at-risk individuals.
Within the women randomized to placebo, those in the Healthy phenotype showed a slight but significant decline in metabolic health over the 5-year trial. However, the metabolic decline was not reflected in cognitive performance. Thus, women in this phenotype provide a representation of low-risk aging. In the High BP phenotype, blood pressure decreased significantly over the course of the trial. This could be explained by initiation of BP medication: 15 women in the High BP phenotype initiated medication to control their blood pressure, compared to 6 Healthy women and 5 Poor Metabolic women. Women in the Poor Metabolic phenotype remained more metabolically stable, only showing a notable decrease in triglycerides. Women in both the High BP and Poor Metabolic phenotypes had a longitudinal increase in cognitive performance comparable to women in the Healthy phenotype.
Although not statistically significant, women in the Healthy and High BP phenotypes trended towards becoming less healthy during the course of the trial, whereas women in the Poor Metabolic phenotype trended towards becoming healthier (Tables 2 and 3). It is possible that women with a Poor Metabolic phenotype received a greater benefit from the increased awareness of their health and lifestyle derived from participating in the clinical trial relative to Healthy or High BP women. However, the Poor Metabolic women did not reach a state of metabolic health comparable to the Healthy women (Table 5). Further, while individual biomarkers showed fluctuations at each trial visit, the three metabolic phenotypes were very stable over the 5-year trial period. These results highlight the power of using a panel of biomarkers: by doing so, we were able to measure overall systemic change rather than focusing on change within one individual biomarker.
In postmenopausal women, diabetes is associated with a longitudinal decline in cognitive function, and previous research has shown that randomization to HT provides some protection against conversion to diabetes (Bonds et al., 2006, Espeland et al., 2011, Margolis et al., 2004). In the current study, randomization to HT provided the greatest metabolic benefit to women in the Poor Metabolic phenotype, as these women showed improvements on nearly every biomarker. Although randomization to HT provided no overall cognitive benefit as measured by the cognitive composite scores, the Trails-B test results indicate that HT ameliorated metabolic effects on executive function for women in the High BP and Poor Metabolic phenotypes. Thus, while HT provided little metabolic or cognitive benefit to women within the Healthy metabolic cluster, it provided metabolic benefit to women classified as Poor Metabolic and some cognitive benefit to women classified as either High BP or Poor Metabolic.
The study results highlight the association between ethnicity, metabolic status, and disease risk, consistent with previous studies (Mayeda et al., 2013, O’Bryant et al., 2013, Zeki et al., 2012). Although the phenotypes were driven solely by metabolic values and derived independently of race, there was a significant difference in the clusters’ racial composition. Epidemiological data indicate that at nearly every age, African Americans and Hispanics have a higher risk of dementia than Caucasians (Barnes and Bennett, 2014, Seshadri et al., 1997). The impact of ethnicity may be due to several factors, including genetically-determined metabolism (Fitten et al., 2014, Kenney et al., 2014) and differences in quality of education (Carvalho et al., 2015). Ethnicity also plays a more general role in lifestyle factors such as nutrition and exercise habits, which may further impact metabolic phenotype (Sheikh and Sharma, 2014).
Although the average age at Alzheimer’s diagnosis is approximately 75 years (Holmans et al., 2005), the prodromal/preclinical state of disease begins decades prior to diagnosis (Sperling et al., 2011), suggesting large populations of people at risk between approximately 55 and 75 years of age. The systems biology approach underlying the development of peripheral-based metabolic biomarkers described herein provides a rapid, clinically deployable strategy to identify persons who may be at risk for later development of cognitive decline and potentially Alzheimer’s disease. This approach utilizes well established clinical indicators of metabolic function that, when combined, provide a strategy for early detection of risk within a population. The strength of this approach is the ability to identify persons who would be considered normal on a single indicator, but who fall at the margin on multiple indicators, indicative of approaching a transition state to abnormal function. The weakness of this approach is the potential for false positives. However, a false positive can be clinically monitored for reversal of an at-risk phenotype. Moreover this biomarker set could be used to detect the impact of metabolic interventions. Confirmation of the validity of this biomarker set in persons who transition to mild cognitive impairment or early Alzheimer’s disease is a critical next step.
Highlights.
Women classified as healthy represented three distinct metabolic phenotypes.
Poor Metabolic phenotype had lower verbal memory performance at baseline.
Hormone therapy provided metabolic benefit to women in the Poor Metabolic phenotype.
Nearly all women had significant increases in global cognition and verbal memory.
Magnitude of cognitive change did not significantly differ between phenotypes.
Acknowledgments
This research was supported by R01AG032236 (to RDB), R01AG024154 (to HH and WM), P01AG026572: Project 4 (to WM and RDB), R01AG033288 (to RDB), F31AG044997 (to JRR), and TL1RR031992 (to JRR). The authors would like to thank Brian Chang, Joseph Fouad, Eduard Babayan, Brandy Riedel, and Sarah Soliman for their assistance with conducting metabolic assays. The authors also acknowledge with gratitude all of the women who participated in ELITE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
None of the authors have a conflict of interest to disclose.
Contributor Information
Jamaica R. Rettberg, Email: jrettberg@gmail.com.
Ha Dang, Email: haminhdang@gmail.com.
Howard N. Hodis, Email: athero@usc.edu.
Victor W. Henderson, Email: vhenderson@stanford.edu.
Jan A. St. John, Email: jstjohn@usc.edu.
Wendy J. Mack, Email: wmack@usc.edu.
REFERENCES
- Alzheimer’s Association. 2014 Alzheimer's disease facts and figures. Alzheimers Dement. 2014;10:e47–e92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Authors. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- Barnes LL, Bennett DA. Alzheimer's disease in African Americans: risk factors and challenges for the future. Health Aff. 2014;33:580–586. doi: 10.1377/hlthaff.2013.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonds DE, Lasser N, Qi L, Brzyski R, Caan B, Heiss G, et al. The effect of conjugated equine oestrogen on diabetes incidence: the Women's Health Initiative randomised trial. Diabetologia. 2006;49:459–468. doi: 10.1007/s00125-005-0096-0. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E. Perimenopause as a neurological transition state. Nat Rev Endocrinol. 2015;11:393–405. doi: 10.1038/nrendo.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo MC, Brashear HR, Logovinsky V, Ryan JM, Feldman HH, Siemers ER, et al. Can we prevent Alzheimer's disease? Secondary "prevention" trials in Alzheimer's disease. Alzheimers Dement. 2013;9:123–131 e1. doi: 10.1016/j.jalz.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Carvalho JO, Tommet D, Crane PK, Thomas ML, Claxton A, Habeck C, et al. Deconstructing racial differences: the effects of quality of education and cerebrovascular risk factors. J Gerontol B Psychol Sci Soc Sci. 2015;70:545–556. doi: 10.1093/geronb/gbu086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42:484–491. doi: 10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Miller ME, Goveas JS, Hogan PE, Coker LH, Williamson J, et al. Cognitive function and fine motor speed in older women with diabetes mellitus: results from the women's health initiative study of cognitive aging. J Womens Health (Larchmt) 2011;20:1435–1443. doi: 10.1089/jwh.2011.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitten LJ, Ortiz F, Fairbanks L, Bartzokis G, Lu P, Klein E, et al. Younger age of dementia diagnosis in a Hispanic population in southern California. Int J Geriatr Psychiatry. 2014;29:586–593. doi: 10.1002/gps.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;71:1218–1227. doi: 10.1001/jamaneurol.2014.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Brinton RD. Menopause and mitochondria: windows into estrogen effects on Alzheimer's disease risk and therapy. Prog Brain Res. 2010;182:77–96. doi: 10.1016/S0079-6123(10)82003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, St John JA, Hodis HN, McCleary CA, Stanczyk FZ, Karim R, et al. Cognition, mood, and physiological concentrations of sex hormones in the early and late postmenopause. Proc Natl Acad Sci U S A. 2013;110:20290–20295. doi: 10.1073/pnas.1312353110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ, Shoupe D, Azen SP, Stanczyk FZ, Hwang-Levine J, et al. Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with Estradiol testing the menopausal hormone timing hypothesis. Menopause. 2015;22:391–401. doi: 10.1097/GME.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmans P, Hamshere M, Hollingworth P, Rice F, Tunstall N, Jones S, et al. Genome screen for loci influencing age at onset and rate of decline in late onset Alzheimer's disease. Am J Med Genet B Neuropsychiatr Genet. 2005;135B:24–32. doi: 10.1002/ajmg.b.30114. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim R, Stanczyk FZ, Brinton RD, Rettberg J, Hodis HN, Mack WJ. Association of endogenous sex hormones with adipokines and ghrelin in postmenopausal women. J Clin Endocrinol Metab. 2015;100:508–515. doi: 10.1210/jc.2014-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna H, Hoeft F, Kelley R, Wroolie T, DeMuth B, Reiss A, et al. Fasting plasma insulin and the default mode network in women at risk for Alzheimer's disease. Neurobiol Aging. 2013;34:641–649. doi: 10.1016/j.neurobiolaging.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney MC, Chwa M, Atilano SR, Falatoonzadeh P, Ramirez C, Malik D, et al. Molecular and bioenergetic differences between cells with African versus European inherited mitochondrial DNA haplogroups: implications for population susceptibility to diseases. Biochim Biophys Acta. 2014;1842:208–219. doi: 10.1016/j.bbadis.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women's Health Initiative Hormone Trial. Diabetologia. 2004;47:1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- Mayeda ER, Haan MN, Kanaya AM, Yaffe K, Neuhaus J. Type 2 diabetes and 10-year risk of dementia and cognitive impairment among older Mexican Americans. Diabetes Care. 2013;36:2600–2606. doi: 10.2337/dc12-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- O'Bryant SE, Xiao G, Edwards M, Devous M, Gupta VB, Martins R, et al. Biomarkers of Alzheimer's disease among Mexican Americans. J Alzheimers Dis. 2013;34:841–849. doi: 10.3233/JAD-122074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani LN, Korenda L, Meigs JB, Driver C, Chamany S, Fox CS, et al. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001–2004. Diabetes Care. 2008;31:1991–1996. doi: 10.2337/dc08-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings AM, Sharrett AR, Schneider AL, Coresh J, Albert M, Couper D, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161:785–793. doi: 10.7326/M14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. 2014;35:8–30. doi: 10.1016/j.yfrne.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RO, Knopman DS, Przybelski SA, Mielke MM, Kantarci K, Preboske GM, et al. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology. 2014;82:1132–1141. doi: 10.1212/WNL.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, et al. Lifetime risk of dementia and Alzheimer's disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- Sheikh N, Sharma S. Impact of ethnicity on cardiac adaptation to exercise. Nat Rev Cardiol. 2014;11:198–217. doi: 10.1038/nrcardio.2014.15. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton W, Gleason CE, Dowling NM, Carlsson CM, Brinton EA, Santoro MN, et al. The KEEPS-Cognitive and Affective Study: Baseline Associations between Vascular Risk Factors and Cognition. J Alzheimers Dis. 2014;40:331–341. doi: 10.3233/JAD-130245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- Yao J, Rettberg JR, Klosinski LP, Cadenas E, Brinton RD. Shift in brain metabolism in late onset Alzheimer's disease: implications for biomarkers and therapeutic interventions. Mol Aspects Med. 2011;32:247–257. doi: 10.1016/j.mam.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Yao J, Sancheti H, Feng T, Melcangi RC, Morgan TE, et al. The perimenopausal aging transition in the female rat brain: decline in bioenergetic systems and synaptic plasticity. Neurobiol Aging. 2015;36:2282–2295. doi: 10.1016/j.neurobiolaging.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki Al, Hazzouri A, Haan MN, Whitmer RA, Yaffe K, Neuhaus J. Central obesity, leptin and cognitive decline: the Sacramento Area Latino Study on Aging. Dement Geriatr Cogn Disord. 2012;33:400–409. doi: 10.1159/000339957. [DOI] [PMC free article] [PubMed] [Google Scholar]