Abstract
Objective
To determine and compare the rates of HIV superinfection and primary HIV infection in high-risk female sex workers in Kampala, Uganda.
Design
A retrospective analysis of individuals who participated in a clinical cohort study among high-risk female sex workers in Kampala, Uganda.
Methods
Plasma samples from HIV-infected female sex workers (FSW) in Kampala, Uganda were examined with next-generation sequencing of the p24 and gp41HIV genomic regions for the occurrence of superinfection. Primary HIV incidence was determined from initially HIV-uninfected FSW from the same cohort, and incidence rate ratios were compared.
Results
The rate of superinfection in these women (7/85; 3.4/100py) was not significantly different from the rate of primary infection in the same population (3.7/100py; IRR=0.91, p=0.42). Seven women also entered the study dual infected (16.5% either dual or superinfected). The women with any presence of dual infection were more likely to report sex work as their only source of income (p=0.05), and trended to be older and more likely to be widowed (p=0.07).
Conclusions
In this cohort of female sex workers, HIV superinfection occurred at a high rate and was similar to that of primary HIV infection. These results differ from a similar study of high-risk female bar-workers in Kenya that found the rate of superinfection to be significantly lower than the rate of primary HIV infection.
Introduction
HIV superinfection (SI) occurs when an HIV-infected individual acquires a distinct new viral strain that is phylogenetically distinct from all previous viral strains within the individual [1-3]. HIV SI can significantly impact HIV disease markers by increasing HIV viral load, possibly accelerating disease progression, and putatively increasing HIV specific antibody responses post-SI [3-7]. Initial examinations of the rate of HIV-SI varied according to detection techniques and target populations, and led to varied results[3, 8]. With the advent of high-throughput next-generation sequencing (NGS) techniques to examine circulating viral populations, which is more sensitive at identifying minor variants and can be designed for large screens, it was possible to accurately determine the rate of SI in multiple populations [1, 2, 9, 10]. A study of HIV-SI using NGS in a rural region of Uganda (Rakai District) found that the rate of SI was comparable to the rate of primary HIV infection (PHI) in a general heterosexual population; as did a study of men who have sex with men (MSM) in California[2, 10]. However, two additional studies found significantly lower rates of SI compared to their respective PHI levels[9, 11, 12].
Understanding the relationship between SI and underlying PHI in populations with differing levels of risk could provide critically important information in regards to HIV vaccine design. The Good Health for Women Project (GHWP) of the Medical Research Council (MRC)-Uganda Virus Research Institute (UVRI) Research Unit recruited a longitudinal cohort of female sex workers (FSW) in Kampala, Uganda with an underlying HIV prevalence of 37%[13]. These prevalently positive women were followed and tested using a highly sensitive NGS assay to determine the level of HIV-SI in this high-risk population, and compared to originally HIV-negative women who seroconverted for HIV [1].
Materials and Methods
Ethics Statement
This study was approved by the Science and Ethics Committee of the Uganda Virus Research Institute and by the Uganda National Council for Science and Technology. Clinical, epidemiological data and blood samples were obtained following informed consent. At each visit, women received counseling for HIV/STI risk reduction and were treated for Sexually Transmitted Infections (STIs). Eligible women received antiretroviral therapy (ART) from accredited collaborating institutions.
Study Population
The GHWP study population has been described in detail previously [13, 14]. Briefly, FSW (n=1027) were invited to attend the GHWP clinic for enrollment and followed at quarterly visits. 382 women who joined the cohort were found to be prevalently infected with HIV[13]. A random selection of 125 women was obtained from these HIV prevalent cases. Specimens were examined from time of recruitment and from the latest follow-up visit recorded or before commencing ART. For women found to be superinfected, the estimated time of SI was determined by analyzing follow-up visits with identical techniques prior to the latest follow-up visit.
HIV pyrosequencing
The women’s samples were examined for SI as previously described[2]. Briefly, viral RNA was extracted from plasma samples, reverse-transcribed, and amplified in a nested-PCR format for a region of the viral p24 (~390 bp) and gp41 (~324 bp) coding regions. These regions were chosen due to their structural stability and relatively limited intra-host evolution. Samples that amplified for both time points in at least one region were sequenced using the 454 DNA Sequencing platform as previously described with some adjustments to use a 2-region format (Roche, Branford, CT)[1, 2]. Pools of samples were processed using emPCR Amplification Manual-Lib-L-LV – June 2013(Roche Branford, CT) using 25% of the recommended amplification primer amount and a 0.2 copy-per-bead ratio[1].
The resulting sequencing reads were analyzed and similar sequences were combined into a single consensus sequence. Consensus sequences that encompassed a cluster of at least ten individual, near-identical sequence reads were determined and used for all subsequent analyses[1, 2].
HIV SI was defined when a woman’s follow-up sample demonstrated two or more distinct consensus sequences forming a phylogenetic cluster that was distinct from the individual’s initial consensus sequences, and was of adequate genetic distance from the baseline sequences to rule out evolutionary drift[1]. Dual-infections were defined as individuals whose enrollment samples contained two phylogenetically distinct viral strains. All SI and dual-infections were verified with either identification in both genomic regions or a second sequencing run[2].
The NGS consensus sequences for gp41 and p24 are available upon request (aredd2@jhmi.edu).
Incidence calculation
The PHI rate was estimated for HIV-uninfected women who enrolled in GHWP and with at least one follow-up sample (n=598), providing 1,738py at risk, and determined as described previously [14].
Statistical Analysis
Individual demographics and behavioral characteristics for SI and PHI populations, total sequence reads and consensus sequences, as well as the dually infected and mono-infected women, were compared with a univariate Chi-square or Mann-Whitney Rank sum test (age). Incidence rate ratios were calculated using STATA 12 (College Station, TX) using an univariate Poisson model.
Results
Amplifiable sequences were obtained for at least one of two genomic regions for both time points in 85 women (68%; 36 had gp41 and gag data, 30 had gp41 data only, and 19 had gag data only). The other 40 women had at least one time point that would not amplify for either region, and therefore were not sequenced. There was a significantly higher number of sequence reads (p=0.003) and consensus sequences (p=0.022) in the first time point when compared to the second time point of the p24 genomic region, but not the gp41 region (Supplementary Table 1).
There were seven cases of HIV-SI detected in the 85 women with amplifiable sequences (Figure 1). SI was detected and confirmed in both genomic regions in three women, in gp41 only in three women, and in gag only in one woman (Supplementary Table 2). In addition, there were seven women who had initial dual infections, none of which became superinfected during the follow-up. Taken together with the SI cases, this demonstrated that 16.5% of women in this population experienced dual infection at some point during their disease. The total amount of follow-up for the 85 women was 206.1 total person years (py) with a median follow-up time of 2.75 person years (IQR=1.12-2.98). The rate of SI in this population was calculated to be 3.40/100py [95% confidence intervals (CI)=1.37-7.00]. The rate of PHI was 3.74/100py (65/1737.8py; 95% CI=2.89-4.77)[14]. This was not significantly different than the rate of SI (IRR=0.91; 95% CI=0.35-1.98; p=0.42).
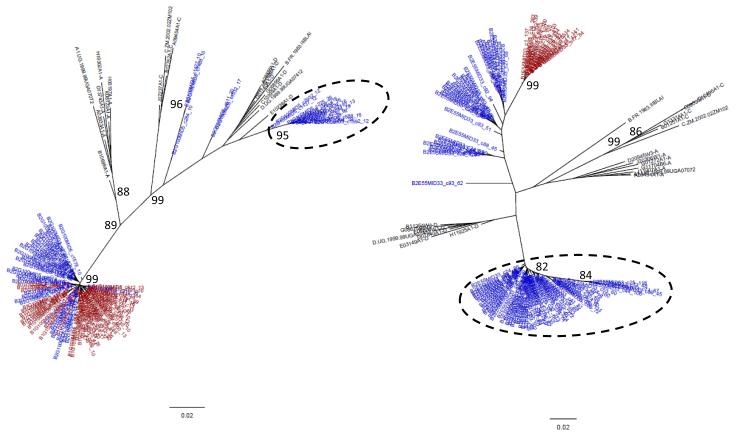
Figure 1. HIV superinfection case.
Phylogenetic tree of consensus (a) p24 and (b) gp41 viral sequences (≥10 reads) derived from 454 pyrosequencing of a subject’s enrollment (red) and last follow-up sample (38 months later, blue) with the superinfecting strain indicated (dashed circle). Number of repeated sequences represented by each consensus sequence is shown at the end of the consensus identifier. Distance is indicated for the tree by the scale at bottom, and samples are grouped with a selection of subtype reference sequences (black). Bootstrap values greater than 80 percent are indicated (1000 replicates).
The two populations examined were relatively similar in most behavior and demographics since they were chosen from the same cohort of high-risk women (Table 1)[13, 14]. However, the women screened for superinfection were older (p=0.02), less likely to consistently use condoms with paying clients (p=0.04), more likely to have ever used alcohol (p=0.04), and had higher prevalence of a variety of other sexually transmitted infections (STI; p<0.01) (Table 1). Due to the relatively limited number of superinfected women in the study these women were combined with the seven who were initially dual infected to examine possible risk-factors[3]. It was found that the dual infected women were significantly more likely to report sex work as their only source of income (p=0.05), and trended older and more likely to be widowed (p=0.07)(Supplementary Table 3).
Table 1.
Behavioral characteristics of HIV-SI and primary incidence cohorts
| Variable | Primary HIV Infection N=598 (%) |
HIV Superinfection N N= 85 (%) |
p-value |
|---|---|---|---|
| Age (median, IQR in years) | 25 (22-29) | 27 (23-30) | 0.02 1 |
|
| |||
| Marital status | 0.10 | ||
| Currently married | 47 (9) | 6 (7) | |
| Widowed | 18 (3) | 5 (6) | |
| Divorced or separated | 368 (61) | 60 (71) | |
| Never married | 165 (27) | 14 (16) | |
|
| |||
| Source of income | 0.66 | ||
| Sex work alone | 184 (31) | 29 (34) | |
| Sex work and other | 387 (65) | 51 (60) | |
| No sex work | 27 (4) | 5 (6) | |
|
| |||
| Number of sexual partners in last month | 0.63 | ||
| None | 50 (8) | 7 (8) | |
| 1-4 | 148 (25) | 22 (26) | |
| 5-19 | 181 (30) | 19 (22) | |
| 20-49 | 135 (23) | 23 (27) | |
| 50+ or can’t remember | 84 (14) | 14 (17) | |
|
| |||
| Condom use with paying clients last month2 | 0.04 | ||
| Consistently | 326 (62) | 37 (49) | |
| Not consistently | 202 (38) | 38 (51) | |
|
| |||
| Ever using alcohol | 0.04 | ||
| No | 144 (24) | 12 (14) | |
| Yes | 454 (76) | 73 (86) | |
|
| |||
| Frequency of alcohol drinking | 0.18 | ||
| Not using | 144 (24) | 12 (14) | |
| Less than once a week | 33 (6) | 5 (6) | |
| At least once a week | 275 (46) | 41 (48) | |
| Daily | 146 (24) | 27 (32) | |
|
| |||
| Use of hormonal contraception | 0.24 | ||
| None or natural methods | 335 (56) | 52 (61) | |
| Oral pill | 59 (10) | 12 (14) | |
| Injectable (Depot) | 148 (25) | 17 (20) | |
| Pregnant | 56 (9) | 4 (5) | |
|
| |||
| HSV2 serology | <0.01 | ||
| Negative | 168 (28) | 7 (8) | |
| Positive | 430 (72) | 78 (92) | |
|
| |||
| Syphilis | 0.008 | ||
| Negative (TPHA−RPR−) | 503 (85) | 61 (72) | |
| Past infection (TPHA+RPR−) | 45 (7) | 14 (16) | |
| Active infection (TPHA+RPR+) | 47 (8) | 10 (12) | |
|
| |||
| N. gonorrhoeae (PCR)3 | 0.005 | ||
| Negative | 543 (91) | 69 (81) | |
| Positive | 54 (9) | 16 (19) | |
|
| |||
| C. trachomatis (PCR)3 | 0.55 | ||
| Negative | 539 (90) | 75 (88) | |
| Positive | 58 (10) | 10 (12) | |
|
| |||
| T. vaginalis (Culture) | <0.01 | ||
| Negative | 517 (86) | 61 (72) | |
| Positive | 81 (14) | 24 (28) | |
|
| |||
| M. genitalium (PCR)4 | 0.40 | ||
| Negative | 524 (88) | 72 (85) | |
| Positive | 72 (12) | 13 (15) | |
|
| |||
| Bacterial vaginosis (Nugent score) | 0.005 | ||
| Negative | 241 (40) | 19 (22) | |
| Intermediate | 55 (9) | 12 (14) | |
| Positive | 302 (51) | 54 (64) | |
|
| |||
| Abnormal vaginal discharge on examination | 0.14 | ||
| No | 254 (42) | 29 (34) | |
| Yes | 344 (58) | 56 (66) | |
|
| |||
| Genital ulcer disease on examination | 0.14 | ||
| No | 566 (95) | 77 (91) | |
| yes | 32 (5) | 8 (9) | |
-t-test;
-Women not reporting paying clients in the past month are excluded;
-1 missing;
-2 missing
Discussion
The initial descriptions of HIV-SI described occurrences in relatively high-risk individuals [6, 15, 16]. Since these seminal studies determining the rate of SI and the role that underlying HIV risk plays on this phenomenon has been a priority for the field [3, 5]. An initial study examining HIV-SI in FSW in Burkina Faso using a less sensitive heteroduplex mobility assay found two SI cases in 147 women screened [3, 8]. With the advent of NGS based systems to screen for SI in a highly sensitive high-throughput manner it has been possible to study large enough populations to fully examine the rate of SI and compare it to primary infection rates in a variety of well-described large longitudinal cohorts around the world [2, 9, 10, 12]. Using similar techniques, two reports found similar rates of SI and primary incidence, and two found significantly lower rates of SI [2, 9, 10, 12]. These data presented here support the findings that the rate of SI and incidence can be similar. This is in contrast to a similar study in Kenyan bar workers where the rate of SI was approximately half the rate of primary incidence [9]. It should be noted that the population screened here are all women who report engaging in commercial sex work, whereas the Kenyan study is of female bar workers. However, both populations are relatively high-risk women, and the Kenyan study identified an increased number of cases of SI (n=21) over a longer total follow-up time [9]. These increased events allowed them to predict the rate of superinfection with a high precision. Interestingly, our study was powered to identify a difference in SI rate of half or double the rate of primary incidence, which was the magnitude of difference seen in the Kenyan study [9]. One other difference between these two groups of women is that in the case of the Kenyan study the women were seen on a monthly basis post-infection where they received full risk-reduction counseling [17]. Women enrolled in GHWP study in Kampala were seen every three months where they also regularly received intensive risk-reduction counseling and STI treatment [13]. It is interesting to speculate that the repeated reinforcement provided by the monthly visits in the Kenyan cohort decreased the risk of HIV superinfection in those women[9, 12, 18]. One other difference is that in the Kampala population women entered into the study prevalently infected, whereas the other three studies examined seroconverters [2, 9, 10, 13].
One of the differences between the populations studied here was the higher rates of other STIs found in the HIV prevalent women. However, the women in this study were treated for bacterial STIs as part of the study so the effect of these infections on HIV risk will most likely be somewhat muted.
A previous analysis of a general heterosexual population in the rural Rakai District, Uganda using virtually identical strategies to detect HIV-SI found that the rate of HIV-SI was 1.4/100py [2]. There was trend towards a higher rate of SI in the female sex workers in Kampala when compared to rate of SI in this rural lower risk population (IRR=2.36; 95% CI=0.71-7.87; p=0.060). Regardless of the relationship between rates of SI and primary infection, this finding suggests that individuals who are participating in higher risk behavior are most likely at an increased risk of both PHI and SI.
These findings add to the growing evidence that HIV-SI occurs at a significant rate throughout the world. A better understanding of this phenomenon and how it affects disease progression and transmission dynamics is critical. SI also provides a unique avenue to explore which aspects of the natural HIV immunological response are important for protecting against a subsequent challenge, which could help to inform ongoing vaccine initiatives.
Supplementary Material
Acknowledgements
The authors would like to thank all the women who participated in the study, and the staff of the Good Health for Women Project. This study was supported by the Division of Intramural Research, the Bench to Bedside Program, and the Office of AIDS Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Cohort recruitment and follow-up were funded by The Medical Research Council, of the United Kingdom, the European and Developing Countries Clinical Trials Partnership (EDCTP) grantnumbersCG_ct_05_33070, TA.2007.40200.011and CG-2007-40200-001 and Wellcome Trust Strategic Award, grant number 084344.
References
- 1.Redd AD, Collinson-Streng A, Martens C, Ricklefs S, Mullis CE, Manucci J, et al. Identification of HIV superinfection in seroconcordant couples in Rakai, Uganda using next generation deep sequencing. J Clin Microbiol. 2011;49 doi: 10.1128/JCM.00804-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redd AD, Mullis CE, Serwadda D, Kong X, Martens C, Ricklefs SM, et al. The Rates of HIV Superinfection and Primary HIV Incidence in a General Population in Rakai, Uganda. J Infect Dis. 2012;206:267–274. doi: 10.1093/infdis/jis325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redd AD, Quinn TC, Tobian AA. Frequency and implications of HIV superinfection. Lancet Infect Dis. 2013 doi: 10.1016/S1473-3099(13)70066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortez V, Odem-Davis K, McClelland RS, Jaoko W, Overbaugh J. HIV-1 superinfection in women broadens and strengthens the neutralizing antibody response. PLoS Pathog. 2012;8:e1002611. doi: 10.1371/journal.ppat.1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith DM, Wong JK, Hightower GK, Ignacio CC, Koelsch KK, Daar ES, et al. Incidence of HIV superinfection following primary infection. JAMA. 2004;292:1177–1178. doi: 10.1001/jama.292.10.1177. [DOI] [PubMed] [Google Scholar]
- 6.Altfeld M, Allen TM, Yu XG, Johnston MN, Agrawal D, Korber BT, et al. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature. 2002;420:434–439. doi: 10.1038/nature01200. [DOI] [PubMed] [Google Scholar]
- 7.Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, Dekosky BJ, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014 doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manigart O, Courgnaud V, Sanou O, Valea D, Nagot N, Meda N, et al. HIV-1 superinfections in a cohort of commercial sex workers in Burkina Faso as assessed by an autologous heteroduplex mobility procedure. AIDS. 2004;18:1645–1651. doi: 10.1097/01.aids.0000131333.30548.db. [DOI] [PubMed] [Google Scholar]
- 9.Ronen K, McCoy CO, Matsen FA, Boyd DF, Emery S, Odem-Davis K, et al. HIV-1 Superinfection Occurs Less Frequently Than Initial Infection in a Cohort of High-Risk Kenyan Women. PLoS Pathog. 2013;9:e1003593. doi: 10.1371/journal.ppat.1003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner GA, Pacold ME, Kosakovsky Pond SL, Caballero G, Chaillon A, Rudolph AE, et al. Incidence and Prevalence of Intrasubtype HIV-1 Dual Infection in At-Risk Men in the United States. J Infect Dis. 2013 doi: 10.1093/infdis/jit633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redd AD, Mullis CE, Wendel SK, Sheward D, Martens C, Bruno D, et al. Limited HIV-1 superinfection in seroconverters from the CAPRISA 004 microbicide trial. J Clin Microbiol. 2013 doi: 10.1128/JCM.03143-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandepitte J, Bukenya J, Weiss HA, Nakubulwa S, Francis SC, Hughes P, et al. HIV and other sexually transmitted infections in a cohort of women involved in high-risk sexual behavior in Kampala, Uganda. Sex Transm Dis. 2011;38:316–323. [PMC free article] [PubMed] [Google Scholar]
- 14.Vandepitte J, Weiss HA, Bukenya J, Nakubulwa S, Mayanja Y, Matovu G, et al. Alcohol use, mycoplasma genitalium, and other STIs associated With HIV incidence among women at high risk in Kampala, Uganda. J Acquir Immune Defic Syndr. 2013;62:119–126. doi: 10.1097/QAI.0b013e3182777167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jost S, Bernard MC, Kaiser L, Yerly S, Hirschel B, Samri A, et al. A patient with HIV-1 superinfection. N Engl J Med. 2002;347:731–736. doi: 10.1056/NEJMoa020263. [DOI] [PubMed] [Google Scholar]
- 16.Ramos A, Hu DJ, Nguyen L, Phan KO, Vanichseni S, Promadej N, et al. Intersubtype human immunodeficiency virus type 1 superinfection following seroconversion to primary infection in two injection drug users. J Virol. 2002;76:7444–7452. doi: 10.1128/JVI.76.15.7444-7452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin HL, Jr., Nyange PM, Richardson BA, Lavreys L, Mandaliya K, Jackson DJ, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178:1053–1059. doi: 10.1086/515654. [DOI] [PubMed] [Google Scholar]
- 18.McClelland L, Wanje G, Kashonga F, Kibe L, McClelland RS, Kiarie J, et al. Understanding the context of HIV risk behavior among HIV-positive and HIV-negative female sex workers and male bar clients following antiretroviral therapy rollout in Mombasa, Kenya. AIDS Educ Prev. 2011;23:299–312. doi: 10.1521/aeap.2011.23.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.