Abstract
Background
Older patients with metastatic pancreatic cancer (mPC) are poorly represented in clinical trials. We compared patterns of care and outcomes of patients with mPC < and > 65 yrs (Group 1 and Group 2, respectively) treated at Fox Chase Cancer Center (FCCC) to identify predictors of survival and better understand the treatment approaches.
Methods
Charts of 579 patients with mPC treated at FCCC from 2000–2010 were reviewed. Group 1 and Group 2 were compared with respect to baseline, treatment characteristics, and overall survival (OS) after diagnosis of metastatic disease.
Results
299 patients in Group 1 (median age 57) and 280 patients in Group 2 (median age 73) were evaluated. Patients in Group2 were less likely to receive any chemotherapy for mPC compared to Group1 (65% vs 75%, p=0.001) and if treated were less likely to receive more than one agent (37% vs 53%, p<0.001). Survival was comparable between the two groups (p=0.16) and Charlson Co-morbidity Index did not emerge as a prognostic factor. Longer OS was associated with higher number of agents used in both groups (p<0.001). Liver metastases conferred worse survival (p=0.02) while lung metastases conferred better survival in both groups (p=0.002).
Conclusions
Older mPC patients are less likely to receive chemotherapy and receive fewer agents yet have similar OS compared to younger patients. OS improves with increasing number of agents, supporting the use of combination chemotherapy in healthy older patients. Our findings encourage enrollment of older patients with mPC with good performance status onto clinical trials with stratification by site of metastases.
Keywords: metastatic pancreatic cancer, older adults, chemotherapy
Introduction
Pancreatic cancer remains a disease with a poor prognosis and relatively limited treatment options. It is the fourth leading cause of cancer related death in the United States, with over 40,000 new cases each year and a nearly similar number of deaths expected (1). The incidence of pancreatic cancer increases with age, with a median age at diagnosis of 71 and over 65% of newly diagnosed patients over the age of 65(2). It is estimated that 60% of the US population will be older than 65 years of age by 2030 and that 70% of cancers will occur in this population (3–5). Metastatic pancreatic cancer has a dismal prognosis and the 5-year survival rate is only 2% (2) with 1-year survival rates of 17 to 23% reported in the gemcitabine monotherapy era (6). The goal of therapy remains palliative with improvement in clinical benefit rate and overall survival with the use of chemotherapy. Gemcitabine remained the mainstay of first line therapy until 2010. The introduction of combination chemotherapy regimens such as FOLFIRINOX and gemcitabine+nab-paclitaxe after 2010, has significantly improved outcomes compared to gemcitabine monotherapy and are considered the first line therapy of choice for good performance status patients (7–9).
The older adult with pancreatic cancer is poorly represented in the oncologic literature. Retrospective studies demonstrate that older patients are treated less aggressively, with less surgical procedures, and less chemotherapy or radiation treatments (10). This may partially explain the data demonstrating lower overall survival of older patients with this disease compared to their younger counterparts (10, 11). In clinical practice, age of the patient can affect treatment decisions due to a concern for treatment related toxicities, but the data supporting or refuting this notion is sparse. Use of chemotherapy improves survival with acceptable toxicities in older patients as indicated in prospective studies in other cancer types (12). For example, in an elderly specific colorectal cancer study, a combination of capecitabine and bevacizumab improved progression free survival with acceptable toxicities, as compared to capecitabine alone (13). Another study showed that with a comprehensive baseline geriatric assessment and reduced starting doses of chemotherapy, even frail and elderly patients can participate in a randomized controlled trial and derive survival benefit from chemotherapy (14). A retrospective analysis of older patients with metastatic Pancreatic Cancer (mPC) found that the majority of the patients received no therapy, although chemotherapy when utilized was associated with superior survival compared to no therapy (15).
The clinical characteristics and treatment outcomes of older patients with mPC have not been extensively studied resulting in lack of data to guide the practicing oncologist. Hence, we conducted this retrospective study to evaluate and compare the disease characteristics and treatment patterns of older adults (Group 2, ≥65 years of age) with mPC as compared to their younger counterparts (Group 1, <65 years of age). Our primary objective was to evaluate outcomes with the goal of identifying predictors of survival in the two groups.
Methods
Through an IRB approved protocol, we performed a retrospective chart review of patients diagnosed with metastatic adenocarcinoma of the pancreas, identified through the tumor registry at Fox Chase Cancer Center, between 2000 and 2010. These included stage IV patients who presented with stage IV disease or patients with earlier stage disease at presentation who subsequently recurred. We included patients who recurred both locally (in the pancreatic bed) and at distant sites, since local recurrence after initial curative therapy has similar outcomes and management strategies as distant metastases. We excluded patients with incomplete medical records or histologic diagnosis other than pancreatic adenocarcinoma.
All charts were reviewed by 2 independent reviewers to ensure accuracy of the data collected. The data recorded included patient characteristics (age, gender, race, weight, height, performance status, co-morbidities, tobacco and alcohol use), tumor characteristics (primary location, stage of disease, grade, site of initial and subsequent metastases, CA 19-9 at diagnosis of metastatic disease and peak level), treatment patterns (therapy received including radiation, surgery, type of chemotherapy], and overall survival (OS) from development of metastatic disease. Performance status was recorded using the ECOG scale as derived from the initial consultation. In order to stratify by co-morbidities, we calculated the age adjusted Charlson Comorbidity Index (CCI) for each patient using the published scoring method (16). We accounted for the diagnosis of metastatic disease for both groups (adds 5 points to CCI). Any tobacco and alcohol consumption that was more than occasional was considered positive. For patients with recurrent/metastatic disease, we calculated total number of agents used over time, excluding any investigational agent. Date of death was obtained from the database Living patients were censored at the time of data cutoff. Given the grave prognosis and limited treatment options available for patients with mPC during the study period (i.e. Gemcitabine, 5 Fluorouracil), we elected to capture any drugs received by patients during the course of their illness.
Statistical Considerations
The primary clinical outcome was overall survival after diagnosis of metastatic disease. Continuous variables were analyzed using the Mann-Whitney test and categorical variables were analyzed using Fisher’s exact test. Univariate log-rank tests and Cox proportional hazards regression were used to examine the association of survival and patient tumor and treatment characteristics between the two age groups. Wherever appropriate, Kaplan-Meier survival curves were computed. All p-values presented are 2-sided and p-values less than 0.05 were considered statistically significant. Multivariate analysis was conducted using variables that were statistically significant in univariate analysis.
Results
We identified 1105 patients with the diagnosis of pancreatic cancer through our tumor registry. Of these, 579 patients with mPC fit our eligibility criteria and were included in the analysis, with 299 patients less than 65 years of age (Group 1) and 280 patients greater than or equal to 65 years of age (Group 2). Baseline patient characteristics are listed in Table 1. The median age at diagnosis was 64 years (range: 31–90 years) for the full cohort, with a median age of 57 years in Group 1 and 73 years in Group 2. The majority of the patients in both groups were male, with pancreatic head tumor, a performance status ≤1, and stage IV disease at diagnosis. Tobacco and alcohol use were more common in Group 1. Median age-adjusted CCI was 9 in Group 1 and 10 in Group 2 (p <0.001). There was no significant difference in the serum albumin level, at diagnosis of metastatic disease, between the groups. Diabetes and renal disease were similarly distributed in the two cohorts but cardiovascular disease was more common in Group 2.
Table 1.
Patient, tumor and treatment characteristics.
| Age < 65 (Group 1) N = 299 (52%) |
Age ≥ 65 (Group 2) N = 280 (48%) |
P value | |
|---|---|---|---|
| Median age | 57 | 73 | |
| Ethnicity | NS | ||
| Caucasian | 239 (80%) | 232 (83%) | |
| African American | 30 (10%) | 15 (5%) | |
| Other/Unknown | 30 (10%) | 33 (12%) | |
| Performance Status | NS | ||
| 0–1 | 222 (74%) | 205 (73%) | |
| >1 | 38 (13%) | 44 (16%) | |
| Unknown | 39 (13%) | 31 (11%) | |
| Co-morbidities | |||
| Charlson Index (age adjusted) |
9 | 10 | 0.0001 |
| Diabetes mellitus | 84 (28%) | 89 (32%) | NS |
| Cardiovascular disease |
108 (36%) | 150 (54%) | <0.001 |
| Renal impairment | 9 (3%) | 17 (6%) | NS |
| Tobacco Use | 194 (65%) | 146 (52%) | 0.001 |
| Alcohol Use | 138 (46%) | 83 (30%) | < 0.001 |
| Serum Albumin | NS | ||
| ≥ 3.5 | 105 (36%) | 55 (40%) | |
| < 3.5 | 171(58%) | 40 (50%) | |
| Unknown | 14 (6%) | 8 (10%) | |
| Stage at diagnosis | NS | ||
| I–III | 135 (45%) | 112 (40%) | |
| IV | 164 (55%) | 168 (60%) | |
|
Site of pancreatic tumor |
NS | ||
| Head | 160 (54%) | 146 (52%) | |
| Body | 54 (18%) | 49 (18%) | |
| Tail | 48 (16%) | 50 (18%) | |
| Unknown | 37 (12%) | 35 (12%) | |
|
Initial site of metastasis |
|||
| Liver | 195 (65%) | 182 (65%) | NS |
| Lung | 24 (8%) | 39 (14%) | NS |
| Peritoneal disease | 62 (21%) | 38 (14%) | 0.002 |
| Local recurrence | 13 (4%) | 18 (6%) | NS |
| CA 19-9 (median) | NS | ||
| At diagnosis | 700 | 918 | |
| Peak level | 2408 | 2806 | |
|
Chemotherapy in met setting |
224 (75%) | 184 (65%) | 0.001 |
| Type of chemo | 64 (21%) | 47 (17%) | NS |
| 5 FU | 192 (64%) | 153 (54%) | 0.004 |
| Gemcitabine | 49 (17%) | 32 (11%) | 0.02 |
| Platinum | 22 (7%) | 10 (4%) | 0.03 |
| Taxanes | 15 (5%) | 4(2%) | 0.04 |
| Irinotecan | 47 (16%) | 27 (10%) | NS |
| EGFR inhibitor >1 agents |
161 (53%) | 105 (37%) | <0.001 |
NS=non-significant p value
Treatment patterns (Table 2)
Table 2.
Univariate analysis of overall survival (stratified by age)
| Group 1, < 65 years (n= 299) |
Group 2, ≥ 65 years (n=280) |
|||
|---|---|---|---|---|
| Variables | Median OS (months) |
P value | Median OS (months) |
P value |
| Overall survival | 6m | NS* | 5m | NS* |
| Renal disease Yes vs. no |
3m vs. 6m | 0.05 | 6m vs. 5m | NS* |
| Performance Status ≤ 1 vs ≥ 2 |
6m vs. 3m | 0.06 | 5m vs. 3.5m | 0.02 |
| Liver metastases Yes vs. no |
5m vs. 7 m | 0.002 | 4m vs. 6m | 0.02 |
| Lung metastases Yes vs. no |
8m vs. 5m | <0.001 | 6m vs. 4m | 0.002 |
| Chemotherapy Yes vs. no |
7m vs. 2m | <0.001 | 6m vs. 2m | <0.001 |
| ≥ 2 chemo agents Yes vs. no |
8m vs. 4m | <0.001 | 7m vs. 4m | <0.001 |
| CCI ≤9 vs. >9 |
6m vs. 3.5m | NS* | 5m vs. 5m | NS* |
| Serum Albumin <3.5 vs. >=3.5 |
4m vs. 7m | 0.012 | 4m vs. 6m | < 0.001 |
For patients diagnosed with Stage I–III disease, rate of curative surgery was not significantly different between Group 1 (25%, n=74) and Group 2 (23%, n=61). In addition, there was no significant difference in the rate of receiving radiation therapy for non-metastatic disease in the two groups [33% (n=100) in Group 1 and 28% (n=74) in Group 2]. Older patients were less likely to receive any chemotherapy for mPC. 73% of patients in Group 1 and 65% in Group 2 received any chemotherapy (Odds Ratio 0.45, p=0.001). Furthermore, older patients who were treated were less likely to receive more than one agent during their treatment course (57% vs. 72%; Odds Ratio 0.50, p<0.001) and the median number of agents was two in Group 1 versus one in Group 2. Use of individual chemotherapy agents (gemcitabine, oxaliplatin, irinotecan and taxanes) was significantly less prevalent among older adults but there was no significant difference in the use of fluoropyrimidines and the oral small molecule tyrosine kinase inhibitor erlotinib between the two groups.
Survival analysis and prognostic factors (Table 3)
Table 3.
Prognostic variables for survival: Multivariate analysis
| Group | Multivariate analysis |
P value |
|---|---|---|
| Group 1 <65 years |
Liver metastases | <0.001 |
| Lung metastases | 0.001 | |
| >/= 2 chemo agents |
<0.001 | |
| S. Albumin | <0.001 | |
| Group 2 ≥ 65 years |
Liver metastases | 0.02 |
| S. Albumin | 0.003 | |
| >/= 2 chemo agents |
<0.001 |
NS= non-significant
RR= relative risk
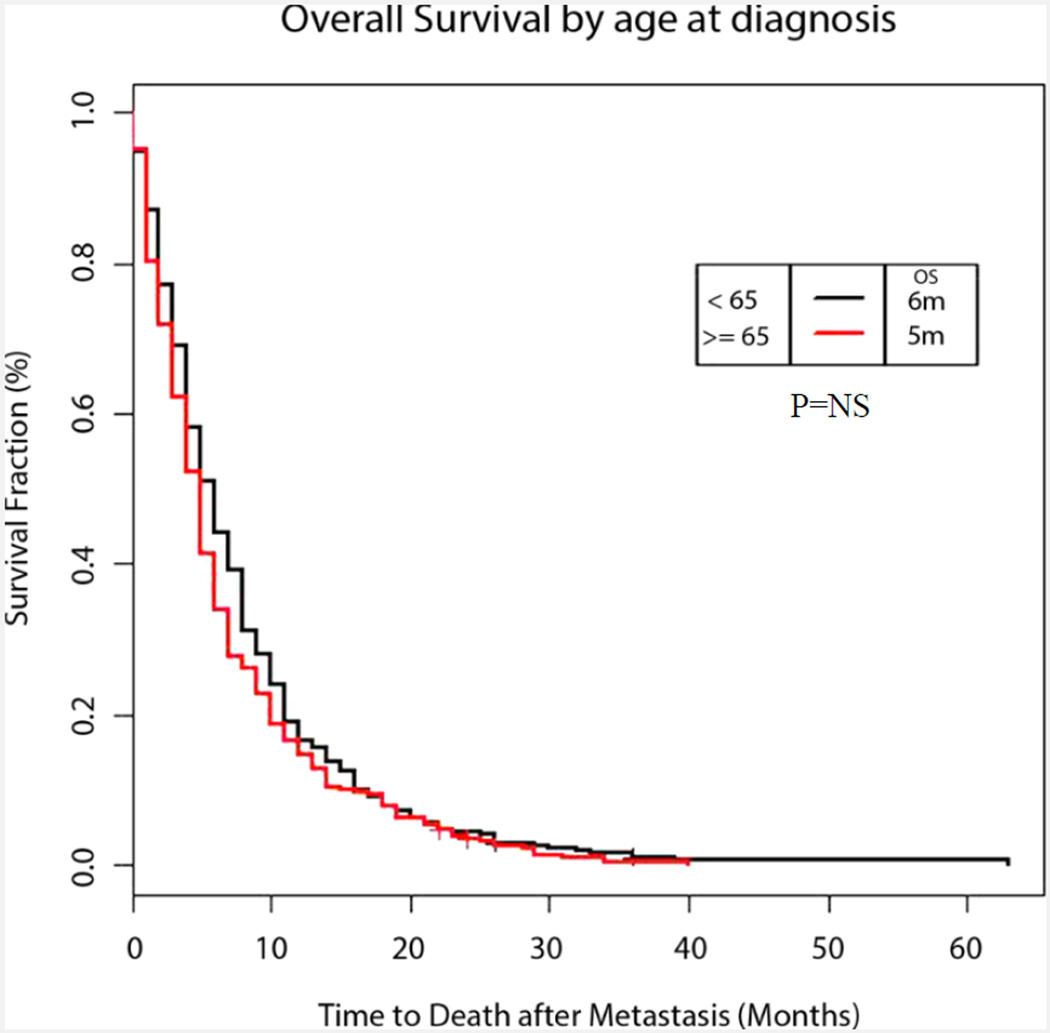
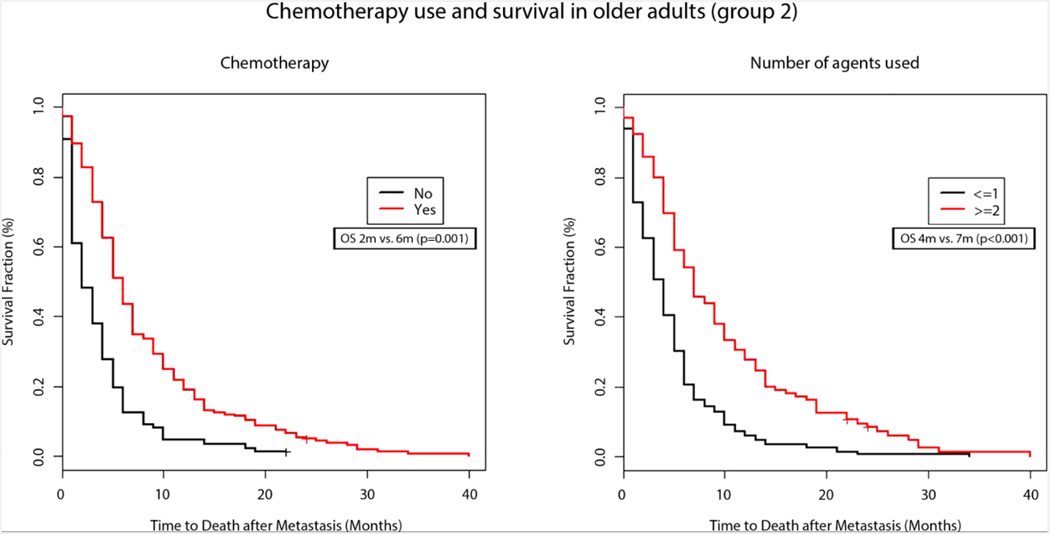
Survival after diagnosis of mPC was comparable between the two groups with median OS being 6 months in Group 1 vs 5 months in Group 2 (p=0.16) (Figure 1). In Group 2, use of chemotherapy was associated with a significantly improved survival (6m vs 2m, p<0.001) (Figure 2A) Univariate analysis also demonstrated improved survival with higher number of chemotherapy agents (relative risk 0.67, p<0.001) among the older patients (Figure 2B). Similar findings were also observed in younger patients, as shown in Table 2.
Figure 1. Overall Survival by age at diagnosis.
Adjusted Kaplan–Meier survival curves for younger vs older patients with metastatic pancreatic cancer (6 months in younger adults and 5 months in older adults, P=0.16)
Figure 2. Chemotherapy use and survival in older adults (group 2).
Adjusted Kaplan–Meier survival curves for older patients with metastatic pancreatic cancer by (a) use of chemotherapy (2 months without versus 6 months with chemotherapy, P=0.001) and (b) number of chemotherapy agents used (4 months for <2 versus 7 months for ≥2 agents, P<0.001).
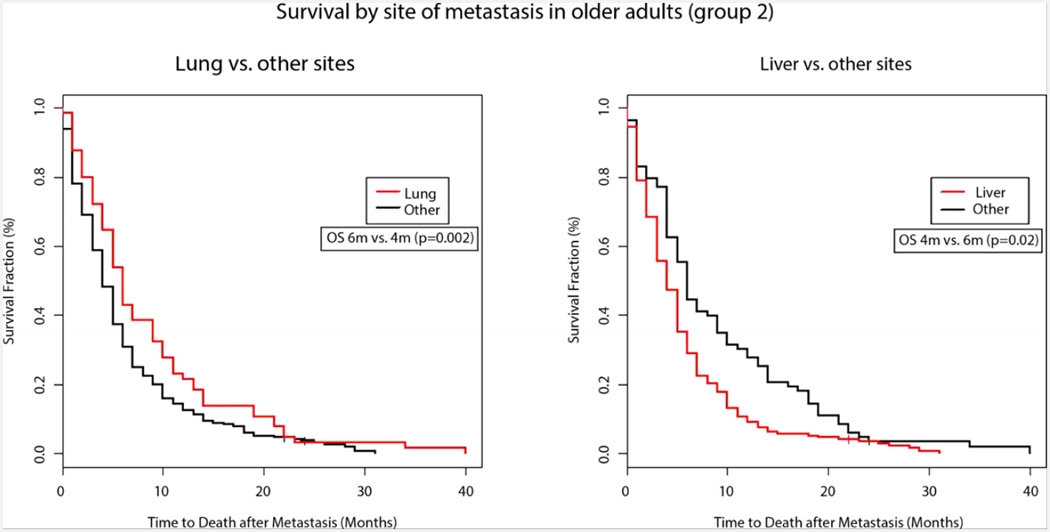
In univariate analysis, the age adjusted CCI did not emerge as a prognostic factor in our analysis for both groups, with a relative risk of 1.04 for Group 1 (p= 0.43) and 1.01 for Group 2 (p= 0.82). Renal disease negatively impacted survival only in younger patients where patients with known kidney disease had a median OS of 3 months compared to 6 months in those without known renal dysfunction (p=0.05). Performance status impacted survival in both groups but was statistically significant only in older patients (p=0.02). A higher serum albumin was an important predictor of improved survival in both groups (Group 1 with a relative risk=0.78, p=0.0032 and Group 2 with relative risk=0.66, p< 0.001). The initial metastatic site affected survival in both groups (Group 1, p=0.02 and Group 2, p=0.01). The presence of liver metastases conferred worse survival compared to other sites in Group 2 (4m vs 6m, p=0.02) while the presence of lung metastases was associated with better survival (6m vs 4m, p=0.002) (Figure 3). This association between site of metastasis and survival was also seen in Group 1 (Table 2)
Figure 3. Survival by site of metastasis in older adults (group 2).
Adjusted Kaplan–Meier survival curves for older patients with metastatic pancreatic cancer by site of metastasis (a) Lung (6 months versus 4 months, P=0.002) and (b) Liver (4 months versus 6 months, P=0.02)
We performed a multivariate analysis (Table 3) including all the prognostic variables that were significant on univariate analysis. The factors associated with poor survival among young patients in this analysis included: liver vs lung as site of metastatic disease, lower number of chemotherapeutic agents used and a lower serum albumin. History of renal dysfunction was not related to survival in this group. The factors associated with worse survival among older patients included: liver vs. other sites of metastases, lower number of chemotherapeutic agents, and lower serum albumin. Performance status did not emerge as a prognostic factor as shown on univariate analysis.
Discussion
Pancreatic cancer is a disease of older adults and as our population ages, the number of older patients with pancreatic cancer will continue to rise and pose challenges to oncology professionals. Despite this large patient population, older patients have often been excluded from clinical trials in the past (17). In one study, only 9% of patients enrolled in FDA drug registration trials were 75 years or older, whereas 31% of patients with cancer are within that age group (18). A recent study noted approximately 5% of older patients with mPC were taking part in clinical trials (19). The data describing outcomes and therapy of older patients with pancreatic cancer is limited to a number of retrospective studies resulting in limited data to guide practicing oncologists as to the optimal treatment approach in this patient population. With increasing awareness of this knowledge gap, more and more emphasis is being put on developing therapies tailored to the needs of older patients with cancer (20).
The effect of age on treatment related outcomes in pancreatic cancer has been studied in some retrospective studies with varying results (21–24). In our study, the survival after diagnosis of metastatic disease was not significantly different between the older and younger patients. It is important to consider the fact that older adults treated at academic institutions often do not represent older patients seen in community oncology practices. These patients are less likely to suffer from multiple geriatric syndromes and may be candidates for aggressive therapy. Yet, these data support the fact that there exists a subpopulation of older patients who have outcomes similar to those seen among younger patients, and may be candidates for a similar treatment approach. Aging is a process not well reflected by the chronologic age of a patient, and performance status and comprehensive geriatric assessments may be better predictors of survival in this patient population (25–28).
The burden of co-morbidities can be assessed by tools like the CCI (16). Nakai, et.al., reported CCI as an important prognostic marker in older pancreatic cancer patients treated with gemcitabine based therapy (24). In contrast, our study did not find a correlation between the age-adjusted CCI and survival in both younger and older patients with mPC. This contradictory finding may be related to the patient population of our study which included older adults referred to an academic center, who are more likely to be fit. However, it is possible that CCI has a lower impact on survival in this aggressive cancer, in which most succumb to the disease rather than other co-morbid conditions. Serum albumin was found to be an important prognostic factor on univariate and multivariate analysis for both younger and older adult patients. Hypoalbuminemia is commonly seen in advanced cancer patients, and is considered a measure of nutritional status and cachexia, and a negative marker of inflammation (29, 30). Inflammation has been shown to be an independent predictor of cancer survival as shown by the Glasgow Prognostic Score (31, 32). Our results are supported by other reports in the literature linking hypoalbuminemia with poorer survival in different cancer types including gastric cancer, renal cell carcinoma, colo-rectal cancer along with pancreatic cancer (33–35), (8).
Prior research has alluded to a differential effect of site of metastasis on survival of patients with pancreatic cancer (36). Our study suggests that initial site of metastasis has a significant influence on survival and improved survival is seen among patients with lung metastases as compared to those with liver metastases. This association persisted among young and old patients alike, pointing to inherent similarities between the two groups. Further research is needed to study the biologic importance of these two different disease presentations.
Advances in chemotherapy and newer combinations have improved survival in mPC. Gemcitabine chemotherapy, the backbone of therapy for mPC, was shown to be tolerable and effective in older and younger patients alike (37). Until 2010, gemcitabine monotherapy was the most widely used first line therapy for metastatic disease. Recently combination chemotherapy of 5-fluorouracil, leucovorin, oxaliplatin and irinotecan (FOLFIRINOX), and gemcitabine+nab-paclitaxel, have demonstrated improved outcomes in mPC (7, 8). Despite encouraging results, data regarding tolerance and efficacy of combination therapy in older adults is not robust.
Studies have shown that use of chemotherapy is less prevalent in older patients with many cancers including pancreatic cancer (38) (39) (40). A retrospective study from the Veterans Affairs Cancer Registry evaluated chemotherapy use among over 400 older patients with mPC (15). The majority of patients (83%) did not receive any chemotherapy. Further analysis revealed that those patients who were treated with chemotherapy had improved overall survival compared to those who were not treated (4.9 months versus 1.7 months). Our study included patients treated before 2011, the year that marked the advent of current treatment paradigm of first line combination chemotherapy for mPC, to select a more homogenously treated population. Similar to the earlier studies, in our experience, older patients were less likely to receive chemotherapy and, when treated, received less number of agents compared to their younger counterparts. Sixty-five percent of the older patients in our study received chemotherapy, which is higher than the 17% in the VA study (15). Gemcitabine monotherapy is a less toxic option compared to the current standard of care (7, 8), and still older patients in our study were less likely to receive it. The disparity in the treatment of older patients with mPC, as seen in the era of single agent gemcitabine, is likely to continue with regards to usage of the newer therapies. On the contrary, among those who received chemotherapy, there was a significant improvement in survival and we found a benefit to using multiple agents, in young and old alike. With the novel combination therapies, this benefit may increase further. While some older adults are not felt to be candidates for aggressive chemotherapy, there are many who may be fit for aggressive treatment approaches. Further research is needed to determine the tolerability of these newer treatment approaches among older patients and the benefit they may derive from it. Our results indicating a longer OS among older patients who were treated with multiple agents would support such research goals.
A study such as ours carries limitation which are a consequence of its retrospective design. Primarily, the study has a potential for recall bias. In an attempt to minimize this effect and ensure accuracy, two investigators independently reviewed each chart. Our clinical outcomes are similar to those reported in large prospective clinical trials, supporting the validity of our sampling. A comprehensive geriatric assessment is highly useful in evaluation of older adults with cancer. Such assessment will include a thorough evaluation of multiple domains including: co-morbidities, polypharmacy, physical activity level and sarcopenia, cognitive and emotional state, and social support. Due to the nature of our study, much of these data was not available, however, we evaluated factors including performance status, albumin level, weight, and co-morbidities, which serve as surrogates for predicting treatment toxicities (31, 41–43) and are a part of a full geriatric assessment (44). As previously noted, our study describes the experience of a single academic institution and may carry additional bias associated with the fitness of the older patients seen in this setting. Interestingly, even the older “fit” patient with mPC was less likely to receive chemotherapy in an academic setting. Finally, our dataset includes patients treated at our center between 2000 and 2010; therefore, the effect of newer combination therapies on older patients could not be assessed in our study. However, we were able to demonstrate a survival benefit of therapy among older patients that is similar to that seen among younger patients, and this may be extrapolated to newer treatment approaches. Given the sparse data available to guide the treatment of this patient population, our data serves as hypothesis generating for future clinical trials evaluating the treatment of older patients with this aggressive cancer. Our conclusion, that older patients with mPC benefit from chemotherapy and should be offered more effective combination therapies if physically fit, is limited by the lack of physician/patient preference information in the database. Some older patients with mPC choose to forgo palliative chemotherapy for fear of adverse effects and slim benefit and the ones who choose to get chemotherapy may be healthier than the average older patient with mPC and expected to have a better survival regardless of therapy. Similarly, patients who recur after initial therapy for early stage pancreatic cancer may be less willing to undergo palliative therapy for this highly incurable and deadly disease. These considerations cannot be ascertain from our data, and pose a limitation to our data. However, the results of our study as presented should support administration of chemotherapy to those fit older patients who are interested in this approach.
In conclusion, our study identifies many similarities between the treatment and clinical outcomes of older and younger patients with mPC. However, older patients with mPC are less likely to receive chemotherapy, and if treated receive fewer agents compared to their younger counterparts. When older patients are treated, they have better outcomes with the use of higher number of active agents. Overall, younger and older patients with liver metastases, lower serum albumin, or who have received fewer active agents have worse outcomes. Our findings support enrollment of elderly patients with mPC with good performance status onto clinical trials. Stratification of patients with mPC by site of metastases and serum albumin to optimize and individualize treatment paradigms for this growing population should be strongly considered.
Acknowledgments
Acknowledgement/Research Support: Cancer Center Support Grant3 P30 CA006927-47S4 and Cancer Center Support Grant P30 CA43703
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous presentations:
Vijayvergia, N., Dotan, E., Cohen, S.J. (2013) Differences in patterns of care and outcomes of elderly versus younger metastatic pancreatic cancer (mPC) patients. J ClinOncol 31, 2013 (suppl; abstr 9546)
Disclosures and Conflict of Interest Statements
The authors have no conflicts of interest to disclose.
Author Contributions
Study Concepts: S Cohen, E Dotan, N Vijayvergia, S Gupta
Study Design: E Dotan, N Vijayvergia, S Cohen
Data Acquisition: N Vijayvergia, K Hatahet, F Rahman, B Lewis, J Ricco
Quality Control of Data and Algorithms: E Dotan, S Cohen, N Vijayvergia
Data Analysis and Interpretation: E Dotan, S Cohen, N Vijayvergia, K Devarajan
Statistical Analysis: K Devarajan
Manuscript Preparation: N Vijayvergia
Manuscript Editing: E Dotan, S Cohen, K Devarajan
Manuscript Review: S Cohen, E Dotan, N Vijayvergia, K Hatahet, F Rahman, B Lewis, J Ricco, S Gupta
REFERENCES
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA: A Cancer Journal for Clinicians. 2014 doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance Epidemiology and End Results Program (SEER) database. [cited 2014 September 11]; Available from: http://seer.cancer.gov/statfacts/html/pancreas.html.
- 3.Hanson LC, Muss HB. Cancer in the oldest old: making better treatment decisions. Journal of Clinical Oncology. 2010;28(12):1975–1976. doi: 10.1200/JCO.2009.27.6022. [DOI] [PubMed] [Google Scholar]
- 4.Gillison TL, Chatta GS. Cancer chemotherapy in the elderly patient. Oncology. 2010;24(1):76–85. [PubMed] [Google Scholar]
- 5.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: A Cancer Journal for Clinicians. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 6.Burris Hr, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. Journal of clinical oncology. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 7.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. New England Journal of Medicine. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. New England Journal of Medicine. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 9.NCCN Clinical Practice Guidelines in Oncology: Pancreatic Cancer Version 2.2015. 2015 Available from: http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. [Google Scholar]
- 10.Jacobson SD, Alberts SR, O’Connell MJ. Pancreatic cancer in the older patient. Oncology. 2001;15:926–932. [PubMed] [Google Scholar]
- 11.Wang P, Meng ZQ, Chen Z, Lin JH, Zhou ZH, Chen H, et al. Survival rate of pancreatic cancer in elderly patients. Hepato-gastroenterology. 2008;55(82–83):681–686. [PubMed] [Google Scholar]
- 12.Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Fermé C, et al. Long-Term Results of the R-CHOP Study in the Treatment of Elderly Patients With Diffuse Large B-Cell Lymphoma: A Study by the Groupe d'Etude des Lymphomes de l'Adulte. Journal of Clinical Oncology. 2005;23(18):4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham D, Lang I, Marcuello E, Lorusso V, Ocvirk J, Shin DB, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. The Lancet Oncology. 2013;14(11):1077–1085. doi: 10.1016/S1470-2045(13)70154-2. [DOI] [PubMed] [Google Scholar]
- 14.Seymour MT, Thompson LC, Wasan HS, Middleton G, Brewster AE, Shepherd SF, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet. 2011;377(9779):1749–1759. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aldoss IT, Tashi T, Gonsalves W, Kalaiah RK, Fang X, Silberstein P, et al. Role of chemotherapy in the very elderly patients with metastatic pancreatic cancer—A Veterans Affairs Cancer Registry analysis. Journal of Geriatric Oncology. 2011;2(3):209–214. [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. New England Journal of Medicine. 1999;341(27):2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 18.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. Jama. 2004;291(22):2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 19.Li D. Treatment, outcomes, and clinical trial participation in very elderly patients (pts) with metastatic pancreas cancer (mPC) Journal of Clinical Oncology. 2014;32:5s. doi: 10.1016/j.clcc.2015.05.005. (suppl; abstr 4119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohile S, Dale W, Magnuson A, Kamath N, Hurria A. Research priorities in geriatric oncology for 2013 and beyond. Cancer forum. 2013;37(3):216–221. [PMC free article] [PubMed] [Google Scholar]
- 21.Park JK, Yoon YB, Kim YT, Ryu JK, Yoon WJ, Lee SH. Survival and prognostic factors of unresectable pancreatic cancer. Journal of clinical gastroenterology. 2008;42(1):86–91. doi: 10.1097/01.mcg.0000225657.30803.9d. [DOI] [PubMed] [Google Scholar]
- 22.Stocken D, Hassan A, Altman D, Billingham L, Bramhall S, Johnson P, et al. Modelling prognostic factors in advanced pancreatic cancer. British journal of cancer. 2008;99(6):883–893. doi: 10.1038/sj.bjc.6604568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tas F, Sen F, Keskin S, Kilic L, Yildiz I. Prognostic factors in metastatic pancreatic cancer: Older patients are associated with reduced overall survival. Molecular and clinical oncology. 2013;1(4):788–792. doi: 10.3892/mco.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakai Y, Isayama H, Sasaki T, Sasahira N, Tsujino T, Kogure H, et al. Comorbidity, not age, is prognostic in patients with advanced pancreatic cancer receiving gemcitabine-based chemotherapy. Critical reviews in oncology/hematology. 2011;78(3):252–259. doi: 10.1016/j.critrevonc.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Reuben DB, Rubenstein LV, Hirsch SH, Hays RD. Value of functional status as a predictor of mortality: results of a prospective study. The American journal of medicine. 1992;93(6):663–669. doi: 10.1016/0002-9343(92)90200-u. [DOI] [PubMed] [Google Scholar]
- 26.Albain KS, Crowley JJ, Hutchins L, Gandara D, O'Bryan RM, Von Hoff DD, et al. Predictors of survival following relapse or progression of small cell lung cancer. Southwest Oncology Group Study 8605 report and analysis of recurrent disease data base. Cancer. 1993;72(4):1184–1191. doi: 10.1002/1097-0142(19930815)72:4<1184::aid-cncr2820720409>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 27.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting Chemotherapy Toxicity in Older Adults With Cancer: A Prospective Multicenter Study. Journal of Clinical Oncology. 2011;29(25):3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanesvaran R, Li H, Koo KN, Poon D. Analysis of prognostic factors of comprehensive geriatric assessment and development of a clinical scoring system in elderly Asian patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(27):3620–3627. doi: 10.1200/JCO.2010.32.0796. [DOI] [PubMed] [Google Scholar]
- 29.Kern K, Norton J. Cancer cachexia. Journal of Parenteral and Enteral Nutrition. 1988;12(3):286–298. doi: 10.1177/0148607188012003286. [DOI] [PubMed] [Google Scholar]
- 30.McMillan DC, Elahi MM, Sattar N, Angerson WJ, Johnstone J, McArdle CS. Measurement of the systemic inflammatory response predicts cancer-specific and non-cancer survival in patients with cancer. Nutrition and cancer. 2001;41(1–2):64–69. doi: 10.1080/01635581.2001.9680613. [DOI] [PubMed] [Google Scholar]
- 31.Forrest L, McMillan D, McArdle C, Angerson W, Dunlop D. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. British journal of cancer. 2004;90(9):1704–1706. doi: 10.1038/sj.bjc.6601789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glen P, Jamieson NB, McMillan DC, Carter R, Imrie CW, McKay CJ. Evaluation of an Inflammation-Based Prognostic Score in Patients with Inoperable Pancreatic Cancer. Pancreatology. 2006;6(5):450–453. doi: 10.1159/000094562. [DOI] [PubMed] [Google Scholar]
- 33.Lien YC, Hsieh CC, Wu YC, Hsu HS, Hsu WH, Wang LS, et al. Preoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardia. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2004;8(8):1041–1048. doi: 10.1016/j.gassur.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 34.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. Journal of Clinical Oncology. 1999;17(8):2530-. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 35.Heys S, Walker L, Deehan D, Eremin E. Serum albumin: a prognostic indicator in patients with colorectal cancer. Age. 1998;38(3) 0.00005. [PubMed] [Google Scholar]
- 36.Chen KT-K. Patterns of recurrence and outcomes in pancreatic cancer. J Clin Oncol. 2013;31 (suppl 4; abstr 234) [Google Scholar]
- 37.Maréchal R, Demols A, Gay F, De Maertelaer V, Arvanitaki M, Hendlisz A, et al. Tolerance and efficacy of gemcitabine and gemcitabine-based regimens in elderly patients with advanced pancreatic cancer. Pancreas. 2008;36(3):e16–e21. doi: 10.1097/MPA.0b013e31815f3920. [DOI] [PubMed] [Google Scholar]
- 38.Emanuel EJ, Young-Xu Y, Levinsky NG, Gazelle G, Saynina O, Ash AS. Chemotherapy use among Medicare beneficiaries at the end of life. Annals of internal medicine. 2003;138(8):639–643. doi: 10.7326/0003-4819-138-8-200304150-00011. [DOI] [PubMed] [Google Scholar]
- 39.Nagrial AM, Chang DK, Nguyen NQ, Johns AL, Chantrill LA, Humphris JL, et al. Adjuvant chemotherapy in elderly patients with pancreatic cancer. Br J Cancer. 2014;110(2):313–319. doi: 10.1038/bjc.2013.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramsey SD, Howlader N, Etzioni RD, Donato B. Chemotherapy Use, Outcomes, and Costs for Older Persons With Advanced Non–Small-Cell Lung Cancer: Evidence From Surveillance, Epidemiology and End Results–Medicare. Journal of Clinical Oncology. 2004;22(24):4971–4978. doi: 10.1200/JCO.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 41.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89(6):1028–1030. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cella D, Eton D, Hensing TA, Masters GA, Parasuraman B. Relationship between symptom change, objective tumor measurements, and performance status during chemotherapy for advanced lung cancer. Clinical lung cancer. 2008;9(1):51–58. doi: 10.3816/CLC.2008.n.009. [DOI] [PubMed] [Google Scholar]
- 43.Frasci G, Lorusso V, Panza N, Comella P, Nicolella G, Bianco A, et al. Gemcitabine plus vinorelbine versus vinorelbine alone in elderly patients with advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18(13):2529–2536. doi: 10.1200/JCO.2000.18.13.2529. [DOI] [PubMed] [Google Scholar]
- 44.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(14):1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]