Abstract
The Morris water maze (MWM) behavioral paradigm is commonly used to measure spatial learning and memory in rodents. It is widely accepted that performance in the MWM declines with age. However, young rats ubiquitously perform very well on established versions of the water maze, suggesting that more challenging tasks may be required to reveal subtle differences in young animals. Therefore, we have used a one-day water maze and novel object recognition to test whether more sensitive paradigms of memory in young animals could identify subtle cognitive impairments early in life that might become accentuated later with senescence. We have found that these two tasks reliably separate young rats into inferior and superior learners, are highly correlated, and that performance on these tasks early in life is predictive of performance at 12 months of age. Furthermore, we have found that repeated training in this task selectively improves the performance of inferior learners, suggesting that behavioral training from an early age may provide a buffer against age-related cognitive decline.
Keywords: Young rats, Morris water maze, Inferior learners, Superior learners, Novel object recognition memory impairment, Hippocampus, Aging, Cognitive impairment
1. Introduction
It is widely accepted that performance in the Morris water maze declines with age [2,8,40], possibly as a result of changes in hippocampal morphology and function [3,8,13,14]. Furthermore, aged rodents show a wide range in individual performance in this task, with some animals demonstrating drastic impairments and other animals retaining a high level of cognitive functioning [4,11,12,26]. Humans demonstrate individual variability in both childhood cognitive ability and the degree of impairments later in life, and it remains unclear whether increased cognition in childhood may have a protective effect against cognitive decline associated with aging [17,18,31]. Due to the time constraints of conducting longitudinal studies in human populations, rodents are a good animal model to study the relationship between cognition in youth and the degree of impairment later in life. However, in order to accurately determine the change in cognitive functioning over time it is necessary to have an accurate baseline measure of performance for each animal at a young age. This is problematic, as young rats generally only demonstrate slight individual variability in established versions of the Morris water maze [27,28,39] and impairments are typically induced by selective drug applications [21,22,25,36]. In addition, currently established versions of the Morris water maze induce memories that can last up to 12 months, preventing repeated testing in the task [7,40]. Therefore, it is important to develop more sensitive testing paradigms that can identify subtle differences in the performance of young rats and be repeated across the lifespan to test the hypothesis that early life performance is predictive of cognitive decline in the senescent brain. This would provide useful information for understanding how performance early in life may impact cognitive abilities with age in human populations.
While condensed versions of the water maze lasting only one or two days have previously been developed [20,23,19] these protocols are either intensive, consisting of multiple blocks of training, or have not attempted to separate naïve young rats based upon individual performance in the task. In order to test our hypothesis that cognitive impairments can be identified early in life, we implemented a modified water maze paradigm that successfully separates young rats into inferior and superior learners with just one day with four trials of hidden platform training. We have found that this task can be repeated in just six weeks, significantly reducing the amount of time in between testing sessions compared to established water maze methods. We have previously shown that young rats display great variability in their performance in the object location memory task [15,16]. Here we used novel object recognition (NOR) to characterize these animals, and our results demonstrate that performance on the one-day water maze correlates highly with performance on NOR. We have used these two independent behavioral tasks to follow the animals from 3–12 months of age, and our results indicate that animals that show inferior learning early in life demonstrate more accentuated behavioral deficits at 12 months of age than animals who displayed high levels of cognitive functioning at a young age. Furthermore, we have found that repeated training selectively improves the performance of inferior learners, suggesting that cognitive training from an early age can provide a buffer against future age-related cognitive decline.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats were purchased from the Harlan rodent colony at 2 months of age. All animals had free access to water and food and were kept on a 12:12 light dark cycle. Behavioral tests were given during the light cycle. In order to ensure the reproducibility of the protocols, two separate cohorts of animals were tested (n = 10 for each cohort). All procedures were approved by the University of Wisconsin Institutional Animal Care and Use Committee and were conducted in accordance with the U.S. National Institutes of Health ‘Guide for the Care and Use of Laboratory Animals’.
2.2. Water maze
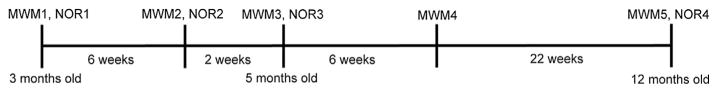
The behavioral regimen is shown in Fig. 1.
Fig. 1.
Behavioral regimen. Behavioral testing began at 3 months of age with MWM1 immediately followed by NOR1. Six weeks following completion of MWM1, animals were tested again in MWM2, immediately followed by NOR2. At 5 months of age, two weeks following completion of MWM2, animals were tested in MWM3 followed by NOR3. Six weeks following completion of MWM3, animals were tested in MWM4. At 12 months of age, animals were tested for a final time in MWM5, followed by NOR4.
2.2.1. MWM1 3 months of age
Water Maze testing began at the age of 3 months. The maze consisted of a dark blue tank 173 cm in diameter, filled with 20–22 °C water. The water was not dyed because both visible and hidden platforms were made of clear Lucite. Both hidden and visible platforms were 10 × 10 cm, with the standing area submerged ~5 cm below the surface of the water.
During the first day of the water maze, animals were acclimated to the task with a visible platform placed in the southeast quadrant of the pool. The platform was made visible by the use of black and white posts protruding from the top of the platform itself. Each animal was dropped from the North, East, South, and West zones over four consecutive trials. Each trial, the animal was placed in the water facing the wall and given 90 s to find the platform. Upon finding the platform, the animal was allowed to sit on the platform for approximately 10 s before being removed and thoroughly dried with a towel. The rat was then taken to the next drop zone for the next trial to begin. If a rat was not able to find the platform at 90 s, he was guided to the platform and allowed to sit on it for 10 s before being removed and dried off. Animals were given four trials on this visible training day. The distance traveled before reaching the platform was analyzed and measured using Videotrack software by ViewPoint Life Sciences (Montreal, Canada). At the end of all 4 trials for a subject on a given day, the rat was dried with a towel, placed in a heated dry-off cage under a blow drier until thoroughly dry, and returned to his home cage. This training trial also served to test for swimming ability and visual acuity, although in young rats no differences in visual acuity were observed.
The hidden platform version of the MWM was performed on day 2 of the task. On hidden platform trials, the platform was always located in the Southeast quadrant of the pool. The hidden platform session was conducted in the same manner as described for visible platform training, with the drop zones completed in a different order and the platform hidden beneath the surface of the water. The probe trial was conducted immediately after completion of the four invisible trials. For the probe trial, the platform was removed immediately after the last hidden trial, the animal was reintroduced to the pool and allowed to swim for 60 s. Percent of total distance covered and percent of time spent in the target quadrant that previously contained the platform was measured. Platform crossings in the probe trial were calculated by tallying the number of times each subject entered the platform zone during the 60 s trial. Rats were classified as superior, intermediate, and inferior learners based on their performance on the probe trial as compared to probe trial performance of the group. Total distance swam and latency to find the platform during the hidden platform trials was also measured.
2.2.2. MWM2—6 weeks
Six weeks following the probe trial of the first round of water maze, the same rats were subjected to the water maze again to test whether they remembered the original location of the platform or if this memory was only short term. This second round of water maze was performed as in MWM1, but with no visible platform training. Hidden platform training lasted only one day. On this day of the task, the hidden platform was submerged beneath the surface of the water in the southeast quadrant of the pool as in MWM1. The rats were placed into the pool from the North, East, South, and West quadrants as described above, and given 90 s to find the platform. The total distance swam on each trial was measured and compared to the learning curve from MWM1. In addition, the average distance swam in all 4 hidden platform trials was measured and compared to the average distance swam in all 4 hidden platform trials of MWM2. To avoid the confounding effects of extinction on retention of the platform location in future MWM trials, a probe trial was not performed in this and following rounds of the water maze.
2.2.3. MWM3—8 weeks
Two weeks following the second water maze trial, the same rats were again subjected to the water maze again to determine if the length of time before re-testing could be further reduced. As in MWM2, the rats did not receive visible platform training. Rats were only subjected to one day of four trials with the hidden platform in the southeast quadrant, as in MWM1 and MMW2. Data was analyzed as in MWM2: the total distance swam in each trial was measured and compared to the learning curve from MWM1 and MWM2, and the average distance swam in MWM3 was compared to the average distance swam in MWM1 and MWM2.
2.2.4. MWM4—14 weeks
Six weeks following MWM3, the same rats were again tested in the water maze to determine whether repeated training in the task would affect retention of the platform location. As in MWM2 and MWM3, the rats were not subjected to visible platform training. The hidden platform was placed in the southeast quadrant, and rats received four hidden trials as in the previous MWM sessions. The total distance swam in each trial was measured and compared to the learning curve from MWM1, MWM2, and MWM3. In addition, the average distance swam in MWM4 was compared to the average distance swam in the first three rounds of MWM.
2.2.5. MWM5—12 months of age
When the rats were 12 months of age, the same rats were again tested in the water maze to determine whether animals that demonstrated deficits earlier in life would have more pronounced cognitive deficits with senescence. As in all previous sessions, the platform was submerged beneath the water in the southeast quadrant of the pool. The animals received two days of hidden platform training, in which the animal was dropped from all four quadrants and given 90 s to find the platform. After the last hidden trial of day 2, the platform was removed from the pool and a probe trial was conducted in which the animal was placed in the pool for 60 s and the number of platform crossings was scored.
2.2.6. MWM in Naïve 12-month old animals
Naïve 12-month old rats were tested in the water maze to compare performance of trained and untrained rats at this time point. These animals were first exposed to the task with 1 day of visible platform training, as described in MWM1. Following visible training, the animals received 2 days of hidden platform training as described in MWM5. Following the last hidden trial of day 2, the platform was removed and the animals received a 60 s probe trial. As in MWM5, the number of platform crossings during the probe trial was scored.
2.3. Novel object recognition (NOR)
2.3.1. NOR1
Testing in novel object recognition began immediately following MWM1, at 3 months of age. The experimental apparatus measured 40.65 × 40.65 × 30.5 cm and corncob bedding was spread ~2″ deep on the floor. To encourage exploration, direct overhead lighting was not used. Instead, knee-level fluorescent lighting was used to provide indirect illumination. On each day of the experiment, the arena and objects were cleaned with 70% ethanol and fresh bedding was put down to limit olfactory cues.
On the first day (training day), rats were trained on the locations of two identical objects. Miniature flamingo figurines were placed in corner locations A and B, approximately, 2.5 cm from the sides of the arena. Rats were allowed to explore the arena and the two objects freely over the course of a 6-min trial. Toys were adhered to the floor of the arena using double-sided tape to ensure that the animals would not be able to disrupt object location over the course of the investigation period. The bedding was stirred and the toys were cleaned with 70% ethanol, before moving on to the next animal.
Testing of object recognition memory occurred 24 h after training. Rats were tested on their preference for a new object compared to the old object. The objects were similar in size and darkness of color, but with slightly different shapes. In each trial, one of the flamingos from the training day was replaced with a miniature figurine of finches. All objects were pre-tested for saliency using a different group of rats to ensure that the rats investigated the finches and flamingos equally, indicating that the objects were equally interesting to the animals. The identity of the objects was counterbalanced, with half the rats presented with the new object on the right, the other half presented with the new object on the left. This was done to reduce potential biases due to a preference for one side of the arena. The orientation of the box is the same as on the previous day, and the rats were again allowed six minutes to explore.
All trials on both the training and testing days were videotaped and analyzed by an experimenter blind to the identity of the rat, using Videotrack software by ViewPoint Life Sciences (Montreal, Canada). Total amount of time spent exploring the novel and familiar objects was recorded for each animal. A rat was scored as exploring an object when it was directly sniffing or rubbing the object with its head or whiskers, biting, or licking the object. Looking without directly touching, sitting on, standing on, or sniffing the air above an object was not scored. The relative exploration time was recorded for each object and expressed as a novelty index (time spent (s) investigating novel object/time spent (s) investigating both objects in total).
2.3.2. NOR2—6 weeks
Six weeks following NOR1, the novel object recognition task was again performed to determine whether performance on the task was consistent for each animal. NOR2 was conducted exactly as NOR1, but miniature Halloween decorations were used to ensure that the animals were forming new memories of novel objects.
2.3.3. NOR3—8 weeks
Two weeks following completion of NOR2, animals were again tested in novel object recognition. The task was conducted exactly as in NOR2, but miniature pumpkins were used to ensure formation of novel memories was occurring.
NOR was not conducted after the MWM4 time point to avoid habituation to unique objects within the testing environment. Thus, the animals were not tested in between the ages of 5 and 12 months to ensure that at the 12 month time point there would be a high interest in the objects presented.
2.3.4. NOR4—12 months of age
At 12 months of age, the animals were tested once more in NOR did to determine whether the individuals who were impaired on this task at an early age also demonstrate impairments later in life. NOR 4 was conducted exactly as in previous NOR sessions, but bird figurines were used to ensure novelty.
2.4. Statistical analysis
All statistical analyses were performed using Prism 5 (Graphpad Software, Inc., La Jolla, CA). Categorization of young animals into inferior and superior learners was based on the total distance swam in hidden platform training of MWM1. The total distance swam was calculated, and animals that swam one standard deviation or more above the mean were classified as inferior whereas animals that performed one standard deviation or more below the mean were classified as superior learners. Pearson’s correlation analysis was conducted to validate the use of total distance swam during the hidden platform trials as a predictor of platform crossings during the probe trial. Repeated measures ANOVA was conducted to determine whether the classification of animals as inferior and superior remained consistent from 3 months of age to 12 months of age. To indicate the length of time required before platform location was forgotten, paired t-tests were conducted to determine if the distance swam changed significantly from the last trial of a given water maze session to the first trial of the next. For example, paired t-tests were conducted to determine if the distance swam on trial 4 was significantly different from the distance swam on trial 5. Repeated measures ANOVA with Bonferroni post-tests was conducted to determine if the total distance swam across all four trials changed significantly across training sessions (MWM1, MWM2, MWM3, and MWM4). For NOR, categorization of animals was based upon the amount of time spent sniffing the novel object relative to the total investigation time (the novelty index). A novelty score of 0.5, indicating that animals investigated the novel object and the familiar object for an equal amount of time, was used as the classification for inferior learners. Animals that performed one standard deviation above chance levels were classified as superior learners. Animals that performed one standard deviation below chance, spending a significantly greater amount of time investigating the familiar object than the novel object, were classified as neophobic and were removed from classification. Repeated measures ANOVA with Bonferroni post-tests was conducted to determine the congruency of performance from NOR1 to NOR4. Pearson’s correlation analysis was used to assess whether the total distance during hidden platform training of MWM1 correlated with the number of platform crossings during the probe trial of MWM1. Pearson’s correlation analysis was also conducted to determine whether performance on MWM1 correlated with NOR1, and whether performance on MWM5 correlated with performance on NOR4. Pearson’s correlation analysis was further used to determine whether performance in MWM1 correlated with performance on MWM5 to assess consistency of performance over the lifespan.
3. Results
3.1. One-day of hidden platform MWM training separates young rats into inferior and superior learners
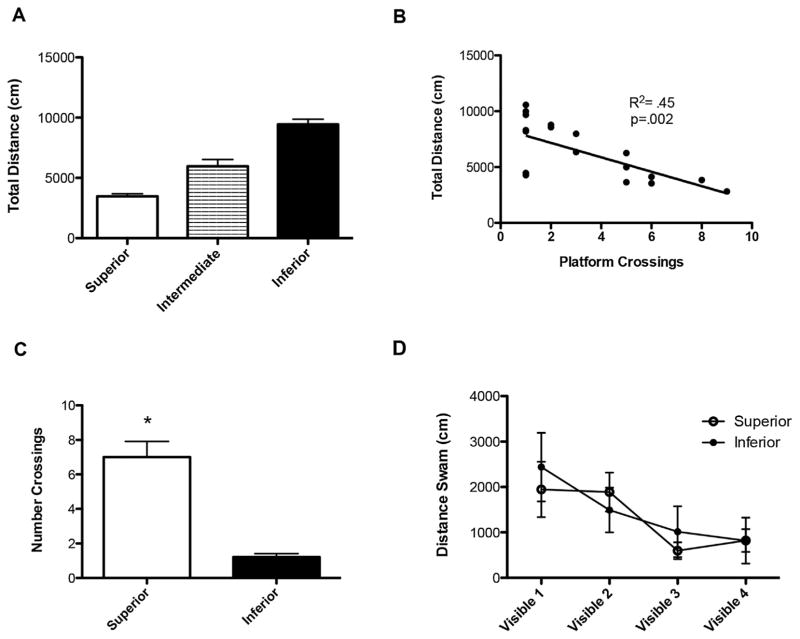
Three-month old rats were subjected to the one-day water maze paradigm (MWM1) and were classified as inferior or superior learners based upon the total distance swam during hidden platform training. We found that there was high individual variability in the distance swam, enabling us to categorize animals based upon hidden training performance. We found that the mean distance swam during hidden platform training in MWM1 was 6354 cm, and that 53% of the rats fell within one standard deviation of the mean to be classified as intermediate learners (n = 10). We found that 21% of the animals performed more than one standard deviation below the mean, swimming an average of 3463 cm and were classified as superior learners (n = 4). Alternatively, 26% of the group swam an average of 9442 cm and were classified as inferior learners (n = 5) (Fig. 2A). Pearson’s correlation analysis reveals a significant correlation between the total distance swam in hidden platform training of MWM1 and the number of platform crossings in the probe trial of MWM1 (Fig. 2B, R2 = 0.45, p = 0.002). Animals classified as superior based upon total distance swam in hidden platform training demonstrated significantly more platform crossings during the probe trial than those classified as inferior (Fig. 2C, unpaired t-test, t = 6.961, df = 7, p < 0.001). Therefore, total distance swam during the hidden platform trials can be used to separate young animals into inferior and superior learners. Importantly, we saw no significant differences in visible platform training between groups, indicating that differences in hidden platform training are not simply due to differences in visual acuity or swimming ability (Fig. 2D).
Fig. 2.
(A) Young rats can be classified into inferior (n = 5), intermediate (n = 10), and superior learners (n = 4) based upon the total distance swam in hidden platform training during MWM1. (B) The number of platform crossings during the probe trial correlates strongly with the total distance swam during the four hidden trials of MWM1, demonstrating that total distance swam during the hidden platform trials can be used to classify animals as impaired and unimpaired (n = 19, R2 = 0.45, p = 0.002). (C) Animals classified as superior based upon hidden platform training demonstrate significantly more platform crossings than animals classified as inferior based upon hidden platform training (p < 0.01). (D) Superior and inferior learners do not show differences in ability to locate the platform during visible platform training.
3.2. Platform location is forgotten after a six week delay without memory carryover, but repeated training results in lasting memory in this task
We wanted to determine whether this version of the MWM could be used repeatedly to track the performance of individual animals at various points in their lifespan. To accomplish this, rats were tested 6 weeks after MWM1 (MWM2), and then again 2 weeks later (MWM3) at 5 months of age. Rats were then tested 6 weeks after MWM3 (MWM4) and were tested last at 12 months of age in MWM5 (see Fig. 1 for experimental timeline).
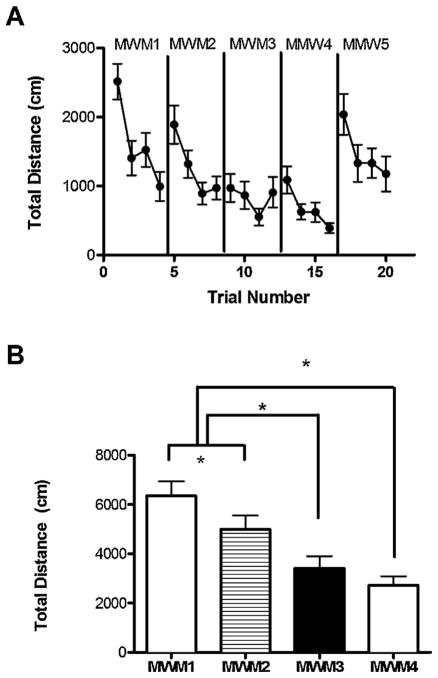
Six weeks after MWM1 animals were re-tested in the hidden platform (MWM2). The distances swam during the four hidden trials of MWM 1 were compared to the distances swam during the four hidden trials of MWM2 (Fig. 3A). As shown in the figure, during the first four trials (representing the entire hidden platform session of MWM1) the distance swam from trial to trial decreases as the animal learns the location of the platform. However, on the first trial of MWM2 (trial 5), the total distance swam is significantly greater than the distance swam in the last trial of MWM1, trial 4 (Fig. 3A, paired t-test, t = 3.684, df = 18, p = 0.0017). This disruption of the learning curve indicates that the rats have forgotten the location of the platform from the last trial of MWM1, suggesting that six weeks is enough time to pass before re-testing the rats in this paradigm. To further test whether previous training in MWM1 impacted performance in MWM2, the total distance swam in hidden platform training of MWM1 was compared to the total distance swam in hidden platform training in MWM2. The total distance swam in MWM2 is significantly less than in MWM1. (Fig. 3B, paired t-test, t = 2.411, df = 18, p = 0.02) This suggests that although initially the rats do not remember the location of the platform from their previous exposure to the maze (as indicated in Fig 3A), previous training facilitates learning during the subsequent hidden platform trials of MWM2.
Fig. 3.
Retention of platform location is dependent on the length of time passed in between testing sessions and the amount of prior training. (A) Location of the platform is forgotten after the six-week inter-trial period between MWM1 and MWM2, but not in the following MWM3 and MWM4. (B) The total distance swam in the hidden trials does not change from MWM1 to MWM2, but it is significantly lower than the total distance swam in MWM3 and MWM4. Asterisk signifies p < 0.05.
Next, we wanted to determine whether rats would retain some memory of the task after only a two-week delay. Rats were retested in the hidden platform in MWM3 two weeks after MWM2 (5 months of age, 2 months after initial testing in MWM1). As shown in Fig. 3A, the distance swam in the first trial of MWM 3 (trial 9) was nearly identical to the distance swam in the last trial of MWM2 (trial 8), suggesting that the rats remember the location of the platform from their MWM2 training. In addition, the total distance swam across all four hidden platform trials was calculated for MWM1, MWM2, and MWM3 to determine if previous exposure to the maze affected performance in MWM3. This total distance is significantly less in the third round than in MWM1 (paired t-test, t = 5.385, df = 18, p < 0.0001) and MWM2 (paired t-test, t = 3.754, df = 18, p = 0.0015), indicating that the location of the platform has not been forgotten in the two weeks in between MWM2 and MWM3 (Fig. 3B). This data indicates that this one-day version of the Morris water maze creates long term memories that persist for two weeks after training, and possibly that there is memory carryover from two previous rounds of MWM training. To further elucidate whether repeated training could enhance retention of the platform location, animals were tested in MWM4 6 weeks following MWM3. We found that the animals showed memory carryover from previous training (Fig. 3A and B, MWM4), suggesting that memory of the platform location is maintained by repeated training in the task.
3.3. Individual performance in MWM is consistent from 3–5 months of age
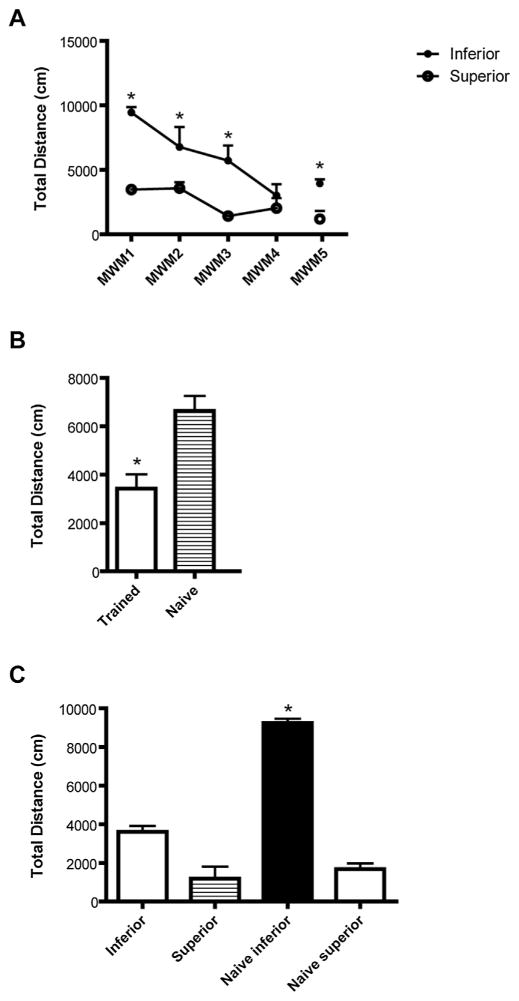
Next, we wanted to determine whether early learning ability is consistent throughout various stages of adulthood. Animals were classified superior or inferior learners based on total distance swam during hidden platform training in MWM1, as shown in Fig. 2A. These assignments into inferior and superior based upon MWM1 were maintained for the duration of MWM testing. The total distance swam during MWM2, MWM3, and MWM4 was calculated for these animals and used to determine whether the separation between inferior and superior animals would be maintained over the course of young adulthood. As shown in Fig. 4A, animals classified as inferior in MWM1 consistently swim a greater distance during the hidden platform trials of MWM2 and MWM3 than the superior learners, demonstrating a high congruency in performance for this water maze paradigm (Fig. 4A, repeated measures ANOVA, F(1,31) = 32.41, p < 0.0001). This indicates that performance is highly consistent up until MWM3, when the animals are 5 months old. However, by MWM4 animals previously classified as inferior learners performed at the same level as superior learners (Fig. 4A). This reflects that increased training improves the performance of impaired animals to the point where they ubiquitously perform quite well in the task. This improvement in performance with training corresponds with the data previously shown in Fig. 3A and B.
Fig. 4.
(A) Individual performance in MWM is consistent from 3–5 months of age. Animals classified as inferior in MWM1 continue to perform significantly worse than superior animals in MWM2 and MWM3 (p < 0.05). By MWM4 there is no distinction between inferior and superior learners. Individuals classified as inferior in MWM1 show greater cognitive deficits in the MWM5 than those classified as superior (p < 0.05), indicating that performance at 3 months of age is predictive of performance at 12 months. (B) Training at an early age prevents cognitive decline at 12 months of age. The total distance swam on day 2 of hidden platform training by naïve 12 month old animals was significantly worse than that of 12 month old animals with previous exposure to the MWM paradigm (p < 0.05). (C) Training specifically improves performance of inferior learners and does not have significant impact on the performance of superior learners.
3.4. Animals classified as impaired early in life continue to show deficits at 12 months of age
Animals were again tested in the water maze at 12 months of age in MWM5 (22 weeks following MWM4). At this age, great variability in performance was observed on the first day of hidden platform training and as such a second day of hidden platform training was conducted. On the second day of hidden platform training, we found that animals that were classified as inferior based upon probe trial performance in MWM1 were also consistently slower to find the platform at 12 months of age compared to those that were classified as superior in MWM1. The total distance swam before finding the platform in MWM5 for inferior learners on day 2 of MWM was an average of 3927 cm, whereas the average for superior learners in MWM5 was 1183 cm (Fig. 4A, MWM5, unpaired t-test, t = 4.325, df = 3, p < 0.05).
For both inferior and superior learners, performance on the water maze task improved with training in MWM1–MWM5. This effect is particularly evident in inferior learners, who improved significantly from MWM1 to MWM5 in hidden platform training (Fig. 4A, paired-test, t = 7.130, df = 2, p < 0.05). This is consistent with other studies that have shown that training can improve performance in the Morris water maze [9,32,41,42]. To investigate the extent to which training could improve performance on the water maze at 12 months of age, we conducted the water maze in naïve 12-month old rats that had not previously been exposed to the maze. These animals were given two days of hidden platform training in order to ensure fair comparison between naïve and trained groups. We found that the total distance swam during the second day of hidden platform training was significantly greater in naïve animals compared to animals that had received previous training in the task (Fig. 4B, unpaired t-test, t = 3.723, df = 34, p < 0.001), providing additional evidence that training at an early age can provide a buffer against age-related deficits in this task. Results from the learning curve shown in Fig. 3A indicate that no memory carryover was present in MWM5 compared to previous exposures to the maze. This data indicates that improved performance in trained animals at 12 months of age compared to naïve animals is not simply due to memory savings from previous exposure to the maze. Importantly, we found that this training only improved performance for inferior learners. Naïve animals were classified into inferior and superior based upon total distance swam in day 2 of hidden platform training. As described previously, animals that swam one standard deviation less than the mean distance were classified as superior and those that swam one standard deviation above the mean distance were classified as inferior learners. The performance of these naïve inferior and superior animals was compared to performance during day 2 of MWM5 (MWM5 in Fig. 4A) in trained animals. These trained animals had previously been classified as inferior and superior based upon their earlier performance in MWM1. We found that trained animals who had been classified as superior learners in MWM1 performed no differently at 12 months of age than naive superior learners (Fig. 4C). This observation could be due to a ceiling effect in this task, the performance of an animal cannot improve beyond a set limit and it is possible that superior learners, regardless of their level of training, are performing at peak levels. However, we found that trained animals who had been classified as inferior based upon their performance in MWM1 performed significantly better than naïve inferior learners, indicating that repeated training in this task specifically improves performance of impaired learners (Fig. 4C, unpaired t-test, t = 11.45, df = 3, p = 0.0014).
3.5. NOR reliably separates young rats into inferior and superior learners and performance correlates with MWM
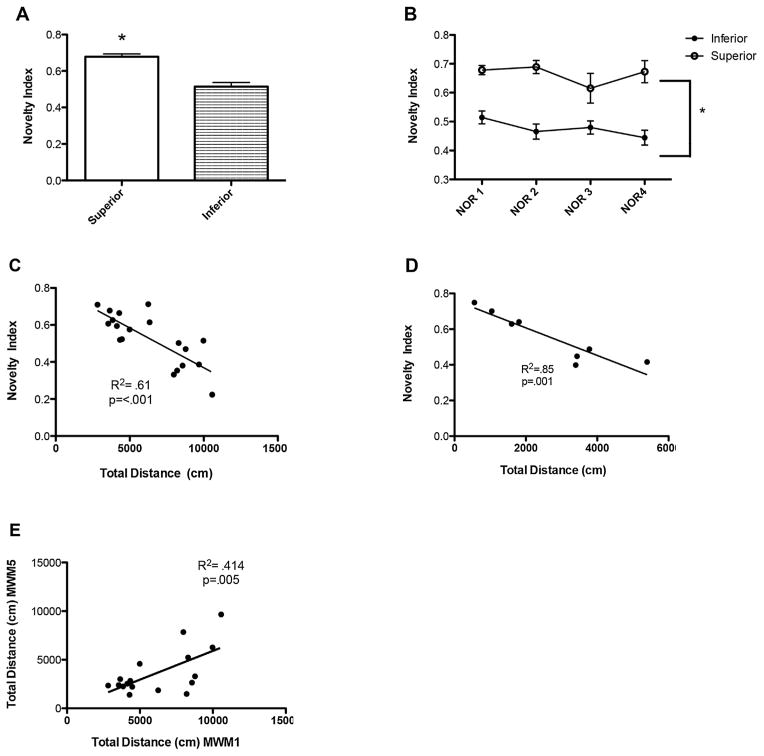
We also wanted to determine whether performance in the MWM was consistent with performance in NOR as an additional test to categorize young rats into inferior and superior learners. 3-month old animals were classified into inferior and superior learners based upon their scores in NOR1. Animals that performed at chance levels, or investigated the novel object and the familiar object for an equal amount of time, were classified as inferior learners (n = 12). Animals that performed one standard deviation above chance levels were classified as superior learners (n = 5). Animals that performed one standard deviation below chance, spending a significantly greater amount of time investigating the familiar object than the novel object, were classified as neophobic and were removed from classification (n = 2). According to these standards, superior learners investigated the novel object a minimum of 68% of the total investigation time whereas inferior learners spent an average of 51% of their investigation time on the novel object (Fig 5A, unpaired t-test, t = 4.464, df = 15, p = <0.001). Importantly, these differences in investigation were not attributed to anxiety as both groups spent roughly 2 min investigating the objects in total. As in performance on the water maze, performance in NOR is consistent from 3 to 5 months of age, with individuals classified as inferior in NOR1 continuing to perform worse than superior learners in NOR2 (6 weeks after NOR1) and NOR3 (age 5 months; Fig. 5B, repeated measures ANOVA, F(1,47) = 62.34, p < 0.0001). This high congruency shows that NOR reliably classifies young rats into inferior and superior learners. Furthermore, we found that performance in NOR at 3 months of age was predictive of performance at 12 months. We found that at 12 months of age, animals that had been previously classified as impaired on NOR1 continued to perform worse than animals that had been classified as superior learners (Fig. 5B, unpaired t-test, t = 4.953, df = 4, p < 0.01).
Fig. 5.
Performance in novel object recognition reliably separates young rats into inferior and superior learners and correlates with the results from the Morris water maze paradigm. (A) Animals can be classified into inferior and superior learners based upon their scores in NOR1. (B) Performance is consistent from 3–12 months of age. (C) Performance in NOR correlates strongly with performance in MWM at 3 months (R2 = 0.61, p < 0.001) and (D) 12 months (R2 = 0.85, p = 0.001) of age. (E) Individual performance in MWM1 is highly correlated to MWM5 (R2 = 0.414, p = 0.005).
In addition, performance in NOR correlates strongly with performance in MWM. Novelty scores from NOR1 were plotted against the average distance swam in the hidden platform training of MWM1 to find that performance on the water maze is highly predictive of performance on NOR (Fig. 5C, R2 = 0.61, p < 0.001). Importantly, the animals classified as inferior in MWM1 were also classified as inferior in NOR1 for 4 out of 5 animals (the 5th inferior learner in MWM1 was excluded from NOR due to potential neophobia) and animals classified as superior in MWM1 were also superior in NOR1 for 3 out of 4 animals. These data demonstrate that NOR and MWM are highly correlated, and that classification into inferior and superior learners is also consistent from MWM to NOR. This high correlation between MWM and NOR performance is also seen at 12 months of age, (Fig. 5D, R2 = 0.85, p < 0.05), demonstrating that these two tasks may be used together to assess cognitive abilities in young and middle aged rats and that performance at a young age is predictive of future performance in the task.
We have also performed a correlation analysis to compare the distance swam in hidden training during MWM1 to that of MWM5 to show that performance in this task at 3 months of age is highly correlated with performance at 12 months. As shown in Fig. 5E, the total distance swam in MWM1 at 3 months of age is highly correlated with the total distance swam in MWM5 at 12 months of age. (R2 = 0.414, p = 0.005), indicating that performance in this task is consistent across the timeframe of testing.
4. Discussion
In this study, we have shown that a novel water maze paradigm with only one day of hidden platform training (4 trials) and the NOR task can be used to separate young rats into inferior and superior learners, and that performance at 3 months of age is predictive of performance in these same tasks at 12 months of age. Furthermore, we have shown that these two tasks are highly correlated, and that the one-day water maze can be repeated once in just 6 weeks. In addition, we have demonstrated that training in the task improves the performance of inferior learners, indicating that cognitive training from an early age can enhance cognition in young-adult animals and therefore provide a protective buffer against age-related cognitive decline.
Other groups have used similar short MWM protocols [20,23] to asses deficits due to brain damage or neurodegeneration, but were not used to separate naïve young rats based upon individual performance in the task. Using a 1 day massed training (5 blocks of 3 trials). Guidi et al. [19] showed that deficits in an episodic memory task emerge at 12 months of age. Our MWM paradigm involves only 4 trials that can be used to categorize rats based on performance as young as 3 months of age.
Longitudinal studies in humans and animals have demonstrated that cognitive abilities on several tasks declines with age; however, the degree of this decline is highly variable among individuals [2,4,8,13,14]. Many factors may play a role in determining the level of cognitive decline seen with age, and a large amount of research is focused on finding variables that can be modified to prevent age related cognitive decline. One possible factor to consider is childhood intelligence; namely whether a high level of cognitive functioning in youth can prevent a large degree of cognitive decline with age. We have developed a modified version of the Morris Water Maze with only one day of hidden platform training that successfully separates young rats into inferior and superior learners and can be repeated after a six-week delay. Given that we tested the animals with a two-week delay only after they had undergone MWM1 and MWM2, we are not able to give insight as to whether the animals would forget the location of the platform in shorter than 6 weeks. Using this task, we have shown that cognitive ability at 3 months is predictive of cognitive performance at 12 months of age, suggesting that performance in youth is the baseline from which cognitive abilities at 12 months of age is determined. However, we have also shown that training in the water maze specifically improved performance in the task, and because of this training at intermediate time points we were unable to determine the relative change in cognition from 3 to 12 months of age. Therefore, it remains unclear whether the high variability in the level of impairment in aged animals is because superior learners have experienced little to no cognitive decline from youth.
To address this issue, we compared the performance of trained 12-month old animals that had been classified as superior and inferior learners at 3 months of age to naïve 12 month olds who had not been previously exposed to the maze. We found that untrained animals classified as superior learners based upon their performance in hidden platform training performed equally well as animals that had been classified as superior learners and received repeated training in the maze from 3 months of age. This suggests that animals that are classified as superior learners at 12 months of age have experienced little cognitive decline over the course of the aging process. However, it is important to note that ceiling effects may impact the ability of animals that are already adept at the task to improve significantly with training. It is possible that young superior rodents are already performing at maximal levels in the task, and that repeated training cannot further improve performance. Interestingly, we found that animals that were classified as inferior learners and received repeated training in the MWM from 3 months of age performed significantly better than naïve 12 month old animals classified as inferior learners based upon their first round of water maze training. Importantly, we have also shown that at 12 months of age, the trained animals did not display memory carryover from their previous exposures to the maze, indicating that differences seen between trained and naïve animals are not simply a reflection of memory savings from prior training. This indicates that inferior learners benefit substantially from repetitive training in the MWM, and that this training can enhance cognitive abilities from an early age to prevent against age-related cognitive decline. This data supports other studies that have shown that previous training can prevent age-related decline in Morris water maze performance [9,32,41,42].
Previous reports have demonstrated that slight individual differences in the water maze do exist in young rats [27,28,39], although this variation is smaller than that reported in aged animals and typically outside measures such as drugs are required to induce large differences in the performance of young rats on the task [21,22,36]. Significant differences in MWM performance have been observed in 3 month-old Wistar rats using a 6 day protocol that consists 2 trials per day. In this cross-sectional study, Ottis et al. [30] also showed that individual cognitive abilities were accentuated by age. In addition, heterogeneous ability in long versions of the MWM or when testing episodic memory in a short massed version of the MWM starts to appear at 12 months of age and increases with aging [19,10]. Heterogeneity in early age-related memory deficits have been described in 8 month old mice when tested for retention of contextual fear memory [24]. At this age 30% of mice performed below criterion and also showed impaired calcium-dependent after hyperpolarization (AHP). Moreover, differences in behavioral ability and AHP in these middle age mice are a result of gene expression differences in TRPC3 [29]. These results point to molecular and cellular changes occurring during middle age. In contrast, deficits in the novel object recognition task have been previously reported in aged animals, but individual variability in performance has not been described in young animals until our study [1,6]. We have shown using two independent tasks of memory that we can categorize rats based on ability at 3 months of age.
It will be important to study a group of animals until late in life since historically categorization into superior and inferior (or impaired) learners has been described at 18–24 month of age, depending on the strain. It has also been shown that learning ability at this age range correlates with functional phenotypes such as hippocampal volume loss, changes in gene expression and synaptic plasticity properties [38,30,35,4]; reviewed in [3]. Future studies will determine if training from young age benefits performance at later points in life when deficits in locomotor activity, exploration, changes in searching strategies, and other functional phenotypes become more accentuated [34,33,4,5]. It will also be important to study whether improved performance on the MWM with training also corresponds to a general improvement in performance in other hippocampal tasks. What we have shown is that training in the water maze specifically enhances performance in this task; however, whether training in the MWM can be used as a means to improve general hippocampal functioning and protect against age-related cognitive decline in other tasks is uncertain. As such, it will be important to determine whether training in the MWM also enhances performance in tasks such as object location memory, contextual fear conditioning, trace eyeblink conditioning, and other hippocampal dependent tasks.
Here, we have shown that performance in the MWM correlates strongly with performance in NOR, and that individual performance in each of these tasks is consistent over time. This is the first known study to classify naïve young rats into superior and inferior learners in young adulthood and follow them to middle age to show that performance at a young age is predictive of performance on these same tasks later in life. This fits with the “cognitive reserve” hypothesis in humans, which suggests that innate intelligence or enriching life experiences such as higher education may explain individual differences in the ability to cope with Alzheimer’s disease pathology and cognitive decline (reviewed in [37]. This suggests that therapeutic strategies based upon cognitive enhancement from a young age may provide a buffer against age related cognitive decline and various forms of dementia, and highlights the significance of early preventative measures in young populations.
Acknowledgments
We would like to thank Greg Rose for his critical reading of an earlier version of the manuscript. This research was supported by funds from the University of Wisconsin Graduate School, School of Medicine and Public Health and Department of Neurology to C.B. R.H. was supported by the University of Wisconsin Neuroscience Training Program Grant NIH/NIGMS T32GM007507 and a NSF National Science Foundation Graduate Research Fellowship Program (GRFP) Fellowship.
Footnotes
Conflict of interest
The authors report no conflict of interest.
References
- 1.Alvarez-Ruiz Y, Carrillo-Mora P. Amyloid beta 25–35 impairs reconsolidation of object recognition memory in rats and this effect is prevented by lithium carbonate. Neurosci Lett. 2013;548:79–83. doi: 10.1016/j.neulet.2013.06.003. http://dx.doi.org/10.1016/j.neulet.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Brandeis R, Brandys Y, Yehuda S. The use of the Morris water maze in the study of memory and learning. Int J Neurosci. 1989;48(1–2):29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- 3.Burger C. Region-specific genetic alterations in the aging hippocampus: implications for cognitive aging. Front Aging Neurosci. 2010;14(2):140. doi: 10.3389/fnagi.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burger C, Cecilia Lopez M, Feller JA, Baker HV, Muzyczka N, Mandel RJ. Changes in transcription within the CA1 field of the hippocampus are associated with age-related spatial learning impairments. Neurobiol Learn Mem. 2007;87(1):21–41. doi: 10.1016/j.nlm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Burger C, Lopez MC, Baker HV, Mendel RJ, Muzyczka N. Genome-wide analysis of aging and learning-related genes in the hippocampal dentate gyrus. Neurobiol Learn Mem. 2008;89:379–396. doi: 10.1016/j.nlm.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA. Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci. 2010;124(5):559–573. doi: 10.1037/a0020893. http://dx.doi.org/10.1037/a0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caprioli A, Ghirardi O, Giuliani A, Ramacci MT, Angelucci L. Spatial learning and memory in the radial maze: a longitudinal study in rats from 4 to 25 months of age. Neurobiol Aging. 1991;12(5):605–607. doi: 10.1016/0197-4580(91)90093-y. [DOI] [PubMed] [Google Scholar]
- 8.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36(1):60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 9.Dellu F, Mayo W, Vallee M, Le Moal M, Simon H. Facilitation of cognitive performance in aged rats by past experience depends on the type of information processing involved: a combined cross-sectional and longitudinal study. Neurobiol Learn Mem. 1997;67(2):121–128. doi: 10.1006/nlme.1996.3750. [DOI] [PubMed] [Google Scholar]
- 10.Fischer W, Chen KS, Gage FH, Bjorklund A. Progressive decline in spatial learning and integrity of forebrain cholinergic neurons in rats during aging. Neurobiol Aging. 1992;31(1):9–23. doi: 10.1016/0197-4580(92)90003-g. [DOI] [PubMed] [Google Scholar]
- 11.Gage FH, Dunnett SB, Bjorklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5(1):43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107(4):618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher M, Nicolle MM. Animal models of normal aging: relationship between cognitive decline and markers in hippocampal circuitry. Behav Brain Res. 1993;57(2):155–162. doi: 10.1016/0166-4328(93)90131-9. [DOI] [PubMed] [Google Scholar]
- 14.Geinisman Y, Detoledo-Morrell L, Morrell F, Heller RE. Hippocampal markers of age-related memory dysfunction: behavioral, electrophysiological and morphological perspectives. Prog Neurobiol. 1995;45(3):223–252. doi: 10.1016/0301-0082(94)00047-l. [DOI] [PubMed] [Google Scholar]
- 15.Gerstein H, Hullinger R, Lindstrom MJ, Burger C. A behavioral paradigm to evaluate hippocampal performance in aged rodents for pharmacological and genetic target validation. PLoS One. 2013;8(5):e62360. doi: 10.1371/journal.pone.0062360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerstein H, Lindstrom MJ, Burger C. Gene delivery of Homer1c rescues spatial learning in a rodent model of cognitive aging. Neurobiol Aging. 2013;34(8):1963–1970. doi: 10.1016/j.neurobiolaging.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gow AJ, Johnson W, Pattie A, Brett CE, Roberts B, Starr JM, Deary IJ. Stability and change in intelligence from age 11 to ages 70, 79, and 87: the Lothian Birth Cohorts of 1921 and 1936. Psychol Aging. 2011;26(1):232–240. doi: 10.1037/a0021072. http://dx.doi.org/10.1037/a0021072. [DOI] [PubMed] [Google Scholar]
- 18.Gow AJ, Johnson W, Pattie A, Whiteman MC, Starr J, Deary IJ. Mental ability in childhood and cognitive aging. Gerontology. 2008;54(3):177–186. doi: 10.1159/000118098. http://dx.doi.org/10.1159/000118098. [DOI] [PubMed] [Google Scholar]
- 19.Guidi M, Kumar A, Rani A, Foster TM. Assessing the emergence and reliability of cognitive decline over the life span in Fisher F 344 rats using the spatial water maze. Front Aging Neurosci. 2014 doi: 10.3389/fnagi.2014.00002. http://dx.doi.org/10.3389/fnagi.2014.00002. [DOI] [PMC free article] [PubMed]
- 20.Gulinello M, Gertner M, Mendoza G, Schoenfeld BP, Oddo S, LaFerla F, Choi CH, McBride SM, Faber DS. Validation of a 2-day water maze protocol in mice. Behav Brain Res. 2009;196(2):220–227. doi: 10.1016/j.bbr.2008.09.002. http://dx.doi.org/10.1016/j.bbr.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han G, An L, Yang B, Si L, Zhang T. Nicotine-induced impairments of spatial cognition and long-term potentiation in adolescent male rats. Hum Exp Toxicol. 2013 doi: 10.1177/0960327113494902. http://dx.doi.org/10.1177/0960327113494902. [DOI] [PubMed]
- 22.Jamialahmadi K, Sadeghnia HR, Mohammadi G, Kazemabad AM, Hosseini M. Glucosamine alleviates scopolamine induced spatial learning and memory deficits in rats. Pathophysiol Off J Int Soc IS Pathophysiol. 2013 doi: 10.1016/j.pathophys.2013.04.003. http://dx.doi.org/10.1016/j.pathophys.2013.04.003. [DOI] [PubMed]
- 23.Kraemer PJ, Brown RW, Baldwin SA, Scheff SW. Validation of a single-day Morris water maze procedure used to assess cognitive deficits associated with brain damage. Brain Res Bull. 1996;39(1):17–22. doi: 10.1016/0361-9230(95)02028-4. [DOI] [PubMed] [Google Scholar]
- 24.Kaczorowski CC, Disterhoft JF. Memory deficits are associated with impaired ability to modulate neuronal excitability in middle-aged mice. Learn Mem. 2009;16:362–366. doi: 10.1101/lm.1365609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li JT, Su YA, Guo CM, Feng Y, Yang Y, Huang RH, Si TM. Persisting cognitive deficits induced by low-dose, subchronic treatment with MK-801 in adolescent rats. Eur J Pharmacol. 2011;652(1–3):65–72. doi: 10.1016/j.ejphar.2010.10.074. http://dx.doi.org/10.1016/j.ejphar.2010.10.074. [DOI] [PubMed] [Google Scholar]
- 26.Markowska AL, Stone WS, Ingram DK, Reynolds J, Gold PE, Conti LH, Pontecorvo MJ, Wenk GL, Olton DS. Individual differences in aging: behavioral and neurobiological correlates. Neurobiol Aging. 1989;10(1):31–43. doi: 10.1016/s0197-4580(89)80008-9. [DOI] [PubMed] [Google Scholar]
- 27.Matzel LD, Grossman H, Light K, Townsend D, Kolata S. Age-related declines in general cognitive abilities of Balb/C mice are associated with disparities in working memory, body weight, and general activity. Learn Mem. 2008;15(10):733–746. doi: 10.1101/lm.954808. http://dx.doi.org/10.1101/lm.954808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matzel LD, Han YR, Grossman H, Karnik MS, Patel D, Scott N, Specht SM, Gandhi CC. Individual differences in the expression of a general learning ability in mice. J Neurosci. 2003;23(16):6423–6433. doi: 10.1523/JNEUROSCI.23-16-06423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neuner SM, Wilmott LA, Hope KA, Hoffmann B, Jayhong CA, Abramowitz J, Birnbaumer L, O’Connell KM, Tryba AK, Greene AS, Chan CS, Kaczorowski CC. TRPC3 channels critically regulate hippocampal excitability and contextual fear memory. Behav Brain Res. 2015;281:69–77. doi: 10.1016/j.bbr.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ottis P, Topic B, Loos M, Li KW, de Souza A, Schulz D, Smit AB, Huston JP, Korth C. Aging-induced proteostatic changes in the rat hippocampus identify ARP3, NEB2, and BRAG2 as a molecular circuitry for cognitive impariment. Plos One. 2013;8(9):e75112. doi: 10.1371/journal.pone.0075112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owens WA., Jr Is age kinder to the initially more able? J Gerontol. 1959;14:334–337. doi: 10.1093/geronj/14.3.334. [DOI] [PubMed] [Google Scholar]
- 32.Pitsikas N, Biagini L, Algeri S. Previous experience facilitates preservation of spatial memory in the senescent rat. Physiol Behav. 1991;49(4):823–825. doi: 10.1016/0031-9384(91)90325-i. [DOI] [PubMed] [Google Scholar]
- 33.Schulz D, Huston JP, Jezek K, Haas HL, Roth-Harer A, Selbach O, Luhmann HJ. Water maze performance, exploratory activity, inhibitory avoidance and hippocampal plasticity in aged superior and inferior learners. Eur J Neurosci. 2002;16:2175–2185. doi: 10.1046/j.1460-9568.2002.02282.x. [DOI] [PubMed] [Google Scholar]
- 34.Schulz D, Kouri C, Huston JP. Behavior on the water maze platform: relationship to learning and open field exploration in aged and adult rats. Brain Res Bull. 2007;74(4):206–215. doi: 10.1016/j.brainresbull.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Shulz D, Sergeeva OA, Ianovskii E, Luhmann HJ, Haas HL, Huston JP. Behavioural parameters in aged rats are related to LTP and gene expression of ChAT and NMDA-NR2 subunits in the striatum. Eur J Neurosci. 2004;19:1373–1383. doi: 10.1111/j.1460-9568.2004.03234.x. [DOI] [PubMed] [Google Scholar]
- 36.Sircar R, Basak A, Sircar D, Wu LC. Effects of gamma-hydroxybutyric acid on spatial learning and memory in adolescent and adult female rats. Pharmacol Biochem Behav. 2010;96(2):187–193. doi: 10.1016/j.pbb.2010.04.028. http://dx.doi.org/10.1016/j.pbb.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stern Y. What is cognitive reserve? theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 38.Sykova E, Mazel T, Hasenorhl RU, Harvey AR, Simonova Z, Mulders WHAM, Huston JP. Learning deficits in aged rats related to decrease in extracellular volume and loss of diffusion anisotropy in hippocampus. Hippocampus. 2002;12(2):269–279. doi: 10.1002/hipo.1101. [DOI] [PubMed] [Google Scholar]
- 39.Tombaugh GC, Rowe WB, Chow AR, Michael TH, Rose GM. Theta-frequency synaptic potentiation in CA1 in vitro distinguishes cognitively impaired from unimpaired aged Fischer 344 rats. J Neurosci. 2002;22(22):9932–9940. doi: 10.1523/JNEUROSCI.22-22-09932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Groen T, Kadish I, Wyss JM. Old rats remember old tricks; memories of the water maze persist for 12 months. Behav Brain Res. 2002;136(1):247–255. doi: 10.1016/s0166-4328(02)00137-7. [DOI] [PubMed] [Google Scholar]
- 41.Vicens P, Bernal MC, Carrasco MC, Redolat R. Previous training in the water maze: differential effects in NMRI and C57BL mice. Physiol Behav. 1999;67(2):197–203. doi: 10.1016/s0031-9384(99)00059-1. [DOI] [PubMed] [Google Scholar]
- 42.Vicens P, Redolat R, Carrasco MC. Effects of early spatial training on water maze performance: a longitudinal study in mice. Exp Gerontol. 2002;37(4):575–581. doi: 10.1016/s0531-5565(01)00217-0. [DOI] [PubMed] [Google Scholar]