Abstract
On-demand release of CO is realized through a novel NIR-responsive nanomedicine in favor of the enhancement of therapy efficacy and bio-safety of CO therapy.
Keywords: drug delivery, controlled release, nanomedicine, graphene, CO therapy
It is well known that the inhalation of excess amount of carbon monoxide (CO) will cause poisoning owing to a reduction in the oxygen-carrying capacity of hemoglobin (Hb). However, low concentration of CO can play a role as messenger to regulate physiological functions of nervous, cardiovascular and immune systems,1 and has remarkable therapeutic potential in the treatment of many relevant diseases, including inflammation,2a organ transplantation and preservation,2b arteriosclerosis,2c stroke,2d and cancer.[2e–g] Controlled administration of CO is therefore vitally important both to enhance the efficacy of CO therapy and to evade the risk of CO poisoning. It is urgently desired to develop nanomedicines which are capable of intracellular delivery and controlled on-demand release of CO.3
To achieve intracellular delivery of CO, several nanomedicine formulas have been constructed by conjugating CORMs (Ru carbonyl, Mn carbonyl, etc.) on various nano-carriers, such as micelles,4a silica nanospheres,4b iron oxide nanoparticles,4c diamond nanoparticles,4d HPMA copolymer nanoparticles,4e diaminobutane dendrimer,4f ferritin and BSA proteins.4g,h However, CO release from these conjugated CORMs is either spontaneous (Ru carbonyl) or only responsive to UV/Visible light (Mn carbonyl). The spontaneous release lacks controllability and therefore has a potential risk of CO poisoning. Furthermore, UV/Vis light has limited tissue penetrability and is also prone to phototoxicity, thus restricting the bio-application of photo-responsive CORMs (photoCORMs). In contrast, near-infrared (NIR) light has a greater depth of tissue penetration and a lower phototoxicity than UV light.5a Therefore, the NIR-responsive nanomedicine for controlled CO release has the potential to mitigate the issues of UV light triggered release of CO, and is worth pursuing through molecular and structural designs but has not been reported so far.5b,c There have been enlightening reports of linking NO-releasing molecules (NORMs) with NIR-sensitive chromophore groups or nanoparticles, which can transfer NIR light energy into chemical energy and consequently cause the photochemical release of NO.6 This strategy is here presumed to work on CORMs for the desired NIR-responsive release of CO.
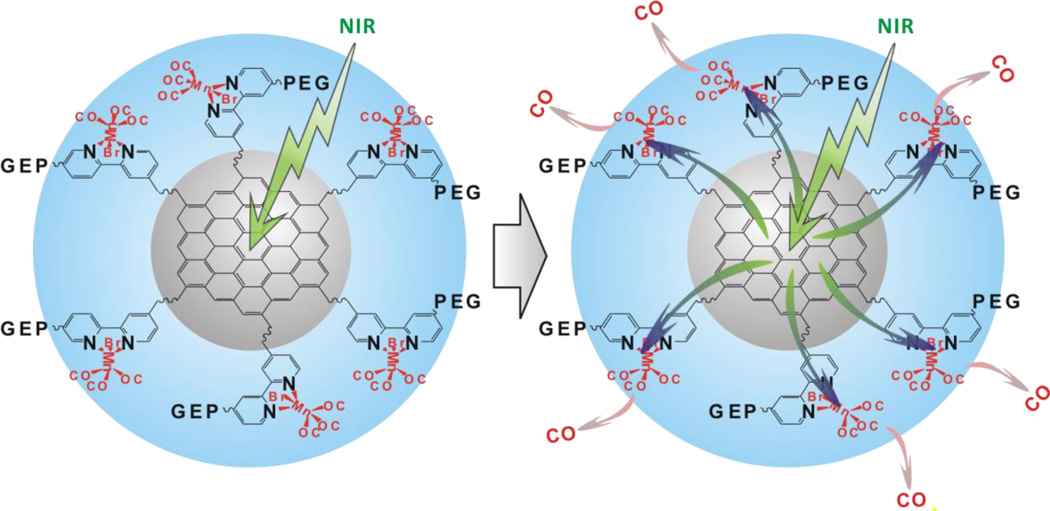
In this work, we constructed a NIR-responsive nanomedicine (MnCO-GO) for the first time by caging Mn-carbonyl CORMs within a small graphene oxide (GO) nanosheet, which was employed as a drug carrier and also as a NIR light energy collector/converter to mediate the photochemical release of CO from caged CORMs. As demonstrated in Scheme 1, GO was used to absorb NIR light (green arrow) and transform photons into active electrons. The electrons were transferred from GO to coordinated Mn-carbonyl molecules (green/blue arrows), and then contested the 3d orbitals of Mn with carbonyls, finally causing the detachment of CO from Mn (pink arrows). The MnCO-GO nanomedicine indeed has high controllability and NIR-responsiveness/sensitivity for CO release, confirming the above-mentioned hypothesis. A proof-of-principle study indicates that the MnCO-GO nanomedicine allows intracellular delivery and controlled on-demand release of CO, and has an anti-inflammation effect.
Scheme 1.
Molecular structure and NIR-responsive CO release mechanism of PEG-BPY[MnBr(CO)3]-GO (abbreviated as MnCO-GO). MnBr(CO)3 is caged in the bipyridine-conjugated GO. See Schemes S1 and S2 in the Supporting Information for construction of MnCO-GO. Under the excitation of NIR light, GO can absorb the energy of NIR light and then transform photons into active electrons, which are transferred from GO to bipyridines and then to coordinated Mn (green/blue arrows) and then contest the 3d orbitals of Mn with carbonyls, finally leading to the detachment of CO from Mn (pink arrows).
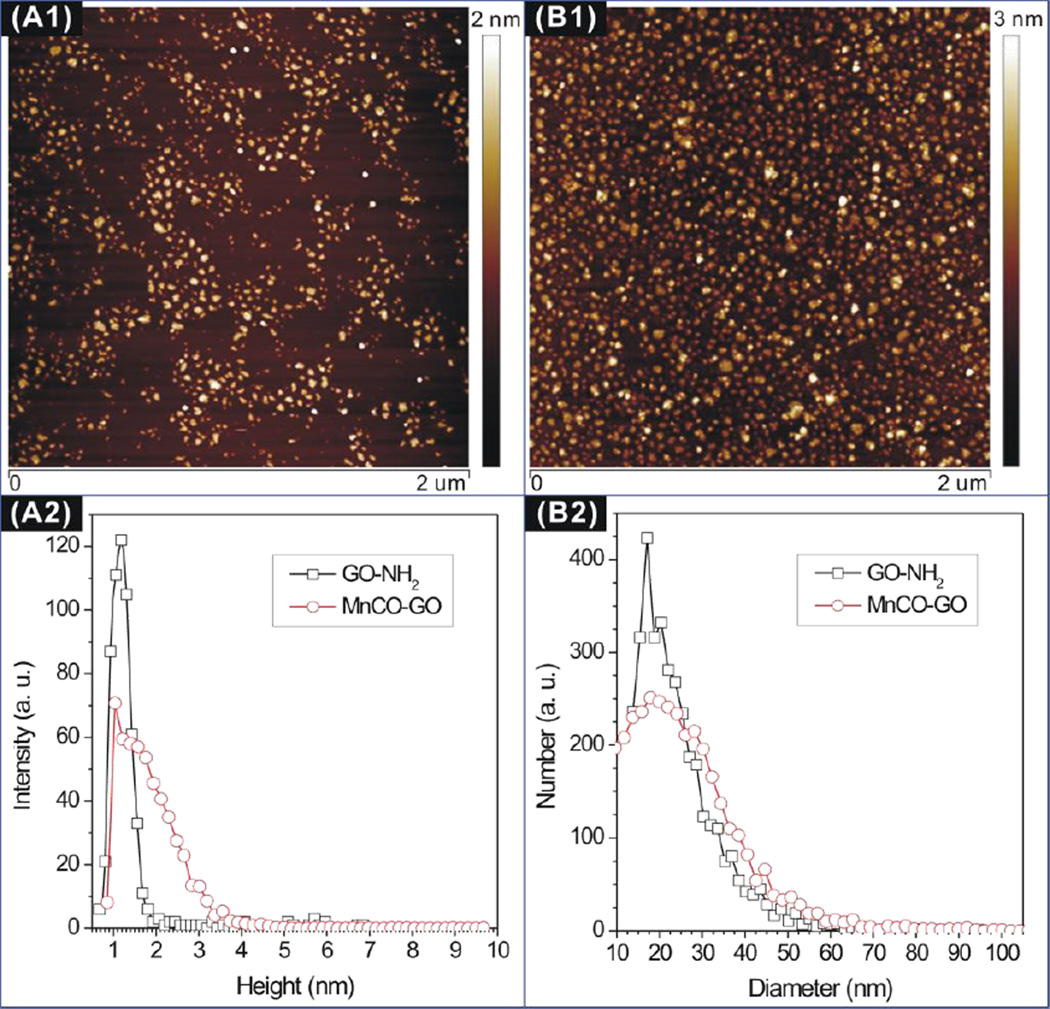
The morphologies of the initial reactant GO-NH2 (armed-aPEG(NH2)8 modified GO) and the final product MnCO-GO were characterized by an atomic force microscope (AFM). From Figures 1A1 and B1, it can be found that both the reactant GO-NH2 and the product MnCO-GO showed uniform particle size and good dispersion in solution. Furthermore, from the dimensional histograms shown in Figures 1A2 and B2, MnCO-GO kept the same nanosheet shape to GO-NH2 but had an increase of about 1 nm both in average height and in average diameter compared with GO-NH2, suggesting the successful molecular coating. In addition, such a small dimension (Ø22 nm×2 nm) of MnCO-GO is expected to favor the cellular uptake and intracellular delivery of CO. Moreover, the UV-Vis-NIR absorption spectrum measurement indicates that both the initial reactant GO-NH2 and the final product MnCO-GO have a broad adsorption band from UV to NIR region, and the maximum adsorption peak is located at 255 nm,7a indicating that the synthesized GO is in a reduced form and therefore has good activity of electron transfer in favor of photochemical decomposition.7b
Figure 1.
AFM images (A1, B1) and dimensional histograms (A2, B2) of GO-NH2 (A) and MnCO-GO (B).
In order to further confirm the chemical modification, the reactants and the final product were characterized by FT-IR (Figure S1). According to the synthesis route as indicated in Schemes S1 in the Supporting Information, 2,2’-bipyridine-4,4’-dicarboxylic acid (BPYDC) was firstly conjugated with GO-NH2 to obtain BPY-GO for the following coordination of Mn carbonyl, and then was modified with PEG to obtain water-dispersible PEG-BPY-GO. To ensure the connection of GO and PEG on both sides of BPYDC through esterification, BPYDC and PEG-NH2 were in access of GO-NH2 and BPYDC, respectively, and particle products were washed multiple times to remove residual reactants. The success of esterification reactions can be confirmed by the disappearance of carboxylic group and the appearance of ester group, as indicated by green and blue arrows in Figure S1, respectively. The obtained PEG-BPY-GO was further coordinated with MnBr(CO)5 by replacing two carbonyl groups to obtain PEG-BPY[MnBr(CO)3]-GO (abbreviated as MnCO-GO, see Scheme S2). A remarkable increase of the absorption peak at 608 cm−1 (Figure S1) indicates the formation of a π-backbonding of Mn–N, and the absorption peak of carbonyl group shifts toward low frequency (as shown by the red arrow), indicative of a weakened bonding of C≡O, suggesting the partial replacement of carbonyl groups coordinated on Mn by bipyridine group as a worse π-acceptor ligand.8 This means that Mn carbonyl had been coordinated with bipyridine (BPY) within PEG-BPY-GO successfully, forming the expected molecular structure of PEG-BPY[MnBr(CO)3]-GO (abbreviated as MnCO-GO, Scheme S2). This molecular coordination does not involve redox reaction or the change in valence states of Mn, bipyridine and the bipyridine-conjugated GO. After NIR-photoinduced release of CO, water and/or oxygen will replace the position of released CO for coordination with Mn. Through elemental analysis, the CORMs-caged capacity of GO was measured to be as high as 881 mg carbonyl per g of GO.
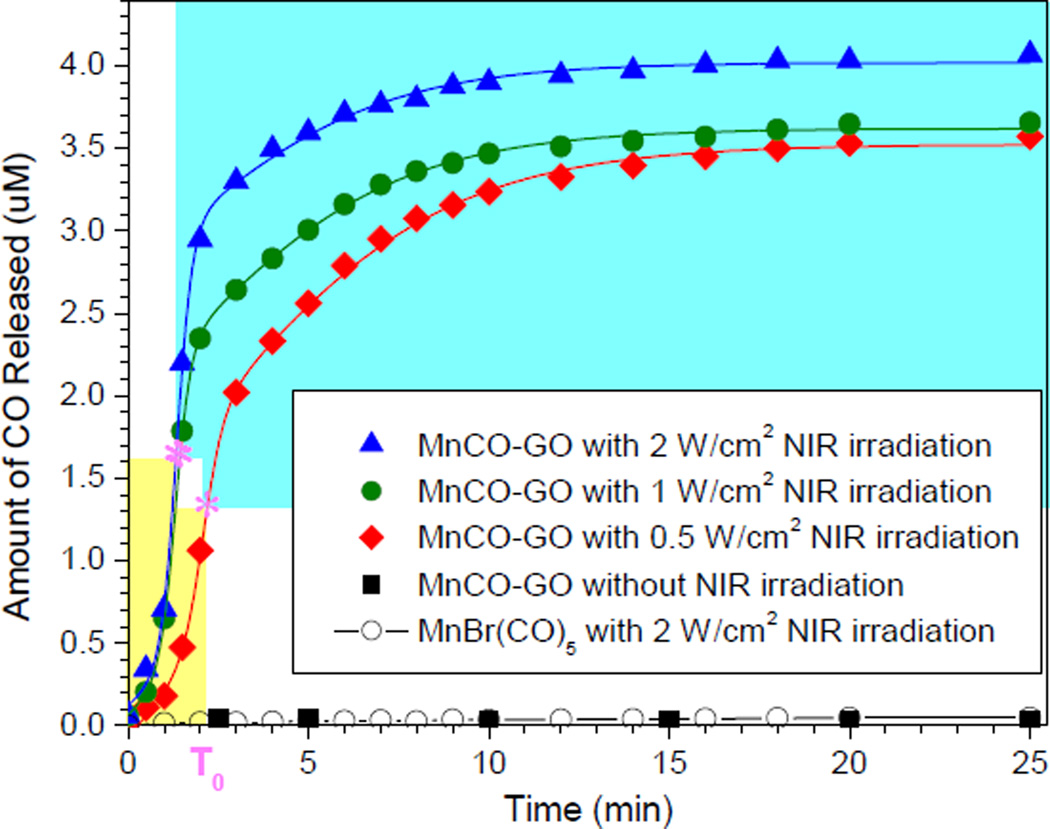
The NIR responsiveness of MnCO-GO for CO release in the PBS was investigated under the excitation of 808-nm NIR light with different power densities (Figure 2). The CO concentration in PBS was measured by an Hb method (Scheme S3) according to the Beer-Lambert law (see the calculation method and Figure S2 in the Supporting Information). It can be found that the precursor MnBr(CO)5 was not responsive to NIR light but to UV light for CO release (Figure S3). By comparison, MnCO-GO was highly responsive to NIR light for CO release (Figure 2). This suggests that GO plays a key role in the NIR-responsive CO release. Although GO in MnCO-GO has a photothermal effect, the increase of temperature induced by MnCO-GO was very marginal (0.5°C) compared with the blank control without MnCO-GO (Figure S4), owing to a considerably low particle concentration (3 µg/mL) and relatively low absorbance at 808 nm compared with the maximum adsorption peak in the UV region (Figure S5). Such a small temperature change did not lead to the decomposition of MnCO-GO for CO release. Even when the concentration of MnCO-GO was increased to 12 µg/mL and NIR irradiation was replaced with direct heating, 5°C of temperature increase did have measurable CO release within 25 min (Figure S6). Therefore, the NIR responsiveness of MnCO-GO for CO release was not related to the photothermal effect of GO even at high particle concentrations. It was therefore thought that the NIR-responsive release of CO from the MnCO-GO nanomedicine was most possibly derived from a photochemical decomposition effect. The NIR absorption and photochemical energy transfer capacities of GO supported the photochemical decomposition of MnCO-GO for the NIR-responsive CO release. Metal carbonyl compounds including Mn carbonly are prone to UV photochemical decomposition and CO release, but are inert/insensitive to NIR light, probably owing to no obvious absorption in the NIR region.5c But after conjugation of Mn carbonyl with GO, GO can absorb NIR light and transform photons into active electrons, which are transferred to coordinated Mn-carbonyl molecules and therefore cause the decomposition of Mn carbonyl and the release of CO (Scheme 1). Moreover, MnCO-GO was also responsive to NIR light in a power density-dependent manner (Figure 2). Higher power densities of NIR light caused faster release of CO from MnCO-GO. Therefore, it is easy to control the CO release rate and amount by adjusting the NIR light power.
Figure 2.
CO release profiles of MnCO-GO (3 µg/mL) in PBS under the excitation of 808-nm NIR light with different power densities (0.5, 1 and 2 W/cm2). MnCO-GO (3 µg/mL) without NIR irradiation and the equivalent molar amount of MnBr(CO)5 with 2 W/cm2 NIR irradiation were used as controls.
Furthermore, the CO release can be well fitted (adjusted R2 > 0.99) by a double-Boltzmann model rather than a single-Boltzmann model or other models, suggesting that two kinds of physical collision among low concentrations of reactant particles play a predominant role (reaction-limited steps) over chemical reactions, as described by the two equations in Figure S7. Through the fitting with a double-Boltzmann equation, several important kinetic parameters are obtained (Table S1). It is very clear that about 30% CO was quickly released from MnCO-GO under excitation of 808-nm laser within several minutes, and then residual CO was released in a sustained way after T0 (inflection point). This kind of drug release profile is thought to be very useful for prolonging drug efficacy and avoiding toxic side effect by quickly achieving an effective drug concentration for therapy and then maintaining the drug concentration within an effective but safe range. Furthermore, T0 is tunable by adjusting the power density of laser. At a relatively high power density of NIR laser, the time to achieve the highest CO-released amount was shorter (T0 in Table S1), the CO release rate was faster (K1 in Table S1) and the CO release amount was higher (Y in Table S1). This subsequently accelerated the collision and adsorption of CO by Hb (K2 in Table S1). Therefore, we can facilely obtain the expected CO release rate and amount by adjusting the power density of NIR laser and exposure time.
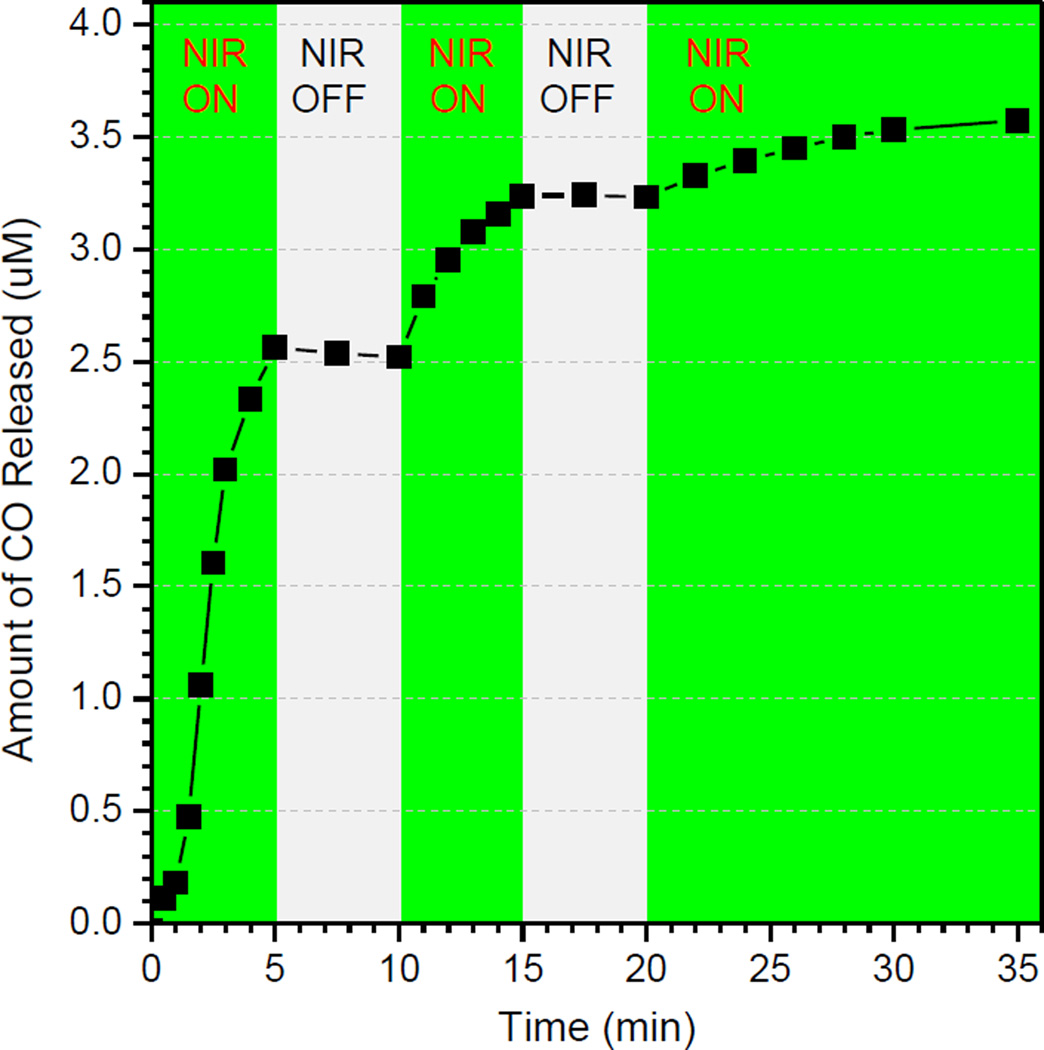
The NIR controllability of MnCO-GO for CO release was investigated, as shown in Figure 3. When NIR light was switched on, the release of CO was initiated; once NIR light was switched off, the release of CO stopped completely. The repeat of switching NIR light could also control the release of CO well in spite of the reduced rate of CO release with the increase of NIR irradiation time. This indicates that MnCO-GO has an excellent NIR controllability for CO release. The CO concentration can be well controlled on demand through controlling the switching and power of NIR light, which is of great significance to maintain the drug concentration within the therapeutic window and also reduce the risk of CO poisoning.
Figure 3.
The NIR controllability of MnCO-GO (3 µg/mL) for CO release by switching on/off 808-nm NIR light (1 W/cm2).
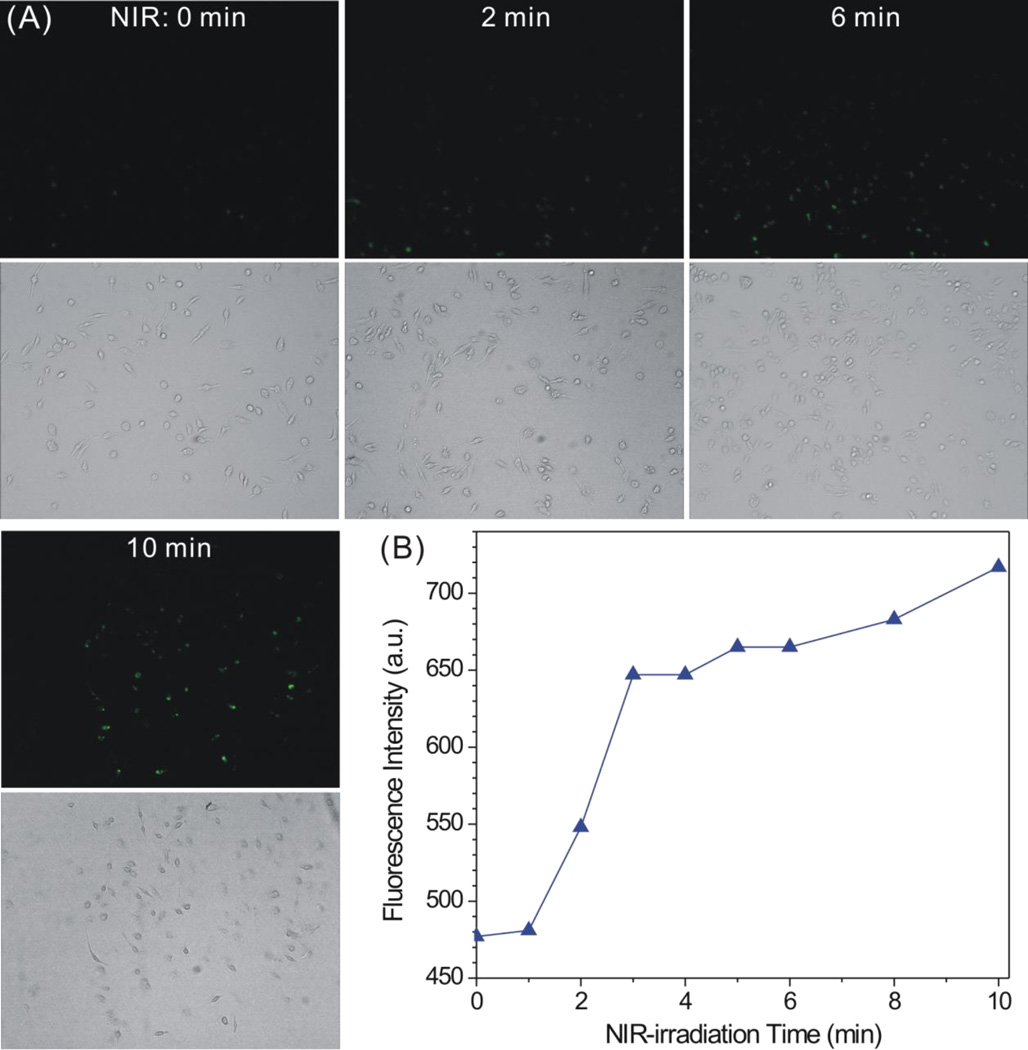
Intracellular CO release profiles of MnCO-GO were measured using a CO probe COP-1 (Scheme S4). COP-1 was synthesized according to the Chang’s method (Scheme S5).9 The intermediate and final products during the synthesis of COP-1 were confirmed by 1H-NMR spectra (Figures S8–S11). In order to check the applicability of COP-1 for detecting CO, COP-1 was firstly used to measure the CO release from MnCO-GO in PBS. From fluorescence monitoring results, the fluorescence intensity of the MnCO-GO solution remarkably enhanced under the irradiation of 808-nm laser, while the control in the absence of MnCO-GO exhibited only minor fluorescence after NIR irradiation (Figure S12). This suggested that CO can indeed be detected using the synthesized COP-1. Furthermore, COP-1 was used to detect the intracellular release of CO. Raw264.7 cells were chose as a model cell line for the measurement of inflammatory response, and were cultured with MnCO-GO and COP-1 in turn. After residual nanoparticles and COP-1 outside cells were washed off, cells were irradiated for different periods of time. As shown in Figure 4A, the green fluorescence gradually enhanced with the increase of NIR irradiation time. By comparison, there was no visible fluorescence in the control in the absence of MnCO-GO even after being irradiated by NIR light 10 min (Figure S13). Meanwhile, the fluorescence of all cells seeded in the plate was measured using a multi-mode microplate reader (Figure 4B). Similarly to the results of qualitative fluorescence observation, the whole fluorescence intensity of Raw264.7 cells treated with the MnCO-GO nanomedicine enhanced with the increase of NIR irradiation time. These results consistently suggest that MnCO-GO can responsively release CO in cells.
Figure 4.
Intracellular CO release profiles of MnCO-GO under the excitation of 808-nm NIR light (0.1 W/cm2) detected with COP-1: (A) qualitative observation under fluorescence microscope (upper: fluorescence images; lower: corresponding bright field images); (B) statistics of the fluorescence intensity of treated Raw264.7 cells.
Unfortunately, it is not accurate to qualify CO concentration according to fluorescence intensity because the CO-responsive fluorescence enhancement of COP-1 depends on incubation/reaction time, probe concentration and their solubility in media according to their reaction dynamics. Therefore, it is necessary to develop more accurate and efficacious probe molecules for intracellular CO detection. Recently, Bernardes et al. used a gas chromatography reduced compound detection (GC-RCP) method to detect CO in tissue digestion solution.4h Alternatively, a free radical analyzer associated with a CO sensor may also be used to more accurately detect CO concentrations in tissue digestion solution.
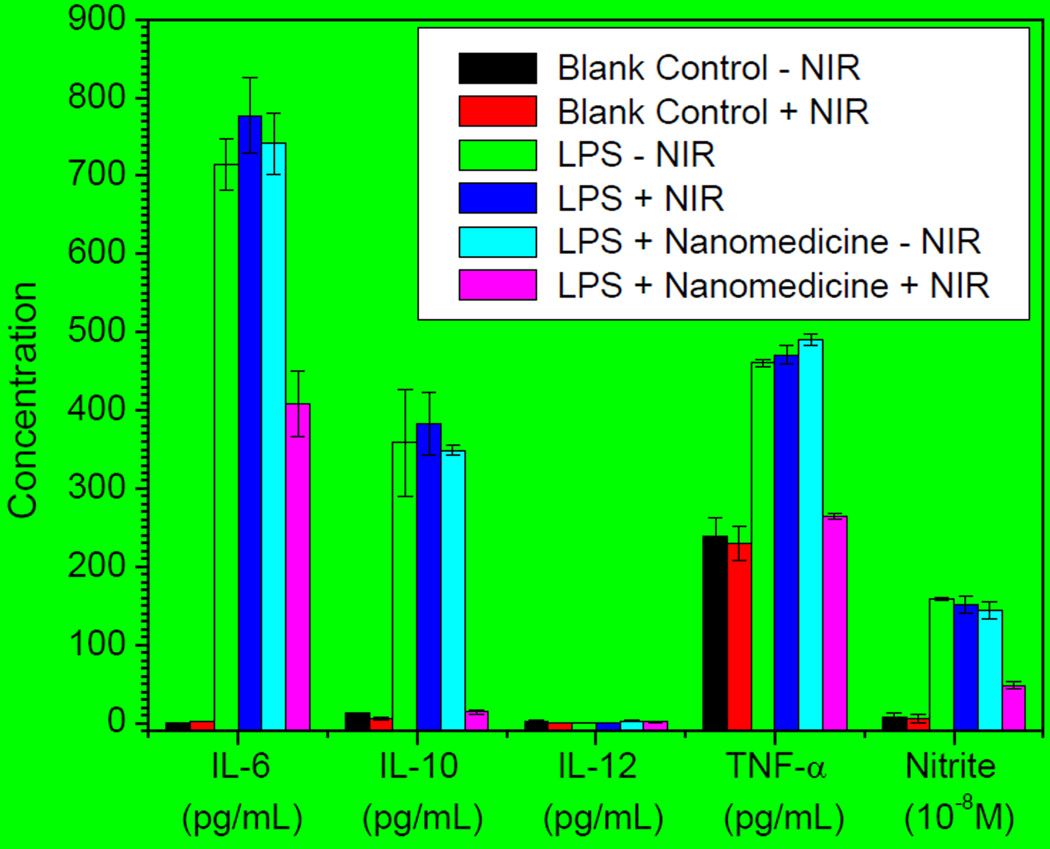
The anti-inflammation effect of MnCO-GO was further investigated using Raw264.7 cells as a model cell line. Several typical inflammation factors, including TNF-α, IL-6, IL-10, IL-12 and nitrite, were evaluated to reflect the inflammatory response of Raw264.7 cells. As shown in Figure 5, Raw264.7 cells exhibited an intensive inflammatory response after stimulation of lipopolysaccharides (LPS) as the levels of IL-6, IL-10, TNF-α and nitrite enhanced remarkably (green bars). NIR irradiation without the treatment of the MnCO-GO nanomedicine had no significant effect on these inflammation factors (red and blue bars). In the absence of NIR irradiation, the treatment of Raw264.7 with MnCO-GO also did not cause significant influence of these inflammation factors (turquoise bars). However, the treatment of Raw264.7 with 50 µg/mL of MnCO-GO plus the irradiation of NIR light effectively suppressed the levels of IL-6, IL-10, TNF-α and nitrite (pink bars), suggesting an obvious anti-inflammation effect of MnCO-GO,10 and thus the anti-inflammation effect of MnCO-GO should be due to NIR-responsive intracellular release of CO. Besides, MnCO-GO had no obvious cytotoxicity to Raw264.7 cells in the concentration range of 3.1–50 µg/mL both before and after NIR irradiation (Figure S14), suggesting that MnCO-GO is safe for NIR-responsive CO release and CO therapy.
Figure 5.
The anti-inflammation effect of CO released from the MnCO-GO nanomedicine under NIR irradiation (1 W/cm2). LPS (1 µg/mL) was used to stimulate the inflammatory response of Raw264.7 cells, and the absence of LPS and nanomedicine was the blank control.
In conclusion, we have successfully constructed a NIR-responsive MnCO-GO nanomedicine by caging CORMs within a photochemical energy converter GO. This energy transfer strategy can be extended to construct other nanomedicines for the responsive release of various therapeutic gases. The MnCO-GO nanomedicine has extraordinarily high NIR-responsiveness and controllability for CO release, and the CO release rate and amount can readily be controlled by adjusting the irradiation time or power density of NIR light. The intracellular release of CO from MnCO-GO has a remarkable anti-inflammation effect.
Supplementary Material
Acknowledgements
We thank the financial support from the National Nature Science Foundation of China (Grant Nos. 51102259, 31271021 and 31222023), Distinguished Young Scholars of Sichuan University (Grant No. 2011SCU04B18), and the Intramural Research Program, National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health.
Footnotes
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Prof. Dr. Qianjun He, Email: nanoflower@126.com.
Zhiyong Qian, Email: anderson-qian@163.com.
Prof. Dr. Xiaoyuan Chen, Email: shawn.chen@nih.gov.
References
- 1.Motterlini R, Otterbein LE. Nat. Rev. Drug Discov. 2010;9:728. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 2.Otterbein LE, Bach FH, Alam J, Soares M, Lu HT, Wysk M, Davis RJ, Flavell RA, Choi AMK. Nat. Med. 2000;6:422. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]; b) Chin BY, Jiang G, Wegiel B, Wang HJ, Macdonald T, Zhang XC, Gallo D, Cszimadia E, Bach FH, Lee PJ, Otterbein LE. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5109. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, Stachulak C, Bodyak N, Smith RN, Csizmadia E, Tyagi S, Akamatsu Y, Flavell RJ, Billiar TR, Tzeng E, Bach FH, Choi AM, Soares MP. Nat. Med. 2003;9:183. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]; d) Nassour I, Kautza B, Rubin M, Escobar D, Luciano J, Loughran P, Gomez H, Scott J, Gallo D, Brumfield J, Otterbein LE, Zuckerbraun BS. Shock. 2015;43:166. doi: 10.1097/SHK.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Wegiel B, Gallo D, Csizmadia E, Harris C, Belcher J, Vercellotti GM, Penacho N, Seth P, Sukhatme V, Ahmed A, Pandolfi PP, Helczynski L, Bjartell A, Persson JL, Otterbein LE. Cancer Res. 2013;73:7009. doi: 10.1158/0008-5472.CAN-13-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Yin H, Fang J, Liao L, Maeda H, Su Q. BMC Cancer. 2014;14:436. doi: 10.1186/1471-2407-14-436. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Vitek L, Gbelcova H, Muchova L, Vanova K, Zelenka J, Konickova R, Suk J, Zadinova M, Knejzlik Z, Ahmad S, Fujisawa T, Ahmed A, Ruml T. Dig. Liver Dis. 2014;46:369. doi: 10.1016/j.dld.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Gallego S, Bernardes G. J. Angew. Chem. Int. Ed. Engl. 2014;53:9712. doi: 10.1002/anie.201311225. [DOI] [PubMed] [Google Scholar]
- 4.a) Hasegawa U, van der Vlies AJ, Simeoni E, Wandrey C, Hubbell JA. J. Am. Chem. Soc. 2010;132:18273. doi: 10.1021/ja1075025. [DOI] [PubMed] [Google Scholar]; (b) Dordelmann G, Pfeiffer H, Birkner A, Schatzschneider U. Inorg. Chem. 2011;50:4362. doi: 10.1021/ic1024197. [DOI] [PubMed] [Google Scholar]; (c) Kunz PC, Meyer H, Barthel J, Sollazzo S, Schmidt AM, Janiak C. Chem. Commun. 2013;49:4896. doi: 10.1039/c3cc41411f. [DOI] [PubMed] [Google Scholar]; (d) Dordelmann G, Meinhardt T, Sowik T, Krueger A, Schatzschneider U. Chem. Commun. 2012;48:11528. doi: 10.1039/c2cc36491c. [DOI] [PubMed] [Google Scholar]; (e) Bruckmann NE, Wahl M, Reiss GJ, Kohns M, Watjen W, Kunz PC. Eur. J. Inorg. Chem. 2011;2011:4571. [Google Scholar]; (f) Govender P, Pai S, Schatzschneider U, Smith GS. Inorg. Chem. 2013;52:5470. doi: 10.1021/ic400377k. [DOI] [PubMed] [Google Scholar]; (g) Fujita K, Tanaka Y, Sho T, Ozeki S, Abe S, Hikage T, Kuchimaru T, Kizaka-Kondoh S, Ueno T. J. Am. Chem. Soc. 2014;136:16902. doi: 10.1021/ja508938f. [DOI] [PubMed] [Google Scholar]; (h) Chaves-Ferreira M, Albuquerque IS, Matak-Vinkovic D, Coelho AC, Carvalho SM, Saraiva LM, Romao CC, Bernardes GJL. Angew. Chem. Int. Edit. 2015;54:1172. doi: 10.1002/anie.201409344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Garcia JV, Yang JP, Shen DK, Yao C, Li XM, Wang R, Stucky GD, Zhao DY, Ford PC, Zhang F. Small. 2012;8:3800. doi: 10.1002/smll.201201213. [DOI] [PubMed] [Google Scholar]; b) Chakraborty I, Carrington SJ, Mascharak PK. Acc. Chem. Res. 2014;47:2603. doi: 10.1021/ar500172f. [DOI] [PubMed] [Google Scholar]; c) Rimmer RD, Pierri AE, Ford PC. Coord. Chem. Rev. 2012;256:1509. [Google Scholar]
- 6.a) Wecksler S, Mikhailovsky A, Ford PC. J. Am. Chem. Soc. 2004;126:13566. doi: 10.1021/ja045710+. [DOI] [PubMed] [Google Scholar]; b) Wecksler SR, Mikhailovsky A, Korystov D, Ford PC. J. Am. Chem. Soc. 2006;128:3831. doi: 10.1021/ja057977u. [DOI] [PubMed] [Google Scholar]; c) Diring S, Wang DO, Kim C, Kondo M, Chen Y, Kitagawa S, Kamei K, Furukawa S. Nat. Commun. 2013;4:2684. doi: 10.1038/ncomms3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Zhang B, Li L, Wang Z, Xie S, Zhang Y, Shen Y, Yu M, Deng B, Huang Q, Fan C, Li J. J. Mater. Chem. 2012;22:7775. [Google Scholar]; c) Mattevi C, Eda G, Agnoli S, Miller S, Mkhoyan KA, Celik O, Mastrogiovanni D, Granozzi G, Garfunkel E, Chhowalla M. Adv. Funct. Mater. 2009;19:2577. [Google Scholar]
- 8.Socrates G. Infrared and Raman characteristic group frequencies: Tables and charts. 3rd Ed. Chichester, New York: John Wiley & Sons Ltd.; 2001. pp. 314–315. [Google Scholar]
- 9.Michel BW, Lippert AR, Chang CJ. J. Am. Chem. Soc. 2012;134:15668. doi: 10.1021/ja307017b. [DOI] [PubMed] [Google Scholar]
- 10.a) Sawle P, Foresti R, Mann BE, Johnson TR, Green CJ, Motterlini R. Br. J. Pharmacol. 2005;145:800. doi: 10.1038/sj.bjp.0706241. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Megias J, Busserolles J, Alcaraz MJ. Br. J. Pharmacol. 2007;150:977. doi: 10.1038/sj.bjp.0707184. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Babu D, Motterlini R, Lefebvre RA. Br. J. Pharmacol. 2015;172:1557. doi: 10.1111/bph.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.