Abstract
Lessons from an animal model that faithfully resembles human membranous nephropathy (MN) have informed our understanding of the pathogenesis of this organ-specific autoimmune disease and common cause of the nephrotic syndrome. Once it was established that the subepithelial immune deposits that characterize experimental MN form in situ when circulating antibodies bind to an intrinsic podocyte antigen, it was merely a matter of time before the human antigen was identified. The M-type phospholipase A2 receptor 1 (PLA2R) represents the major target antigen in primary MN, and thrombospondin type 1 domain-containing 7A (THSD7A) was more recently identified as a minor antigen. Serological tests for anti-PLA2R as well as kidney biopsy specimen staining for PLA2R exhibit more than 90% specificity and 70% to 80% sensitivity for the diagnosis of primary MN in most populations. The assays distinguish most cases of primary MN from MN associated with other systemic diseases, and sequential titers of anti-PLA2R are useful to monitor treatment response. A positive pre-transplantation test for anti-PLA2R is also helpful for predicting the risk of post-transplantation recurrence. Identification of target epitopes within PLA2R as well as the genetic association of primary MN with class II major histocompatibility and PLA2R1 variants are 2 additional examples of our evolving understanding of this disease.
Keywords: Membranous nephropathy (MN), nephrotic syndrome, M-type phospholipase A2 receptor 1 (PLA2R), PLA2R1, thrombospondin type 1 domain-containing 7A (THSD7A), organ-specific autoimmunity, class II major histocompatibility, genetic polymorphisms, post-transplant recurrent disease, epitope
In the late 1970s, 2 groups of investigators, one based in the Netherlands and the other in Boston, Massachusetts, were studying Heymann nephritis, a rat model of experimental membranous nephropathy (MN), and made an observation that changed our understanding about the pathogenesis of MN 1–3. Until that time, it was believed that all forms of immune-complex glomerulonephritis (ie, those characterized by granular immune deposits on immunofluorescence (IF) and electron densities on electron microscopy (EM)) were due to circulating immune complexes passively trapped in the glomeruli. However, the new work conclusively showed that the deposits in experimental MN formed in situ when circulating immunoglobulin (Ig) G antibodies bound to a yet to be characterized intrinsic glomerular antigen 3. Although the location of the intrinsic antigen on the glomerular capillary wall was not defined, we speculated that it might be a component of the visceral epithelial cell (podocyte) surface 1. Some years later, a confluence of studies from several groups identified megalin, a large transmembrane protein member of the low-density lipoprotein receptor family, as the intrinsic antigen and showed that it is expressed on the soles of podocyte foot processes where it can be engaged by circulating IgG antibodies 4–8. This finding led to a search for anti-megalin antibodies in patients with MN, which proved futile: human podocytes do not express megalin even though it is abundant in the human proximal tubular brush border 8. This, and the failure by several investigators to identify another candidate podocyte antigen, created some doubt as to whether the in situ paradigm established in Heymann nephritis also applied to human MN. This doubt was finally dispelled in 2002: interest in the identity of one or more podocyte antigens in human MN was rekindled when Debiec and colleagues9 described a remarkable case of neonatal MN in which transplacental passage of anti-neutrophil endopeptidase (NEP) antibodies from a presensitized NEP-deficient mother bound to NEP on the baby’s podocytes.
MN is a common cause of nephrotic syndrome in adults with a peak occurrence in the 5–6th decades, although the age range of onset is broad 10. Most cases (~80%) are primary (formerly called idiopathic MN), but MN may be secondary to systemic lupus erythematosus (class V lupus nephritis), infection with hepatitis B virus or other agents, solid cancers, and various drugs or toxins. MN may also occur as the result of an alloimmune response, for example de novo MN after kidney transplantation and during chronic graft versus host disease after allogeneic stem cell transplantation, and also as neonatal MN as noted previously. Primary MN is an organ-specific autoimmune disease. The characteristic pathological features are a non-inflammatory glomerular lesion with glomerular basement membrane (GBM) thickening (often seen as spike-like extrusions or craters in the GBM) on Jones silver stain, granular capillary wall deposits of IgG and complement on IF, and subepithelial immune deposits on electron microscopy with extensive effacement of the podocyte foot processes. Primary MN may be distinguished from secondary MN by its predominant IgG4 and absent C1q. The clinical course is quite variable, with spontaneous remission reported in up to one third of cases and progression to end-stage renal disease (ESRD) in a similar number. Recurrence of primary MN after kidney transplantation is common and may lead to allograft loss, as we discuss later.
In 2009, we reported on the identification of the M-type phospholipase A2 receptor (PLA2R) as a major target antigen in human primary MN 11. This finding followed several years of experimentation with extracts of normal human glomeruli and MN patient sera using western blotting and mass spectrometry, and the serendipitous discovery that human MN autoantibodies only recognize the antigen, PLA2R, under non-reducing conditions (ie, the epitope[s] identified by anti-PLA2R are conformation dependent). Our initial studies also showed that PLA2R is expressed on podocytes, and that anti-PLA2R autoantibodies are present in a high proportion, but not all, cases of primary MN; are predominantly of the IgG4 subclass (as is true of the immune deposits from which they can be eluted); are uniquely present in primary and not secondary MN; and are correlated with disease activity. As in the Heymann nephritis model, we also found that the distribution of PLA2R shifted to become readily detectable in the immune deposits in primary but not secondary MN (Figure 1), a finding that has been adopted by nephropathologists to define PLA2R-associated MN 12,13. Interestingly, our initial studies identified another putative antigen in a small number of anti-PLA2R–negative cases that ultimately proved to be a second podocyte antigen: thrombospondin type 1 domain-containing 7A (THSD7A) 14. Like PLA2R, THSD7A is expressed on podocytes and redistributes to form the subepithelial immune deposits 14,15. It may account for up to 5% of cases of primary MN in Western countries but has been reported in 9.1% of Japanese patients with primary MN, with an equal prevalence among women and men16 (Box 1).
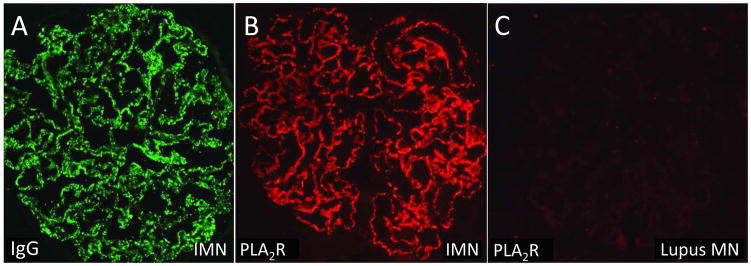
Figure 1.
Immunofluorescence micrographs of kidney biopsy specimens from patients with (A, B) primary membranous nephropathy and (C) membranous lupus nephritis stained for (A) immunoglobulin (Ig) G and (B, C) phospholipase A2 receptor (PLA2R). Note the bright staining for PLA2R in the immune deposits in primary MN but not in lupus MN. Abbreviation: IMN, idiopathic membranous nephropathy.
Box 1. Membranous Nephropathy.
A leading cause of nephrotic syndrome in adults
Antibody-mediated podocytopathy
-
Primary MN (majority)
organ-specific autoimmune disease
variable course – remission, persistent nephrotic syndrome, ESRD
PLA2R-associated ~80%
THSD7A-associated ~5%–10% (possibly population-dependent)
recurrence post-transplantation
Secondary – lupus, infection with hepatitis B virus or other agents, drugs, cancer
Alloimmune – neonatal MN (anti-NEP), de novo post-kidney and stem cell transplantation MN
Our 2009 report11 stimulated renewed interest in primary MN and led to development of diagnostic tests, investigation of the genetic basis of MN, and exploration of the target epitope(s). As commercial immunoassays for anti-PLA2R become more widely used and more nephropathologists perform immunostaining of kidney biopsy specimens for PLA2R, the value for these tests will become increasingly clear for the diagnosis and treatment of primary MN, the exclusion of secondary causes (especially cancer), and the prediction of post-transplantation recurrence. In this review, we focus on developments since the initial discovery of PLA2R as a major target antigen in primary MN. The reader is referred elsewhere for updated discussions of the treatment of MN17–19.
Are the Available Serum Assays for Anti-PLA2R Sensitive and Specific in Predicting Primary MN?
Anti-PLA2R antibody has proved to be a valuable biomarker for the diagnosis of primary MN. It is also useful for monitoring disease activity and predicting disease recovery and relapse. Depending on the state of disease activity, and as discussed in the following, between 50% and 80% of patients will test positive for anti-PLA2R antibody with any of the available tests 12,13,20–22. This variability in sensitivity has more to do with the biology of the disease and perhaps ethnicity (for example, Japanese patients with primary MN have a lower rate of anti-PLA2R positivity 22) rather than the characteristics of the assays used.
The original discovery of PLA2R and early clinical studies relied on western blotting, which is both sensitive and very specific for the detection of anti-PLA2R when recombinant human PLA2R is used as the antigen; however, the technique is costly, labor intensive, and impractical for routine clinical use. In 2014, the US Food and Drug Administration approved the first commercially available tests for anti-PLA2R, an indirect immunofluorescence assay (IIFA) and an enzyme-linked immunosorbent assay (ELISA), two tests that had first become available in Europe and were widely used in the workup of patients with nephrotic syndrome 23. Although the IIFA is relatively high throughput, the titers of anti-PLA2R are semi-quantitative and observer dependent, and reactivity with other baseline cell antigens may occasionally predispose to equivocal results. It is generally used as an initial screening assay, much like antineutrophil cytoplasmic antibody assays, before proceeding to the high throughput and more quantitative and specific ELISA. As illustrated in Figure 2, when compared with the western blot assay for anti-PLA2R, the commercial IIFA and ELISA tests are highly specific for primary MN versus secondary MN and other forms of glomerular disease, although the ELISA is somewhat less sensitive using the recommended cutoff for positivity. These results are similar to those reported by other investigators with respect to these commercial assays 13,24 and an independent ELISA 25 and luminex assay 26. We should point out, however, that occasional cases of MN with a positive test for anti-PLA2R have been described in patients with cancer, lupus, hepatitis B virus infection, and other inflammatory and autoimmune diseases 27–30. Whether or not these are real secondary cases of MN or the chance association of PLA2R-associated primary MN in patients with other common diseases must await the test of time. A notable exception is a study from Shanghai that found a high proportion of patients with MN with hepatitis B surface antigen who were also positive for anti-PLA2R and exhibited co-localization of the viral antigen and PLA2R in the glomerular immune deposits 31. We await confirmation of these findings with interest.
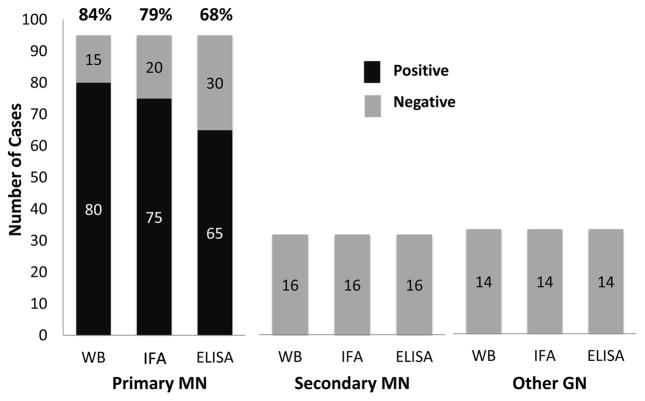
Figure 2.
Comparison of serologic tests for anti–phospholipase A2 receptor (PLA2R) in patients with primary and secondary membranous nephropathy (MN) and other forms of glomerulonephritis (GN). The numbers within the bars represent the number of cases in each group. Results based on data collected by 2 of the authors (LHB, DJS). Abbreviation: WB, western blot; IFA, indirect immunofluorescence assay performed using cells transfected with a recombinant PLA2R-expressing vector; ELISA, enzyme-linked immunosorbent assay.
Perhaps most important is the fact that seropositivity for anti-PLA2R has not been found in any proteinuric kidney diseases other than MN 11,27,29,32. In other words, any patient with nephrotic syndrome that tests positive for anti-PLA2R is almost certain to have MN. This is important in patients at high risk for kidney biopsy, such as those needing anticoagulation for thromboembolic disease and patients with a single kidney.
Immunostaining for PLA2R on Kidney Biopsy: Another Sensitive and Specific Test for Primary MN
PLA2R is expressed on the cell body of human podocytes and their foot processes11. Once anti-PLA2R antibodies bind, the immune complexes are thought to aggregate and then be shed into the subepithelial space between the podocyte and GBM. The detection by immunostaining of PLA2R in the subepithelial deposits in kidney tissue is a very sensitive and specific technique to diagnose PLA2R-associated MN and correlates well with the serological tests.13 However, the absence of circulating anti-PLA2R antibodies in the serum of patients with biopsy-proven MN does not rule out the diagnosis of PLA2R-associated MN. As we discuss later, some patients may have positive PLA2R immunostaining of the subepithelial immune deposits on kidney biopsy, supporting the diagnosis of PLA2R-associated MN despite the absence of circulating anti-PLA2R antibodies 12,33,34. Interestingly, a minority of patients who are seropositive for anti-PLA2R antibodies exhibit no staining for PLA2R within the immune deposits of their corresponding biopsy 12. This may represent masking by autoantibodies of epitopes recognized by the commercial antibody used for immunofluorescence staining, but has not yet been fully explored.
The absence of PLA2R deposits in kidney tissues from most patients with secondary MN makes this test very specific for primary MN. However, a few patients diagnosed with secondary MN on the basis of concurrent lupus, cancer, sarcoidosis, or viral hepatitis have been reported to test positive for serum anti-PLA2R antibodies, or have positive immunostaining test for PLA2R on kidney biopsy 29,31,35. Interestingly, in almost all of these patients, the deposited antibodies are predominantly IgG4, a feature classically associated with primary MN 29,35. Although more studies are needed to explain this phenomenon, we favor the probability that such patients have primary PLA2R-associated MN concomitant with, rather than secondary to, sarcoidosis, cancer, or hepatitis. However, we recognize the possibility that the associated conditions may represent a disease-precipitating “second hit” in a patient genetically and immunologically predisposed to develop MN (see below).
Clinical Use of Anti-PLA2R Seropositivity and Glomerular Immunostaining for PLA2R
To better understand the utility of anti-PLA2R antibody for the diagnosis and follow up of PLA2R-associated MN, it is helpful to consider the lag between antibody deposition, seropositivity, and clinical activity as measured by proteinuria 33. Early in the course of the disease, when anti-PLA2R antibodies are first produced, podocyte injury and proteinuria are induced when the antibodies bind to the antigen on podocytes and immune deposits containing both PLA2R and anti-PLA2R antibodies are formed. At this time, circulating anti-PLA2R antibodies might not be detectable using standard assays despite their presence in the glomerular deposits, because the kidney acts as a “sink” absorbing all detectable circulating anti-PLA2R antibodies36,37. In such cases, PLA2R will be detectable in the immune deposits by immunofluorescence staining, thus providing evidence that the MN is PLA2R-associated. With ongoing immunological activity and disease progression, the renal tissue becomes saturated with anti-PLA2R antibodies and becomes and seropositive, whereupon the available tests become valuable tools for the diagnosis and monitoring of disease activity 37,38. As patients enter an immunologic remission, anti-PLA2R disappears from the circulation but proteinuria may remain unresolved. If a kidney biopsy were to be performed at this stage, it is very likely that staining for PLA2R in the immune deposits would still be present. These relationships are illustrated in Figure 3.
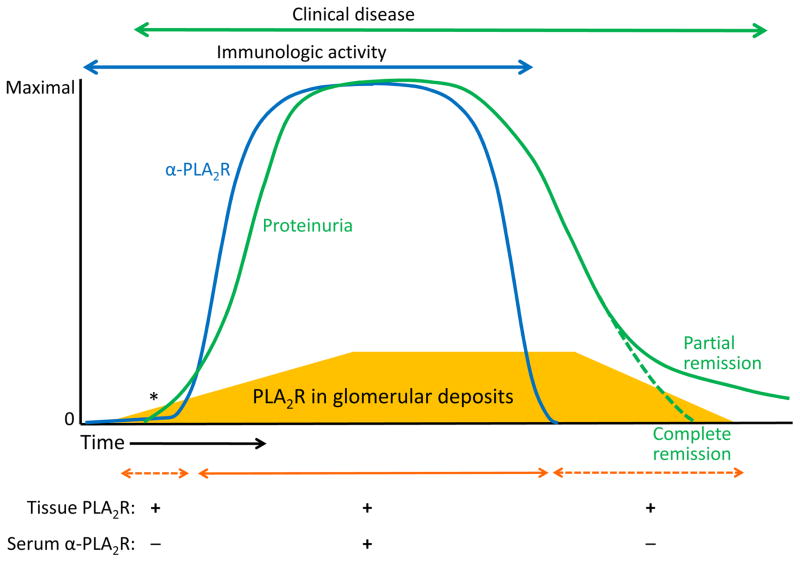
Figure 3.
Schematic representation of the temporal association of serologic tests for anti–phospholipase A2 receptor (PLA2R), tissue staining for PLA2R, and clinical disease activity as represented by proteinuria. The mass of PLA2R within the deposits is represented by the gold-colored trapezoid at the bottom of the graph. Note that tissue staining for PLA2R may precede the appearance of circulating anti-PLA2R antibodies, as the kidney likely acts as a “sink” for these autoantibodies early in the disease. At some point (denoted by an asterisk), the subepithelial PLA2R- and immunoglobulin (Ig) G–containing deposits will cause sufficient podocyte injury to lead to detectable proteinuria, which increases with disease progression. Once anti-PLA2R antibody (α-PLA2R) levels decline and disappear, immune deposits will slowly start to recede, allowing resolution of proteinuria (partial and complete remissions). Note that PLA2R will continue to be detected in tissue well after the circulating antibody disappears.
Given the association of MN with cancer, it is general practice to screen patients with MN for the presence of solid tumors. Although observations from prospectively followed patient cohorts are lacking, current evidence suggests that most patients with cancer-associated MN do not have tissue or serological evidence of PLA2R-associated MN, and the biopsy features are more consistent with secondary rather than primary MN 29. Conversely, when patients with PLA2R-associated MN develop cancer, it tends to occur months to years after the onset of MN, suggesting that it is unrelated 39. This means that a patient with PLA2R-associated MN need not undergo an extensive workup for malignancy beyond routine age-appropriate health maintenance tests, whereas PLA2R-negative cases require closer scrutiny.
Correlation of Anti-PLA2R Antibodies With Clinical Activity and Prognostic Value of Titers
Anti-PLA2R antibodies have emerged as an excellent biomarker of disease activity in primary MN. At presentation, 70% to 80% of patients with primary MN will test positive for serum anti-PLA2R antibodies 11,25,29,34,40. Several studies have shown a temporal relationship between the presence and the levels of anti-PLA2R antibodies and the disease activity 20,41,42. Usually, the spontaneous or treatment-induced decline or disappearance of circulating anti-PLA2R antibodies (so-called “immunologic remission”) precedes a corresponding clinical remission by a period of several months 41. Therefore, monitoring of anti-PLA2R antibody levels in patients with PLA2R-associated primary MN might help anticipate a spontaneous remission and avoid immunosuppression if the levels are declining, or avoid unnecessarily prolonged treatment in those with residual proteinuria in whom the circulating antibodies have disappeared. Although formal prospective studies are needed to determine if there is value in monitoring anti-PLA2R antibody levels in patients in remission, existing retrospective studies on stored samples showed that clinical relapse is usually associated with the re-appearance of anti-PLA2R antibodies 20.
Antibody titers may also be informative. In a study involving 82 patients with PLA2R-associated MN (as determined by ELISA and IIFA) reported by Hofstra and colleagues, the antibody titer correlated well with baseline proteinuria, and spontaneous remission occurred most often in those with the lowest titers of anti-PLA2R IgG4 42. A recent prospective study from Hoxha et al showed a correlation between baseline anti-PLA2R antibody levels and disease activity as measured by proteinuria 43. The rate of remission and time to achieve clinical remission strongly correlated with baseline anti-PLA2R antibody levels, while a fall in anti-PLA2R antibody levels was associated with an improvement in proteinuria 43. Likewise, among 81 patients with PLA2R-associated MN studied by Ruggenenti et al, lower anti-PLA2R antibody titer at baseline and complete antibody depletion 6 months after induction treatment with rituximab strongly predicted remission 44. A 2009 study45 had reported that reduction of anti-PLA2R antibody titer precedes the decline in proteinuria by several months. In the Ruggenennti et al cohort, total reduction of anti-PLA2R was noted in all 25 patients that had a complete remission and, of those cases, 18 remained in clinical and serological remission 44. On the other hand, the reappearance of circulating anti-PLA2R antibodies preceded disease relapse.
The levels of anti-PLA2R antibody at the end of a therapeutic intervention may also predict the long-term clinical outcome. In a study by Bech et al, 46 almost 60% of the patients who had undetectable anti-PLA2R antibodies at the end of the therapy remained in clinical remission, while all the patients who had detectable anti-PLA2R antibody after therapy experienced clinical relapse. A potential refinement in testing for anti-PLA2R antibody was recently reported by Seitz-Polski et al47 who examined the reactivity of MN patient sera with PLA2R from different species by ELISA and found that rabbit PLA2R was as effective as human PLA2R in detecting anti-PLA2R antibody in patients with active disease, however reactivity with mouse PLA2R was better at identifying those patients at greater risk of progression.
Whereas a low or falling titer of anti-PLA2R antibodies correlates with remission and a favorable prognosis, the prognostic significance of antibody titer at the time of diagnosis prior to treatment is less clear. High titers at the time of diagnosis are more likely to be associated with or development of nephrotic syndrome rather than asymptomatic proteinuria 25,40,48; however, it remains uncertain if they forecast a poor outcome. In a retrospective ELISA study25 of stored sera, prevalent patients with the highest titers at the time of diagnosis were most likely to have active disease and were at greater risk of experiencing declines in kidney function during follow up. On the other hand, a prospective Korean study40 found that high levels of anti-PLA2R antibodies, as measured by western blotting at the time of kidney biopsy, did not predict the probability of progressive kidney failure. The different conclusions of these 2 studies may have to do with lead-time bias in the former and/or the different assay methods. A prospective study49 of 118 patients with primary MN assessed the risk of kidney function decline (conservatively defined as an increase in serum creatinine by 25% or more, to a level ≥1.3 mg/dl) in terms of anti-PLA2R antibody titer measured within 6 months of kidney biopsy and prior to any immunosuppressive therapy. Multivariate analysis showed that high anti-PLA2R levels independently predicted loss of kidney function. Future prospective analyses should determine the whether time-averaged autoantibody levels or the trajectory of these levels would be better indicators of risk than absolute titer at baseline.
In summary, the measurement of anti-PLA2R antibody levels supports the diagnosis of primary MN; predicts who might have spontaneous remission; helps to monitor the disease activity and response to therapy; identifies those at risk of progression; and, importantly, might help clinicians decide when to minimize or stop treatment.
Genetics of Primary MN
Although MN is not a typical Mendelian hereditary disease, it—like other systemic and organ-specific autoimmune diseases—has long been known to be associated with certain class II major histocompatibility (HLA-II) immune response genes50–54. In addition, soon after our report on PLA2R as the target antigen,11 studies55,56 of Asian patients with primary MN showed strong associations with certain coding variants in PLA2R1 that might have explained conformational changes in PLA2R and susceptibility to MN were it not for the fact that these variants are common in the general population (Figure 4). Likewise the results of a genome-wide association study57 in Europeans showed remarkably strong associations with HLA-DQA1 and a single-nucleotide polymorphism (SNP) in a non-coding portion of PLA2R1, but as yet no unique coding variants have been found in PLA2R1 that explain the association despite sequencing of the exons and splice sites and comparing the results to the National Center for Biotechnology Information’s short genetic variations database (dbSNP) and the 1000 Genomes Project database58. While the HLA-DQA1 and PLA2R1 risk alleles were reported to be strongly associated with anti-PLA2R antibody seropositivity and glomerular PLA2R immunostaining in individuals of Chinese or European ancestry59,60, the SNPs were the same common variants previously identified in earlier reports. Additional studies have suggested that the genetic influence of the HLA-II and PLA2R1 variants might manifest in the severity of disease rather than in its initiation25. Alternatively, we have proposed that the genetic susceptibility to primary MN may depend not on the presence of unique rare SNPs but instead on the concurrence of common genetic variants in PLA2R1 and the predisposition to autoimmunity conferred by HLA-DQA1 in concert with an external trigger61.
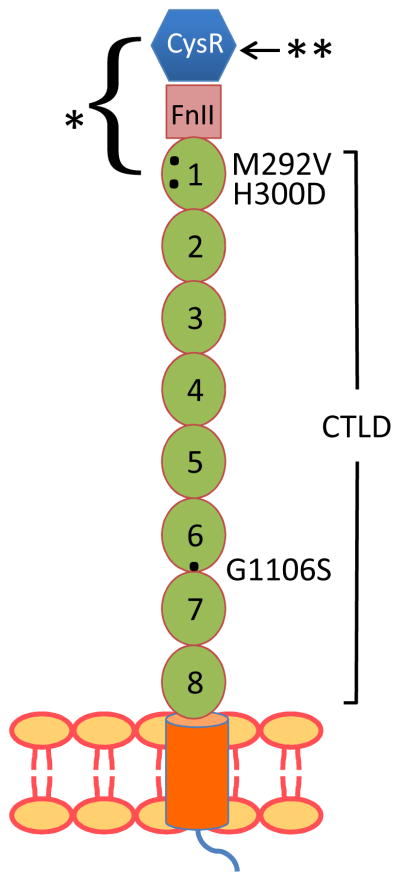
Figure 4.
Schematic of PLA2R structure, showing the sites of polymorphisms associated with primary membranous nephropathy (represented by black dots) and the location of common epitopes identified in Coenen et al58 (single asterisk) and Svobodova et al33 (double asterisk). Abbreviations and definitions: CysR, cysteine-rich (ricin B) domain; FnII, fibronectin II-like domain; CTLD, C-type lectin-like domains 1–8; M292V, methionine to valine substitution at amino acid 292; H300D, histidine to aspartate substitution at amino acid 300; G1106S, glycine to serine substitution at amino acid 1,106.
Defining the PLA2R Epitope(s)
When we first identified PLA2R as a major target antigen in primary MN and found that all reactive antisera identified the antigen only under non-reducing conditions, we reasoned that there might be a single conformation-dependent epitope. This seemed to fit with the observation that other members of the mannose receptor (MR) family are known to exist in either extended or bent configurations62. It also seemed to derive support from genetic studies that showed association of primary MN with coding variants in the amino-terminal region of PLA2R1 that is involved in the configuration changes found in other MR family members. The implications of this were potentially exciting. If there is indeed a single epitope, one could envisage making small peptides containing the epitope to immunoabsorb the offending antibodies or to serve as decoy antigens in vivo that block the antibodies from binding PLA2R—or even to restore self-tolerance by oral or nasal immunization. Evidence that there is indeed a universal epitope in the amino-terminal region was documented by 2 groups of investigators. Fresquet et al36 identified a 31–amino acid sequence in the ricin B (cysteine-rich) domain that blocked most of the anti-PLA2R antibody reactivity in patient sera, while Kao et al63 found that MN patient sera specifically recognized a protein complex consisting of the cysteine-rich, fibronectin-like type II and first C-type lectin-like domain of PLA2R (Figure 4). Unfortunately, although all patients have reactivity to the epitope contained within the cysteine-rich domain, epitope spreading to other parts of the molecule will have occurred by the time patients present with clinical symptoms64,65. Thus, the hope of a magic bullet to block reactivity or restore tolerance in MN has receded for the present, but defining the spread of epitopes may provide information on disease severity and prognosis 65.
Predictive Value of Anti-PLA2R for Recurrence of MN After Kidney Transplantation
Primary MN may recur after kidney transplantation. The incidence of recurrence ranges from 10% to 45%, reflecting differences between centers that perform protocol biopsies and those that only perform biopsies for clinical indication 66–72. The presence of circulating anti-PLA2R antibodies at the time of kidney transplantation is associated with a high risk of disease recurrence in the kidney graft 73–77.
Testing for anti-PLA2R antibodies at the time of transplantation might provide valuable information regarding the risk of primary MN recurrence in the kidney allograft. The first case report,73 published in 2010, demonstrated that the presence of anti-PLA2R antibodies in a patient at time of kidney transplantation was associated with recurrence of primary MN. Treatment with rituximab resulted in clinical improvement with a progressive decline of both proteinuria and anti-PLA2R antibody levels. This case report also highlighted the timeline of immunological remission, which usually precedes clinical remission by a few months. Kattah et al76 reported that the presence of circulating anti-PLA2R antibodies at the time of kidney transplantation has a 83% positive predictive value for recurrent MN as assessed by protocol biopsy. Worsening clinical course and increasing proteinuria were seen in patients with persistent or reappearing anti-PLA2R antibodies after kidney transplantation, whereas little or no proteinuria was noted in patients with biopsy-proven recurrent MN, in whom the anti-PLA2R antibodies disappeared with standard transplant immunosuppression 76,78. Similar findings were recently reported in another cohort77 of patients with primary MN in which the presence of anti-PLA2R antibodies at the time of kidney transplantation had a positive predictive value of disease recurrence of 91%. However, the probability that anti-PLA2R antibody seropositivity at the time of transplantation will lead to clinically significant recurrent MN in the allograft is likely influenced not only by the presence of the autoantibodies but also by their titer.77 Donor and recipient relatedness, class II major histocompatibility complex interactions,79 and the potency of transplant immunosuppression78 are also likely to be influential.
While the probability of histological recurrence is high in patients with high titers of anti-PLA2R antibodies at the time of transplantation, some patients may experience recurrence despite a negative pre-transplantation test for anti-PLA2R antibodies. Some of these individuals become seropositive after transplantation, perhaps due to the awakening of memory cells; recurrence in other cases could be due to primary MN from causes unrelated to PLA2R, including anti-THSD7A antibodies. At present, it is difficult to assign a negative predictive value (NPV) to a negative pre-transplantation test for anti-PLA2R antibodies, and the NPV of 42% reported by Kattah et al76 may actually underestimate the value of a negative test. This is because it has been difficult to ascertain how many patients that tested negative at the time of transplantation actually had PLA2R-associated MN. Such information is likely to become available as more patients with MN are tested for anti-PLA2R antibodies before they develop ESRD. Thus, testing for anti-PLA2R antibodies prior to transplantation in patients with MN has merit in that a positive test heightens surveillance for recurrent disease but is not in itself a contraindication to transplantation. Conversely, a negative test does not ensure that the disease will not recur.
Role of Anti-PLA2R Antibodies in Distinguishing Recurrent From De Novo MN
Testing for anti-PLA2R antibodies is also useful for distinguishing recurrent primary MN from de novo MN after kidney transplantation. De novo development of MN after kidney transplantation in patients who did not have MN as a cause of ESRD occurs occasionally and may be associated with evidence of antibody-mediated rejection and circulating donor-specific antibodies 80–82. Whereas IgG4 is usually the dominant or co-dominant IgG subclass deposited in recurrent MN, the IgG1 subclass predominates in de novo MN 83. Assays for circulating anti-PLA2R antibodies or staining for PLA2R on kidney biopsies almost always gives negative results in patients with de novo MN 84. These findings suggest different pathogenic processes between recurrent primary MN and de novo MN after kidney transplantation. De novo MN is most likely the result of alloantibodies in the allograft rather than the autoantibodies responsible for recurrent primary MN. PLA2R positivity is strongly associated with recurrent MN, with a sensitivity of 83% and specificity over 90% 84, making this test an ideal tool for differentiating between recurrent and de novo MN in patients in whom the original cause of ESRD was unknown.
Future Directions
The discovery of autoantibodies to PLA2R, and more recently to THSD7A, has added a new dimension to the monitoring and treatment of MN as it has opened a window onto the immunologic course of disease (Box 2). We anticipate that future research will investigate whether a high positive predictive value of anti-PLA2R seropositivity will obviate the need for kidney biopsy in such patients, assist in the selection of appropriate, immunologically-active patients for therapeutic research studies, and ultimately help tailor the type and duration of therapy on an individualized basis. Understanding the repertoire of epitopes targeted in an individual may help predict disease severity, and may someday lead to the development of small inhibitory peptides or even to induction of tolerance to the autoantigen. Although patients with primary MN for which no association with PLA2R or THSD7A has been detected appear to behave similarly to their counterparts in terms of baseline characteristics, prognosis, and response to treatment, we anticipate that future research will identify the responsible antigen-autoantibody pairs in these individuals as well.
Box 2. Future Directions.
Assess the diagnostic utility of PLA2R and THSD7A immunoassays vs kidney biopsy
Select immunologically active individuals for therapeutic trials
Determine if the titer and repertoire of PLA2R or THSD7A epitopes in individuals has prognostic implications
Investigate if immunodominant epitopes of PLA2R or THSD7A can be used as molecular decoys or tolerogens
Define the target antigens in the 15–20% cases that are currently considered idiopathic
Prospectively validate the utility of serological monitoring before and after transplantation for recurrence of MN
Acknowledgments
Support: This work was supported by research grants R01 DK097053 (to Dr Beck), R01 DK090029 (to Dr Salant), and NRSA T32 DK 007053 from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Financial Disclosure: Drs Beck and Salant are co-inventors on a patent entitled “Diagnostics for Membranous Nephropathy”. Dr Salant has received grant support from Alnylam, Alexion, and Pfizer for studies of experimental nephritis and has served as a consultant for Chugai, Actelion, and Astellas. Dr Francis declares that he has no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Couser WG, Steinmuller DR, Stilmant MM, Salant DJ, Lowenstein LM. Experimental glomerulonephritis in the isolated perfused rat kidney. J Clin Invest. 1978;62(2):1275–1287. doi: 10.1172/JCI109248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Damme BJ, Fleuren GJ, Bakker WW, Vernier RL, Hoedemaeker PJ. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. V. Fixed glomerular antigens in the pathogenesis of heterologous immune complex glomerulonephritis. Lab Invest. 1978;38(4):502–510. [PubMed] [Google Scholar]
- 3.Couser WG, Salant DJ. In situ immune complex formation and glomerular injury. Kidney Int. 1980;17(1):1–13. doi: 10.1038/ki.1980.1. [DOI] [PubMed] [Google Scholar]
- 4.Makker SP, Singh AK. Characterization of the antigen (gp600) of Heymann nephritis. Lab Invest. 1984;50(3):287–293. [PubMed] [Google Scholar]
- 5.Raychowdhury R, Nile JL, McCluskey RT, Smith JA. Autoimmune target in Heymann nephritis is a glycoprotein with homology to the LDL receptor. Science. 1989;244(4909):1163–1165. doi: 10.1126/science.2786251. [DOI] [PubMed] [Google Scholar]
- 6.Kerjaschki D, Farquhar MG. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proceedings of the National Academy of Sciences of the United States of America. 1982;79(18):5557–5581. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerjaschki D, Farquhar MG. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerjaschki D, Miettinen A, Farquhar MG. Initial events in the formation of immune deposits in passive Heymann nephritis. J Exp Med. 1987;166(1):109–128. doi: 10.1084/jem.166.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debiec H, Guigonis V, Mougenot B, et al. Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. The New England journal of medicine. 2002 Jun 27;346(26):2053–2060. doi: 10.1056/NEJMoa012895. [DOI] [PubMed] [Google Scholar]
- 10.Ayalon R, Beck LH., Jr Membranous nephropathy: not just a disease for adults. Pediatric nephrology. 2015 Jan;30(1):31–39. doi: 10.1007/s00467-013-2717-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck LH, Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009 Jul 2;361(1):11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med. 2011 Feb 17;364(7):689–690. doi: 10.1056/NEJMc1011678. [DOI] [PubMed] [Google Scholar]
- 13.Hoxha E, Kneissler U, Stege G, et al. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney international. 2012 Oct;82(7):797–804. doi: 10.1038/ki.2012.209. [DOI] [PubMed] [Google Scholar]
- 14.Tomas NM, Beck LH, Jr, Meyer-Schwesinger C, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014 Dec 11;371(24):2277–2287. doi: 10.1056/NEJMoa1409354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godel M, Grahammer F, Huber TB. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. The New England journal of medicine. 2015 Mar 12;372(11):1073. doi: 10.1056/NEJMc1500130. [DOI] [PubMed] [Google Scholar]
- 16.Iwakura T, Ohashi N, Kato A, Baba S, Yasuda H. Prevalence of Enhanced Granular Expression of Thrombospondin Type-1 Domain-Containing 7A in the Glomeruli of Japanese Patients with Idiopathic Membranous Nephropathy. PLoS One. 2015;10(9):e0138841. doi: 10.1371/journal.pone.0138841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofstra JM, Fervenza FC, Wetzels JF. Treatment of idiopathic membranous nephropathy. Nat Rev Nephrol. 2013 Aug;9(8):443–458. doi: 10.1038/nrneph.2013.125. [DOI] [PubMed] [Google Scholar]
- 18.Ponticelli C, Glassock RJ. Glomerular diseases: membranous nephropathy--a modern view. Clinical journal of the American Society of Nephrology: CJASN. 2014 Mar;9(3):609–616. doi: 10.2215/CJN.04160413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salant DJ, Cattran DC. Membranous Nephropathy. In: Johnson RJ, Feehally J, Floege J, editors. Comprehensive Clinical Nephrology. 5. Philadelphia: Elsevier; 2014. p. 239. [Google Scholar]
- 20.Hofstra JM, Beck LH, Jr, Beck DM, Wetzels JF, Salant DJ. Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011 Jun;6(6):1286–1291. doi: 10.2215/CJN.07210810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoxha E, Harendza S, Pinnschmidt HO, et al. Spontaneous remission of proteinuria is a frequent event in phospholipase A2 receptor antibody-negative patients with membranous nephropathy. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2015 Nov;30(11):1862–1869. doi: 10.1093/ndt/gfv228. [DOI] [PubMed] [Google Scholar]
- 22.Akiyama S, Akiyama M, Imai E, Ozaki T, Matsuo S, Maruyama S. Prevalence of anti-phospholipase A2 receptor antibodies in Japanese patients with membranous nephropathy. Clinical and experimental nephrology. 2015 Aug;19(4):653–660. doi: 10.1007/s10157-014-1054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlumberger W, Hornig N, Lange S, et al. Differential diagnosis of membranous nephropathy with autoantibodies to phospholipase A2 receptor 1. Autoimmun Rev. 2014 Feb;13(2):108–113. doi: 10.1016/j.autrev.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Dahnrich C, Komorowski L, Probst C, et al. Development of a standardized ELISA for the determination of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Clin Chim Acta. 2013 Jun 5;421:213–218. doi: 10.1016/j.cca.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Kanigicherla D, Gummadova J, McKenzie EA, et al. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 2013;83(5):940–948. doi: 10.1038/ki.2012.486. [DOI] [PubMed] [Google Scholar]
- 26.Behnert A, Schiffer M, Muller-Deile J, Beck LH, Jr, Mahler M, Fritzler MJ. Antiphospholipase A(2) receptor autoantibodies: a comparison of three different immunoassays for the diagnosis of idiopathic membranous nephropathy. J Immunol Res. 2014;2014:143274. doi: 10.1155/2014/143274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ronco P, Debiec H. Pathogenesis of membranous nephropathy: recent advances and future challenges. Nat Rev Nephrol. 2012 Apr;8(4):203–213. doi: 10.1038/nrneph.2012.35. [DOI] [PubMed] [Google Scholar]
- 28.Huang X, Qin W, Zhang M, Zheng C, Zeng C, Liu Z. Detection of anti-PLA2R autoantibodies and IgG subclasses in post-allogeneic hematopoietic stem cell transplantation membranous nephropathy. The American journal of the medical sciences. 2013 Jul;346(1):32–37. doi: 10.1097/MAJ.0b013e318267b5cd. [DOI] [PubMed] [Google Scholar]
- 29.Qin W, Beck LH, Jr, Zeng C, et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol. 2011 Jun;22(6):1137–1143. doi: 10.1681/ASN.2010090967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dauvergne M, Moktefi A, Rabant M, et al. Membranous Nephropathy Associated With Immunological Disorder-Related Liver Disease: A Retrospective Study of 10 Cases. Medicine (Baltimore) 2015 Jul;94(30):e1243. doi: 10.1097/MD.0000000000001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Q, Li Y, Xue J, et al. Renal Phospholipase A2 Receptor in Hepatitis B Virus-Associated Membranous Nephropathy. Am J Nephrol. 2015;41(4–5):345–353. doi: 10.1159/000431331. [DOI] [PubMed] [Google Scholar]
- 32.Hoxha E, Harendza S, Zahner G, et al. An immunofluorescence test for phospholipase-A(2)-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2011 Aug;26(8):2526–2532. doi: 10.1093/ndt/gfr247. [DOI] [PubMed] [Google Scholar]
- 33.Svobodova B, Honsova E, Ronco P, Tesar V, Debiec H. Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2013 Jul;28(7):1839–1844. doi: 10.1093/ndt/gfs439. [DOI] [PubMed] [Google Scholar]
- 34.Hofstra JM, Wetzels JF. Phospholipase A2 receptor antibodies in membranous nephropathy: unresolved issues. J Am Soc Nephrol. 2014 Jun;25(6):1137–1139. doi: 10.1681/ASN.2014010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsen CP, Messias NC, Silva FG, Messias E, Walker PD. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2013 May;26(5):709–715. doi: 10.1038/modpathol.2012.207. [DOI] [PubMed] [Google Scholar]
- 36.Fresquet M, Jowitt TA, Gummadova J, et al. Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J Am Soc Nephrol. 2015 Feb;26(2):302–313. doi: 10.1681/ASN.2014050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van de Logt AE, Hofstra JM, Wetzels JF. Serum anti-PLA2R antibodies can be initially absent in idiopathic membranous nephropathy: seroconversion after prolonged follow-up. Kidney international. 2015 Jun;87(6):1263–1264. doi: 10.1038/ki.2015.34. [DOI] [PubMed] [Google Scholar]
- 38.Ramachandran R, Kumar V, Nada R, Jha V. Serial monitoring of anti-PLA2R in initial PLA2R-negative patients with primary membranous nephropathy. Kidney international. 2015 Nov;88(5):1198–1199. doi: 10.1038/ki.2015.310. [DOI] [PubMed] [Google Scholar]
- 39.Timmermans SA, Ayalon R, van Paassen P, et al. Anti-phospholipase A2 receptor antibodies and malignancy in membranous nephropathy. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2013 Dec;62(6):1223–1225. doi: 10.1053/j.ajkd.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 40.Oh YJ, Yang SH, Kim DK, Kang SW, Kim YS. Autoantibodies against phospholipase A2 receptor in Korean patients with membranous nephropathy. PLoS One. 2013;8(4):e62151. doi: 10.1371/journal.pone.0062151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beck LH, Jr, Fervenza FC, Beck DM, et al. Rituximab-Induced Depletion of Anti-PLA2R Autoantibodies Predicts Response in Membranous Nephropathy. J Am Soc Nephrol. 2011 Aug;22(8):1543–1550. doi: 10.1681/ASN.2010111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hofstra JM, Debiec H, Short CD, et al. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012 Oct;23(10):1735–1743. doi: 10.1681/ASN.2012030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoxha E, Thiele I, Zahner G, Panzer U, Harendza S, Stahl RA. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol. 2014 Jun;25(6):1357–1366. doi: 10.1681/ASN.2013040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruggenenti P, Debiec H, Ruggiero B, et al. Anti-Phospholipase A2 Receptor Antibody Titer Predicts Post-Rituximab Outcome of Membranous Nephropathy. J Am Soc Nephrol. 2015 Oct;26(10):2545–2558. doi: 10.1681/ASN.2014070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beck LH, Fervenza FC, Beck DM, Bonegio RGB, Erickson SB, Salant DJ. Association of anti-PLA2R antibody level with clinical response to rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol. 2009;20:3A–4A. [Google Scholar]
- 46.Bech AP, Hofstra JM, Brenchley PE, Wetzels JF. Association of anti-PLA(2)R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clinical journal of the American Society of Nephrology: CJASN. 2014 Aug 7;9(8):1386–1392. doi: 10.2215/CJN.10471013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seitz-Polski B, Dolla G, Payre C, et al. Cross-reactivity of anti-PLA2R1 autoantibodies to rabbit and mouse PLA2R1 antigens and development of two novel ELISAs with different diagnostic performances in idiopathic membranous nephropathy. Biochimie. 2015 Nov;118:104–115. doi: 10.1016/j.biochi.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Hoxha E, Harendza S, Pinnschmidt H, Panzer U, Stahl RA. PLA2R antibody levels and clinical outcome in patients with membranous nephropathy and non-nephrotic range proteinuria under treatment with inhibitors of the renin-angiotensin system. PLoS One. 2014;9(10):e110681. doi: 10.1371/journal.pone.0110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoxha E, Harendza S, Pinnschmidt H, Panzer U, Stahl RA. M-type phospholipase A2 receptor autoantibodies and renal function in patients with primary membranous nephropathy. Clin J Am Soc Nephrol. 2014 Nov 7;9(11):1883–1890. doi: 10.2215/CJN.03850414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klouda PT, Manos J, Acheson EJ, et al. Strong association between idiopathic membranous nephropathy and HLA-DRW3. Lancet. 1979 Oct 13;2(8146):770–771. doi: 10.1016/s0140-6736(79)92118-4. [DOI] [PubMed] [Google Scholar]
- 51.Le Petit JC, Laurent B, Berthoux FC. HLA-DR3 and idiopathic membranous nephritis (IMN) association. Tissue Antigens. 1982 Sep;20(3):227–228. doi: 10.1111/j.1399-0039.1982.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 52.Vaughan RW, Demaine AG, Welsh KI. A DQA1 allele is strongly associated with idiopathic membranous nephropathy. Tissue Antigens. 1989 Nov;34(5):261–269. doi: 10.1111/j.1399-0039.1989.tb01741.x. [DOI] [PubMed] [Google Scholar]
- 53.Ogahara S, Naito S, Abe K, Michinaga I, Arakawa K. Analysis of HLA class II genes in Japanese patients with idiopathic membranous glomerulonephritis. Kidney international. 1992 Jan;41(1):175–182. doi: 10.1038/ki.1992.24. [DOI] [PubMed] [Google Scholar]
- 54.Chevrier D, Giral M, Perrichot R, et al. Idiopathic and secondary membranous nephropathy and polymorphism at TAP1 and HLA-DMA loci. Tissue Antigens. 1997 Aug;50(2):164–169. doi: 10.1111/j.1399-0039.1997.tb02855.x. [DOI] [PubMed] [Google Scholar]
- 55.Kim S, Chin HJ, Na KY, et al. Single nucleotide polymorphisms in the phospholipase A2 receptor gene are associated with genetic susceptibility to idiopathic membranous nephropathy. Nephron Clin Pract. 2011;117(3):c253–258. doi: 10.1159/000320194. [DOI] [PubMed] [Google Scholar]
- 56.Liu YH, Chen CH, Chen SY, et al. Association of phospholipase A2 receptor 1 polymorphisms with idiopathic membranous nephropathy in Chinese patients in Taiwan. J Biomed Sci. 2010;17:81. doi: 10.1186/1423-0127-17-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stanescu HC, Arcos-Burgos M, Medlar A, et al. Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med. 2011 Feb 17;364(7):616–626. doi: 10.1056/NEJMoa1009742. [DOI] [PubMed] [Google Scholar]
- 58.Coenen MJ, Hofstra JM, Debiec H, et al. Phospholipase A2 receptor (PLA2R1) sequence variants in idiopathic membranous nephropathy. J Am Soc Nephrol. 2013 Mar;24(4):677–683. doi: 10.1681/ASN.2012070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lv J, Hou W, Zhou X, et al. Interaction between PLA2R1 and HLA-DQA1 variants associates with anti-PLA2R antibodies and membranous nephropathy. J Am Soc Nephrol. 2013 Jul;24(8):1323–1329. doi: 10.1681/ASN.2012080771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larsen CP, Beggs ML, Walker PD, Saeed M, Ambruzs JM, Messias NC. Histopathologic effect of APOL1 risk alleles in PLA2R-associated membranous glomerulopathy. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2014 Jul;64(1):161–163. doi: 10.1053/j.ajkd.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 61.Salant DJ. Genetic variants in membranous nephropathy: perhaps a perfect storm rather than a straightforward conformeropathy? J Am Soc Nephrol. 2013 Mar;24(4):525–528. doi: 10.1681/ASN.2013020166. [DOI] [PubMed] [Google Scholar]
- 62.Llorca O. Extended and bent conformations of the mannose receptor family. Cellular and molecular life sciences: CMLS. 2008 May;65(9):1302–1310. doi: 10.1007/s00018-007-7497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kao L, Lam V, Waldman M, Glassock RJ, Zhu Q. Identification of the immunodominant epitope region in phospholipase A2 receptor-mediating autoantibody binding in idiopathic membranous nephropathy. J Am Soc Nephrol. 2015 Feb;26(2):291–301. doi: 10.1681/ASN.2013121315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beck LH, Sandor DG, Ma H, Salant DJ. Identification of distinct dominant and subdominant humoral epitopes within PLA2R1 in primary membranous nephropathy. J Am Soc Nephrol. 2014;25:17A. [Google Scholar]
- 65.Seitz-Polski B, Dolla G, Payré C, et al. Epitope spreading in PLA2R1 is associated with bad prognosis in membranous nephropathy. J Am Soc Nephrol. 2016;27(5) doi: 10.1681/ASN.2014111061. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cosyns JP, Couchoud C, Pouteil-Noble C, Squifflet JP, Pirson Y. Recurrence of membranous nephropathy after renal transplantation: probability, outcome and risk factors. Clinical nephrology. 1998 Sep;50(3):144–153. [PubMed] [Google Scholar]
- 67.Josephson MA, Spargo B, Hollandsworth D, Thistlethwaite JR. The recurrence of recurrent membranous glomerulopathy in a renal transplant recipient: case report and literature review. American journal of kidney diseases: the official journal of the National Kidney Foundation. 1994 Nov;24(5):873–878. doi: 10.1016/s0272-6386(12)80685-8. [DOI] [PubMed] [Google Scholar]
- 68.Floege J. Recurrent glomerulonephritis following renal transplantation: an update. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2003 Jul;18(7):1260–1265. doi: 10.1093/ndt/gfg102. [DOI] [PubMed] [Google Scholar]
- 69.Moroni G, Gallelli B, Quaglini S, et al. Long-term outcome of renal transplantation in patients with idiopathic membranous glomerulonephritis (MN) Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2010 Oct;25(10):3408–3415. doi: 10.1093/ndt/gfq223. [DOI] [PubMed] [Google Scholar]
- 70.Dabade TS, Grande JP, Norby SM, Fervenza FC, Cosio FG. Recurrent idiopathic membranous nephropathy after kidney transplantation: a surveillance biopsy study. Am J Transplant. 2008 Jun;8(6):1318–1322. doi: 10.1111/j.1600-6143.2008.02237.x. [DOI] [PubMed] [Google Scholar]
- 71.El-Zoghby ZM, Grande JP, Fraile MG, Norby SM, Fervenza FC, Cosio FG. Recurrent idiopathic membranous nephropathy: early diagnosis by protocol biopsies and treatment with anti-CD20 monoclonal antibodies. Am J Transplant. 2009 Dec;9(12):2800–2807. doi: 10.1111/j.1600-6143.2009.02851.x. [DOI] [PubMed] [Google Scholar]
- 72.Floege J, Regele H, Gesualdo L Group E-EIW. The ERA-EDTA Database on Recurrent Glomerulonephritis following renal transplantation. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2014 Jan;29(1):15–21. doi: 10.1093/ndt/gft299. [DOI] [PubMed] [Google Scholar]
- 73.Stahl R, Hoxha E, Fechner K. PLA2R autoantibodies and recurrent membranous nephropathy after transplantation. N Engl J Med. 2010 Jul 29;363(5):496–498. doi: 10.1056/NEJMc1003066. [DOI] [PubMed] [Google Scholar]
- 74.Debiec H, Martin L, Jouanneau C, et al. Autoantibodies specific for the phospholipase A2 receptor in recurrent and De Novo membranous nephropathy. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011 Oct;11(10):2144–2152. doi: 10.1111/j.1600-6143.2011.03643.x. [DOI] [PubMed] [Google Scholar]
- 75.Blosser CD, Ayalon R, Nair R, Thomas C, Beck LH., Jr Very early recurrence of anti-Phospholipase A2 receptor-positive membranous nephropathy after transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012 Jun;12(6):1637–1642. doi: 10.1111/j.1600-6143.2011.03957.x. [DOI] [PubMed] [Google Scholar]
- 76.Kattah A, Ayalon R, Beck LH, Jr, et al. Anti-phospholipase A(2) receptor antibodies in recurrent membranous nephropathy. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015 May;15(5):1349–1359. doi: 10.1111/ajt.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quintana LF, Blasco M, Seras M, et al. Antiphospholipase A2 Receptor Antibody Levels Predict the Risk of Posttransplantation Recurrence of Membranous Nephropathy. Transplantation. 2015 Aug;99(8):1709–1714. doi: 10.1097/TP.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 78.Seitz-Polski B, Payre C, Ambrosetti D, et al. Prediction of membranous nephropathy recurrence after transplantation by monitoring of anti-PLA2R1 (M-type phospholipase A2 receptor) autoantibodies: a case series of 15 patients. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2014 Dec;29(12):2334–2342. doi: 10.1093/ndt/gfu252. [DOI] [PubMed] [Google Scholar]
- 79.Andresdottir MB, Wetzels JF. Increased risk of recurrence of membranous nephropathy after related donor kidney transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012 Jan;12(1):265–266. doi: 10.1111/j.1600-6143.2011.03818.x. [DOI] [PubMed] [Google Scholar]
- 80.El Kossi M, Harmer A, Goodwin J, et al. De novo membranous nephropathy associated with donor-specific alloantibody. Clin Transplant. 2008 Jan-Feb;22(1):124–127. doi: 10.1111/j.1399-0012.2007.00741.x. [DOI] [PubMed] [Google Scholar]
- 81.Honda K, Horita S, Toki D, et al. De novo membranous nephropathy and antibody-mediated rejection in transplanted kidney. Clin Transplant. 2011 Mar-Apr;25(2):191–200. doi: 10.1111/j.1399-0012.2010.01213.x. [DOI] [PubMed] [Google Scholar]
- 82.Lim BJ, Kim MS, Kim YS, Kim SI, Jeong HJ. C4d deposition and multilayering of peritubular capillary basement membrane in posttransplantation membranous nephropathy indicate its association with antibody-mediated injury. Transplant Proc. 2012 Apr;44(3):619–620. doi: 10.1016/j.transproceed.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 83.Ponticelli C, Glassock RJ. De novo membranous nephropathy (MN) in kidney allografts. A peculiar form of alloimmune disease? Transpl Int. 2012 Dec;25(12):1205–1210. doi: 10.1111/j.1432-2277.2012.01548.x. [DOI] [PubMed] [Google Scholar]
- 84.Larsen CP, Walker PD. Phospholipase A2 receptor (PLA2R) staining is useful in the determination of de novo versus recurrent membranous glomerulopathy. Transplantation. 2013 May 27;95(10):1259–1262. doi: 10.1097/TP.0b013e31828a947b. [DOI] [PubMed] [Google Scholar]