Abstract
Purpose
A hospital-based epidemiology study to describe herpes zoster ophthalmicus (HZO) prevalence, and identify risk factors for recurrent and chronic disease.
Design
Retrospective, hospital-based cohort study
Participants
All patients evaluated in the Broward and Miami VA Healthcare System (MIAVHS) during the study period.
Methods
Retrospective medical record review of patients seen in the MIAVHS from January 1, 2010 and December 31, 2014 with a HZO clinical diagnosis. Assessment of the patient's clinical course was defined by the following: an acute episode of HZO was defined as quiescence of disease within 90 days of initial presentation; HZO recurrence was defined as any recurrent eye disease or rash >90 days after quiescence disease was noted off therapy; chronic HZO was defined as active disease persisting greater than 90 days from initial presentation.
Main Outcome Measures
1) Frequency of HZ involving the V1 dermatome (HZO) with and without eye involvement; 2) HZO recurrence rates 3) Risk factors for recurrent or chronic HZO.
Results
90 patients with HZO were included in the study. The mean age at incident episode of HZO was 68±13.8 years (range, 27-95 years). The majority of patients were white (73%), immune competent (79%), and did not receive zoster vaccination at any time point in their follow up (82%). Patients were followed for a mean of 3.9±5.9 years, (range, 0-33 years). The period prevalence of HZ in any dermatome was 1.1%, the frequency of HZ involving V1 (HZO) was 0.07% and the frequency of HZO with eye involvement was 0.05%. The overall 1, 3, and 5-year recurrence rates for either recurrent eye disease or rash were 8%, 17%, 25%, respectively. Ocular hypertension (HR 4.6, 95% Cl 1.3-16.5; OR 6.7, 95% Cl 1.5-31.2) and uveitis (HR 5.7, 95%CI 1.7-19.0; OR 6.7, 95% C1 1.5-31.2) increased the risk of recurrent and chronic disease.
Conclusion
This study supports newer data that a significant proportion of patients experience recurrent and chronic HZO. Further study is needed to guide preventative and therapeutic approaches to recurrent and chronic HZO.
Keywords: varicella zoster virus, herpes zoster ophthalmicus, ocular complications, recurrence, chronicity, frequency
Introduction
Herpes zoster (HZ) is defined as the emergence from latency of the varicella zoster virus (VZV). As HZ is not a reportable condition in the United States, its incidence is inferred. When age-adjusted to the 2000 U.S. population, the CDC estimates that there are 1 million cases of zoster annually and that 32% of persons in the United States will experience zoster during their lifetime.1 Observed rates have varied across individual studies ranging from 3.2-4.2 per 1000 population per year.2-7 Immunosuppression and the immunosenescence of aging have been associated with an increased risk of developing HZ.8-10 The most common long term complication of HZ is postherpetic neuralgia (PHN), or persistent neuropathic pain lasting beyond three months after initial presentation of HZ. PHN can negatively affect quality of life to a degree similar to congestive heart failure, depression, acute myocardial infarction, diabetes. PHN is a leading cause of suicide in patients over 70 with chronic pain.11,12
Herpes zoster ophthalmicus (HZO) is defined as zoster within the ophthalmic division of the fifth cranial nerve (V1).12 HZO accounts for 10-20% of HZ cases12, and can be further categorized as HZO with or without eye involvement. The most common presenting ophthalmic manifestations in HZO are keratitis, uveitis and conjunctivitis. Other presentations include episcleritis and scleritis, acute retinal necrosis, cranial nerve involvement and/or meningoencephalitis.13 Long-term structural complications including glaucoma, cataract, corneal scarring, and PHN can have devastating outcomes on visual function and/or quality of life. In the pre-antiviral era, approximately 50% of patients with HZO developed ocular involvement.14 With antiviral therapy, lower frequencies of eye involvement have been reported, ranging from 2% (4 of 202)15 to 29% (25 of 85).16
What is less understood is the course of HZ after its initial presentation. Traditionally studied and treated in the acute phase,17-19 recent data suggest that some patients experience a chronic or recurrent disease course. In a population based study of individuals living in Olmsted County, Minnesota from 1996-2001, a total of 1669 cases of HZ were identified, with an incidence of 3.6 per 1000 person-years.2 To evaluate for recurrence, these patients were then followed through 2007, and 105 recurrences of HZ, defined as a characteristic vesicular rash accompanied by pain or dysesthesia in a dermatomal pattern, ≥3 months from the index episode, were identified. The Kaplan-Meier estimated recurrence rate of HZ at 8 years was 6% overall. Interestingly, 86% (90 of 105) of HZ recurrences were immunocompetent individuals,20 and in 45% of the recurrences, the site of the recurrence was in a different region of the body than the site of the index episode.20 Recurrences were more likely in persons with zoster-associated pain of 30 days or longer, immunocompromised status, female gender, and older age (≥50 years) on initial presentation.20 Data on ocular manifestations of HZO is likewise available for an Olmsted County cohort. In a medical record review from 1980-2007, 2035 patients with HZ in any dermatome were identified and of these individuals, 9% (12 of 84) had HZO with eye involvement.13 Of these 184 patients, 6.5% were immunocompromised at time of eye involvement and 6.6% had permanent vision decrement at 8 year follow-up. Recurrent eye disease >3 months from initial presentation of HZO was observed in the form of keratitis (6.9%) and iritis (7.4%) at the 8 year follow-up.13 In more recent studies of HZO, even higher frequencies of recurrent eye disease have been suggested. In a retrospective review of 45 patients with HZO and eye involvement, recurrent disease was observed in 51% of patients over a mean follow-up of 24.9 ± 18.2 months (range, 12-72 months), in the form of stromal (n=9) and epithelial keratitis (n=3), keratouveitis (n=4), and anterior uveitis (n=6).21 In a survey of 100 ophthalmologists, 87% reported treating recurrent or chronic cases of ocular disease in the setting of HZO in the previous year.22
Based on this data, it is clear that more information is needed on the long term clinical course of HZO. The purpose of this study was to characterize the epidemiology of recurrent and chronic HZO in a unique South Florida population, with an ethnically and racially mixed, predominately male population.
Methods
Study methodology
This retrospective review of medical records examined all patients in the South Florida VA Healthcare system (MIAVHS) seen between January 1, 2010 to December 31, 2014 with a clinically documented HZO diagnosis, defined as characteristic vesicular rash and dermatomal pain in the V1 dermatome . Miami VA Institution Review Board approval was obtained to allow the retrospective evaluation of charts. The study was conducted in accordance to the principles of the Declaration of Helsinki and complied with the requirements of the United States Health Insurance Portability and Accountability Act.
Setting
The MIAVHS provides an excellent hospital-based study population due to its age, racial, and ethnically diverse constituency. The electronic medical record system in place incorporates all notes from providers including details of every visit to clinic, emergency department, hospitalizations and all correspondence concerning each patient, providing an accurate and comprehensive view into a patient's disease course.
Subjects for potential inclusion
Between January 1, 2010 and December 31, 2014, 119,569 patients total were evaluated within the MIAVHS. 1,358 of these patients had ICD-9 codes 53.0 – 53.9 designating HZ infection anywhere in the body. 124 had ICD-9 codes 53.20 – 53.29 designating HZO, defined as HZ involvement of the V1 dermatome. Of that group, 90 patients had a clinically documented episode of a vesicular rash and dermatomal pain involving V1 in the medical record. 62 of these patients had documented HZO with eye involvement diagnosed by a treating ophthalmologist based on characteristic ophthalmic findings in patients with typical dermatomal pain and vesicular rash.
Data collected
The information collected from each HZO ophthalmic related visit included: recorded diagnoses, procedures, medications, and where possible, the extent of HZO ophthalmic involvement (e.g. episcleritis/conjunctivitis, epithelial, stromal, endothelial disease, uveitis), pain (e.g. PHN), and ophthalmic complications (e.g. ocular hypertension, corneal scar, cataract). Patients were split into two groups based on whether signs of ocular involvement were present or absent within 30 days of initial presentation of V1 rash (HZO with versus without eye involvement).
Demographic data included age, gender, and race. The patient's immune status at the time of initial presentation of HZ was assessed and patients were deemed immunocompromised if they had human immunodeficiency virus (HIV), actively treated malignancies (systemic chemotherapy), hematological malignancies, end stage renal disease, and/or were on immunosuppressive drug therapy. Corticosteroid medications were considered immunosuppressive if they were given at levels equivalent to 10 mg or greater of prednisone daily. If none of the conditions were documented at the time of or during the year prior to the HZ eye diagnosis, the patient was assumed to be immunocompetent.
Clinical course
Assessment of the patient's clinical course was defined by the following: an acute episode of HZO was defined as quiescence of disease within 90 days of initial presentation and complete termination of all antiviral and antiinflammatory treatment. Chronic HZO was defined as active disease requiring antiviral and/or anti-inflammatory therapy for greater than 90 days from initial presentation. HZO recurrence was defined as any eye disease and/or recurrent rash in any dermatome.
Statistical analysis
A period prevalence was calculated by dividing (1) the number of patients with HZ involving any dermatome; (2) the number of patients with HZ involving the V1 dermatome (HZO); and (3) those with HZO and ocular complications, by the total number of patients seen over the study period. Frequencies were calculated for the various manifestations of HZO and compared using Chi-squared tests; means were compared with two-sample t-tests. Kaplan-Meier survival analysis was used to characterize the recurrence rate of disease over time. At risk patients included those with HZ rash without initial eye involvement, those with initial eye involvement which acutely resolved, and those with chronic eye involvement that were eventually able to wean off medication for at least 90 days with quiescence. Time zero for the survival analysis was the time point at which disease was quiescent off all medications. Potential risk factors for recurrent and chronic disease (demographics, immune status, vaccination status, ophthalmic involvement, PHN i.e. persistent pain lasting beyond three months after HZ onset, and prior history of recurrences) were evaluated with Cox proportional hazards ratio and logistic regression methodology, respectively.
Results
Study population
General characteristics of the study population are summarized in Table 1. A total of 90 patients (87 male and 3 female) were included in the study. The mean age at initial presentation of HZO was 68 ± 13.8 years (range, 27 – 95 years). The majority of patients were white (73%), immune competent (79%), and did not receive zoster vaccination at any time point in their follow up (82%). Patients were followed for a mean of 3.9 ± 5.9 years, (range, 0-33 years).
Table 1. Demographic and clinical characteristics of study population.
| Demographic | n (%) |
|---|---|
| Gender | |
| Male | 87 (96.7) |
| Female | 3 (3.3) |
| Age of Presentation | |
| Mean | 68+/-13.8 |
| Range | 27-95 |
| <65 | 35 (38.9) |
| >65 | 55(61.1) |
| Race/Ethnicity | |
| White | 66 (73.3) |
| Black | 13(14.4) |
| Hispanic | 11 (13.3) |
| Asian | 1 (1.1) |
| Other | 0(0) |
| Immune Status | |
| Competent | 71 (78.9) |
| Compromised | 19(21.1) |
| Vaccination Status | |
| Vaccinated* | 16(17.8) |
| Not Vaccinated | 74 (82.2) |
| Duration of follow-up | |
| Mean (years) | 3.9 +/- 5.9 |
| Range (years) | 0-33 |
At any time point throughout follow-up
Frequency of HZO in our population
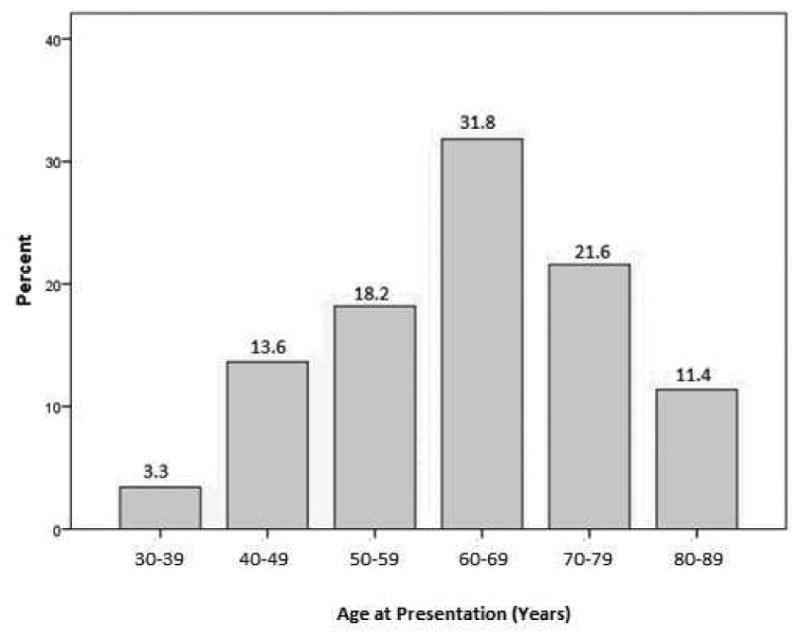
In the MIAVHS population, the period prevalence of HZ in any dermatome was 1.1%, the frequency of HZ involving V1 (HZO) was 0.07% and the frequency of HZO with eye involvement was 0.05%. Considering all patients with V1 dermatomal zoster, the disease most frequently presented in individuals between the ages of 60 to 69, followed by in those between 70 and 79, followed by in those between 50 and 59 (32%, 22%, 18% respectively). Thirty-five percent of patients were under the age of 60 when they presented with their V1 dermatomal rash (Figure 1). The demographic characteristics of those with HZO, including both those with and without ocular disease (n=90), were different than the general MIAVHS population. Specifically, HZO patients were more frequently older (>65 years), white, and male, compared to their counterparts without HZO seen during the same time period (61% vs 49%, p=0.05, 86% vs 75%, p=0.05, and 97% vs 90%, p=0.05), respectively.
Figure 1. Age distribution of patients at first diagnosis of Herpes Zoster Ophthalmicus.
Presenting findings
Of the patients with HZO, 31% (28 of 90) had rash only, and 70% (62 of 90) also had eye involvement within the first month of initial presentation. The eye findings on first occurrence of HZO were, in order of frequency, conjunctivitis/ episcleritis (53%, 33 of 62), epithelial keratitis (31%, 19 of 62), uveitis (32%, 20 of 62), stromal keratitis (14.5%, 9 of 62), and endotheliitis (6.5%, 4 of 62). Nine patients (all of whom had concomitant uveitis) presented with ocular hypertension at initial presentation.
Clinical course
Of the 28 patients with HZO without eye involvement at initial presentation, 5 had recurrent disease (2 rash in the same V1 dermatome, 1 with cranial nerve involvement, 1 with episcleritis, stromal keratitis, and uveitis, and 1 with meningoencephalitis). The mean time to first recurrence in these 5 patients was 6.5 years (SD 7.4, range 0.5-16 years). Of the 62 HZO patients with eye involvement at initial presentation, 48 (77%) had an acute course and 14 (23%) had a chronic course (disease that required either greater than 90 days to resolution, and/or never resolved). Of the 48 with an acute course, 6 (13%) had recurrent disease on follow-up (1 with epithelial keratitis and uveitis, 1 with epithelial keratitis, 2 with stromal keratitis, 1 with uveitis). The mean time to first recurrence in these 6 patients was 5.3 years (SD 3.8, range 1-10 years). In addition, 7 of 14 patients with an initially chronic course were eventually weaned off all medications, and after being off medications for at least 90 days, 5 patients had recurrent disease (1 rash, 2 epithelial keratitis, 1 with conjunctivitis, epithelial keratitis, endotheliitis, and uveitis, 1 uveitis, 1 stromal keratitis and endotheliitis). With time zero being the point at which disease was quiescent off all medications, the mean time to first recurrence in these 5 patients was 2.9 years (SD 4.0, range 0.83-10 years). The remaining 7 of 14 patients required chronic treatment.
Zoster vaccination status
Sixteen patients received the zoster vaccine; 14 after an episode of HZO and 2 prior to an episode. Of the 14 patients in the first group, 1 recurred with rash 8 months after vaccination. Of the 2 patients in the latter group, one presented with rash and eye involvement which recurred at 4 and 8 months after initial presentation while the other patient had no disease recurrence.
Recurrent disease
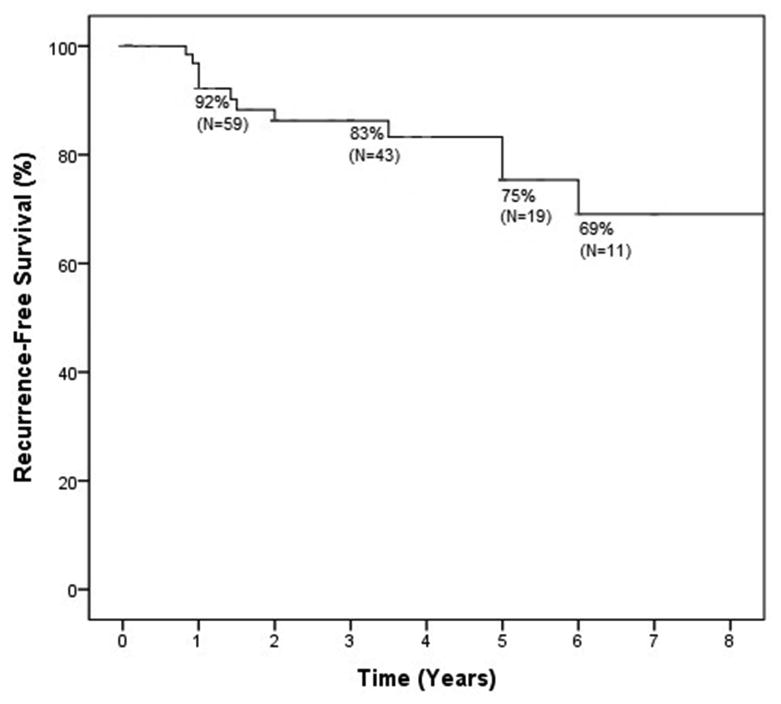
As such, 83 patients (28 without initial eye involvement, 48 with acute eye involvement, and 7 with chronic eye involvement weaned off medication at least 90 days) were included in the KM analysis with time zero being the point at which disease was quiescent off all medications. The overall 1, 3, and 5-year recurrence rates for either recurrent eye disease or rash were 8% (5 events total, n=59 remain at risk), 17% (8 events total, n=43 remain at risk), 25% (11 events total, n=19 remain at risk), respectively (Figure 2). Even after 5 years, the recurrence rate continued to increase with a 6-year recurrence rate of 31% (12 events total, n=11 remain at risk).
Figure 2. Kaplan-Meier survival analysis demonstrating cumulative recurrence-free survival over time.
Risk factors for recurrent disease
Several ocular findings on initial presentation increased the risk of recurrent disease. These risk factors included ocular hypertension (HR 4.6, 95% CI 1.3-16.5), uveitis (HR 5.7, 95% CI 1.7-19.0) and an initially chronic disease course (HR 6.3, 95% CI 2.1-19.1).
Risk factors for chronic disease
Several ocular findings on initial presentation similarly increased the risk of having a chronic disease course. These risk factors included ocular hypertension (OR 6.7, 95% Cl 1.5-31.2) and uveitis (OR 6.7, 95% CI 1.5-31.2). Forward step-wise multivariable analyses were performed to examine whether uveitis, ocular hypertension, or both remained significantly associated with recurrent and chronic disease. When adjusting for gender and age, only ocular hypertension remained a significant risk factor for recurrent (HR 4.57, 95% CI 1.26-16.50) and chronic (OR 6.74, 95% CI 1.46-31.16) disease. Immune status, gender, age, vaccination status, and PHN did not significantly increase the risk of recurrent or chronic disease.
Discussion
To summarize, we found that a significant proportion of patients with HZO had disease recurrences over time. Our data on recurrence is different from older published studies where recurrence frequencies were reported at 1.3% -14% (most between 4-5%) over varying follow-up durations.5,23-26 Our findings of significant recurrences in HZO is similar to that found in an Italian cohort of 45 patients with HZO followed over a mean of 24.9 ± 18.2 months (range, 12-72 months), which reported a recurrence frequency of 51%,21 with stromal keratitis and uveitis being the most common manifestation of recurrent disease, followed by epithelial keratitis.21 Similar to the Italian cohort, the clinical profile of recurrent disease in this cohort was different from that of the initial presentation, with uveitis, stromal keratitis, and epithelial keratitis more common on recurrence, and conjunctivitis/episcleritis more common on presentation. While direct comparisons of recurrence rates is difficult due to differing study methodologies, our study adds to the existing body of literature that HZO does not appear to be a monophasic illness. Rather it appears to have a recurrent or chronic course in some patients.
Considering the demographics of our population, we found that HZO was most frequent in individuals between the the ages of 60-69 years.2,9,27 This is in contrast to other studies that have found the disease most frequent in those 50-59 years old.20,21 The older demographics of the VA patient population28-30 may account for these differences. However it is important to note that in our population approximately 1/3 of patients presented with disease at an age younger than 60 years, and similar to previous study,20 there was no statistically significant difference in risk of recurrence found in younger versus older patients. Although HZ is more common and severe in immunocompromised people, 92% of people with HZ are not immunocompromised,2 as was seen in the majority of cases in our study. Regarding risk factors, we identified uveitis and ocular hypertension as risk factors for both recurrent and chronic disease. In addition, patients with active eye disease for > 90 days after initial presentation (chronic disease) were more likely to have subsequent disease recurrence after a period of quiescence. We were unable to replicate previously reported risk factors including immunosuppression, female gender, older age, and absence of zoster vaccination.8-10,13,31-34 The low numbers of females, immunosuppressed and vaccinated patients in our cohort may have limited our power to demonstrate differences in recurrence risk.
There is biologic plausibility that both chronic and recurrent disease can be caused by active viral replication and infection and/or by an inflammatory response to the virus. Studies suggest that clinical latency is not a true period of latency but an active period of subclinical viral transcription and translation held in check by an intact 7cell mediated immune response.35-37 In both animal models and human subjects, VZV proteins, subclinical viremia, and upregulation of cell-mediated immunity have been detected in those without clinical manifestations of disease.38-45 When immune surveillance is reduced, as in the case of acquired immune deficiency syndrome (AIDS), immunosuppressant therapy, and aging, this fine balance can be tipped in favor of viral replication, and clinically, disease recurrence in the setting of active viral replication is observed. Active virus (via PCR) has been detected in recurrent epithelial dendritifoim lesions, and these lesions have been reported to respond to antiviral therapy.46-48 Host keratectomy specimens have likewise demonstrated VZV DNA positivity which correlated to the clinical findings of uveitis and the histopathological features of chronic stromal keratitis.49,50 As such, recurrent disease may represent an active infection, an immune response, or both and thus both anti-viral and anti-inflammatory agents may be useful in the treatment of chronic and recurrent VZV.
Our study raises more questions on the best strategies to prevent and treat recurrent and chronic HZO. This is of importance as the incidence and prevalence of HZO seemed to be increasing.1 Reasons cited include an older population with inherent immune senescence, immunosuppression by pharmacotherapy, immunocompromising diseases such as AIDS, and universal varicella vaccination in the young leading to fewer “booster” exposures within the community to maintain cell mediated immunity. 12,25,51,52 Information regarding recommended antiviral treatment for acute HZO and its relationship to recurrent or chronic disease is lacking. As such, with increasing numbers of patients expected to present with disease, studying the role of suppressive antiviral treatment to reduce recurrent and chronic HZO is an important avenue of future study.
One important question is how zoster vaccination affects disease course and recurrence. The Shingles Prevention Study demonstrated the safety and efficacy of the zoster vaccine in decreasing both the incidence of HZO and risk of PHN.53 However, it has also been published that patients with a history of HZO may have recurrent ophthalmic, dermatologic, or disseminated disease after vaccination.54-58 Despite this possible risk, these events are not common, and a history of HZO is not a contraindication to zoster vaccination. 12,53,59,60 Another important question is whether a longer duration of anti-viral therapy will change the course of disease. Current practice is to treat individuals with a new episode of HZ with 7-14 days of antivirals.61-65 However, Miserocchi et al. found a decreased frequency of recurrent episodes from 3.4 episodes per year to 2.1 episodes per year in patients treated with prophylactic oral acyclovir or valacyclovir for at least one year.21 Unfortunately, neither one of these questions can be fully answered by our data as only 2 patients received the zoster vaccine prior to first episode of HZO and those on chronic suppressive or prophylactic therapy were few in number.
As with all studies our findings need to be considered bearing in mind our study limitations, which include the retrospective nature, limited number of patients, male predominance of our study population, and reliance on chart information. The predominantly male population is a limitation of the generalizability of these findings to a female population. Chronic suppressive antiviral therapy was not used in a frequent, systematic, nor consistent manner in our study population, thus we were unable to evaluate its effect on disease recurrence. This lack of consensus in the prolonged use of antiviral therapy is similar to the findings in a previous survey of HZO treatment practice patterns across the U.S, indicative of the confusion that plagues many practitioners treating recurrent and chronic disease.22 A strength of our study, however, was the long-term information provided by the electronic medical system that allowed a comprehensive means to evaluate long-term HZO disease course, and provide important and new information on recurrent and chronic HZO.
To summarize, this is the first study in the U.S. to document the frequency of recurrent ocular inflammation after initial resolution of clinically diagnosed herpes zoster ophthalmicus. Frequency data and knowledge of risk factors for recurrence will help in planning clinical trials to test new management strategies such as prolonged anti-viral treatment or post-HZO vaccination.
Acknowledgments
Funding: Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research and Development's Career Development Award CDA-2-024-10S (Dr. Galor), NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, Department of Defense (DOD- Grant#W81XWH-09-1-0675 and Grant# W81XWH-13-1-0048 ONOVA) (institutional), The Ronald and Alicia Lepke Grant, The Lee and Claire Hager Grant, The Jimmy and Gaye Bryan Grant, The Gordon Charitable Trust, and the Richard Azar Family Grant(institutional grants).
Footnotes
Propriety Interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harpaz R, Ortega-Sanchez IR, Seward JF, (CDC) AColPACfDCaP Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR RecoMm Rep. 2008;57(RR-5):1–30. quiz CE32-34. [PubMed] [Google Scholar]
- 2.Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341–1349. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 3.Insinga RP, Itzler RF, Pellissier JM. Acute/subacute herpes zoster: healthcare resource utilisation and costs in a group of US health plans. Pharmacoeconomics. 2007;25(2):155–169. doi: 10.2165/00019053-200725020-00007. [DOI] [PubMed] [Google Scholar]
- 4.Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992-2002. J Infect Dis. 2005;191(12):2002–2007. doi: 10.1086/430325. [DOI] [PubMed] [Google Scholar]
- 5.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155(15):1605–1609. [PubMed] [Google Scholar]
- 6.Richards P. Shingles in one family practice. Arch Fam Med. 1996;5(1):42–46. doi: 10.1001/archfami.5.1.42. [DOI] [PubMed] [Google Scholar]
- 7.Mullooly JP, Riedlinger K, Chun C, Weinmann S, Houston H. Incidence of herpes zoster, 1997-2002. Epidemiol Infect. 2005;133(2):245–253. doi: 10.1017/s095026880400281x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borkar DS, Tham VM, Esterberg E, et al. Incidence of herpes zoster ophthalmicus: results from the Pacific Ocular Inflammation Study. Ophthalmology. 2013;120(3):451–456. doi: 10.1016/j.ophtha.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghaznawi N, Virdi A, Dayan A, et al. Herpes zoster ophthalmicus: comparison of disease in patients 60 years and older versus younger than 60 years. Ophthalmology. 2011;118(11):2242–2250. doi: 10.1016/j.ophtha.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Edell AR, Cohen EJ. Herpes simplex and herpes zoster eye disease: presentation and management at a city hospital for the underserved in the United States. Eye Contact Lens. 2013;39(4):311–314. doi: 10.1097/ICL.0b013e31829a3b47. [DOI] [PubMed] [Google Scholar]
- 11.McElhaney JE. Herpes zoster: a common disease that can have a devastating impact on patients' quality of life. Expert Rev Vaccines. 2010;9(3 Suppl):27–30. doi: 10.1586/erv.10.31. [DOI] [PubMed] [Google Scholar]
- 12.Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115(2 Suppl):S3–12. doi: 10.1016/j.ophtha.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Yawn BP, Wollan PC, St Sauver JL, Butterfield LC. Herpes zoster eye complications: rates and trends. Mayo Clin Proc. 2013;88(6):562–570. doi: 10.1016/j.mayocp.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harding SP, Upton JR, Wells JC. Natural history of herpes zoster ophthalmicus: predictors of postherpetic neuralgia and ocular involvement. Br J Ophthalmol. 1987;71(5):353–358. doi: 10.1136/bjo.71.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Severson EA, Baratz KH, Hodge DO, Burke JP. Herpes zoster ophthalmicus in olmsted county, Minnesota: have systemic antivirals made a difference? Arch Ophthalmol. 2003;121(3):386–390. doi: 10.1001/archopht.121.3.386. [DOI] [PubMed] [Google Scholar]
- 16.Hoang-Xuan T, Buchi ER, Herbort CP, et al. Oral acyclovir for herpes zoster ophthalmicus. Ophthalmology. 1992;99(7):1062–1070. doi: 10.1016/s0161-6420(92)31849-4. discussion 1070-1061. [DOI] [PubMed] [Google Scholar]
- 17.McKendrick MW, McGill Jl, White JE, Wood MJ. Oral acyclovir in acute herpes zoster. Br Med J (Clin Res Ed) 1986;293(6561):1529–1532. doi: 10.1136/bmj.293.6561.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood MJ, Johnson RW, McKendrick MW, Taylor J, Mandal BK, Crooks J. A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med. 1994;330(13):896–900. doi: 10.1056/NEJM199403313301304. [DOI] [PubMed] [Google Scholar]
- 19.Huff JC, Bean B, Balfour HH, et al. Therapy of herpes zoster with oral acyclovir. Am J Med. 1988;85(2A):84–89. [PubMed] [Google Scholar]
- 20.Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86(2):88–93. doi: 10.4065/mcp.2010.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miserocchi E, Fogliato G, Bianchi I, Bandello F, Modorati G. Clinical features of ocular herpetic infection in an italian referral center. Cornea. 2014;33(6):565–570. doi: 10.1097/ICO.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 22.Sy A, McLeod SD, Cohen EJ, et al. Practice patterns and opinions in the management of recurrent or chronic herpes zoster ophthalmicus. Cornea. 2012;31(7):786–790. doi: 10.1097/ICO.0b013e31823cbe6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epstein E. Recurrences in herpes zoster. Cutis. 1980;26(4):378–379. [PubMed] [Google Scholar]
- 24.HOPE-SIMPSON RE. THE NATURE OF HERPES ZOSTER: A LONG-TERM STUDY AND A NEW HYPOTHESIS. Proc R Soc Med. 1965;58:9–20. [PMC free article] [PubMed] [Google Scholar]
- 25.Ragozzino MW, Melton LJ, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 1982;61(5):310–316. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Bowsher D. The lifetime occurrence of Herpes zoster and prevalence of postherpetic neuralgia: A retrospective survey in an elderly population. Eur J Pain. 1999;3(4):335–342. doi: 10.1053/eujp.1999.0139. [DOI] [PubMed] [Google Scholar]
- 27.Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20(8):748–753. doi: 10.1111/j.1525-1497.2005.0150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Statistics. VNCfVAa. Special Reports: State Summaries Florida. Washington, D.C.: VA National Center for Veterans Analysis and Statistics; 2014. [Google Scholar]
- 29.1L T, editor. (VetPop2014) OotAVPPM. Projected Veteran Population 2013 to 2043. Washington, D.C.: 2014. [Google Scholar]
- 30.Millenials Outnumber Baby Boomers and Are Far More Diverse [press release] Washington, D.C.: 2015. [Google Scholar]
- 31.Yoshida M, Hayasaka S, Yamada T, et al. Ocular findings in Japanese patients with varicella-zoster virus infection. Ophthalmologica. 2005;219(5):272–275. doi: 10.1159/000086110. [DOI] [PubMed] [Google Scholar]
- 32.Puri LR, Shrestha GB, Shah DN, Chaudhary M, Thakur A. Ocular manifestations in herpes zoster ophthalmicus. Nepal J Ophthalmol. 2011;3(2):165–171. doi: 10.3126/nepjoph.v3i2.5271. [DOI] [PubMed] [Google Scholar]
- 33.Opstelten W, Eekhof J, Neven AK, Verheij T. Treatment of herpes zoster. Can Fam Physician. 2008;54(3):373–377. [PMC free article] [PubMed] [Google Scholar]
- 34.Hayward AR, Herberger M. Lymphocyte responses to varicella zoster virus in the elderly. J Clin Immunol. 1987;7(2):174–178. doi: 10.1007/BF00916011. [DOI] [PubMed] [Google Scholar]
- 35.Cohrs RJ, Mehta SK, Schmid DS, Gilden DH, Pierson DL. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J Med Virol. 2008;80(6):1116–1122. doi: 10.1002/jmv.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devlin ME, Gilden DH, Mahalingam R, Dueland AN, Cohrs R. Peripheral blood mononuclear cells of the elderly contain varicella-zoster virus DNA. J Infect Dis. 1992;165(4):619–622. doi: 10.1093/infdis/165.4.619. [DOI] [PubMed] [Google Scholar]
- 37.Wilson A, Sharp M, Koropchak CM, Ting SF, Arvin AM. Subclinical varicella-zoster virus viremia, herpes zoster, and T lymphocyte immunity to varicella-zoster viral antigens after bone marrow transplantation. J Infect Dis. 1992;165(1):119–126. doi: 10.1093/infdis/165.1.119. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy PG, Grinfeld E, Bontems S, Sadzot-Delvaux C. Varicella-Zoster virus gene expression in latently infected rat dorsal root ganglia. Virology. 2001;289(2):218–223. doi: 10.1006/viro.2001.1173. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy PG, Grinfeld E, Bell JE. Varicella-zoster virus gene expression in latently infected and explanted human ganglia. J Virol. 2000;74(24):11893–11898. doi: 10.1128/jvi.74.24.11893-11898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy PG, Grinfeld E, Gow JW. Latent Varicella-zoster virus in human dorsal root ganglia. Virology. 1999;258(2):451–454. doi: 10.1006/viro.1999.9745. [DOI] [PubMed] [Google Scholar]
- 41.Cohrs RJ, Randall J, Smith J, et al. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J Virol. 2000;74(24):11464–11471. doi: 10.1128/jvi.74.24.11464-11471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohrs RJ, Barbour M, Gilden DH. Varicella-zoster virus (VZV) transcription during latency in human ganglia: detection of transcripts mapping to genes 21, 29, 62, and 63 in a cDNA library enriched for VZV RNA. J Virol. 1996;70(5):2789–2796. doi: 10.1128/jvi.70.5.2789-2796.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadzot-Delvaux C, Debrus S, Nikkels A, Piette J, Rentier B. Varicella-zoster virus latency in the adult rat is a useful model for human latent infection. Neurology. 1995;45(12Suppl8):S18–20. doi: 10.1212/wnl.45.12_suppl_8.s18. [DOI] [PubMed] [Google Scholar]
- 44.Brunell PA, Ren LC, Cohen Jl, Straus SE. Viral gene expression in rat trigeminal ganglia following neonatal infection with varicella-zoster virus. J Med Virol. 1999;58(3):286–290. doi: 10.1002/(sici)1096-9071(199907)58:3<286::aid-jmv15>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 45.Meier JL, Holman RP, Croen KD, Smialek JE, Straus SE. Varicella-zoster virus transcription in human trigeminal ganglia. Virology. 1993;193(1):193–200. doi: 10.1006/viro.1993.1115. [DOI] [PubMed] [Google Scholar]
- 46.Pavan-Langston D, Yamamoto S, Dunkel EC. Delayed herpes zoster pseudodendrites. Polymerase chain reaction detection of viral DNA and a role for antiviral therapy. Arch Ophthalmol. 1995;113(11):1381–1385. doi: 10.1001/archopht.1995.01100110041023. [DOI] [PubMed] [Google Scholar]
- 47.Aggarwal S, Cavalcanti BM, Pavan-Langston D. Treatment of pseudodendrites in herpes zoster ophthalmicus with topical ganciclovir 0.15% gel. Cornea. 2014;33(2):109–113. doi: 10.1097/ICO.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 48.Hu AY, Strauss EC, Holland GN, Chan MF, Yu F, Margolis TP. Late varicella-zoster virus dendriform keratitis in patients with histories of herpes zoster ophthalmicus. Am J Ophthalmol. 2010;149(2):214–220. doi: 10.1016/j.ajo.2009.08.030. e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wenkel H, Rummelt C, Rummelt V, Jahn G, Fleckenstein B, Naumann GO. Detection of varicella zoster virus DNA and viral antigen in human cornea after herpes zoster ophthalmicus. Cornea. 1993;12(2):131–137. doi: 10.1097/00003226-199303000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Mietz H, Eis-Hubinger AM, Sundmacher R, Font RL. Detection of varicella-zoster virus DNA in keratectomy specimens by use of the polymerase chain reaction. Arch Ophthalmol. 1997;115(5):590–594. doi: 10.1001/archopht.1997.01100150592003. [DOI] [PubMed] [Google Scholar]
- 51.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833. doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rimland D, Moanna A. Increasing incidence of herpes zoster among Veterans. Clin Infect Dis. 2010;50(7):1000–1005. doi: 10.1086/651078. [DOI] [PubMed] [Google Scholar]
- 53.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 54.Galea SA, Sweet A, Beninger P, et al. The safety profile of varicella vaccine: a 10-year review. J Infect Dis. 2008;197(Suppl 2):S165–169. doi: 10.1086/522125. [DOI] [PubMed] [Google Scholar]
- 55.Bhalla P, Forrest GN, Gershon M, et al. Disseminated, persistent, and fatal infection due to the vaccine strain of varicella-zoster virus in an adult following stem cell transplantation. Clin Infect Dis. 2015;60(7):1068–1074. doi: 10.1093/cid/ciu970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gershon AA, Gershon MD. Pathogenesis and current approaches to control of varicella-zoster virus infections. Clin Microbiol Rev. 2013;26(4):728–743. doi: 10.1128/CMR.00052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levin MJ, DeBiasi RL, Bostik V, Schmid DS. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198(10):1444–1447. doi: 10.1086/592452. [DOI] [PubMed] [Google Scholar]
- 58.Lai YC, Yew YW. Severe Autoimmune Adverse Events Post Herpes Zoster Vaccine: A Case-Control Study of Adverse Events in a National Database. Drugs Dermatol. 2015;14(7):681–684. [PubMed] [Google Scholar]
- 59.Cheetham TC, Marcy SM, Tseng HF, et al. Risk of Herpes Zoster and Disseminated Varicella Zoster in Patients Taking Immunosuppressant Drugs at the Time of Zoster Vaccination. Mayo Clin Proc. 2015;90(7):865–873. doi: 10.1016/j.mayocp.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 60.Tseng HF, Lewin B, Hales CM, et al. Zoster Vaccine and the Risk of Postherpetic Neuralgia in Patients Who Developed Herpes Zoster Despite Having Received the Zoster Vaccine. J Infect Dis. 2015 doi: 10.1093/infdis/jiv244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wood MJ, Kay R, Dworkin RH, Soong SJ, Whitley RJ. Oral acyclovir therapy accelerates pain resolution in patients with herpes zoster: a meta-analysis of placebo-controlled trials. Clin Infect Dis. 1996;22(2):341–347. doi: 10.1093/clinids/22.2.341. [DOI] [PubMed] [Google Scholar]
- 62.Beutner KR, Friedman DJ, Forszpaniak C, Andersen PL, Wood MJ. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39(7):1546–1553. doi: 10.1128/aac.39.7.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jackson JL, Gibbons R, Meyer G, Inouye L. The effect of treating herpes zoster with oral acyclovir in preventing postherpetic neuralgia. A meta-analysis. Arch Intern Med. 1997;157(8):909–912. [PubMed] [Google Scholar]
- 64.Crooks RJ, Jones DA, Fiddian AP. Zoster-associated chronic pain: an overview of clinical trials with acyclovir. Scand J Infect Dis Suppl. 1991;80:62–68. [PubMed] [Google Scholar]
- 65.Li Q, Chen N, Yang J, et al. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2009;(2):CD006866. doi: 10.1002/14651858.CD006866.pub2. [DOI] [PubMed] [Google Scholar]


