Abstract
Purpose
To investigate the differences in the frequency of optic disc hemorrhage (DH) and prevalence of beta-zone parapapillary atrophy (βPPA) between individuals of African (AD) and European descent (ED).
Design
Prospective, multicenter observational cohort.
Participants
1,950 eyes of 1,172 participants of the African Descent and Glaucoma Evaluation Study (ADAGES).
Methods
Stereoscopic disc photographs of subjects with and without glaucomatous optic neuropathy (GON) followed during the first 13 years of the ADAGES underwent masked review searching for DH and βPPA. 928 eyes (non-GON, 581; GON, 347) of 551 AD patients (non-GON, 334; GON, 217), and 1,022 eyes (non-GON, 568; GON, 454) of 611 ED patients (non-GON, 334; GON, 277) were included. We compared the number of eyes with detected DH at any time during follow-up and eyes with βPPA between the AD and ED groups. The analyses were then adjusted for clinical parameters using multivariable logistic regression.
Main Outcome Measures
Differences in frequency of DH and prevalence of βPPA.
Results
9,395 stereoscopic disc photos were reviewed. More ED eyes experience DH than AD eyes (49/1022 (4.8%) vs. 10/928 eyes (1.1%), respectively, P<0.001), whereas βPPA had higher prevalence in AD eyes (675 eyes (72%) vs. 659 eyes (64%), P<0.001). In the final multivariable model, after controlling for confounders, AD eyes were less likely to have at least one detected DH than ED eyes (odds ratio, OR=0.21; 95% CI=0.10–0.45; P <0.001) but were more likely to have βPPA than ED eyes (OR=1.55; 95% CI=1.12–2.14; P=0.008).
Conclusions
ED subjects are at higher risk for developing DH compared to AD subjects while AD subjects have greater prevalence of βPPA. These findings suggest that there are structural differences within the optic nerve complex between these groups. Further research is needed to determine whether racial differences in the frequency of DH and prevalence of βPPA affect the likelihood of glaucomatous progression.
Disc hemorrhage (DH) and beta-zone parapapillary atrophy (βPPA) are well-described features of glaucomatous optic neuropathy (GON).1–15 DH has been reported to occur in every glaucoma disease phenotype, regardless of disease stage, and has been repeatedly demonstrated to be an independent risk factor for disease progression in virtually every study in which it has been assessed.3,5,9,16,17 DH occurs in tandem with localized optic nerve injury,18 and represents evidence of prior and continued rapid localized optic nerve damage.2,5,11 βPPA, although associated with myopia in eyes without glaucoma, occurs in higher frequency, may widen, and also increases the risk of disease progression in eyes with GON.12–14, 19–28
African descent is known to be associated with increased frequency, prevalence, severity, and progression of glaucoma.29–32 However, it remains unclear whether race is an independent risk factor for glaucoma or whether differences in clinical, socioeconomic, ocular characteristics or other factors between individuals of European descent (ED) and African descent (AD) are confounding variables that may help explain different susceptibilities.11 For instance, in the Ocular Hypertension Treatment study (OHTS),33 participants of AD were at increased risk of conversion to primary open angle glaucoma (POAG), but this association was no longer statistically significant after adjusting for differences in central corneal thickness (CCT) and baseline status of glaucomatous damage.34–35 The African Descent and Glaucoma Evaluation Study (ADAGES) is a longitudinal clinical study that aims to address this question and to determine why individuals of AD are at increased risk of glaucoma onset and progression. Participants of both ancestry groups (AD and ED) were included in the ADAGES cohort so that comparisons could be made.36
In this study, we hypothesized that given the positive and independent associations between DH, βPPA, and progressive VF loss in glaucoma, individuals of AD would be more likely to exhibit these features during clinical examination. The long term, prospective, multicenter, design of ADAGES, which includes normal, glaucoma suspect and glaucomatous participants, provides a unique opportunity to test this hypothesis. We aimed to compare the frequency of DH and prevalence of βPPA between eyes of participants of AD and ED, which could account for differences in their susceptibility to glaucoma.
METHODS
The three-site ADAGES collaboration includes the Hamilton Glaucoma Center at the Department of Ophthalmology, University of California-San Diego (UCSD) (data coordinating center), the New York Eye and Ear Infirmary, and the Department of Ophthalmology, University of Alabama-Birmingham (UAB). The institutional review boards at all three sites approved the study methodology, which adheres to the tenets of the Declaration of Helsinki and to the Health Insurance Portability and Accountability Act. All participants gave written informed consent. ADAGES is registered as a cohort clinical trial (clinicaltrials.gov). Enrollment began in January 2003 and ended in July 2006. Methodological details have been described previously. In brief, participants of both African and European ancestry were included in the cohort. The main goal was to identify factors accounting for differences in glaucoma onset and rate of progression between individuals of AD and ED.36
Data are centrally processed and analyzed at UCSD through established reading centers. The data from ADAGES were compared and combined with data on an additional group of ED normal subjects and patients obtained through 2 ongoing prospectively designed longitudinal studies at UCSD, which together make up the Diagnostic Innovations in Glaucoma Study (DIGS).37 With the exception of the targeted population for ADAGES, the protocols for ADAGES and DIGS are identical and patients are followed longitudinally. DIGS does not limit enrollment based on race or ethnicity, but only persons of AD and ED are included in this report.
Participants
Participants were asked to identify their race by self-report using the National Eye Institute inclusion/enrollment system describing ethnicity and race (http://orwh.od.nih.gov/pubs/outreach.pdf [pages 120–121]). Information regarding a family history of glaucoma (biological mother, father, sibling, aunt, uncle, and grandparent) was also obtained. Normal and patient participants were recruited from the glaucoma clinics and ophthalmic practices at each of the three recruiting sites, by advertisement and community presentations, and by referral from other ophthalmologists and optometrists in the community.
Inclusion criteria at baseline
All participants had open angles, a best-corrected visual acuity ≥ 20/40, and a refractive error <5.0 diopters sphere and 3.0 diopters cylinder. We required at least 1 good-quality stereophotograph and 2 reliable standard automated perimetry (SAP) Humphrey 24-2 field test results at baseline, defined as < 33% false positives, false negatives, and fixation losses. Both eyes were included, except in cases where only one eye met the study criteria. All participants were older than 18 years. Diabetic participants without evidence of retinopathy were included.
Each participant underwent SAP using the 24-2 program on the Humphrey Field Analyzer II, with the Swedish Interactive Thresholding Algorithm (SITA), 33 version 4.1 (Carl Zeiss Meditec, Inc., Dublin, California). The VF mean deviation (MD) from tests performed closest to the date of baseline photographs was used in the analysis. Eyes with and without GON (defined below) were included in the present study.
Exclusion criteria
Participants were excluded if they had a history of intraocular surgery (except for uncomplicated cataract surgery or glaucoma surgery), secondary causes of glaucoma (eg., iridocyclitis, trauma), other systemic or ocular diseases known to affect the VF (eg., pituitary lesions, demyelinating diseases, etc.), significant cognitive impairment, history of stroke, Alzheimer disease, or dementia, problems other than glaucoma affecting color vision, an inability to perform visual field examinations reliably, or a life-threatening disease that precluded retention in the study. Because DH and βPPA are identified in different frequency between normal and glaucomatous subjects,15,22 we separated all eligible participants into those with and without GON based on masked stereo photograph review.
Evaluation of the optic nerve complex
All data were processed through the ADAGES Coordinating Center, the VisFACT (Visual Field Assessment Center), and the IDEA (Imaging Data Evaluation and Analysis) Center housed at the Hamilton Glaucoma Center, UCSD. The IDEA Center processed and reviewed the quality of all simultaneous stereophotographs. These reading centers also handled all data from DIGS and other National Eye Institute– or industry-sponsored trials. Both centers are responsible for certifying VF and imaging technicians and photo graders, processing any data-related queries to and from each site, and requesting that tests be repeated when needed. For the present study, disc photographs obtained from April 2003 through August 2014 were included.
Glaucomatous optic neuropathy (GON) was defined as excavation, neuroretinal rim thinning or notching, localized or diffuse retinal nerve fiber layer defect, or vertical cup-disc ratio (VCDR) asymmetry > 0.2 between eyes based on masked grading of stereophotographs by two graders at the IDEA Center. DH and βPPA were not considered criteria for classification as GON. Disagreement in GON status was resolved by adjudication by a third experienced grader or by consensus. DH was defined as a splinter or flame-shaped hemorrhage on or within the retinal nerve fiber layer or neuroretinal rim with a proximal edge no further than 1/2 disc diameter from the disc margin, or hemorrhages within the cup area.15 βPPA was defined as an area adjacent to the disc margin with a notable atrophy of the retinal pigment epithelium, visible sclera and visible large choroidal vessels (as opposed to alpha-zone atrophy, a more peripheral region with irregular pigmentation).21 Stereophotographs were reviewed for DH and βPPA by two independent glaucoma specialists at New York Eye and Ear Infirmary (CGDM and AS) masked to participant diagnosis, GON grading results from the IDEA Center, race and all other identifying characteristics. Cases of disagreement were adjudicated by a third, experienced grader (JML).
Statistical analysis
Categorical variables were compared using the Fisher’s exact-test, and continuous variables were compared using the 2-tailed, unpaired t-test. To test for differences in frequency of DH and prevalence of βPPA between AD and ED participants, logistic regression was performed using univariable and multivariable models. The latter were adjusted for covariates: age, gender, presence of GON, central corneal thickness (CCT), baseline visual field mean deviation (MD), spherical equivalent (SE), and self-reported history of diabetes mellitus and systemic hypertension. Interactions between age, racial group, and presence of GON were also evaluated. Kaplan-Meier with log-rank test was performed to test for differences in frequency of DH between racial groups. Generalized estimating equation (GEE) was used to adjust for potential inter-eye associations when both eyes of the same patient were entered in the analyses. Statistical analyses were performed using commercially available software (STATA, version 12; StataCorp LP, College Station, TX). Statistical significance defined at P< 0.05.
RESULTS
9,395 stereoscopic disc photos of 1,950 eyes of 1,172 participants were included. The study groups’ demographic and clinical data are shown in Table 1. Regardless of the GON status, AD patients were younger, had thinner corneas, had larger VCDR, and presented with worse VF MD than ED patients. Follow-up time between disc photos was similar between the two ancestry groups.
Table 1.
Comparison of clinical characteristics of study patients based on race and presence of glaucomatous optic neuropathy.
| GON | p | Non-GON | p | |||
|---|---|---|---|---|---|---|
| African Descent |
European Descent |
African Descent |
European Descent |
|||
| N (patients) | 217 | 277 | NA | 334 | 334 | NA |
| N (eyes) | 347 | 454 | NA | 581 | 568 | NA |
| Age (yrs) | 58±13 | 64±12 | <0.001 | 54±13 | 58±13 | <0.001 |
| CCT (μ) | 527.3±38.2 | 545.2±38.8 | <0.001 | 532.5±38.1 | 559.7±37.8 | <0.001 |
|
Spherical equivalent (D) |
−0.44±1.8 | −0.50±1.9 | 0.638 | −0.25±1.9 | −0.49±1.6 | 0.0209 |
| VCDR | 0.76±0.1 | 0.76±0.1 | 0.648 | 0.52±0.1 | 0.47±0.1 | <0.001 |
| MD (dB) | −3.81±5.9 | −2.90±4.6 | 0.017 | −1.80±3.2 | −1.11±2.1 | <0.001 |
|
Number of Eyes with βPPA (%) |
280 (80) | 338 (74) | 0.0415 | 395 (67.9) | 321 (56.6) | <0.001 |
|
Follow-up time (years) |
6.3±3.4 | 6.7±3.4 | 0.0994 | 5.4±3.7 | 5.4±3.7 | 1.00 |
Abbreviations: GON= glaucomatous optic neuropathy, VCDR= vertical cup-to-disc ratio, MD= visual field mean deviation, CCT= central corneal thickness.
DH was more common in ED eyes (49/1022 (4.8%) vs. 10/928 (1.1%) eyes, P<0.001), whereas βPPA was more common in AD eyes (675 (72%) vs. 659 (64% eyes), P<0.001). Agreement between graders for detection of DH and βPPA was excellent; adjudication was required in five (0.053%) of photographs for DH and in 37 (0.39%) of photographs for βPPA.
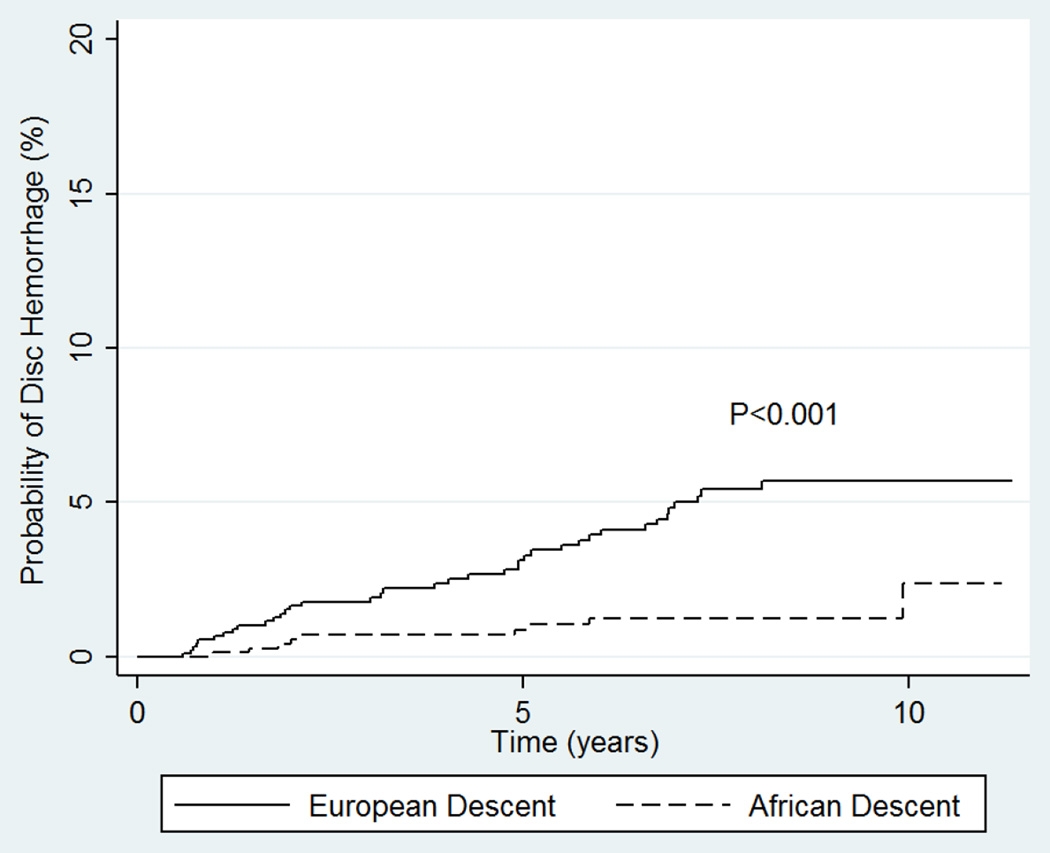
Figure 1 depicts the Kaplan-Meier curves associated with the frequency of DH between AD and ED eyes. Log-rank test revealed a significantly higher frequency of DH in ED eyes (P<0.001). 96% (47/49) of all DH in ED eyes were detected in the inferotemporal optic disc, whereas 60% (6/10) among AD eyes were detected inferotemporally and 30% (3/10) inferonasally. Six ED eyes had more than one DH on a photograph; three had two or more simultaneous DH in more than one location. In addition, 3 had recurrent DH in a different location on subsequent photographs during the follow-up period. No AD eye experienced more than one DH. 79.6% (39/49) of ED eyes with DH had GON at baseline compared to 60% (6/10) of the AD eyes (P=0.400).
Figure 1.
Kaplan-Meier survival curves showing the probabilities of disc hemorrhage detection over time between participants of African and European descent.
In the univariable logistic regression analysis, AD subjects were less likely of having at least one detected DH than ED subjects (OR=0.18; 95% CI=0.08–0.38; P<0.001), and were more likely to have βPPA (OR=1.38; 95% CI =1.06–1.81; P=0.016) detected on any of their photographs. Other predictors significantly associated with DH were presence of GON (OR=4.56; 95% CI= 2.45–8.49; P<0.001), older age (OR=1.06/year; 95% CI=1.03–1.08; P<0.001), and more severe visual field MD (OR =0.94/dB; 95% CI=0.90–0.98; P=0.012). Predictors significantly associated with βPPA were presence of GON (OR=1.97; 95% CI=1.52–2.55; P<0.001), older age (OR=1.02/year, 95% CI=1.01–1.03, P<0.001), thinner cornea (OR=0.99/micron; 95% CI=0.99–0.99; P=0.001), and more severe visual field MD (OR=0.95/dB; 95% CI=0.91–0.99; P=0.014) (Tables 2 and 3).
Table 2.
Univariable and multivariable logistic regression investigating the association between different predictors and detection of disc hemorrhage.
| Univariable | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | OR | 95% CI | P | OR | 95% CI | P | |||
| Race (African) |
0.18 | 0.08 | 0.38 | <0.001 | 0.21 | 0.10 | 0.45 | <0.001 | |
| GON | 4.56 | 2.45 | 8.49 | <0.001 | 3.24 | 1.65 | 6.36 | 0.001 | |
| Gender (female) |
1.05 | 0.55 | 2.02 | 0.863 | 1.29 | 0.66 | 2.52 | 0.453 | |
| Age (years) | 1.06 | 1.03 | 1.08 | <0.001 | 1.03 | 1.00 | 1.06 | 0.031 | |
| Spherical equivalent (diopters) |
1.14 | 0.98 | 1.32 | 0.073 | 1.10 | 0.95 | 1.29 | 0.189 | |
| CCT (microns) |
1.00 | 0.99 | 1.00 | 0.979 | 1.00 | 0.99 | 1.01 | 0.972 | |
| Baseline MD (dB) |
0.94 | 0.90 | 0.98 | 0.012 | 0.95 | 0.90 | 1.01 | 0.118 | |
| Diabetes | 0.61 | 0.26 | 1.41 | 0.252 | 0.78 | 0.32 | 1.88 | 0.588 | |
| Systemic hypertension |
0.77 | 0.38 | 1.59 | 0.492 | 1.58 | 0.78 | 3.22 | 0.199 | |
Abbreviations: OR= odds ratio; CI= confidence intervals; CCT= central corneal thickness; MD= 24-2 visual field mean deviation.
Table 3.
Univariable and multivariable logistic regression investigating the association between different predictors and the prevalence of beta-zone parapapillary atrophy.
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictors | OR | 95% CI | P | OR | 95% CI | P | ||
| Race (African) |
1.38 | 1.06 | 1.81 | 0.016 | 1.55 | 1.12 | 2.14 | 0.008 |
| GON | 1.97 | 1.52 | 2.55 | <0.001 | 1.72 | 1.30 | 2.27 | <0.001 |
| Gender (female) |
1.20 | 0.92 | 1.57 | 0.170 | 1.21 | 0.90 | 1.63 | 0.184 |
| Age (years) | 1.02 | 1.01 | 1.03 | <0.001 | 1.02 | 1.01 | 1.04 | <0.001 |
| Spherical equivalent (diopters) |
0.99 | 0.93 | 1.06 | 0.977 | 0.91 | 0.85 | 0.99 | 0.031 |
| CCT (microns) |
0.99 | 0.99 | 0.99 | 0.001 | 0.99 | 0.99 | 1.00 | 0.231 |
| Baseline MD (dB) |
0.95 | 0.91 | 0.99 | 0.014 | 0.97 | 0.94 | 1.01 | 0.283 |
| Diabetes | 1.04 | 0.70 | 1.54 | 0.833 | 0.82 | 0.54 | 1.24 | 0.351 |
| Systemic hypertension |
1.20 | 0.90 | 1.58 | 0.197 | 0.96 | 0.71 | 1.31 | 0.835 |
Abbreviations: OR= odds ratio; CI= confidence intervals; CCT= central corneal thickness; MD= 24-2 visual field mean deviation
We performed multivariable analysis testing the associations between racial groups, DH, and βPPA after controlling for other covariates described above. AD was found to be less likely than ED to have at least one detected DH (OR=0.21; 95% CI=0.10–0.45; P<0.001) and was found to be more likely than ED to have βPPA (OR=1.55; 95% CI=1.12–2.14; P=0.008). Other covariates with significant association with DH were presence of GON (OR=3.24; 95% CI=1.65–6.36; P=0.001) and older age (OR=1.03/year; 95% CI=1.00–1.06; P=0.031). Covariates associated with βPPA were presence of GON (OR=1.72; 95% CI=1.30–2.27; P<0.001), older age (OR=1.02/year; 95% CI=1.01–1.04; P<0.001), and higher myopia (OR=0.91; 95% CI=0.85–0.99; P=0.031) (Tables 2 and 3).
To further test whether the effect of age on DH frequency differed between the two racial groups, we ran an additional multivariable analysis with the interaction term ‘Age*Race’. Older age was not found to differentially increase the odds for having a DH (P=0.312) in one racial group. We performed similar analysis testing whether the effect of age on βPPA prevalence differed between groups, using the same interaction term. Nevertheless, when evaluating the effect of aging on βPPA, older age was found to have a stronger effect on the likelihood of having βPPA in AD than in ED (OR=1.03; 95% CI=1.01–1.05; P=0.011). This means that for each year older, the likelihood of having βPPA is greater among AD than ED patients after controlling for confounders. We also tested whether the effect of visual field severity on DH frequency different between groups by testing the interaction term ‘MD*Race’. We found no significant effect (P=0.159). A similar result was found when testing the same interaction on the prevalence of βPPA (P=0.690).
We found a borderline association between DH frequency and βPPA prevalence when looking at the two groups together (P=0.057); yet, this association was significant among ED patients alone (P=0.021) but not among AD patients (P= 0.475).
DISCUSSION
DH and βPPA are independent risk factors of glaucoma progression.1,4,6,8,16,18–19,21,23,28 In eyes at risk for but without frank GON at baseline, the OHTS reported DH to occur in 0.5% of subjects per year.10 The rate of glaucomatous VF loss in these eyes was more than twice as fast in eyes with DH than those without it (−0.17±0.27 dB/yr vs. −0.07±0.19 dB/yr, respectively, P<0.01),33 emphasizing the clinical importance and prognostic relevance of DH in non-glaucomatous ocular hypertensive individuals. Higher DH frequency, of 15–20%, has been reported in individuals with POAG,38–39 and especially in normal tension glaucoma (NTG) compared to other phenotypes.40–41 The Early Manifest Glaucoma Trial (EMGT) identified DH in approximately 55% of all patients - whether by ophthalmoscopy or review of photographs - during a median follow-up of 8 years. Moreover, the frequency of DH over time at any of the follow-up visits was approximately 12.5% based on photographs. EMGT patients, with newly diagnosed glaucoma and established baseline VF loss, were at increased risk of further VF deterioration if DH was detected.11 DH are often seen in eyes with βPPA.12–14 The association between βPPA and glaucoma has been suggested to be independent of ethnicity.23
In this study, we compared the frequency of DH and prevalence of βPPA between individuals of African and European descent followed in a long term, prospective, observational cohort. We refuted our initial hypothesis that AD subjects would have a higher frequency of DH and βPPA during masked photograph review given their known increased susceptibility to glaucoma onset and more rapid progression.31–32 In fact, ED eyes had a higher frequency of DH, while AD eyes had a greater prevalence of βPPA. Noteworthy, the relationship between ED and DH was stronger than that between AD and βPPA. As expected, the majority of eyes with DH from both groups had clinically defined GON. In addition, the most common DH location was the inferotemporal region of the disc, as these hemorrhages most often occur adjacent to regions of prior tissue damage, such as the edge of a disc notch or nerve fiber layer defect.7
These findings add new information to our current understanding as of how these clinical features (race, DH, and βPPA) interact with glaucoma status and prognosis. There is a wide consensus among glaucoma specialists that the identification of DH and βPPA are key components in the optic disc examination and that they have important roles in the disease process and estimation of future outcomes.43 It can be inferred from our study that despite the known increased susceptibility to glaucoma and worse prognosis once the disease is diagnosed,29–32 AD subjects are less likely to present with DH, which is a very strong predictor of progression is all studies.
An explanation for this finding can be that other risk factors previously shown to be associated with worse glaucoma, e.g.:, higher intraocular pressure (IOP), decreased CCT, worse baseline VF status, and decreased retinal nerve fiber layer thickness,9,44–48 may play a more meaningful role than DH on the susceptibility to glaucoma among individuals of African descent than those of European descent. In fact, previous ADAGES reports confirmed that even among participants with statistically normal IOP and normal optic discs and achromatic perimetry, AD subjects showed worse VF global indices49 and thinner nerve fiber layer measurements than ED subjects.50 In addition, greater prevalence of βPPA in AD participants, as shown in our study, may contribute more to their increased susceptibility, as βPPA has been demonstrated to be correlated with increased risk for disease progression.6,21 It is possible, however, that a higher prevalence of βPPA among AD eyes could be an artifact due to differences in background retinal pigmentation (from the retinal pigmented epithelium, RPE), which increases the contrast between areas with and without RPE in AD eyes. It should be noted, however, that since DH detection is not affected by background pigmentation, it is unlikely that this finding is caused by underestimation of DH in the AD participants compared to ED participants.
Our βPPA prevalence findings are consistent with the literature. βPPA was reported by Jonas et al. to occur in 20% of normal individuals and in two-thirds of glaucoma patients.22 Teng et al. reported βPPA to present in 65% of glaucomatous eyes.21 No description of the study populations’ ancestries was reported in these publications. To the best of our knowledge, this is the first report comparing the prevalence of βPPA in AD and ED eyes.
Our results suggest that βPPA has different impacts on DH development in ED as opposed to the AD eyes. Radcliffe et al12 reported that DH is more commonly seen in eyes with βPPA, and that the region of the widest βPPA often appears at the region of the thinnest neuroretinal rim,22 and therefore is more often accompanied by DH.12,14 It has also been suggested that βPPA enlarges as glaucoma progresses,19 and microstructural changes within the optic nerve complex may predispose to a faster rate of future structural loss.51 However, to the best of our knowledge, no other study has evaluated whether the association between βPPA and DH varies by race. Moreover, no study has evaluated whether there is a difference in the microstructural susceptibility between AD and ED eyes, which might explain the differences in the frequency of DH, and βPPA in this study.
There are several possible limitations to our study. Although possible recruitment differences between sites, ADAGES guarded against any site-specific differences by using the same study protocol, training, and certification procedures to ensure uniformity across study sites. There was a significant age difference between AD and ED participants, as AD participants were younger on average (Table 1). Nonetheless, similar differences have been reported in major clinical trials52,53 and a population-based study,30 and most likely reflect an earlier disease onset in AD subjects. In addition, we used the current definition of βPPA widely employed in clinical practice, rather than spectral domain optical coherence tomography (OCT) (e.g. based on the relationship between Bruch’s membrane opening and end of the RPE). Based upon OCT images, Dai et al. differentiated βPPA between a peripheral β-zone defined as Bruch's membrane devoid of RPE and a more centrally-located γ-zone where there is no choroid or Bruch's membrane overlying the parapapillary sclera.54 Their results showed that OCT-defined βPPA was significantly associated with the presence of glaucoma, whereas the γ-zone was associated with the absence of glaucoma. Therefore, a simple clinical evaluation of βPPA has possible limitations as it does not provide insight of microstructural features that could better differentiate glaucoma vs. non-glaucoma-related βPPA. Nonetheless, there is currently no evidence that OCT-based classifications systems should replace clinical standards. In addition, since we required agreement between masked reviewers (and adjudication by a third grader) in order to define the presence of DH and βPPA, it could cause potential underestimation (by increasing our specificity at the expense of lower sensitivity). However, agreement between graders was excellent. Moreover, since the same definitions and review methods were applied to both racial groups masked to race, they likely did not affect the interpretation of our results. We did not assess longitudinal change in βPPA over time, which might be different between the groups.
In summary, we found a significantly higher frequency of DH among individuals of ED compared to AD and higher prevalence of βPPA among individuals of AD compared to ED. Individuals of European descent who have βPPA were found to be at higher risk for developing DH during their follow up, compared to AD subjects. These findings may help clarify the role of these important structural features and might help improve our ability to detect and predict the development of glaucomatous progression in ED and ED glaucoma patients.
Acknowledgments
Financial support:
A. Skaat: None.
C.G. De Moraes: National Institutes of Health, National Eye Institute; Research to Prevent Blindness, New York, NY.
J.M. Liebmann: C; Alcon Laboratories Inc., Allergan Inc., Carl Zeiss Meditec Inc., Dyopsis Inc., Pfizer Inc., Topcon Medical Systems Inc.
R. Ritch: F; Diopsys Inc., Topcon Medical Systems Inc.
C; Dyopsis Inc., Pfizer Inc., Topcon Medical Systems Inc.
C. Bowd: F: Pfizer Inc.
P.A. Sample: F; Carl Zeiss Meditec Inc., Haag-Streit,
C.A. Girkin: C; Alcon Laboratories Inc., Allergan Inc., Pfizer Inc.
R; Heidelberg Engineering GmbH, Optove Inc., Merck Inc., Topcon Medical Systems, Inc., Carl Zeiss Meditec, Inc.
F.A. Medeiros: F; Alcon Laboratories Inc., Carl Zeiss Meditec Inc., Pfizer Inc.
C; Alcon Laboratories Inc., Allergan Inc., Pfizer Inc. R; Alcon Laboratories Inc., Allergan Inc., Carl Zeiss Meditec Inc., Pfizer Inc., Reicherts Inc.
R.N. Weinreb: F; Carl Zeiss Meditec Inc., Heidelberg Engineering GmbH, Optovue Inc., Topcon Medical Systems, Nidek Inc.
C; Alcon Laboratories Inc., Allergan Inc., Carl Zeiss Meditec Inc., Optovue Inc., Pfizer Inc., Merck Inc.
L.M. Zangwill: F; Carl Zeiss Meditec Inc., Heidelberg Engineering GmbH, Optovue Inc., Topcon Medical Systems Inc.
R: Heidelberg Engineering
Funding/support:
Supported by National Eye Institute grants U10EY14267, EY08208, EY11008, EY019869, EY13959; Eyesight Foundation of Alabama; Alcon Laboratories Inc.; Allergan Inc.; Pfizer Inc.; Merck Inc.; Santen Inc.; and the Edith C. Blum Foundation Fund of the New York Glaucoma Research Institute, New York, NY (Dr. Skaat).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: clinicaltrials.gov Identifier: NCT00221923
Conflict of Interest: No conflicting relationship exists for any author related to materials described in this article.
REFERENCES
- 1.Susanna R, Drance SM, Douglas GR. Disc hemorrhages in patients with elevated intraocular pressure. Occurrence with and without field changes. Arch Ophthalmol. 1979;97:284–285. doi: 10.1001/archopht.1979.01020010136007. [DOI] [PubMed] [Google Scholar]
- 2.Drance SM. Disc hemorrhages in the glaucomas. Surv Ophthalmol. 1989;33:331–337. doi: 10.1016/0039-6257(89)90010-6. [DOI] [PubMed] [Google Scholar]
- 3.Siegner SW, Netland PA. Optic disc hemorrhages and progression of glaucoma. Ophthalmology. 1996;103:1014–1024. doi: 10.1016/s0161-6420(96)30572-1. [DOI] [PubMed] [Google Scholar]
- 4.Rasker MT, van den Enden A, Bakker D, Hoyng PF. Deterioration of visual fields in patients with glaucoma with and without optic disc hemorrhages. Arch Ophthalmol. 1997;115:1257–1262. doi: 10.1001/archopht.1997.01100160427006. [DOI] [PubMed] [Google Scholar]
- 5.Ishida K, Yamamoto T, Sugiyama K, Kitazawa Y. Disk hemorrhage is a significantly negative prognostic factor in normal-tension glaucoma. Am J Ophthalmol. 2000;129:707–714. doi: 10.1016/s0002-9394(00)00441-4. [DOI] [PubMed] [Google Scholar]
- 6.De Moraes CG, Juthani VJ, Liebmann JM, et al. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol. 2011;129:562–568. doi: 10.1001/archophthalmol.2011.72. [DOI] [PubMed] [Google Scholar]
- 7.Sonnsjo B, Dokmo Y, Krakau T. Disc haemorrhages, precursors of open angle glaucoma. Prog Retin Eye Res. 2002;21:35–56. doi: 10.1016/s1350-9462(01)00019-2. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Park KH. The relationship between recurrent optic disc hemorrhage and glaucoma progression. Ophthalmology. 2006;113:598–602. doi: 10.1016/j.ophtha.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Miglior S, Torri V, Zeyen T, et al. Intercurrent factors associated with the development of open-angle glaucoma in the European glaucoma prevention study. Am J Ophthalmol. 2007;144:266–275. doi: 10.1016/j.ajo.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 10.Budenz DL, Anderson DR, Feuer WJ, et al. Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology. 2006;113:2137–2143. doi: 10.1016/j.ophtha.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bengtsson B, Leske MC, Yang Z, Heijl A. Disc hemorrhages and treatment in the early manifest glaucoma trial. Ophthalmology. 2008;115:2044–2048. doi: 10.1016/j.ophtha.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 12.Radcliffe NM, Liebmann JM, Rozenbaum I, et al. Anatomic relationships between disc hemorrhage and parapapillary atrophy. Am J Ophthalmol. 2008;146:735–740. doi: 10.1016/j.ajo.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa T, Sugiyama K, Tomita G, et al. Correlation of the peripapillary atrophy area with optic disc cupping and disc hemorrhage. J Glaucoma. 1998;7:306–311. [PubMed] [Google Scholar]
- 14.Ahn JK, Kang JH, Park KH. Correlation between a disc hemorrhage and peripapillary atrophy in glaucoma patients with a unilateral disc hemorrhage. J Glaucoma. 2004;13:9–14. doi: 10.1097/00061198-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Jonas JB, Iester M. Disc hemorrhage and glaucoma. Ophthalmology. 1995;102:365–366. doi: 10.1016/s0161-6420(13)30831-8. [DOI] [PubMed] [Google Scholar]
- 16.Gordon J, Piltz-Seymour JR. The significance of optic disc hemorrhages in glaucoma. J Glaucoma. 1997;6:62–64. [PubMed] [Google Scholar]
- 17.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 18.De Moraes CG, Prata TS, Liebmann CA, et al. Spatially consistent, localized visual field loss before and after disc hemorrhage. Invest. Ophthalmol Vis Sci. 2009;50:4727–4733. doi: 10.1167/iovs.09-3446. [DOI] [PubMed] [Google Scholar]
- 19.Uchida H, Ugurlu S, Caprioli J. Increasing peripapillary atrophy is associated with progressive glaucoma. Ophthalmology. 1998;105:1541–1545. doi: 10.1016/S0161-6420(98)98044-7. [DOI] [PubMed] [Google Scholar]
- 20.Park SC, De Moraes CG, Tello C, et al. In-vivo microstructural anatomy of beta-zone parapapillary atrophy in glaucoma. Invest Ophthalmol Vis Sci. 2010;51:6408–6413. doi: 10.1167/iovs.09-5100. [DOI] [PubMed] [Google Scholar]
- 21.Teng CC, De Moraes CG, Prata TS, et al. Beta-Zone parapapillary atrophy and the velocity of glaucoma progression. Ophthalmology. 2010;117:909–915. doi: 10.1016/j.ophtha.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Jonas JB, Naumann GO. Parapapillary chorioretinal atrophy in normal and glaucoma eyes. II. Correlations. Invest Ophthalmol Vis Sci. 1989;30:919–926. [PubMed] [Google Scholar]
- 23.Jonas JB. Clinical implications of peripapillary atrophy in glaucoma. Curr Opin Ophthalmol. 2005;16:84–88. doi: 10.1097/01.icu.0000156135.20570.30. [DOI] [PubMed] [Google Scholar]
- 24.Wilensky JT, Kolker AE. Peripapillary changes in glaucoma. Am J Ophthalmol. 1976;81:341–345. doi: 10.1016/0002-9394(76)90251-8. [DOI] [PubMed] [Google Scholar]
- 25.Buus DR, Anderson DR. Peripapillary crescents and halos in normal-tension glaucoma and ocular hypertension. Ophthalmology. 1989;96:16–19. doi: 10.1016/s0161-6420(89)32930-7. [DOI] [PubMed] [Google Scholar]
- 26.Jonas JB, Fernandez MC, Naumann GO. Glaucomatous parapapillary atrophy. Occurrence and correlations. Arch Ophthalmol. 1992;110:214–222. doi: 10.1001/archopht.1992.01080140070030. [DOI] [PubMed] [Google Scholar]
- 27.Nevarez J, Rockwood EJ, Anderson DR. The configuration of peripapillary tissue in unilateral glaucoma. Arch Ophthalmol. 1988;106:901–903. doi: 10.1001/archopht.1988.01060140047021. [DOI] [PubMed] [Google Scholar]
- 28.Park KH, Tomita G, Liou SY, Kitazawa Y. Correlation between peripapillary atrophy and optic nerve damage in normal-tension glaucoma. Ophthalmology. 1996;103:1899–1906. doi: 10.1016/s0161-6420(96)30409-0. [DOI] [PubMed] [Google Scholar]
- 29.Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991;325:1412–1417. doi: 10.1056/NEJM199111143252004. [DOI] [PubMed] [Google Scholar]
- 30.Tielsch JM, Sommer A, Katz J, et al. Racial variations in the prevalence of primary open-angle glaucoma: the Baltimore Eye Survey. JAMA. 1991;266:369–374. [PubMed] [Google Scholar]
- 31.Racette L, Wilson MR, Zangwill LM, et al. Primary open angle glaucoma in blacks: a review. Surv Ophthalmol. 2003;48:295–313. doi: 10.1016/s0039-6257(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 32.Girkin CA. Primary open-angle glaucoma in African Americans. Int Ophthalmol Clin. 2004;44:43–60. doi: 10.1097/00004397-200404420-00006. [DOI] [PubMed] [Google Scholar]
- 33.Demirel S, De Moraes CG, Gardiner SK, et al. The rate of visual field change in the ocular hypertension treatment study. Invest Ophthalmol Vis Sci. 2012;53:224–227. doi: 10.1167/iovs.10-7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 35.Higginbotham EJ, Gordon MO, Beiser JA, et al. The Ocular Hypertension Treatment Study: topical medication delays or prevents primary open-angle glaucoma in African American individuals. Arch Ophthalmol. 2004;122:813–820. doi: 10.1001/archopht.122.6.813. [DOI] [PubMed] [Google Scholar]
- 36.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127:1136–1145. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boden C, Hoffmann EM, Medeiros FA, et al. Intereye concordance in locations of visual field defects in primary open-angle glaucoma: diagnostic innovations in glaucoma study. Ophthalmology. 2006;113(6):918–923. doi: 10.1016/j.ophtha.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Lan YW, Wang IJ, Hsiao YC, et al. Characteristics of disc hemorrhage in primary angle-closure glaucoma. Ophthalmology. 2008;115:1328–1333. doi: 10.1016/j.ophtha.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 39.Medeiros FA, Alencar LM, Sample PA, et al. The relationship between intraocular pressure reduction and rates of progressive visual field loss in eyes with optic disc hemorrhage. Ophthalmology. 2010;117:2061–2066. doi: 10.1016/j.ophtha.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Kitazawa Y, Shirato S, Yamamoto T. Optic disc hemorrhage in low-tension glaucoma. Ophthalmology. 1986;93:853–857. doi: 10.1016/s0161-6420(86)33658-3. [DOI] [PubMed] [Google Scholar]
- 41.Krupin T, Liebmann JM, Greenfield DS, et al. The Low-pressure Glaucoma Treatment Study (LoGTS) study design and baseline characteristics of enrolled patients. Ophthalmology. 2005;112:376–385. doi: 10.1016/j.ophtha.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 42.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 43.Bowd C, Weinreb RN, Zangwill LM. Evaluating the optic disc and retinal nerve fiber layer in glaucoma. I: Clinical examination and photographic methods. Semin Ophthalmol. 2000;15:194–205. doi: 10.3109/08820530009037871. [DOI] [PubMed] [Google Scholar]
- 44.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 45.AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:490–491. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 46.Musch DC, Gillespie BW, Lichter PR, et al. Visual field progression in the Collaborative Initial Glaucoma Treatment Study the impact of treatment and other baseline factors. Ophthalmology. 2009;116:200–207. doi: 10.1016/j.ophtha.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medeiros FA, Sample PA, Zangwill LM, et al. Corneal thickness as a risk factor for visual field loss in patients with preperimetric glaucomatous optic neuropathy. Am J Ophthalmol. 2003;136:805–813. doi: 10.1016/s0002-9394(03)00484-7. [DOI] [PubMed] [Google Scholar]
- 48.Lalezary M, Medeiros FA, Weinreb RN, et al. Baseline optical coherence tomography predicts the development of glaucomatous change in glaucoma suspects. Am J Ophthalmol. 2006;142:576–582. doi: 10.1016/j.ajo.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Racette L, Liebmann JM, Girkin CA, et al. African Descent and Glaucoma Evaluation Study (ADAGES): III. Ancestry differences in visual function in healthy eyes. Arch Ophthalmol. 2010;128:551–559. doi: 10.1001/archophthalmol.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Girkin CA, Sample PA, Liebmann JM, et al. African Descent and Glaucoma Evaluation Study (ADAGES): II. Ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Arch Ophthalmol. 2010;128:541–550. doi: 10.1001/archophthalmol.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curcio CA, Saunders PL, Younger PW, Malek G. Peripapillary chorioretinal atrophy: Bruch’s membrane changes and photoreceptor loss. Ophthalmology. 2000;107:334–343. doi: 10.1016/s0161-6420(99)00037-8. [DOI] [PubMed] [Google Scholar]
- 52.The Advanced Glaucoma Intervention Study (AGIS): 3. Baseline characteristics of black and white patients. Ophthalmology. 1998;105:1137–1145. doi: 10.1016/s0161-6420(98)97012-9. [DOI] [PubMed] [Google Scholar]
- 53.Gordon MO, Kass MA. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–583. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 54.Dai Y, Jonas JB, Huang H, et al. Microstructure of parapapillary atrophy: beta zone and gamma zone. Invest Ophthalmol Vis Sci. 2013;54:2013–2018. doi: 10.1167/iovs.12-11255. [DOI] [PubMed] [Google Scholar]