Abstract
In the mouse telencephalon, Dlx homeobox transcription factors are essential for the tangential migration of subpallial-derived GABAergic interneurons to neocortex. However, the mechanisms underlying this process are poorly understood. Here, we demonstrate that Dlx1&2 have a central role in restraining neurite growth of subpallial-derived immature interneurons at a stage when they migrate tangentially to cortex. In Dlx1−/−;Dlx2−/− mutants, neurite length is increased and cells fail to migrate. In Dlx1−/−;Dlx2+/− mutants, while the tangential migration of immature interneurons appears normal, they develop dendritic and axonal processes with increased length and decreased branching, and have deficits in the their neocortical laminar positions. Thus, Dlx1&2 are required for coordinating programs of neurite maturation and migration. In this regard, we provide genetic evidence that in immature interneurons Dlx1&2 repression of the p21-activated kinase PAK3, a downstream effector of the Rho-family of GTPases, is critical in restraining neurite growth and promoting tangential migration.
Keywords: neuronal differentiation, cortical development, cytoskeleton, medial ganglionic eminence
INTRODUCTION
The cerebral cortex consists of two main neuronal classes, pyramidal neurons (projection neurons) and interneurons (local-circuit neurons). They differ in their embryonic origin, migration properties, cell morphology, and neurotransmitter production. Pyramidal neurons are generated in the proliferative zones of the pallium from progenitors expressing the transcription factors Emx1, Neurogenin1 and 2, Pax6, and Tlx1 and 2, and migrate radially into the cortical plate (Guillemot et al., 2006). Substantial progress has been made in identifying the mechanisms that control radial migration and differentiation of pyramidal cells (Kriegstein and Noctor, 2004; Bielas et al., 2004; Guillemot et al., 2006). However, relatively less is known about the cellular and molecular mechanisms that control the migration and differentiation of cortical GABAergic interneurons. Cortical interneurons are born in the proliferative zones of the subpallium (ventral telencephalon) from progenitors expressing transcription factors including Nkx2.1 Mash1, Dlx1 and 2, and Lhx6 (Campbell, 2003; Marin and Rubenstein, 2003; Guillemot, 2005). Postmitotic immature interneurons exit the subpallial subventricular zone (SVZ) and take tangential migratory routes to enter the cortex. Within the cortex, they change their mode of migration from tangential to radial to invade the cortical plate and acquire their laminar positions, where they differentiate into a variety of interneuron cell types (Marin and Rubenstein, 2003; Metin et al., 2006; Wonders and Anderson, 2006; Ayala et al., 2007). Thus, postmitotic subpallial-derived interneurons must delay their maturation while they are migrating to their final cortical positions. The mechanisms that coordinate their migration and neuronal maturation are unknown.
Dlx homeobox genes have major roles in regulating GABAergic differentiation in the forebrain (Panganiban and Rubenstein, 2002). Analysis of Dlx loss-of-function mouse mutants has demonstrated that these genes regulate the production of GABAergic neurons from progenitors located in the SVZ (Anderson et al., 1997a,b; Marin et al., 2000; Anderson et al., 2001; Yun et al., 2002). In Dlx1−/−;Dlx2−/− double mutants (Dlx1/2−/−), tangential migration of GABAergic cortical interneurons from the subpallial SVZ to neocortex and hippocampus is nearly abolished. As a result, interneuron precursors accumulate in the SVZ of the medial and caudal ganglionic eminences (MGE, CGE) (Anderson et al., 1997a). These findings raised the question of whether Dlx genes simply regulate cell motility or have further functions in neuronal differentiation. Expression data is consistent with additional functions of Dlx genes in neuronal differentiation: cortical interneurons express Dlx1&2 genes during all developmental stages and into adulthood, with robust expression of Dlx1&2 in SVZ, high expression of Dlx1&2 in migrating cells, and moderate expression of Dlx1 and low expression of Dlx2 in post-migratory cells (Liu et al., 1997; Yun et al., 2002; Cobos et al., 2005, 2006). Moreover, recent analysis of Dlx1 single knockouts showed that Dlx1 cell autonomously regulates dendrite maturation of at least a subset of cortical interneurons (Cobos et al, 2005).
Here, we investigate Dlx1&2 functions in regulating migration and neuronal differentiation of cortical interneurons. Using Dlx1/2−/− double mutants, we demonstrate that Dlx genes regulate the growth of neuronal processes of immature presumptive GABAergic interneurons. Mutant interneurons exhibited longer neurites. In Dlx1−/−;Dlx2+/− compound mutants tangential migration takes place, but immature neocortical interneurons show defects in the acquisition of their final laminar positions, and have dendritic and axonal processes with increased length and decreased branching. To dissect the molecular pathways underlying these defects, we used microarray analysis comparing gene expression of Dlx1/2−/− and wild-type MGE-derived cells. We focused on PAK3, a member of the p21-activated serine/threonine kinases (PAKs) family. PAK kinases are major downstream effectors of the Rho-family GTPases Rac1 and Cdc42 (Bokoc, 2003; Hofmann et al., 2004; Kumar et al., 2006). Rho GTPases integrate different extracellular and intracellular signals to orchestrate the changes in the cytoskeleton that are ultimately responsible for cell motility, neurite outgrowth, and axon and dendrite guidance (Luo, 2000; Govek et al., 2005). We show that in wild-type embryos, PAK3 expression is low in migrating interneurons, and is up-regulated once they have arrived to cortex. We provide genetic evidence that PAK3 repression by Dlx1&2 in MGE-derived immature interneurons is critical in restraining the growth of their neurites and promoting their tangential migration.
RESULTS
Interneuron precursors from Dlx1/2−/− double mutants have longer neuronal processes
Precursors of mouse cortical GABAergic neurons are generated in the proliferative zones (VZ/SVZ) of the subpallium from where they migrate tangentially to cortex. Lack of Dlx1/2 function produces a cell-autonomous block in this migration that results in accumulation of interneuron precursors in the subpallial SVZ (Anderson et al, 1997a; 2001; Marin et al., 2000). The Dlx1/2−/− SVZ contains both proliferating cells and cells that express markers of differentiating neurons, such as microtubule associated protein-2 (MAP2). These cells extend neurites when dissociated and grown in vitro (Anderson et al, 1997b). These observations show that Dlx1/2 function is required for initiating tangential migration of interneurons, but is not required for neurogenesis or for initiating neurite outgrowth. To characterize the ability of interneuron precursors lacking Dlx1/2 to extend and branch their neurites, we analyzed the morphology of individual MGE-derived neurons using an in vitro cell transplantation assay (Xu et al., 2004). Donor cells were dissociated from the MGE and plated at low density over high-density feeder cells prepared from P0 wild-type cortices. The experiment was performed using E15.5 MGE, an age when it is primarily producing cells that will become cortical interneurons. Green fluorescent protein (GFP) expression was used to visualize the morphology of donor cells using the Lhx6-GFP BAC transgene (http://www.gensat.org). This transgene is a good reporter for cortical interneurons in our experimental paradigm because: i) Lhx6 is expressed by a large number of GABAergic neurons in embryonic and postnatal cortex (Cobos et al., 2006; Liodis et al., 2007); ii) nearly all Lhx6-positive neurons migrating throughout the embryonic cerebral cortex are Dlx-positive (Figure S1 in Supplemental data); and iii) expression levels of Lhx6 mRNA in the telencephalon are not greatly changed in Dlx1/2 mutants (Cobos et al., 2005).
First, we analyzed neurite outgrowth in GFP-expressing interneurons between 1 and 3 days in vitro (DIV), from cultures prepared with low numbers of E15.5 donor cells (1%). We obtained confocal images of randomly selected GFP-expressing cells, and measured in each cell the number of neurites, total neurite length (TNL), average neurite length (ANL), and length of longest neurite (LN). Our analysis showed that, by 3 DIV, neurite length (TNL, ANL and LN) had significantly increased in mutant cells as compared to control cells (TNL: ~45%; ANL: ~35%; LN: ~65%), whereas the number of neurites was similar in mutants and controls (Figure 1A–D). We performed a similar analysis using E12.5 MGE donor cells, when cortical interneurons are beginning to migrate from MGE, and found results that were similar to those obtained with E15.5 MGE donors (Figure S2 in Supplementary data). Thus, our in vitro analysis showed that MGE-derived neurons from Dlx1/2−/− mutants develop longer neurites.
Figure 1. Interneuron precursors from Dlx mutant MGE cultures develop longer neuronal processes.
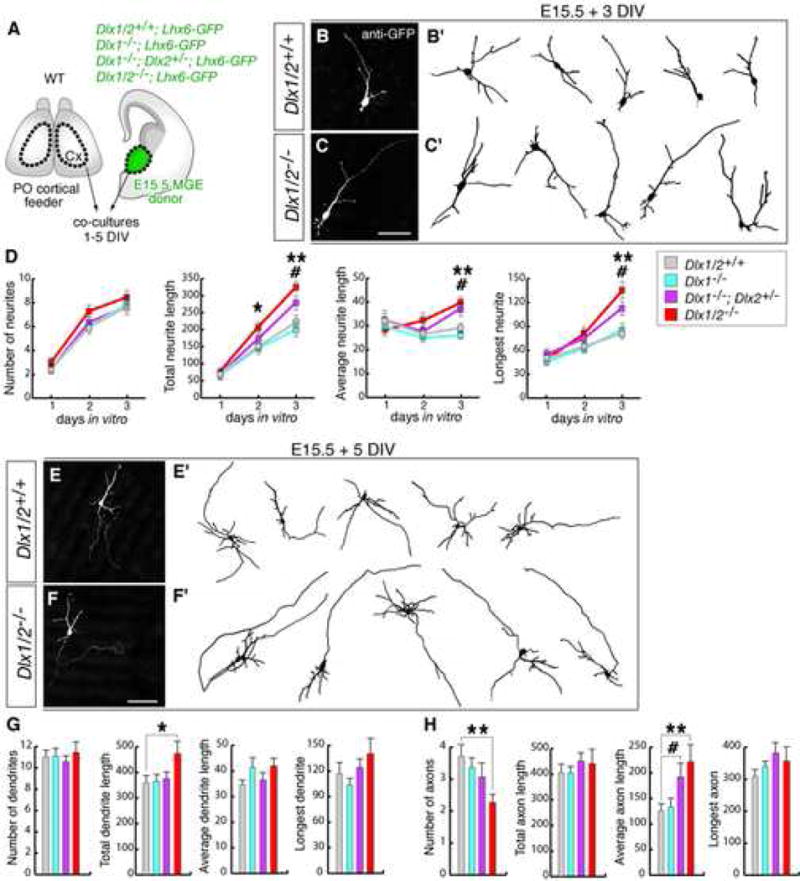
(A) Experimental assay used to characterize the morphology of interneuron precursors derived from progenitors of the MGE from Dlx1−/−, Dlx1−/−;Dlx2+/−, and Dlx1/2−/− mutants.
(B, C) Examples of Dlx1/2−/− mutant and control neurons from E15.5 MGE cultures stained with anti-GFP antibody after 3 DIV. (B′, C′) are drawings of representative neurons.
(D) Quantification of number of neurites, total neurite length (TNL), average neurite length (ANL), and length of longest neurite (LN) from control (gray lines), and Dlx mutants (color lines) after 1–3 DIV. Tukey-Kramer test revealed significant increases of TNL, ANL and LN in Dlx1/2−/− and in Dlx1−/−;Dlx2+/− mutant cells as compared to control cells by 3 DIV [Dlx1/2−/−: (in μm) TNL: 325 ± 12.8, ANF: 39.8 ± 2.1, LN: 134.2 ± 10; Dlx1−/−;Dlx2+/−: TNL: 280 ± 20.2, ANF: 37.8 ± 2.8, LN: 112.9 ± 8.2; control: TNL: 228.5 ± 19.3, ANF: 30.9 ± 1.9, LN: 80.3 ± 3.9; n = 25, each genotype]. * p < 0.05, ** p < 0.01 (Dlx1/2−/− versus controls); # p < 0.05 (Dlx1−/−;Dlx2+/− versus controls).
(E, F) Examples of Dlx1/2−/− mutant and control neurons after 5 DIV. (E′, F′) are drawings of representative neurons.
(G, H) Quantification of axonal and dendritic morphology in control (gray bars) and Dlx mutants (color bars) after 5 DIV. (Average dendrite length: Dlx1/2−/−: 42 ± 3.1 μm; control: 33.3 ± 2.4 μm; average axon length: Dlx1/2−/−: 221.1 ± 33.7 μm; Dlx1−/−;Dlx2+/−: 190.7 ± 27.8 μm; control: 122.2 ± 14.3 μm; number of axonal branches: Dlx1/2−/: 2.27 ± 0.26; control: 3.88 ± 0.38; n = 25 each genotype). * p < 0.05, ** p < 0.01 (Dlx1/2−/− versus controls); # p < 0.05 (Dlx1−/−;Dlx2+/− versus controls; Tukey-Kramer test).
Scale bars = 50 μm (B,C) and 100 μm (E,F).
Next, we analyzed E15.5 cultures grown in vitro for 5 days. At this stage we were able to distinguish reliably axons and dendrites in both wild-type and mutant neurons, based on the more segregated expression of Tau1 into axons and MAP2 into nascent dendrites (Dehmelt and Halpain, 2005). Analysis of dendrite morphogenesis showed increased (~30%) average dendrite length in mutant cells compared to controls, and similar number of dendritic branches in both genotypes. Analysis of axon morphogenesis showed increased (~75%) average axon length and reduced (~40%) number of axonal branches in mutant cells (Figure 1E–H). Thus, Dlx1/2−/− immature interneurons developed longer dendrites, and axons that were longer and had fewer branches.
Interneuron precursors from Dlx1/2−/− undergo apoptosis
Previous studies have shown that the Dlx genes are required for cell survival (De Melo et al., 2004; Cobos et al., 2005). In our in vitro cell transplantation assay using MGE-derived interneuron precursors, there was a dramatic reduction in numbers of GFP-expressing Dlx1/2−/− cells after 5DIV; those remaining frequently had shrunken cell bodies with retracting processes and pyknotic nuclei. Quantification of numbers of GFP+ cells revealed that the number of Dlx1/2−/− mutant cells decreased to 80% by 5 DIV, and were further decreased to 7% by 10 DIV (Figure S3 in Supplemental data). To directly assess apoptosis, we used TUNEL staining and anti-active caspase-3 immunocytochemistry. Whereas double TUNEL+/GFP+ and double caspase3+/GFP+ cells were rarely observed in control cultures, they were increased ~10-fold in mutants at the time when there was a reduction in GFP+ cells. Furthermore, TUNEL assay of sections from Dlx1/2−/− neonatal brains showed a robust increase in the number of TUNEL+ cells in the basal ganglia (SVZ and mantle) (Figure S3). Thus, our results support a requirement of Dlx1/2 for the survival of MGE-derived GABAergic neurons.
Interneurons from Dlx1−/−;Dlx2+/− mutants tangentially migrate to cortex but show defects in dendritic and axonal growth
Development of the forebrain, branchial arches and limbs are sensitive to Dlx dosage (Depew et al., 2005). Because postnatal Dlx1−/− mice have mild defects in dendrite morphogenesis in a subset of cortical interneurons (Cobos et al., 2005), we tested whether Dlx1−/−;Dlx2+/− compound mutants exhibited a phenotype intermediate to the single and double Dlx mutants in interneuron differentiation.
Dlx1−/−;Dlx2+/− compound mutant pups died shortly after birth, probably because they have cleft palate. To first verify that Dlx1−/−;Dlx2+/− embryos are hypomorphic relative to Dlx1/2−/−, we studied key phenotypic features of the double null mutants, i.e., disruption of development of the striatal SVZ, reduced production of striatal projection neurons, and reduced migration of interneurons to olfactory bulb and to cerebral cortex (Anderson et al., 1997a,b; Marin et al., 2000; Yun et al., 2002). Consistent with a disruption of striatal SVZ differentiation through altered Notch signaling (Yun et al., 2002), E13.5 compound heterozygous embryos showed moderate increases in mRNA expression of the Notch ligand Delta-like1 (Dll1), the transcription factor Hes5, and the bHLH transcription factor Mash1. As a result, the striatal mantle of E17.5 embryos was smaller (Figure S4 in Supplemental data). Consistent with defects in migration of interneurons to olfactory bulb, we detected reduced numbers of cells expressing several interneuron markers (i.e., GAD67, ER81, Arx, and Reelin) in the granular and periglomerular layers. Finally, we found that cortical interneuron precursors were able to migrate to cerebral cortex, based on expression of GAD67 and other interneuron markers (Figures S4 and S5 in Supplemental data). Thus, the Dlx1−/−;Dlx2+/− compound mutants exhibit a hypomorphic phenotype relative to Dlx1/2−/− double mutants, but maintain tangential migration.
To compare the effects of the Dlx1−/−, Dlx1−/−;Dlx2+/−, and Dlx1/2−/− mutations on interneuron morphology, we used our in vitro cell transplantation assay (Figure 1A). E15.5 MGE donors from all genotypes were analyzed (all were Lhx6-GFP+). This analysis showed that Dlx1−/−;Dlx2+/− cells, by 3 DIV, had increased neurite length (TNL, ANL and LN) as compared to control cells (TNL: ~25%; ANL: ~25%; LN: ~40%), and similar number of neurites (Figure 1D). By 5 DIV, the average length of axons was increased (~50%) (Figure 1G,H). These defects were similar but milder than in Dlx1/2−/− cells. By contrast, Dlx1−/− cells did not show significant differences in dendrite or axon morphogenesis (Figure 1D,G,H).
We next prepared dissociated neuronal cultures from P0 cortices (Lhx6-GFP+) of Dlx1−/−;Dlx2+/− mutants and littermate controls (Figure 2A). At 1–3 DIV, GFP+ cells from Dlx1−/−;Dlx2+/− cortices showed increased (~20%) average neurite length and increased (~30%) length of their longest neurite, as compared with controls (data not shown). At 5 DIV, the Dlx1−/−;Dlx2+/− mutant cells had reduced numbers of both dendritic and axonal branches (~25%) (Figure 2B–E). Thus, Dlx1−/−;Dlx2+/− cells showed defects in neurite growth (i.e., increased length and decreased branching) that were similar but milder than Dlx1/2−/− cells. Together, these results show that Dlx genes restrain neurite growth in immature interneurons, and additionally control their axonal and dendritic growth at later stages of maturation in the neocortex.
Figure 2. Interneurons from neocortex of Dlx1−/−;Dlx2+/− compound mutants develop neuronal processes with fewer branches.
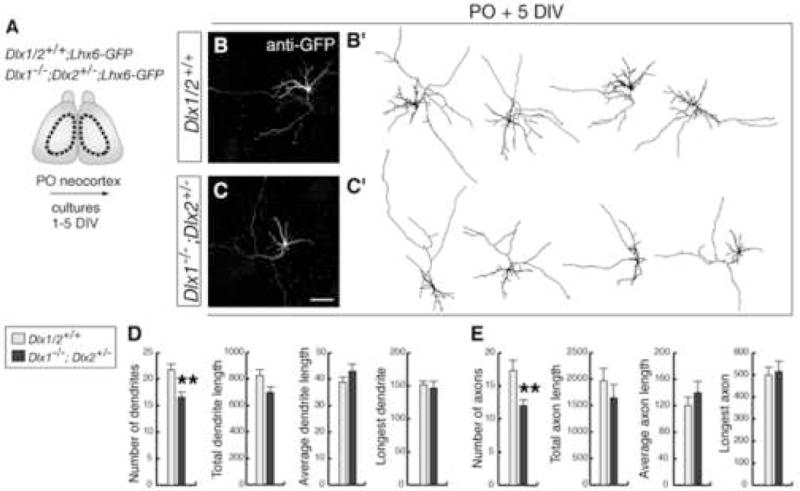
(A) Experimental assay used to characterize the morphology of interneurons.
(B, C) Examples of interneurons isolated from P0 neocortex and immunostained with anti-GFP after 5 DIV. (B′, C′) are drawings of representative neurons.
(D, E) Quantification of axonal and dendritic morphology from control (gray bars) and Dlx1−/−;Dlx2+/− (black bars) interneurons after 5 DIV. Student’s t-test revealed significantly decreases in the numbers of dendritic and axonal branches in mutant cells (dendrites: mutant: 16.6 ± 1; control: 21.8 ± 1.2, p < 0.01; axons: mutant: 12 ± 0.8; control: 17.3 ± 1.6, p < 0.01; n = 30). ** p < 0.01.
Scale bar = 100 μm (B,C).
Interneurons from Dlx1−/−;Dlx2+/− mutants show defects in neocortical layer acquisition
At perinatal stages, neocortical interneurons radially migrate “inward” from the marginal zone (MZ) to the cortical plate, and “outward” from the VZ/SVZ and intermediate zone (IZ) to the cortical plate, to adopt their laminar identities (Nadarajah et al., 2002; Tanaka et al., 2003; Hevner et al., 2004). We tested whether Dlx1−/−;Dlx2+/− mutation affected the ability of neocortical interneurons to migrate to their laminar positions. First, we analyzed the expression of interneuron markers in P0 cortices (Figure 3A–D). We observed that, although the total numbers of GAD67+ neurons in neocortex was normal, there were increased numbers of GAD67+ neurons in the marginal and subventricular zones (MZ, SVZ; Figure 3 B–D, and Supplemental Figure 5). Next, we used the Lhx6-GFP BAC allele to visualize the interneurons by GFP immunofluorescence. At P0, the numbers of GFP+ neurons in both MZ and VZ/SVZ were increased in the mutant brains (Figure 3E, F). In superficial neocortical layers of control brains, interneurons were radially-oriented, consistent with an inward migration of Lhx6+ interneurons from superficial to middle/deeper layers. By contrast, GFP+ cells in superficial neocortical layers of compound mutants lacked this radial orientation (Figure 3G). Furthermore, organotypic cultures prepared from P0 telencephalic slices of Dlx1−/−;Dlx2+/−;Lhx6-GFP mice that were maintained up to 7 DIV, showed defects in the laminar distribution of GFP+ cells (Figure 3H–K). At 7 DIV, ectopic GFP+ cells were frequently observed in layer I, and there was a 2-fold increase in GFP+ cells in superficial layers (II–III), a ~40% decrease in middle layers (IV–V), and a ~60% increase in deep layers (VI). Thus, Dlx1&2 regulate the acquisition of laminar positions of neocortical interneurons. Together, these results show that Dlx genes promote both tangential and radial migration of neocortical interneurons, while they restrain their neurite growth.
Figure 3. Dlx1−/−;Dlx2+/− compound mutants have defects in the laminar position of immature cortical interneurons.
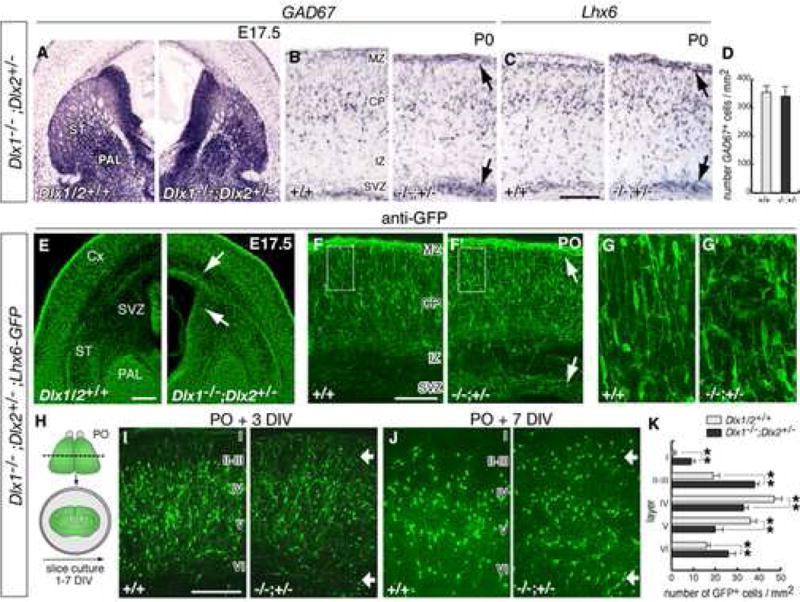
(A) mRNA in situ hybridization for GAD67 in coronal sections though the basal ganglia of a Dlx1−/−;Dlx2+/− mutant and a control embryo at E17.5 (abbreviations: Cx, cortex; PAL: globus pallidus; ST, striatum).
(B, C) GAD67 and Lhx6 expression in coronal sections through neocortex at P0, showing accumulation of GAD67+ cells and Lhx6+ cells in the SVZ and MZ (black arrows; abbreviations: SVZ, subventricular zone; IZ, intermediate zone; CP, cortical plate; MZ, marginal zone).
(D) Quantification of total numbers of GAD67+ neurons in control and mutant P0 neocortex.
(E–G) Histological analysis of Dlx1−/−;Dlx2+/−;Lhx6-GFP+ embryos. (E) shows GFP expression in the globus pallidus (PAL) and in interneurons migrating to neocortex. (F, F′) are coronal sections through neocortex at P0. Arrows in (E, F) indicate increased numbers of GFP+ cells in the subpallial SVZ, and in the cortical SVZ and MZ. (G, G′) are higher magnifications of the areas boxed in (F, F′), showing the morphology of GFP+ cells in the cortical plate.
(H) Experimental assay used to examine the laminar positions of interneurons in neocortex.
(I–J) Coronal sections through the neocortex from organotypic cultures prepared from P0 brains and maintained 3 and 7 DIV. Roman numbers approximate the position of cortical layers. Arrows indicate accumulation of GFP+ neurons in superficial (I–III) and deep (VI) layers of mutant brains.
(K) Quantification of the distribution of GFP+ cells in neocortical layers of control and Dlx1−/−;Dlx2+/− organotypic cultures after 7 DIV (controls: I: 0.15 ± 0.13; II–III: 19.1 ± 2.3; IV: 47.3 ± 4.9; V: 36.6 ± 3.7; VI: 16.1 ± 2.1; mutants: I: 9.3 ± 1.9, p < 0.01; II–III: 38.5 ± 2.5, p < 0.01; IV: 33.4 ± 2.8, p < 0.01; V: 19.9 ± 4.0, p < 0.01; VI: 26.2 ± 4.8, p < 0.01; n = 7, each genotype). ** p < 0.01 (Student’s t test). Scale bars = 500 μm (A,E) and 250 μm (B,C,F,G and I,J).
Microarray expression analysis from Dlx1/2−/− interneuron precursors reveals molecular pathways affecting neuronal differentiation and migration
To identify molecular pathways that regulate interneuron differentiation, which are deregulated in Dlx1/2−/− mutants, we carried out microarray analysis comparing gene expression of cells derived from the MGE of Dlx1/2−/− versus Dlx1/2+/? embryos. For that, we used dissociated cells that were obtained from the VZ/SVZ of the MGE at E15.5 and grown in vitro for 3 days, a stage of differentiation in which we observed abnormal, longer neurites, but did not detect significant neuronal death. The data can be found at the NIH Neuroscience Microarray Consortium website (http://arrayconsortium.tgen.org). We concentrated on a set of genes that are known to regulate neuronal differentiation and cell migration, and verified that their expression is indeed altered in vivo based on in situ hybridization and/or immunocytochemistry (Figure S6 and Table I in Supplemental data). We identified signaling molecules that are relevant for cell communication and cell adhesion, and for the regulation of the cytoskeleton, and that were deregulated in Dlx1/2 mutants. Altered functions of these molecules often result in phenotypes affecting cell morphology and migration (Luo, 2000; Guan and Rao, 2003; Govek et al., 2005).
Notably, a number of genes encoding molecules that regulate the cytoskeleton were up-regulated in Dlx1/2−/− mutants. These include Mtap2 (MAP2), Mapt (Tau1), GAP43 (growth associated protein 43), and PAK3 (p21-activated kinase 3) (Figure 4). The increased mRNA expression resulted in increased protein levels in neurites, as determined using immunocytochemical detection of MAP2 (2a + 2b), Tau1, GAP43 and PAK3 in primary cell cultures derived from E15.5 MGE of Dlx1/2−/− and control littermates. Immunoblots from mutant cultures lysates confirmed the increase in protein expression (MAP2: ~50%; Tau1: ~30%; GAP43: 3-fold; PAK3: 3-fold) (Figure 4A–G). We next examined MAP2, Tau1, GAP43 and PAK3 expression in sections from Dlx1/2−/− and littermate control embryos at E17.5. Whereas in controls their expression was low/undetectable in the SVZ of the MGE, in the mutants they were prematurely expressed in the SVZ (Figure 4H–M). These results are consistent with our phenotypic analysis showing increased neurite length of Dlx1/2 mutant interneuron precursors. We focused our next studies on the expression and function of PAK3 in interneurons.
Figure 4. MGE-derived neurons from Dlx1/2−/− show increased expression of several proteins that regulate the cytoskeleton.
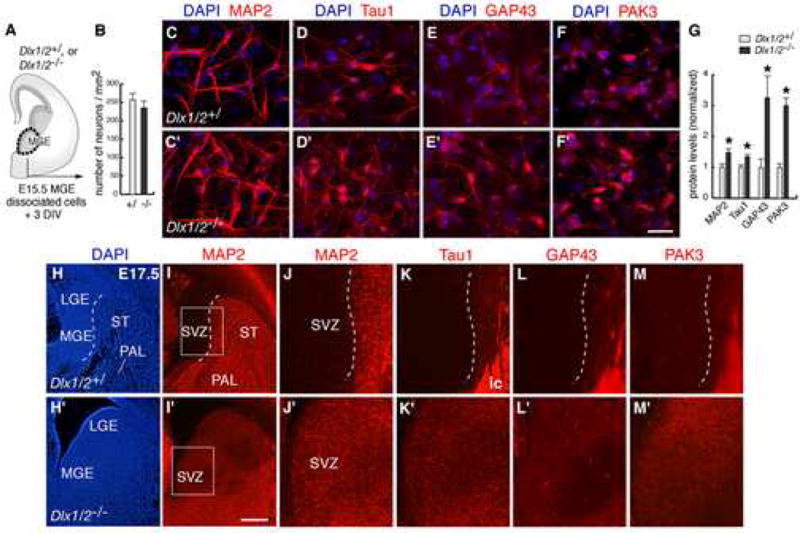
(A) In vitro experimental assay used to analyze neuronal differentiation of Dlx1/2−/− MGE.
(B) Quantification of numbers of neurons from control and mutant MGE cultures after 3 DIV.
(C–F) Immunofluorescent staining for MAP2 (2a + 2b), Tau1, GAP43, and PAK3 in mutant (C′–F′) and control (C–F) MGE cultures after 3 DIV.
(G) Quantification of protein levels from immunoblots of control and mutant MGE cultures after 3 DIV. * p < 0.05 (Student’s t test).
(H–M) Immunofluorescent staining for MAP2, Tau1, GAP43 and PAK3 in histological sections from E17.5 Dlx1/2−/− (H′–M′) and controls (H–M), showing increased levels of these proteins in the SVZ of the MGE. (J–M) are higher magnifications of the areas boxed in (I). Dashed lines approximate the boundary between SVZ and mantle (ST: striatum; PAL: globus pallidum). Strong expression of Tau1, GAP43 and PAK3 in (K–M) labels fibers of the internal capsule (ic).
Scale bars = 50 μm (C–F) and 500 μm (H,I).
Dlx1/2 genes control growth of neurites and cell migration in part through negative regulation of PAK3 expression
PAK serine/threonine kinases are critical for coordinating the dynamics of the actin and microtubule cytoskeletons during cell motility and cell growth and/or transformation (Bokoc, 2003; Hofmann et al., 2004; Kumar et al., 2006). We tested the hypothesis that PAK3 overexpression in Dlx1/2−/− neurons contributed to the excessive neurite growth and block in migration in MGE-derived interneurons.
First, we analyzed the developmental expression of PAK3 in the wild-type mouse telencephalon (Figure 5A–C). At early and mid-gestational stages (E11.5–E15.5), PAK3 mRNA expression was nearly undetectable in the MGE and cells migrating out of the MGE (Figure 5A, B). At P0 and postnatal stages, MGE-derived cells, including projection neurons of the globus pallidus, striatal interneurons, and cortical and hippocampal interneurons, showed high expression of PAK3 mRNA (Figure 5C, D, and Supplemental Figure 7). PAK3 was also expressed by neocortical and hippocampal pyramidal neurons, as described in previous studies (Allen et al., 1998). In Dlx1/2−/− mutant brains, PAK3 expression was robust in the SVZ of the MGE (Figure 5A′–C′). The Dlx1−/−;Dlx2+/− mutant MGE showed a slight elevation in PAK3 expression (Supplemental Figure 8). Immunocytochemistry in cell cultures prepared from wild-type E15.5 MGE showed a diffuse PAK3 distribution throughout the soma and neuronal processes (Figure 5E–G). Notably, PAK3 expression in wild-type cultures was up-regulated as neurons differentiated, as assessed by immunocytochemistry (3–7 DIV; Figure 5E–G) and immunoblots from culture lysates (Figure 5H).
Figure 5. Analysis of PAK3 expression in the mouse telencephalon.
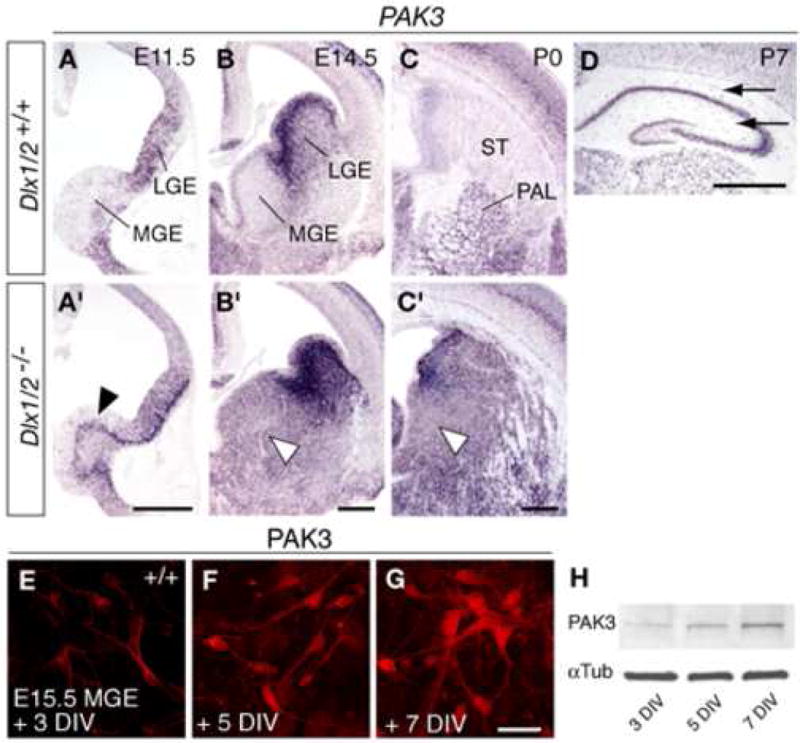
(A–D) RNA in situ hybridization for PAK3 in coronal sections through the telencephalon of wild-type (A–D) and Dlx1/2−/− mutant (A′–C′) embryos at E11.5, E14.5, and P0. Note the absence of detectable PAK3 expression in the MGE SVZ of wild-type embryos, and increased expression in the MGE SVZ of Dlx1/2−/− mutants (arrowheads in A′–C′). (D) PAK3 expression in wild-type postnatal (P7) hippocampus. Black arrows point to PAK3 expression in hippocampal layers that contain interneurons.
(E–G) Immunofluorescent staining for PAK3 protein in cultures from wild-type E15.5 MGE after 3, 5 and 7 DIV.
(H) Immunoblot analysis of PAK3 from cell lysates of MGE cultures after 3, 5 and 7 DIV. Scale bars = 500 μm (A,B,C,D) and 50 μm (E–G).
To test whether PAK3 was necessary for neurite growth in MGE-derived neurons, we reduced its expression ~8-fold by transfecting dissociated MGE cells with PAK3 siRNA oligos [see Figure S9 in Supplementary data for the siRNA efficiency in PAK3 down-regulation, and Boda et al., (2004) for the specificity of PAK3 down-regulation by PAK3 siRNA]. Dissociated cells from E15.5 wild-type MGE (VZ/SVZ) were transfected with PAK3 siRNA (or non-silencing siRNA for controls), and plated over cortical feeders prepared from wild-type E18.5/P0 brains (Figure 6). Co-electroporation of a GFP-expressing DNA plasmid served as a reporter for visualizing the morphology of transfected cells. Morphometric analysis of randomly selected GFP-expressing cells after 3 DIV showed significant decreases in total neurite length, average neurite length and longest neurite length in PAK3 siRNA-treated neurons (TNL: ~15%-; ANL: ~15%; LN: ~25%), whereas the number of neurites was unchanged (Figure 6 A–C, H). Next, to test whether PAK3 was mediating the overgrowth of neurites in Dlx1/2−/− neurons, we performed the same experiments in mutant cells. Our results showed that after 3 DIV, PAK3 siRNA-treated neurons showed a significant decrease in average neurite length and length of longest neurite when compared to control cells (ANL: ~15%; LN: ~25%), and similar number of neurites (Figure 6 D,E, H).
Figure 6. Dlx1/2 genes regulate growth of neurites through PAK3.
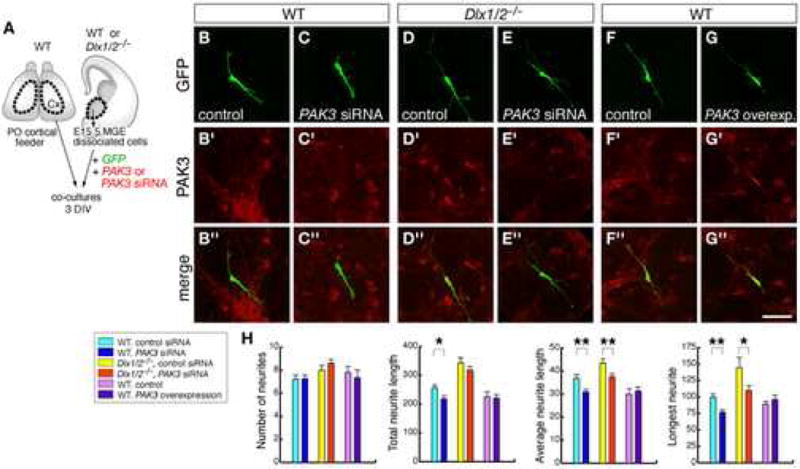
(A) Experimental assay used to test the effects of down-regulating PAK3 expression on neurite growth in wild-type and in Dlx1/2 MGE neurons, and of up-regulating PAK3 in wild-type MGE neurons.
(B–G) Representative neurons stained with double immunofluorescence for GFP and PAK3 from samples that were co-transfected with PAK3 siRNA and a GFP-expressing vector (and the corresponding controls), in either wild-type or Dlx1/2 samples, or that were co-transfected with full-length PAK3 and GFP-expression vectors (and controls) in wild-type samples.
(H) Quantification of number of neurites, total neurite length, average neurite length, and length of longest neurite from each type of experiments. Student’s t-test revealed significant decreases in TNL, ANL and LN in wild-type neurons treated with PAK3 siRNA [siRNA: (in μm) TNL: 218.4 ± 10.5, ANF: 30.9 ± 1.2, LN: 77 ± 3.4; control: TNL: 256.9 ± 10.4, p < 0.05, ANF: 36.9 ± 1.5, p < 0.01, LN: 99.8 ± 4.6, p < 0.01; n = 40], and significant decreases in ANL and LN in Dlx1/2−/− neurons treated with PAK3 siRNA [siRNA: (in μm) ANF: 37.5 ± 1.3, LN: 109.7 ± 7.5; control: ANF: 43.5 ± 1.8, p < 0.01, LN: 144.7 ± 15.7, p < 0.05; n = 40]. * p < 0.05; ** p < 0.01.
Scale bar = μm (B–G).
To test whether PAK3 overexpression could increase the length of neurites in wild-type MGE-derived neurons, we transfected dissociated cells with an expression vector coding the full-length sequence of PAK3; this resulted in ~4-fold increased PAK3 expression (Figure S9). Analysis of neurite growth from cells co-transfected with PAK3 and GFP after 3 DIV did not show significant differences in either length or number of neurites (Figure 6F–H). Thus, our results revealed that PAK3 contributes to the lengthening of neuronal processes in MGE-derived cells, both in wild-type and Dlx1/2 mutants, and its overexpression is not sufficient alone to promote neurite growth.
PAK proteins regulate cell motility (Bokoch et al., 2003). Thus, we asked whether PAK3 overexpression in the Dlx1/2−/− MGE contributed to the defect in interneuron migration. To test this possibility, we used a slice tissue culture assay (Figure 7). Brain slices were prepared from E14.5 wild-type embryos, focally co-electroporated with PAK3 and GFP expression vectors in the MGE, and allowed to develop in culture for 3 days (Figure 7A–D). Quantification of GFP+ cells in the cortex showed a ~70% reduction in cell migration after 3 DIV in PAK3 electroporations, compared to control electroporations (Figure 7E). These results suggest that MGE-derived interneurons have to maintain PAK3 expression at low levels while they tangentially migrate to the cortex.
Figure 7. Dlx1/2 genes promote tangential migration of interneurons to neocortex in part through negative regulation of PAK3 and MAP2 expression.
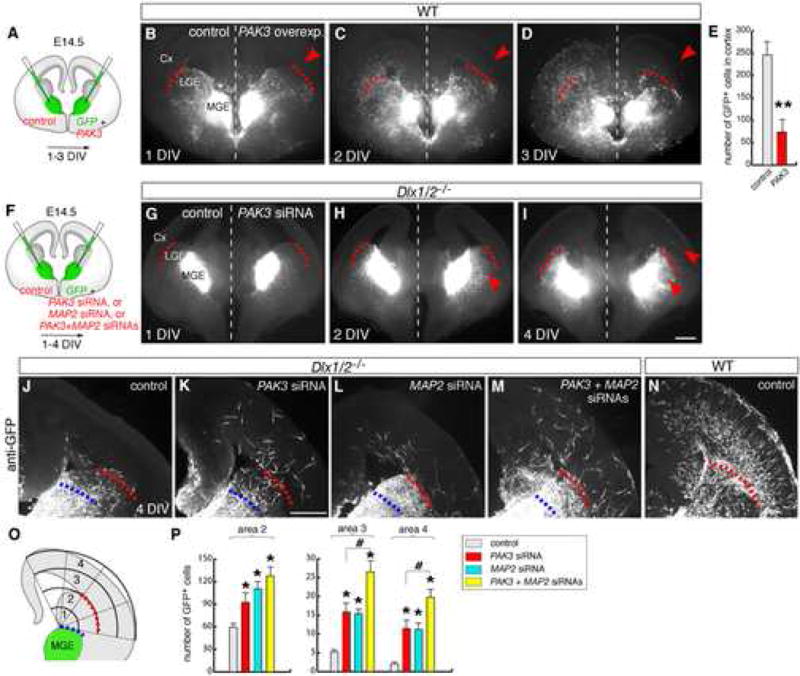
(A,F) Experimental assay used to test the consequences of overexpressing PAK3 in wild-type MGE neurons, and of down-regulating PAK3 and MAP2 in Dlx1/2−/− MGE neurons.
(B–D) Representative example of focal electroporations into the MGE in organotypic cultures of E14.5 forebrain slices, showing in vivo labeling of GFP neurons after co-electroporation with full-length PAK3 and GFP-expression vectors (right hemisphere of the brain), and control plasmids (left hemisphere). The same slice was photographed after 1, 2 and 3 DIV. Red dashed lines demarcate the subpallial/pallial boundary. Note the decrease of migration from MGE to neocortex in the hemisphere electroporated with PAK3 (red arrowheads).
(E) Quantification of numbers of GFP+ cells migrated to cortex after 3 DIV [control: (number of GFP+ cells in cortex): 246.4 ± 40.2; PAK3: 74.4 ± 35.4, n = 25 slices). ** p < 0.01 (Student’s t test).
(G–I) In vivo labeling of GFP neurons from one Dlx1/2−/− embryo after co-electroporation with PAK3 siRNA and GFP-expressing vector (right hemisphere), and siRNA control and GFP-expressing vector (left hemisphere). Arrowheads in (H, I) indicate increased migration to dorsal LGE and cortex.
(J–N) Anti-GFP imunohistochemistry in representative Dlx1/2−/− slices that were electroporated with siRNAs: control (J), PAK3 (K), MAP2 (L), or PAK3+MAP2 (M) siRNAs. The slices were fixed and re-sectioned (75 μm). Note increased migration of GFP+ cells to cortex after PAK3 and MAP2 siRNA electroporations. Migration in one representative wild-type slice that was electroporated with siRNA control is shown in (N).
(O, P) Quantification of numbers of GFP+ cells migrated to cortex in Dlx1/2−/− slices after 4 DIV. A grid with concentric circles was superimposed to each microphotograph, with its center placed at the dorsal limit of the electroporated area (typically between MGE and LGE; blue dash lines). The number of GFP+ cells in areas 2 (dorsal LGE), 3 (ventrolateral cortex) and 4 (dorsal cortex) were counted [control: 59.3 ± 4.9 (area 2), 5.2 ± 0.7 (area 3), 2 ± 0.5 (area 4); PAK3: 92.4 ± 12.3 (area 2), 15.8 ± 2.5 (area 3), 11.4 ± 2.2 (area 4); MAP2: 110.0 ± 9.2 (area 2), 15.4 ± 1.2 (area 3), 11.2 ± 1.7 (area 4); PAK3 + MAP2: 127.5 ± 12.3 (area 2), 26.5 ± 3 (area 3), 19.7 ± 2.1 (area 4); n = 15 slices for each type). * p < 0.05 versus control; # p < 0.05 versus PAK3 siRNA group (Tukey-Kramer test).
Scale bars = μm (B–D, G–I, and J–N).
To test whether reducing PAK3 was sufficient to rescue the block in migration in Dlx1/2−/−, we focally co-electroporated PAK3 siRNA and a GFP expression vector in MGE of Dlx1/2−/− slices. Electroporations with control siRNA showed very few GFP+ cells in the cortex; these cells were located in the cortical subventricular zone adjacent to the subpallial/pallial boundary (Figure 7J). By contrast, PAK3 siRNA resulted in a ~4-fold increase in the number of cells that tangentially migrated into the mantle zone of the dorsolateral cortex (control siRNA: ~7 cells, PAK3 siRNA ~28 cells; versus ~250 cells in wild-type slices, Figure 7J,K,P).
Finally, we tested whether reducing MAP2 in the MGE of Dlx1/2−/− could also contribute to rescue the migration defect [see Figure S9 for the siRNA efficiency in MAP2 down-regulation]. MAP2 is expressed primarily in post-migratory neurons where it exerts important functions in stabilizing microtubules during dendrite growth (Fujimori et al., 2002; Dehmelt and Halpain, 2005). In Dlx1/2−/− mutant brains, MAP2 was prematurely expressed in the SVZ of the MGE (Figure 4J; Anderson et al., 1997b). We found that electroporating MAP2 siRNA into the mutant MGE resulted in a ~4-fold increase in the number of tangentially migrating cells reaching the cortex (~28 cells; Figure 7L,P). Moreover, co-electroporation of PAK3 and MAP2 siRNAs further increased (~8-fold) the number of GFP+ cells that tangentially migrated into the dorsolateral cortex (~47 cells; Figure 7M–P). This represented a ~12% rescue of the tangential migration defect in Dlx1/2−/− mutants, when compared to the migration in wild-type slices (~326 cells; Figure 7N). Thus, these results indicate that reducing PAK3 (and MAP2) contributes, at least partially, to rescue the tangential migration defect in Dlx1/2−/− mutants.
DISCUSSION
Precursors of mouse neocortical GABAergic interneurons originate in the subpallial telencephalon, from where they tangentially migrate to reach the cortical plate, and then radially migrate to acquire their laminar positions. Within neocortex, they develop their dendritic and axonal arborizations. Previous studies have shown that Dlx transcription factors are required for the tangential migration of subpallial-derived GABAergic interneurons (Anderson et al., 1997a; Panganiban and Rubenstein, 2002). In the present study, we demonstrate a central role of Dlx1&2 genes in inhibiting neurite growth in immature interneurons at a stage while they migrate tangentially to cortex. Using Dlx1−/−;Dlx2+/− mutants, we demonstrate that developing interneurons also require Dlx genes for migrating to their neocortical laminar positions, and for regulating the growth and branching of their dendrites and axons in neocortex. We provide evidence that premature and excessive expression of several proteins that regulate the cytoskeleton (i.e., PAK3, MAP2) in subpallial-derived Dlx1/2−/− neurons contributes to the excessive neurite growth and the block in tangential migration.
Evidence that Dlx genes regulate neuronal differentiation and migration of cortical GABAergic interneurons
Dlx1 and 2 genes are co-expressed by progenitor cells of the subpallial subventricular zone, where they have redundant functions in controlling the development of GABAergic neurons (Liu et al., 1997; Anderson et al., 1997b, Yun et al., 2002). In Dlx1/2−/− embryos, interneuron precursors fail to migrate out of the SVZ, where they partially differentiate (Anderson et al., 1997a,b). We provide three lines of evidence that Dlx genes have a central role in controlling neuronal differentiation that may contribute to their migration defects. First, we show that primary cultures from Dlx1/2−/− MGE produce neurons with increased neurite length. As neurons further differentiate, they show increased dendritic and axonal length, and decreased axonal branching. Second, we confirmed this phenotype by analyzing neocortical interneurons from Dlx1−/−;Dlx2+/− mutants, which maintain tangential migration but exhibit abnormalities in the growth and branching of their neuronal processes. Third, there was precocious and robust expression of several proteins that regulate the cytoskeleton (i.e, MAP2, Tau1, GAP43, and PAK3) both in the MGE of Dlx1/2−/− embryos and in MGE cultures (see also Anderson et al. 1997b). In vivo, these proteins are found primarily in post-migratory cells, where they exert important functions in controlling the growth of axons and dendrites (Figure 4–6, and Aigner et al., 1995; Fujimori et al., 2002; Dehmelt and Halpain, 2005). Because loss of Dlx1&2 produces neurons with increased complexity (i.e, longer neurites) and up-regulation of genes that are normally expressed by more mature, post-migratory interneurons, we hypothesize that Dlx genes have a central role in repressing molecular pathways in migrating interneurons that promote their later differentiation steps. Thus, in the absence of Dlx function, immature interneurons are unable to maintain the undifferentiated state that is necessary for their migration (see model in Figure 8).
Figure 8. Model for Dlx1&2 functions in the migration and neuronal differentiation of GABAergic interneurons.
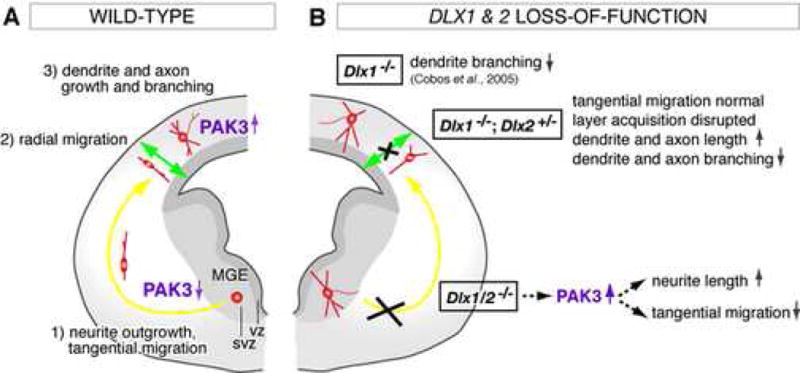
(A) Most mouse precursors for cortical GABAergic interneurons (red) are generated in the medial ganglionic eminence (MGE) in the ventral telencephalon. Their differentiation program involves the outgrowth of neurites and tangential migration (yellow arrow) towards the cortical plate (Marin and Rubenstein, 2003; Metin et al., 2006; Wonders and Anderson, 2006). Within the cortical plate, they change their mode of migration from tangential to radial (green arrow) to acquire their laminar positions (Nadarajah et al., 2002; Tanaka et al., 2003; Hevner et al., 2004; Pla et al., 2006). In neocortex, they grow and pattern their dendritic and axonal arbors, at the time that they up-regulate expression of the PAK3 kinase. Reducing PAK3 levels using siRNA against PAK3 inhibits the lengthening of neuronal processes.
(B) Dlx dosage has a profound effect on the migration and differentiation of GABAergic interneurons. In Dlx1/2−/− double mutants, interneuron precursors fail to migrate tangentially; instead they partially differentiate within the subventricular zone (SVZ) of the MGE (Anderson et al., 1997a). They show increased neurite length. In Dlx1−/−;Dlx2+/− compound mutants, interneurons are able to migrate tangentially to neocortex, but show defects in the acquisition of their laminar positions, and exhibit dendritic and axonal processes with increased length and decreased branching. In Dlx1−/− single mutants, a subset of neocortical interneurons show subtle defects in dendritic morphogenesis; these mice survive to adulthood and develop epilepsy (Cobos et al., 2005). Complete loss of Dlx1&2 results in premature expression of several proteins that regulate the cytoskeleton (i.e, MAP2, Tau1, GAP43 and PAK3) in the SVZ of the MGE. Overexpression of PAK3 in the MGE is sufficient to block tangential migration. Reducing PAK3 and MAP2 levels in the MGE of Dlx1/2−/− mutants partially rescues the tangential migration defect.
An alternative interpretation for this phenotype could be a switch in cell fate. In this context, it could be possible that Dlx loss-of-function favored the differentiation of specific neuronal subtypes characterized by increased neurite length and decreased neurite branching. This seems unlikely, because changes in cell fate have not been observed in the Dlx1/2−/− double mutant MGE (Anderson et al., 1997b; Marin et al., 2000; Yun et al., 2002; De Melo et al, 2005; Cobos et al., 2005). Furthermore, the MGE of the Dlx1/2−/− embryos maintains expression of several interneuron markers including somatostatin and neuropeptide Y (Cobos et al., 2005). In addition, analysis of interneuron markers in the neocortex of Dlx1−/−;Dlx2+/− neonates and in primary cultures from Dlx1−/−;Dlx2+/− neocortex do not show obvious changes in GABAergic interneuron cell fate (Cobos and Rubenstein, unpublished data).
We have previously shown that postnatal Dlx1−/− single mutant mice have decreased branching of dendrites in subsets of cortical interneurons (e.g. bitufted somatostatin+ interneurons; Cobos et al., 2005). Here we report progressively more severe morphological defects in Dlx1/2−/− > Dlx1−/−;Dlx2+/− > Dlx1−/− GABAergic interneurons. Dlx1+/−;Dlx2−/− compound mutants have defects in forebrain development that are similar to those observed in the Dlx1−/−;Dlx2+/− mutants (Cobos and Rubenstein, unpublished data). These findings suggest redundant functions of Dlx1 and Dlx2 in GABAergic differentiation, and are consistent with previous studies showing that Dlx dosage is critical in development of neural and non-neural structures (Anderson et al., 1997b; Robledo et al., 2002; Depew et al., 2005). It does remain possible that Dlx1 and Dlx2 also have distinct functions in GABAergic neurons.
In addition to the role of Dlx genes in controlling neuronal morphogenesis in GABAergic interneurons, we present the first evidence that Dlx affect the acquisition of their normal laminar positions in neocortex. In Dlx1−/−;Dlx2+/− mice, excess Lhx6+ interneurons accumulate in deep and superficial layers of neocortex, and fail to sufficiently populate middle (IV–V) layers. Neocortical interneurons have been shown to migrate radially to adopt their laminar identities (Nadarajah et al., 2002; Tanaka et al., 2003; Hevner et al., 2004; Pla et al., 2006). Future studies will be aimed at elucidating the molecular mechanisms regulating radial migration of GABAergic interneurons by Dlx genes.
Finally, our results show that Dlx function is required for the survival of GABAergic neurons developing in vivo and in vitro. Previous studies have reported a requirement of Dlx1&2 for cell survival in the retina (De Melo et al., 2004), and of Dlx1 in mature interneurons of the neocortex and hippocampus (Cobos et al., 2005). The mechanisms underlying cell death in Dlx1/2−/− are unknown, although we propose that it has a cell autonomous basis because mutant MGE cells show apoptosis when transplanted onto wild type cortical cells (Supplemental Figure 3). Whether Dlx genes regulate the expression of trophic factor receptors and/or pre- and pro-apoptotic genes remains to be determined.
Dlx genes coordinate neuronal differentiation and migration through regulation of cytoskeletal proteins: role of PAK3
We show that immature MGE-derived Dlx1/2−/− interneurons exhibit premature and excessive expression of several cytoskeletal proteins, including MAP2, Tau1, GAP43 and PAK3. We focused on the analysis of PAK3 because of the central role of PAK kinases in cytoskeletal reorganization and cell behavior. PAK kinases are major downstream effectors of the Rho-family of GTPases (RhoA, Rac1, Cdc42), a family of key signaling proteins that integrate different extracellular and intracellular signals to regulate the actin cytoskeleton (Govek et al., 2005; Rossman et al., 2005). PAKs usually interact with and are activated by Rac1 and Cdc42, although they can also be activated by a variety of Rho GTPase-independent mechanisms (Bokoc, 2003). In Drosophila, PAK kinases have essential roles in axon guidance (Hing et al., 1999; Fan et al., 2003). In the mammalian brain, they play crucial roles in regulating neurite outgrowth, cell migration, spine morphogenesis, and synapse formation (Bokoc, 2003; Hofmann et al., 2004; Boda et al., 2006). We found that in MGE primary cultures PAK3 expression is up-regulated as neurons differentiate. In vivo, PAK3 expression is up-regulated in cortical interneurons once they have migrated into the cortex, a stage when they develop their dendritic and axonal arborizations. By reducing PAK3 levels using RNA interference, we show that PAK3 promotes the lengthening of neuronal processes. These results are in agreement with previous studies showing that PAK proteins regulate the cytoskeleton during neurite growth and remodeling. For example, PAK1 is implicated in neurite outgrowth (Rashid et al., 2001; Daniels et al., 1998), and GEFT, a guanine nucleotide exchange factor that activates the Rac1/Cdc42-PAK signaling pathway, promotes dendrite growth in primary hippocampal neurons (Bryan et al., 2004).
On the other hand, PAK3 overexpression in MGE neurons was insufficient to increase the lengthening of neuronal processes. This is in line with previous studies in cell lines showing that wild-type PAK and cytoplasmically expressed PAK do not cause efficient neurite outgrowth and/or elongation, while membrane-targeted forms of PAK are able to increase neurite extension. Local recruitment and activation of PAK to the membrane, together with Paxillin and GIT1, form a signaling complex that appear to be necessary for mediating polarized actin polymerization and subsequent neurite extension (Daniels et al., 1998; Obermeier et al., 1998; Nayal et al., 2006).
Major in vivo functions of PAK3 in mammalian neurons involve dendrite spine morphogenesis and synapse formation and plasticity (Allen et al., 1998; Boda et al., 2004; Zhang et al., 2005; Meng et al., 2005). Here we provide evidence that PAK3 promotes neurite growth in cortical GABAergic interneurons. Notably, we found that in Dlx1/2−/− mutants, PAK3 is prematurely expressed in the MGE, and that down-regulating PAK3 in the mutant MGE is sufficient to rescue the excessive growth of neurites in the mutants. These findings support our hypothesis that Dlx1&2 have a major role in immature interneurons for repressing molecular pathways that promote axon and dendrite maturation.
PAK proteins also regulate cell motility (Bokoc, 2003). In fibroblasts and epithelial cells, PAK (PAK1, PAK3) is a key effector for adhesion turnover and protrusions dynamics. In these cells, PAK is targeted with Paxillin and GIT1 near their leading edge to increase adhesion turnover, protrusion and motility (Zhao et al., 2000; Nayal et al., 2006). Although the functions of PAK in the cytoskeletal structure are well established, its mechanism in regulating neuronal migration is less understood. In the mammalian cortex, PAK1 co-localizes with p35/Cdk5 and Rac1 at the leading edges of axonal growth cones. p35/Cdk5 is essential for the radial migration of immature pyramidal neurons and is required for cortical lamination (Chae et al, 1997; Nikolic et al, 1998). p35/Cdk5 hyperphosphorylate and subsequently down-regulate PAK1, which is crucial for the proper regulation of the cytoskeleton during neurite growth and migration (Nikolic et al, 1998; Rashid et al., 2001). Thus, these studies indicate that in mouse neocortex PAK1 activity has to be inhibited for the proper regulation of cytoskeletal dynamics during neuronal migration. In agreement with this concept, we found that PAK3 expression was low/absent in wild-type MGE-derived migratory interneurons, and that PAK3 overexpression in the MGE was sufficient to arrest the tangential migration of interneurons to neocortex. Moreover, down-regulating PAK3 with RNA interference in the MGE of Dlx1/2−/− tissue culture slices was sufficient to increase the numbers of neurons migrating tangentially to neocortex. Tangential migration in Dlx1/2−/− tissue culture slices was further increased using RNA interference against MAP2 together with PAK3. Although the rescue of migration was incomplete, these results demonstrate that Dlx genes promote tangential migration at least in part through negative regulation of neurite growth. Deficits in additional signaling pathways in Dlx1/2−/− embryos may well also contribute to the migration failure. For instance, changes in expression of the Slit receptor Robo1, the Semaphorin receptor Neuropilin2, or the Neuregulin1 receptor ErbB4 (Supplemental Figure 6), which have been implicated in cortical interneuron migration (Marin et al., 2001; Flames et al., 2004; Andrews et al., 2006), may also explain the tangential migration defect of Dlx1/2−/− mutants.
METHODS
Mice
The mouse mutant strains with double null alleles of Dlx1 and Dlx2 (Dlx1/2) and single null allele of Dlx1 (Qiu et al., 1997) were used. Dlx1−/−;Dlx2+/− compound mutant mice were generated by mating Dlx1+/− with Dlx1/2+/− animals. The transgenic line Lhx6-GFP BAC (NINDS GENSAT project) was bred to the Dlx1+/− and Dlx1/2+/− lines to generate Dlx1+/−;Lhx6-GFP and Dlx1/2+/−;Lhx6-GFP animals, respectively. Mating of these mice to either Dlx1 or Dlx1/2 mice were used to generate Dlx1−/−;Lhx6-GFP, Dlx1−/−;Dlx2+/−;Lhx6-GFP, and Dlx1/2−/−;Lhx6-GFP embryos. Littermate mice (WT or, when not possible, heterozygous) were used as controls for all experiments. Currently, no phenotype has been identified in the brain of Dlx1/2+/− mice. Mouse colonies were maintained at the University of California, San Francisco, in accordance with National Institutes of Health and UCSF guidelines.
Histology
Pregnant females and neonatal pups were deeply anesthetized in a CO2 chamber and sacrificed by cervical dislocation or decapitation. Mouse pups (P5–P7) were anesthetized with Avertin (0.2cc/10g body weight), perfused intracardiacally with 4% paraformaldehyde (PFA) in phosphate-buffered solution (PB 0.1 M, pH 7.4), and their brains removed and post-fixed overnight in the same fixative. E13.5-P0 brains were dissected and fixed by immersion in 4% PFA in PB. The tissue was cryoprotected by immersion in 30% sucrose, embedded in OCT (Tissue-Tek), and cryostat sectioned (10–20 μm).
Immunohistochemistry on cryostat sections was performed according to standard methods. The antibodies used were as follows: mouse anti-Tuj1 (1:1000, Covance), mouse anti-MAP2 (2a + 2b; 1:2000, Sigma), mouse anti-Tau1 (1:1000, Chemicon), rabbit anti-GAP43 (1:4000, Chemicon), goat anti-PAK3 (1:100, Santa Cruz), rabbit anti-pan-Dll antibody (1:500, G. Panganiban), rabbit anti-GFP (1:2000, Molecular Probes), and mouse anti-GFP (1:500, Molecular Probes). Fluorescent secondary antibodies (goat anti-rabbit, goat anti-mouse, or donkey anti-goat), conjugated with either Alexa-488 or Alexa-594 (1:300; Molecular Probes), were used.
In situ RNA hybridization on cryostat sections was performed according to standard methods. The probes used and their sources were as follows: GAD67 (B. Condie), Lhx6 (V. Pachnis) Dll1 and Hes5 (G. Weinmaster), Mash1 (F. Guillemot), ErbB4 (C. Lai), Robo1, Nrp2 and Sema3A (M. Tessier-Lavigne), and Mef2c (E. Olson). Some probes were made from EST clones (Invitrogen): Elmo1 (BI694830), CD47 (BG964433), DSCAM (CO043786), PAK3 (AW049432), IGSF4a (BF021469), and Rapgef5 (BE310387). Our expression data was in agreement with public electronic databases [BGEM (http://www.stjudebgem.org), NINDS GENSAT project (http://www.gensat.org)] and published data (Supplemental Figure 6).
Primary cell cultures
Dlx1/2−/− and Dlx1/2+/? control brains were used for primary MGE cultures (E15.5), and for transplantation of MGE donor cells (E15.5) over cortical feeders following the method of Xu et al. (2004). The brains were removed in ice-cold Hanks buffer (HBSS; Invitrogen), and the proliferative zones (VZ/SVZ) from the anterior part of the MGE were isolated (Cobos et al. 2005). The cells were dissociated mechanically in Neurobasal/B27 medium (NB/B27, Invitrogen) containing 1 U/ml DNase I (Roche). Then, they were pelleted, resuspended in DMEM (Invitrogen) with 10% fetal bovine serum (FBS, HyClone), and plated at a density of 2.5 × 105 cells per cm2, in either 16-well, 8-well or 2-well chamber slides (0.4 cm2, 0.8 cm2, and 4 cm2, respectively; Lab-Tek, Nalgene). The slides were previously coated with poly-lysine (10 μg/ml; Sigma) followed by laminin (5 μg/ml; Invitrogen). Primary cortical cultures were prepared from E18.5 or P0 brains. The neocortex was dissected in ice-cold HBSS, macerated using fine forceps, trypsinized (0.05% trypsin, 37°C, 20 min), and further triturated using a fire-polished glass pipette. For transplantations of MGE donor cells over neocortical feeders, we isolated Dlx1−/−, Dlx1−/−;Dlx2+/−, Dlx1/2−/−, and control cells (all Lhx6-GFP+), and plated them on E18.5/P0 wild-type feeders prepared 1 day earlier. One thousand donor cells were added per well (8-well slides; 0.8 cm2) in experiments designed to image and analyze cell morphology; ten thousand donor cells were used in experiments designed to quantify cell survival.
Image analysis and quantification of neurite growth
Quantification of neurite growth was performed in GFP-expressing immature interneurons from primary cultures prepared with either Dlx1−/−, Dlx1−/−;Dlx2+/−, or Dlx1/2−/− mutants and littermate controls (all Lhx6-GFP+). Transplantation of donor cells at low density, and dilution of GFP cortices with WT, non-GFP cortices, as described above, allowed identification of individual GFP+ cells so that their processes could be clearly distinguished from those of other GFP+ cells. For sampling of cultured neurons, we used an unbiased, non-stereological method that gave every cell an equal chance of being sampled. We defined a systematic series of fields of view in the culture area, and randomly chose 1–3 cells in each field for imaging. The experimenter was blinded to genotype during sampling, image analysis, data collection and statistical analysis. We sampled 20–40 interneurons for each time point and each genotype, from at least 6 different wells obtained from 3 independent experiments. Images were acquired on a confocal microscope (Radiance 2000, BioRad) with a 20× or 40× objective. The confocal settings were chosen so that the GFP fluorescence was within the brightness range required to clearly visualize the entire cell, and were maintained for all experiments of the same category. Series of z-stack images (3–10 optical series, depending on the age) were collected encompassing all neuronal processes of each cell. Digitized images were imported to Image J (National Institute Health software; Neuron J plugin) for tracing and quantifying neurites. Total number of branches (filopodia longer than 5 μm were considered branch tips), total neurite length, average neurite length, and length of longest neurite were determined for each cell. Statistical analyses were performed with SPSS 10.0. Differences between means were assessed by ANOVA, followed by Tukey-Kramer post hoc test. Significance level was taken as p < 0.05.
DNA plasmids and siRNA
The expression vector coding the full-length sequence of the mouse PAK3 was generated subcloning PAK3 (clone ID 30060082, MGC full-lengh) into pCAGGS, a chicken β-actin promoter expression vector (Stuhmer et al., 2002). An expression vector encoding enhanced green fluorescent protein (GFP) cloned into pCAGGS (Stuhmer et al., 2002) was used for co-transfection experiments. siRNA targeting the mouse PAK3 and MAP2 genes were used. siRNA was obtained from IDT (duplexed RNAi oligonucleotides). The target sequence for PAK3 previously published by Boda et al. (2004) (5′ TAGCAGCACATCAGTCGAATA), and for MAP2 used by Fontaine-Lenoir et al. (2006) (5′ TTCGCTGAGCCTTTAGACA), resulted in a consistent reduction of ~90% of PAK3 and MAP2 protein levels, respectively (n = 5 each; as measured by western blot analysis). The target sequence used as non-silencing control was 5′ AATTCTCCGAACGTGTCACGT (Boda et al., 2004).
Transient transfection of primary neurons
For transfections of MGE cells with either siRNA or DNA plasmids we utilized Nucleofector technology (Amaxa biosystems). The VZ/SVZ of the MGE from Dlx1/2−/− or control embryos, aged E15.5, was dissected and their cells dissociated as described above. We conducted several control experiments to determine the optimal volume of sample (number of cells) and substrate (siRNA and/or DNA plasmid) being transfected for maximum gene transfer efficiency and cell viability. Based on these results, we used 3 × 106 cells (pooled from 3–5 embryos) for each transfection, and 0.2 nmol of siRNA or 2 μg of DNA, which resulted in > 90% efficiency in gene delivery. Transfections were performed according to the manufacturer guidelines (Amaxa biosystems), using Program O-05 and the primary mouse neuron kit. Identical conditions were used for transfections of DNA, RNA, and co-transfections with DNA and RNA together. After transfections, the cells were resuspended in DMEM/10% FBS and plated at high density. For analysis of cell morphology, MGE cells were co-transfected with either PAK3 siRNA or the PAK3 expressing vector, and the GFP expressing vector, and plated over wild-type neocortical feeders prepared 1 day previously.
Slice tissue cultures and electroporations
Preparation of slice tissue cultures from embryonic mouse brains followed the methods of Anderson et al. (1997a). To achieve focal electroporations of MGE cells, we pressure injected, through a glass micropipette, PAK3 siRNA (200 μM), MAP2 siRNA (200 μM) or the PAK3 expressing vector (1 μg/μl), and the corresponding controls in the contralateral brain hemisphere. In all cases we co-electroporated the GFP-expression vector to assess the efficiency of the electroporations, and to quantify the migration of the targeted cells to the cortex. Electroporations followed the methods of Stuhmer et al. (2002). Five square wave pulses of 100 volts and 5 ms duration were applied using a T820 Electro Square Porator (BTX). Cell viability was assessed using 25 μg/ml propidium iodine (Molecular Probes) added to the medium. Each slice was examined for GFP expression under an epifluorescent dissecting microscope and photographed at 1, 2 and 3 days in vitro (DIV). After 3–4 DIV, the slices were fixed in 4% PFA in PB, cryoprotected in 30% sucrose, embedded in OCT compound, and re-sectioned on a cryostat (20–75 μm) for immunohistrochemistry.
Microarrays
RNA was extracted from E15.5 MGE cells (Dlx1/2−/− and Dlx1/2+/? littermates) that were dissociated and grown in vitro for 3 days. Genotypes were determined based on the presence of cleft palate in mutants and PCR. Total RNA was isolated using Absolutely RNA Miniprep kit (Stratagene). RNA from at least 6 embryos, each genotype, was pooled to average genetic and experimental variations. 6–20 μg of RNA, each genotype, were used. cDNA reverse transcription, amplification, labeling and hybridization to Affymetrix GeneChip 430 2.0 arrays was performed by the NIH Neuroscience Microarray Consortium.
Western blots
For western blot analysis, cells from primary MGE cultures were lysed in RIPA buffer (50 mM Tris HCl ph7.5, 150 mM NaCl, 0.5% DOC, 0.1% SDS, 1% NP-40) supplemented with protease inhibitors (protease inhibitors cocktail, Roche). Protein samples (10 μg each) were resolved on polyacrylamide gels and transferred to nitrocellulose membranes by electroblotting. Membranes were pre-incubated in 5% nonfat dry milk in TBT (Tris-buffered solution and 0.1% Tween-20), and incubated O/N with the primary antibodies in the same solution. Membranes were probed with HRP-couple anti-mouse, -rabbit or -goat antibodies (1:5000, Bio-Rad) and developed using ECL reagent (GE Healthcare). Each experiment was done at least three times. Quantification of optical density was done using NIH Image software.
Supplementary Material
Acknowledgments
This work was supported by funds to JLRR (Nina Ireland, Larry L. Hillblom Foundation, NIMH RO1 MH49428 and K05 MH065670), IC (Autism Speaks), and UB (Human Frontiers, Autism Speaks, NARSAD). The microarray experiments were completed through the NINDS NIMH Microarray Consortium (http://arrayconsortium.tgen.org) with the assistance of Winnie Liang. We thank members of the Rubenstein laboratory for helpful discussions, and Sam Pleasure, and Andy Peterson for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–78. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Allen KM, Gleeson JG, Bagrodia S, Partington MW, McMillan JC, Cerione RA, Mulley JC, Walsh CA. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet. 1998;20:25–30. doi: 10.1038/1675. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997a;278:474–6. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997b;19:27–37. doi: 10.1016/s0896-6273(00)80345-1. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–63. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Andrews W, Liapi A, Plachez C, Camurri L, Zhang J, Mori S, Murakami F, Parnavelas JG, Sundaresan V, Richards LJ. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–52. doi: 10.1242/dev.02379. [DOI] [PubMed] [Google Scholar]
- Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Bielas S, Higginbotham H, Koizumi H, Tanaka T, Gleeson JG. Cortical neuronal migration mutants suggest separate but intersecting pathways. Annu Rev Cell Dev Biol. 2004;20:593–618. doi: 10.1146/annurev.cellbio.20.082503.103047. [DOI] [PubMed] [Google Scholar]
- Boda B, Alberi S, Nikonenko I, Node-Langlois R, Jourdain P, Moosmayer M, Parisi-Jourdain L, Muller D. The mental retardation protein PAK3 contributes to synapse formation and plasticity in hippocampus. J Neurosci. 2004;24:10816–25. doi: 10.1523/JNEUROSCI.2931-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boda B, Nikonenko I, Alberi S, Muller D. Central Nervous System Functions of PAK Protein Family: From Spine Morphogenesis to Mental Retardation. Mol Neurobiol. 2006;34:67–80. doi: 10.1385/mn:34:1:67. [DOI] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–81. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Bryan B, Kumar V, Stafford LJ, Cai Y, Wu G, Liu M. GEFT, a Rho family guanine nucleotide exchange factor, regulates neurite outgrowth and dendritic spine formation. J Biol Chem. 2004;279:45824–32. doi: 10.1074/jbc.M406216200. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Campbell K. Dorsal-ventral patterning in the mammalian telencephalon. Curr Opin Neurobiol. 2003;13:50–6. doi: 10.1016/s0959-4388(03)00009-6. [DOI] [PubMed] [Google Scholar]
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–68. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Cobos I, Long JE, Thwin MT, Rubenstein JL. Cellular patterns of transcription factor expression in developing cortical interneurons. Cereb Cortex. 2006;16(Suppl 1):i82–8. doi: 10.1093/cercor/bhk003. [DOI] [PubMed] [Google Scholar]
- Daniels RH, Hall PS, Bokoch GM. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J. 1998;17:754–64. doi: 10.1093/emboj/17.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo J, Du G, Fonseca M, Gillespie LA, Turk WJ, Rubenstein JL, Eisenstat DD. Dlx1 and Dlx2 function is necessary for terminal differentiation and survival of late-born retinal ganglion cells in the developing mouse retina. Development. 2005;132:311–22. doi: 10.1242/dev.01560. [DOI] [PubMed] [Google Scholar]
- Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005;6:204. doi: 10.1186/gb-2004-6-1-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depew MJ, Simpson CA, Morasso M, Rubenstein JL. Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development. J Anat. 2005;207:501–61. doi: 10.1111/j.1469-7580.2005.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Labrador JP, Hing H, Bashaw GJ. Slit stimulation recruits Dock and Pak to the roundabout receptor and increases Rac activity to regulate axon repulsion at the CNS midline. Neuron. 2003;40:113–27. doi: 10.1016/s0896-6273(03)00591-9. [DOI] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JL, Marin O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–61. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Fontaine-Lenoir V, Chambraud B, Fellous A, David S, Duchossoy Y, Baulieu EE, Robel P. Microtubule-associated protein 2 (MAP2) is a neurosteroid receptor. Proc Natl Acad Sci U S A. 2006;103:4711–6. doi: 10.1073/pnas.0600113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori K, Takauji R, Tamamaki N. Differential localization of high- and low-molecular-weight variants of microtubule-associated protein 2 in the developing rat telencephalon. J Comp Neurol. 2002;449:330–42. doi: 10.1002/cne.10286. [DOI] [PubMed] [Google Scholar]
- Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- Guillemot F. Cellular and molecular control of neurogenesis in the mammalian telencephalon. Curr Opin Cell Biol. 2005;17:639–47. doi: 10.1016/j.ceb.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Molnar Z, Tarabykin V, Stoykova A. Molecular mechanisms of cortical differentiation. Eur J Neurosci. 2006;23:857–68. doi: 10.1111/j.1460-9568.2006.04626.x. [DOI] [PubMed] [Google Scholar]
- Guan KL, Rao Y. Signalling mechanisms mediating neuronal responses to guidance cues. Nat Rev Neurosci. 2003;4:941–56. doi: 10.1038/nrn1254. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Daza RA, Englund C, Kohtz J, Fink A. Postnatal shifts of interneuron position in the neocortex of normal and reeler mice: evidence for inward radial migration. Neuroscience. 2004;124:605–618. doi: 10.1016/j.neuroscience.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Hing H, Xiao J, Harden N, Lim L, Zipursky SL. Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell. 1999;97:853–63. doi: 10.1016/s0092-8674(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Hofmann C, Shepelev M, Chernoff J. The genetics of Pak. J Cell Sci. 2004;117:4343–54. doi: 10.1242/jcs.01392. [DOI] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27:392–9. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459–71. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JK, Ghattas I, Liu S, Chen S, Rubenstein JL. Dlx genes encode DNA-binding proteins that are expressed in an overlapping and sequential pattern during basal ganglia differentiation. Dev Dyn. 1997;210:498–512. doi: 10.1002/(SICI)1097-0177(199712)210:4<498::AID-AJA12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–83. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Marin O, Anderson SA, Rubenstein JL. Origin and molecular specification of striatal interneurons. J Neurosci. 2000;20:6063–76. doi: 10.1523/JNEUROSCI.20-16-06063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JL. Sorting of striatal and cortical interneurons regulated by semaphorin-neuropilin interactions. Science. 2001;293:872–5. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- Meng J, Meng Y, Hanna A, Janus C, Jia Z. Abnormal long-lasting synaptic plasticity and cognition in mice lacking the mental retardation gene Pak3. J Neurosci. 2005;25:6641–50. doi: 10.1523/JNEUROSCI.0028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metin C, Baudoin JP, Rakic S, Parnavelas JG. Cell and molecular mechanisms involved in the migration of cortical interneurons. Eur J Neurosci. 2006;23:894–900. doi: 10.1111/j.1460-9568.2006.04630.x. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Alifragis P, Wong RO, Parnavelas JG. Ventricle-directed migration in the developing cerebral cortex. Nat Neurosci. 2002;5:218–24. doi: 10.1038/nn813. [DOI] [PubMed] [Google Scholar]
- Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J Cell Biol. 2006;173:587–9. doi: 10.1083/jcb.200509075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai LH. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395:194–8. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- Obermeier A, Ahmed S, Manser E, Yen SC, Hall C, Lim L. PAK promotes morphological changes by acting upstream of Rac. EMBO J. 1998;17:4328–39. doi: 10.1093/emboj/17.15.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganiban G, Rubenstein JL. Developmental functions of the Distal-less/Dlx homeobox genes. Development. 2002;129:4371–86. doi: 10.1242/dev.129.19.4371. [DOI] [PubMed] [Google Scholar]
- Pla R, Borrell V, Flames N, Marin O. Layer acquisition by cortical GABAergic interneurons is independent of Reelin signaling. J Neurosci. 2006;26:6924–34. doi: 10.1523/JNEUROSCI.0245-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M, Bulfone A, Ghattas I, Meneses JJ, Christensen L, Sharpe PT, Presley R, Pedersen RA, Rubenstein JL. Role of the Dlx homeobox genes in proximodistal patterning of the branchial arches: mutations of Dlx-1, Dlx-2, and Dlx-1 and -2 alter morphogenesis of proximal skeletal and soft tissue structures derived from the first and second arches. Dev Biol. 1997;185:165–84. doi: 10.1006/dbio.1997.8556. [DOI] [PubMed] [Google Scholar]
- Rashid T, Banerjee M, Nikolic M. Phosphorylation of Pak1 by the p35/Cdk5 kinase affects neuronal morphology. J Biol Chem. 2001;276:49043–52. doi: 10.1074/jbc.M105599200. [DOI] [PubMed] [Google Scholar]
- Robledo RF, Rajan L, Li X, Lufkin T. The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev. 2002;16:1089–101. doi: 10.1101/gad.988402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–80. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Stuhmer T, Anderson SA, Ekker M, Rubenstein JL. Ectopic expression of the Dlx genes induces glutamic acid decarboxylase and Dlx expression. Development. 2002;129:245–52. doi: 10.1242/dev.129.1.245. [DOI] [PubMed] [Google Scholar]
- Tanaka D, Nakaya Y, Yanagawa Y, Obata K, Murakami F. Multimodal tangential migration of neocortical GABAergic neurons independent of GPI-anchored proteins. Development. 2003;130:5803–5813. doi: 10.1242/dev.00825. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–96. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–22. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun K, Fischman S, Johnson J, Hrabe de Angelis M, Weinmaster G, Rubenstein JL. Modulation of the notch signaling by Mash1 and Dlx1/2 regulates sequential specification and differentiation of progenitor cell types in the subcortical telencephalon. Development. 2002;129:5029–40. doi: 10.1242/dev.129.21.5029. [DOI] [PubMed] [Google Scholar]
- Zhang H, Webb DJ, Asmussen H, Niu S, Horwitz AF. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J Neurosci. 2005;25:3379–88. doi: 10.1523/JNEUROSCI.3553-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZS, Manser E, Loo TH, Lim L. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol Cell Biol. 2000;20:6354–63. doi: 10.1128/mcb.20.17.6354-6363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


