Abstract
Acute kidney injury (AKI) is a devastating complication commonly occurring in the critically ill population with devastating short- and long-term consequences. Despite standardization of the definition and staging of AKI, early recognition remains challenging given that serum creatinine (Scr) is a marker—albeit imperfect—of kidney function and not kidney injury. Furthermore, the delay in rise of Scr after loss of glomerular filtration also prevents timely detection of decreased kidney function in patients with AKI. Over the past decade, numerous clinical investigations have evaluated the utility of several biomarkers in the early diagnosis and risk stratification of AKI. In 2014, the US Food and Drug Administration (FDA) approved the marketing of a test based on the combination of the urine concentrations of tissue inhibitor of metalloproteinase 2 and insulin-like growth factor binding protein 7 ([TIMP-2]x[IGFBP7]) to determine if certain critically ill patients are at risk of developing moderate to severe AKI. The optimal role of this biomarker in diagnosis, management, and prognosis of AKI in different clinical settings requires further clarification. In this perspective, we summarize the biological actions of these two cell-cycle arrest biomarkers, and present important considerations regarding the clinical application, interpretation, and limitations of this novel test for the early detection AKI.
Keywords: acute kidney injury (AKI), biomarker, [TIMP-2] × [IGFBP7], diagnosis, critically ill, tissue inhibitor of metalloproteinase 2, insulin-like growth factor binding protein 7, NephroCheck, early detection, risk assessement, renal dysfunction, decreased kidney function
Overview of Biomarker Development in AKI
Acute kidney injury (AKI) is a common but complex clinical syndrome in hospitalized patients associated with substantial inpatient complications, increased risk for end-stage renal disease (ESRD), and significant mortality.1–4 The nascent phase of field of AKI biomarkers dates to the early 21st century and was further propelled by recommendations from the American Society of Nephrology Renal Research Group in 2005.5 Biomarkers were prioritized to decrease reliance on serum creatinine (SCr) and urine output, as these two long-standing, “gold-standard” functional markers are insensitive for early recognition of kidney injury and real-time evaluation of AKI.6, 7 This search for the “renal troponin” was aided by the development of consensus definitions of AKI over the past decade, which allowed for standardization across biomarker trials.8–11 As a result, numerous AKI clinical investigations have been conducted evaluating biomarkers such as plasma and urine neutrophil gelatinase-associated lipocalin (NGAL), urine interleukin 18 (IL-18), urine liver-type fatty acid binding protein (L-FABP), and urine kidney injury molecule 1 (KIM-1) in high-risk patients.8–12 A few of these biomarkers have been approved for clinical use outside the United States (US) (Table 1). However, it was not until September 2014, following the publication of two multi-center intensive care unit (ICU) cohort studies, that the combination of urine tissue inhibitor of metalloproteinase 2 and insulin-like growth factor binding protein 7, known as [TIMP-2] × [IGFBP7], was allowed for marketing by the US Food and Drug Administration (FDA). This is the first biomarker for risk assessment of AKI to become available for clinical use in the US.11, 13, 14
Table 1.
Urine biomarker performance for prediction of AKI
| Urine biomarker | clinical trial Setting | Clinical Use | ||||
|---|---|---|---|---|---|---|
| ICU | CPB surgery | Kidney Tx | IV contrast | ER | ||
| NGAL12, 66–68 | + | + | + | + | + | Approved in Europe and Canada |
| IL-1812, 66, 67, 69, 70 | + | + | + | ? | ? | Not approved for clinical use |
| L-FABP12, 71–74 | + | + | ? | ? | + | Approved in Japan |
| KIM-112, 68, 75–78 | ? | + | -- | ? | + | Not approved for clinical use |
| [TIMP-2]x[IGFBP7]14, 31, 36, 37 | + | + | ? | ? | ? | Approved in United States and Europe |
Abbreviations and definitions: NGAL: neutrophil gelatinase-associated lipocalin; IL-18: interleukin 18; L-FABP: liver-type fatty acid binding protein; KIM-1: kidney injury molecule 1; TIMP-2: tissue inhibitor of metalloprotease 2; IGFBP7: insulin-like growth factor binding protein 7; ICU: intensive care unit; ER: emergency room; CPB: cardiopulmonary bypass; Tx: transplant; IV: intravascular; AKI: acute kidney injury; + associated with risk of AKI in clinical trials; -- did not correlate with AKI ; ? data is inconclusive or biomarker has not been extensively studied in that setting
Biological Functions of TIMP-2 and IGFBP7
TIMP-2 and IGFBP7 are cell-cycle arrest proteins expressed in renal tubular cells during periods of cellular stress or injury. TIMP-2 inhibits matrix metalloproteinase (MMP) activity, and exhibits a number of MMP-independent effects, many centering on regulation of the cell cycle. TIMP-2 binds to α3β1 integrin on the surface of endothelial cells, thus potently blocking endothelial cell proliferation and angiogenesis.15 This effect is mediated by induction of p27KIP1 expression, a cyclin-dependent kinase inhibitor that induces G1 cell-cycle arrest.16 TIMP-2 is also capable of stimulating cell division, emphasizing that the cellular effects of TIMPs are highly context dependent.17 In kidneys, TIMP-2 is strongly expressed in renal cell carcinoma, indicating that renal epithelia are capable of expressing it.18 IGFBP7 is a secreted protein that is a member of the IGFBP superfamily, also known as IGFBP-related proteins (IGFBPrP).19 Insulin-like growth factors (IGFs) exhibit pleiotropic effects in development and disease, and IGFBP7 regulates the bioavailability of IGFs through direct, low-affinity binding.19 Several IGF-independent effects, such as tumor suppression in melanoma and colon cancer, and induction of cell senescence in breast cancer lines, are also ascribed to IGFBP7.20,21
Uregulation of TIMP-2 and IGFBP7 in patients with AKI has been proposed to reflect their growth inhibitory functions because G1 cell cycle arrest is a known consequence of AKI.11 Certainly p27KIP1 and p21 can be induced by TIMP-2 and IGFBP7, respectively, and this would potently induce G1 arrest. On the other hand, there is much that remains poorly understood regarding the biological role for these proteins in AKI, beyond their utility as biomarkers. Their presumed role as inducers of G1 cell cycle arrest in the kidney remains speculative given that these proteins are capable of inducing a wide variety of cellular responses.
Premarketing Clinical Trials
Even though multiple plasma and urine biomarkers have been studied for prediction of AKI in critically ill patients, prior to 2014 no biomarker had been allowed to be marketed in the US for either diagnosis or risk assessment of AKI.22–30 The four pre-marketing [TIMP-2]x[IGFBP7] studies are (1) Discovery (discovery study to identify novel biomarkers) (2) Sapphire (multi-center validation study) (3) Opal (analysis to determine appropriate cut-offs) and (4) Topaz (multi-center study using clinical platform for [TIMP-2]x[IGFBP7] measurement and three experts for clinical adjudication).11, 14, 31 To better understand the appropriate use of this biomarker, these studies are summarized below and in Table 2.
Table 2.
Overview of [TIMP-2]x[IGFBP7] trials leading to FDA approval
| Study Name | Purpose of study | Patient population | N | Result | Other |
|---|---|---|---|---|---|
| Discovery | Identify novel protein biomarkers for AKI | ICU patients with sepsis or 1 risk factor for AKI | 522 | TIMP-2 and IGFBP7 were the best-performing markers (AUCs of 0.75 and 0.77 respectively) | 340 potential biomarkers were tested, including urine NGAL, KIM-1, IL-18 and L-FABP |
| Sapphire | Validation study | ICU patients over 21, with either respiratory or CV impairment; patients with moderate or severe AKI were excluded | 728 | 14% reached primary end point of moderate or severe AKI; risk of AKI was significantly elevated with [TIMP-2]x[IGFBP7] > 0.3 | Risk for MAKE significantly elevated with [TIMP-2]x[IGFBP7] > 0.3 |
| Opal | Derivation and validation study to confirm accuracy and clinical utility of 2 different cut-off values | ICU patients over 21; majority had respiratory or CV impairment; indwelling Foley catheters | 154 | 18% reached primary end point of moderate or severe AKI; AUC was 0.79; for 0.3 cut-off, NPV was 97%, PPV was 49% | 0.3 is highly sensitive but has very low specificity; baseline Scr was elevated in patients who reached primary end |
| Topaz | Validation study with clinical adjudication of primary end point | ICU patients over 21; all had either respiratory or CV impairment; indwelling Foley catheters; 83% had nephrotoxic medication exposure | 420 | 17.4% reached primary end point of moderate or severe AKI; AUC was 0.82; at cut-off of 0.3, sensitivity was 92% and specificity was 46%; at cut-off of 2.0, sensitivity was 46% and specificity was 95% | [TIMP-2]x[IGFBP7] remained significant even when combined with clinical model |
FDA: Food and Drug Administration; AKI: acute kidney injury; ICU: intensive care unit; NGAL: neutrophil gelatinase-associated lipocalin; IL-18: interleukin 18; L-FABP: liver-type fatty acid binding protein; KIM-1: kidney injury molecule 1; TIMP-2: tissue inhibitor of metalloprotease 2; IGFBP7: insulin-like growth factor binding protein 7; CV: cardiovascular; MAKE: major adverse kidney events; AUC: area under curve; NPV: negative predictive value; PPV: positive predictive value;
In the Discovery study, 340 candidate biomarkers were assessed for their ability to predict risk of AKI in a cohort of 522 critically ill adults. Urine TIMP-2 and IGFBP7 were identified as the best-performing biomarkers.11 The subsequent multicenter validation Sapphire study prospectively enrolled 728 critically ill adults with respiratory and/or cardiovascular impairment. Patients were enrolled within 24 hours of ICU admission, and those with KDIGO (Kidney Disease: Improving Global Outcomes) stage 2 or 3 AKI were excluded..32 The performance of urine concentrations of TIMP-2 and IGFBP7, measured within 24 hours of ICU admission, was tested against a panel of existing biomarkers in predicting the primary endpoint of of KDIGO stage 2 or 3 AKI within 12 hours. The concentration of TIMP-2 multiplied by the concentration of IGFBP7 ([TIMP-2]x[IGFBP7]) was superior to the other biomarkers (P<0.002) with an area under the receiver operating characteristic curve (AUC) of 0.80. Of note, an AUC of 0.70 is generally considered to be the cut off for a clinically useful biomarker.33 [TIMP-2]x[IGFBP7] was not elevated at baseline among patients with chronic kidney disease, and it performed well in patients with sepsis (AUC, 0.82) and high-risk surgery (AUC, 0.85). In the Sapphire study, [TIMP-2]x[IGFBP7] was superior for risk assessment of KDIGO stage 2 or 3 AKI (AUC, 0.80; 95% CI, 0.76–0.88) when compared to simultaneously measured plasma and urine NGAL (AUCs of 0.69 (95% CI, 0.64–0.73) and 0.72 (95% CI, 0.68–0.76), respectively), plasma cystatin C (AUC, 0.71; 95% CI, 0.66–0.75), urine IL-18 (AUC, 0.69; 95% CI, 0.65–0.73), KIM-1 (AUC, 0.70; 95% CI, 0.65–0.74) and L-FABP (AUC, 0.61; 95% CI, 0.56–0.66).
Using data from the Sapphire study, two cut-offs for [TIMP-2]x[IGFBP7], 0.3 (ng/mL)2/1000 and 2.0 (ng/mL)2/1000, were developed based on sensitivity and specificity for assessment of risk for AKI. These thresholds were prospectively tested and verified in the multicenter Opal study. The Opal study enrolled 154 critically ill adults, of whom 18% developed KDIGO stage 2 or 3 AKI within 12 hours of testing.31 This study confirmed the high sensitivity and high negative predictive values of 89% and 97%, respectively, for the cut-off of 0.3, and the high specificity and moderate positive predictive value of 95% and 49%, respectively, for the cut-off of 2.0.31
Topaz was a multicenter prospective study of 420 critically ill patients conducted in the US. Unlike prior AKI biomarker studies, the endpoint was independently adjudicated by three nephrologists who were blinded to the test results. The pre-selected high sensitivity value cut-off for [TIMP-2]x[IGFBP7] of 0.3 was validated for the prediction of clinically adjudicated KDIGO stage 2 or 3 AKI within 12 hours of testing. Urine [TIMP-2]x[IGFBP7] had an AUC of 0.82 in this population, and the proposed threshold of 0.3 discriminated between patients at high and low risk for AKI.14 Approximately 25% of patients with a [TIMP-2]x[IGFBP7] above 0.3 developed AKI, while only 4% with a [TIMP-2]x[IGFBP7] less than 0.3 developed AKI. The absolute risk of AKI with a [TIMP-2]x[IGFBP7] above 0.3 was seven times higher than the absolute risk among patients with values below this cut-off.
Other Clinical Trials
Additional studies have examined the use of [TIMP-2]x[IGFBP7] to predict increased risk of AKI in patients after cardiopulmonary bypass (CPB) and other major non-cardiac surgeries. Patients undergoing CPB surgery constitute one of the highest risk populations for development of AKI.34 A recent clinical trial in 240 patients undergoing CPB surgery found that elevated ([TIMP-2]x[IGFBP7] ≥ 0.5) four hours after surgery correlated with post-operative AKI development.35 An additional smaller study among CPB surgery patients showed a sensitivity of 0.92 and a specificity of 0.81 for a cut-off value of 0.5 measured within 24 hours postoperatively.36 In both these studies, a value of 0.5 (not 0.3) was used as a predictor of developing AKI. In another small study with 42 participants, in those patients who went on to develop AKI, [TIMP-2]x[IGFBP7] levels were not significantly elevated 4 hours post-operatively, and did not become elevated until post-operative day 1.37 This demonstrates that in addition to the actual test result, timing of measurement is very important in its interpretation.
In the pediatric population, a prospective cohort study explored the ability of [TIMP-2]x[IGFBP7] to predict risk for AKI in 51 children undergoing CPB surgery. The study compared [TIMP-2]x[IGFBP7], NGAL, and KIM-1 levels in 12 children with AKI (defined as a decrease in the estimated creatinine clearance by 25% from baseline) and 39 without AKI.38 The AUC for [TIMP-2]x[IGFBP7] was 0.85 (95% CI, 0.72–0.94) at 4 hours after CPB, similar to NGAL (AUC, 0.87; 95% CI, 0.74–0.95) and better than KIM-1 (AUC, 0.64; 95% CI, 0.49–0.77). Interestingly, the baseline levels of [TIMP-2]x[IGFBP7] were high (~1.0) in all children. Unexpectedly, the [TIMP-2]x[IGFBP7] values of those without AKI decreased at 4 hours after surgery, and the best cut-off to predict AKI at 4 hours was 0.7 (lower than the baseline value and higher than the approved cut-off of 0.3). The reason for the elevated baseline levels as well as the subsequent decline in the biomarker in children without AKI is unclear. At present, the biomarker is not approved for use in those under the age of 21.
The utility of [TIMP-2]x[IGFBP7] has also been investigated in high-risk patients undergoing major non-cardiac surgery. In a recent single-center study, this biomarker was measured in 107 high-risk patients on ICU admission approximately 4 hours after undergoing major surgery, with a pre-defined cut-off value of [TIMP-2]x[IGFBP7] > 0.3 to predict risk for AKI. In this cohort, the AUC for predicting risk of developing AKI was 0.85 and that for predicting need for RRT within 48 hours was 0.83.39
The furosemide stress test (FST) has been investigated for its utility to predict outcomes in AKI. Urine output less than 200 mL in the first two hours after administration of 1 to 1.5 mg/kg of furosemide has been shown to predict AKI progression with a sensitivity of 87.1% and specificity of 84.1%.40 The combination of [TIMP-2]x[IGFBP7] and FST has demonstrated synergy in prognosticating progression of AKI, need for RRT, and inpatient mortality in patients with early AKI. In this 77 participant cohort, [TIMP-2]x[IGFBP7] alone performed modestly in predicting these outcomes (AUCs of 0.61 to 0.69)41; however, when the FST results were analyzed in 32 participants with a pre-furosemide [TIMP-2]x[IGFBP7] >0.3, the stress test provided an AUC of 0.90 (p<0.001) for AKI progression and 0.91 for inpatient RRT.41 Utilizing an elevated [TIMP-2]x[IGFBP7] as the “renal troponin” trigger for a “kidney stress test” may improve risk stratification in patients with early AKI.
Clinical Application of [TIMP-2]x[IGFBP7]
The FDA allowed Astute Medical to market the [TIMP-2]x[IGFBP7] test under the brand name NEPHROCHECK on September 5, 2014.42 The package insert specifies that the test is to be used in ICU patients greater than 21 years of age, with cardiovascular and or respiratory compromise within the prior 24 hours.45 The test is “an aid in the risk assessment for moderate or severe acute kidney injury” (emphasis added). The insert also states that test results are “intended to be used in conjunction with clinical evaluation.” The FDA decision summary clarifies that [TIMP-2]x[IGFBP7] is not a standalone test and should not be used as point of care testing in the US.43 The FDA also requires that the manufacturer provide appropriate end-user training to “mitigate the risk of failure to correctly interpret test results”.43
As noted above, a [TIMP-2]x[IGFBP7] value >0.3 had a sensitivity of 92% for moderate or severe AKI in the next 12 hours, and was associated with approximately seven times the risk compared to values < 0.3 in the Topaz study.14 Importantly, the specificity of this cut-off to detect AKI was only 46% (95% CI, 41%–52%), with a positive predictive value of 27% (95% CI, 21%–32%). Therefore, false positive results will be quite common and will be magnified if the test is used inappropriately in low-risk patients. Like other tests with a high sensitivity and low specificity, the predictive value of the test is dependent on the likelihood that disease is present. For example, serum troponin is useful to assess for the presence of myocardial infarction when tested in the appropriate clinical setting (e.g., chest pain and known clinical risk factors) and likely to predict myocardial infarction if positive; however, in a low-risk patient, a positive test is less meaningful. Similarly, a [TIMP-2]x[IGFBP7] value in excess of 0.3 will have greater predictive value in patients at high risk for AKI, but will be less useful in patients at low risk for AKI. To put this into clinical context, cardiac surgery is associated with approximately 18% incidence of AKI and might be an appropriate setting in which to use this biomarker.44
Clinicians should be mindful of using [TIMP-2]x[IGFBP7] in settings where it has not been studied (Box 1). This biomarker should not be used in ambulatory practices and it is not beneficial in patients with established KDIGO stage 2 or 3 AKI, as it is unknown how long elevations in [TIMP-2]x[IGFBP7] might persist and, therefore, whether or not levels would predict worsening of AKI or kidney recovery. The [TIMP-2]x[IGFBP7] test should not be considered as a substitute for measurement of SCr. Serial measurements of the biomarker have not been established as a means of assessing progression of AKI, and therefore routine daily measurements are not recommended. A subsequent measurement in a critically ill patient may be considered if a change in clinical situation in a stable patient (e.g. hypotension, blood loss etc.) puts him or her at risk for AKI. Higher levels of [TIMP-2]x[IGFBP7] levels are more specific for assessing kidney injury; in a secondary analysis of the Topaz study, a cut-off of 2.0 was associated with specificity for moderate to severe AKI of 95%, although the sensitivity fell to 37%.14 High levels of urine bilirubin and albumin interfere with the test, and clinicians should be aware of this limitation since many critically ill patients have significant co-morbidities such as diabetic nephropathy and end-stage liver disease that lead to proteinuria and bilirubinuria (Box 1).45
Box 1. Summary of use of [TIMP-2]x[IGFBP7] in the clinical setting.
|
Appropriate patient population ICU patients ≥ 21 years of age with:
|
Appropriate use
|
Unapproved uses and limitations
|
Implications of positive test (>0.3)
|
TIMP-2: tissue inhibitor of metalloprotease 2; IGFBP7: insulin-like growth factor binding protein 7; ICU: intensive care unit; AKI: acute kidney injury; KDIGO: Kidney Disease: Improving Global Outcomes
Testing Process and Cost
According to the manufacturer, 10 mL of fresh urine should be collected in a sterile container and centrifuged by the laboratory within an hour of collection.45 Approximately 100 μL of urine is placed in the ASTUTE140 Meter (Astute Medical) and the readout is printed after 20 minutes. Thus, with prompt handling, results may reasonably be expected within one hour of sample collection. The result is reported as a single value (calculated by the machine) referred to as the “AKIRISK™ Score” which is the concentration of TIMP-2 (ng/mL) multiplied by the concentration of IGFBP7 (ng/mL) divided by 1000. The result is reported without any units or the concentrations of the individual biomarkers. A value of > 0.3 identifies patients with a high likelihood to have moderate to severe AKI within 12 hours, while a value of ≤ 0.3 identifies patients with a low risk to develop moderate to severe AKI within 12 hours. In Europe, an second cut-off of 2.0, which is associated with higher specificity, has been approved.46
Currently, the machine that runs the biomarker test costs approximately $5,000.46 The cost to the hospital for the cartridge for one [TIMP-2]x[IGFBP7] test is listed at $85, but a $15 discount is expected to be given to centers with “high usage”.46 When comparing reagent cost alone, it is significantly higher than i-STAT system test cartridges (Abbott)® for creatinine ($4), the “basic metabolic panel” (about $10), and troponin ($3). The cost of the test will be included in the inpatient diagnosis related group (DRG) since it does not have a separate code.46 An important logistical issue with the current [TIMP-2]x[IGFBP7] testing kit is that one machine can perform only one test at a time, so at 20 minutes per test, only 3 tests can be done in an hour. This might change if [TIMP-2]x[IGFBP7] is integrated into large chemistry platforms.
The cost benefit of [TIMP-2]x[IGFBP7] testing is unknown. It is well established that AKI contributes substantially to the cost of clinical care. In one analysis, the cost attributable to mild and severe post-operative AKI using the RIFLE (Risk Injury Failure Loss ESRD) score was $10,700 in RIFLE-R versus $21,400 in RIFLE-I.47 Thus, if early recognition and treatment led to reduced severity of AKI, a favorable cost benefit ratio of [TIMP-2]x[IGFBP7] might be anticipated. Currently, there are no data to support the premise that early recognition of kidney injury with [TIMP-2]x[IGFBP7] or other AKI biomarkers will prevent of progression of AKI or will be associated with any cost benefit to the patient or the institution. In addition, a false positive test may lead to unnecessary and expensive diagnostic and therapeutic evaluations.
Management Options for Patients With a Positive Result
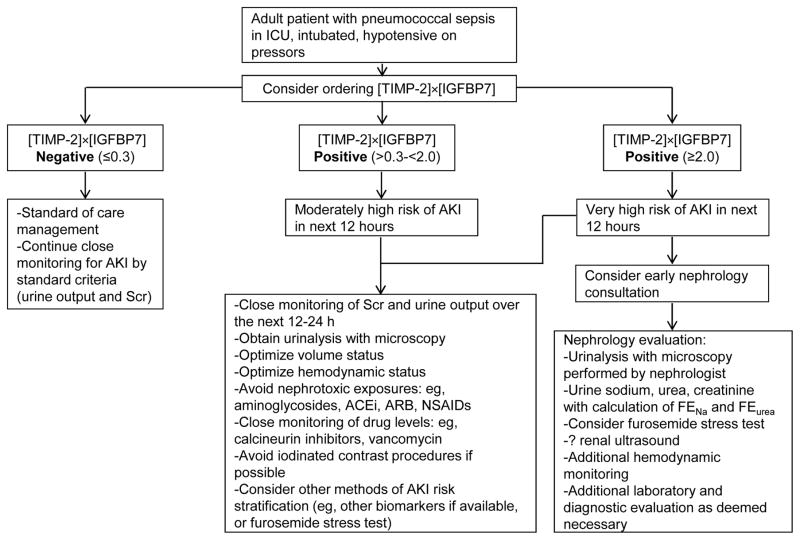
Nephrologists may not be involved in ordering [TIMP-2]x[IGFBP7] testing, but they must be familiar with the use and interpretation of the results, as positive results are likely to lead to nephrology consultation (Box 1). In patients with values >0.3, clinicians should consider standard preventative approaches similar to those taken with rising SCr, as recommended in KDIGO clinical guideline for prevention and treatment of AKI (Figure 1).48 These may include early renal consultation, volume resuscitation, maintenance of adequate blood pressure, and judicious avoidance of nephrotoxins such as aminoglycosides, iodinated contrast etc. The overall goal is to reduce further kidney injury, and potentially prevent progression of AKI.
Figure 1.
Hypothetical clinical scenario showing a potential use for [TIMP-2]x[IGFBP7] in a critically ill patient. ICU: intensive care unit; Scr; serum creatinine; AKI: acute kidney injury; FENa: fractional excretion of sodium; FEurea: fractional excretion of urea; ACEi: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; NSAIDs: non-steroidal anti-inflammatory drugs. Note: The authors have presented this hypothetical case illustration to demonstrate a possible use for this biomarker. To date, this algorithm has not been standardized and is not in use at any of medical centers that is affiliated with the authors. We recommend that each center institute its own protocol to ensure the appropriate use of this test.
Newer standardized AKI definitions have enabled the use of electronic alert systems (EAS) for early detection of AKI with the goal to warn clinicians early and optimize intervention.49–51 In a recent randomized single-blind study in a large US teaching hospital, EAS using SCr results was not associated with improved outcomes in patients with AKI.52 Possible reasons for the disappointing result include alert fatigue, physicians already aware of rising SCr, and the fact that the alert was not associated with any specific management recommendations. Since [TIMP-2]x[IGFBP7] is a novel biomarker that can detect kidney injury early, an elevated value might trigger early renal consultation, similar to cardiology consultation for patients with elevated troponin levels. There are data to suggest that early nephrology involvement is associated with improved outcomes in patients with AKI.53–56 Thus, we believe that it is essential for nephrologists to be involved in the interpretation and response to an [TIMP-2] x [IGFBP7] test. Numerous actions could be taken including optimizing fluid balance, discontinuation of nephrotoxic medications, and earlier diagnostic evaluation (Figure 1). Nephrologists are trained to perform urine microscopy, and presence of granular casts and tubular epithelial cells calculated as the urine sediment score has been shown to be of diagnostic and prognostic benefit.57 The combination of urine sediment score and positive [TIMP-2]x[IGFBP7] could potentially have higher sensitivity and specificity compared to their individual results.58
For institutions exploring the option of adding [TIMP-2]x[IGFBP7] to their laboratory panel, we suggest that center-specific protocols be created and implemented to ensure appropriate response to the test result. The protocol should ensure collaboration among nephrologists, surgeons, and critical care physicians in appropriate interpretation of the test and management of the patient, not just to utilize appropriate preventive measures, but also to avoid unnecessary additional testing. Nephrologists, in turn, should become familiar with validity and utility of the test and pursue appropriate management strategies, like urine microscopy, FST, etc.
Future Directions
Drug-induced nephrotoxicity has been implicated in up to 20% of in-hospital and community acquired AKI, and 25% of AKI cases occurring in the ICU.59, 60 Early detection of nephrotoxicity is critical so that the responsible agent may be discontinued and supportive therapy begun in order to minimize kidney damage. Even though 83% of patients in the Topaz study had nephrotoxicity contributing to AKI, there have been no clinical studies demonstrating the utility of [TIMP-2]x[IGFBP7] in detecting drug toxicity when it might be the sole cause for AKI. Given that TIMP-2 and IGFBP7 are involved in cell-cycle arrest of renal tubular cells in response to injury, it is possible that they may be useful for monitoring the development of nephrotoxicity related to known tubular toxins such as aminoglycosides, amphotericin B, cisplatin, calcineurin inhibitors, and iodinated contrast. However, the use of [TIMP-2]x[IGFBP7] to detect drug-induced nephrotoxicity outside of the ICU cannot be recommended at this time and should be considered as a potential area for future research.
The lack of efficacy of various non-dialytic interventions for AKI have been attributed to several factors including heterogeneity of AKI, complex patient population, poor trial design, and delay in recognition of AKI using SCr measurements.61 Trials evaluating IGF-1, anaritide, and fenoldopam have used elevation in SCr or development of oliguria as an entry criterion.62–64 By the time the SCr becomes elevated, AKI is fully established in the extension or maintenance phase, and delayed interventions may not be particularly beneficial in restoring tubular function. The NIDDK (National Institute of Diabetes and Digestive and Kidney Diseases) Kidney Research National Dialogue recommended the pursuit of discovery efforts of biomarkers that can not only help in clinical decision making, but also guide therapeutic interventional trials in AKI.65 Future interventional trials in AKI may consider using elevated [TIMP-2]x[IGFBP7] as a criterion for early enrollment.
In conclusion, the approval of the novel biomarker combination of [TIMP-2]x[IGFBP7] is a positive step in the search for robust and accurate means of early diagnosis of kidney injury. However, it is imperative that clinicians and laboratory directors understand the utility and limitations of this test before deciding whether to make it available at their institution. To translate this advancement in AKI biomarkers to meaningful improvement in clinical outcomes, standardization of care and early nephrology involvement will be important. Additional trials to study the role of this biomarker in preventive strategies and interventional trials are needed to assess its effectiveness in improving outcomes in AKI.
Acknowledgments
The ASN Acute Kidney Injury Advisory Group (AKIAG) comprises Sarah Faubel, MD (through 2016), David J. Askenazi, MD, MPH (2016), Rajit K. Basu, MD (2018), Udayan Y. Bhatt, MD, MPH (2018), Azra Bihorac, MD (2017), Jorge Cerda, MD, FASN (2017), Michael J. Connor, Jr., MD (2018), Alan J. Davidson, PhD (2016), Mark P. de Caestecker, MBBS, PhD (2017), Kent Doi, MD, PhD (2018), William Henry Fissell, MD (2016), Ladan Golestaneh, MD (2018), Michael Heung, MD (2017), Benjamin D. Humphreys, MD, PhD (2017), Jay L. Koyner, MD (2018), Kathleen D. Liu, MD, PhD (2016), Girish K. Mour, MD, MBBS (2017), Prabhleen Singh, MD, MPH (2018), Charuhas V. Thakar, MD (2018), and Anitha Vijayan, MD (2017).
Support: None. Financial Disclosure: Dr Vijayan was a clinical adjudicator for Topaz trial and has research support from Spectral Medical Inc. and AM-Pharma. Dr Askenazi is a speaker for the AKI foundation. Dr Heung reports receiving grant funding from Astute Medical Inc, the manufacturer of NEPHROCHECK. Dr Humphreys is a Co-Principal Investigator on an NIH grant to develop novel biomarkers of CKD (U01DK104308). Dr Koyner reports receiving grant funding from Abbott and Astute Medical Inc. for enrolling patients in observational AKI biomarker studies, and consulting fees from Astute Medical Inc. Dr Liu was a clinical adjudicator for the Topaz trial and reports consultancy agreements with Achaogen Inc., Astute Medical Inc., Chemocentryx Inc., and Durect Corporation. She is a Co- Principal Investigator on an NIH grant to test novel biomarkers of CKD (U01DK085649). Dr Nolin is member of the Independent Data Monitoring Committee for Thrasos Innovation Inc. Dr Bihorac is supported by Center for Sepsis and Critical Illness Award P50 GM-111152 from the National Institute of General Medical Sciences and has received research grants from the Society of Critical Care Medicine, Astute Medical Inc, and the I. Heermann Anesthesia Foundation Inc. The remaining authors declare that they have no relevant financial interests. Peer Review: Evaluated by 2 external peer reviewers, a Co-Editor, the Education Editor, and the Editor-in-Chief.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clinical journal of the American Society of Nephrology : CJASN. 2014;9:448–456. doi: 10.2215/CJN.02440213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chertow G, Levy E, Hammermeister K, Grover F, Daley J. Independent association between acute renal failure and mortality following caridac surgery. Am J Med. 1998;104:343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. Jama. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 4.Koyner JL, Cerda J, Goldstein SL, et al. The daily burden of acute kidney injury: a survey of U.S. nephrologists on World Kidney Day. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64:394–401. doi: 10.1053/j.ajkd.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Nephrology TASo. The American Society of Nephrology Renal Research Report. J Am Soc Nephrol. 2005;16:1886–1903. doi: 10.1681/ASN.2005030285. [DOI] [PubMed] [Google Scholar]
- 6.Moran SM, Myers BD. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int. 1985;27:928–937. doi: 10.1038/ki.1985.101. [DOI] [PubMed] [Google Scholar]
- 7.Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol. 2012;23:13–21. doi: 10.1681/ASN.2010111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parikh CR, Devarajan P, Zappitelli M, et al. Postoperative Biomarkers Predict Acute Kidney Injury and Poor Outcomes after Pediatric Cardiac Surgery. J Am Soc Nephrol. 2011;22:1737–1747. doi: 10.1681/ASN.2010111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parikh CR, Coca SG, Thiessen-Philbrook H, et al. Postoperative Biomarkers Predict Acute Kidney Injury and Poor Outcomes after Adult Cardiac Surgery. J Am Soc Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siew ED, Ware LB, Gebretsadik T, et al. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol. 2009;20:1823–1832. doi: 10.1681/ASN.2008070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Critical care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59:246–255. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration News Release. FDA allows marketing of the first test to assess risk of developing acute kidney injury. 2014 Sep 5; http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm412910.htm.
- 14.Bihorac A, Chawla LS, Shaw AD, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014;189:932–939. doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 15.Seo DW, Li H, Guedez L, et al. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- 16.Seo DW, Li H, Qu CK, et al. Shp-1 mediates the antiproliferative activity of tissue inhibitor of metalloproteinase-2 in human microvascular endothelial cells. The Journal of biological chemistry. 2006;281:3711–3721. doi: 10.1074/jbc.M509932200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barasch J, Yang J, Qiao J, et al. Tissue inhibitor of metalloproteinase-2 stimulates mesenchymal growth and regulates epithelial branching during morphogenesis of the rat metanephros. The Journal of clinical investigation. 1999;103:1299–1307. doi: 10.1172/JCI4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engers R, Springer E, Michiels F, Collard JG, Gabbert HE. Rac affects invasion of human renal cell carcinomas by up-regulating tissue inhibitor of metalloproteinases (TIMP)-1 and TIMP-2 expression. The Journal of biological chemistry. 2001;276:41889–41897. doi: 10.1074/jbc.M105049200. [DOI] [PubMed] [Google Scholar]
- 19.Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocrine reviews. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 20.Wajapeyee N, Kapoor V, Mahalingam M, Green MR. Efficacy of IGFBP7 for treatment of metastatic melanoma and other cancers in mouse models and human cell lines. Molecular cancer therapeutics. 2009;8:3009–3014. doi: 10.1158/1535-7163.MCT-09-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuo S, Liu C, Wang J, et al. IGFBP-rP1 induces p21 expression through a p53-independent pathway, leading to cellular senescence of MCF-7 breast cancer cells. Journal of cancer research and clinical oncology. 2012;138:1045–1055. doi: 10.1007/s00432-012-1153-y. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Guo W, Zhang J, et al. Urinary interleukin 18 for detection of acute kidney injury: a meta-analysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;62:1058–1067. doi: 10.1053/j.ajkd.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrology, Dialysis, Transplantation. 2013;28:254–273. doi: 10.1093/ndt/gfs380. [DOI] [PubMed] [Google Scholar]
- 24.Ostermann M, Philips BJ, Forni LG. Clinical review: Biomarkers of acute kidney injury: where are we now? Critical care. 2012;16:233. doi: 10.1186/cc11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wasung ME, Chawla LS, Madero M. Biomarkers of renal function, which and when? Clinica Chimica Acta. 2015;438:350–357. doi: 10.1016/j.cca.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 26.Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrology Dialysis Transplantation. 2014;29:1301–1311. doi: 10.1093/ndt/gft510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koyner JL, Parikh CR. Clinical Utility of Biomarkers of AKI in Cardiac Surgery and Critical Illness. Clinical Journal of the American Society of Nephrology. 2013;8:1034–1042. doi: 10.2215/CJN.05150512. [DOI] [PubMed] [Google Scholar]
- 28.de Geus HR, Betjes MG, Bakker J. Biomarkers for the prediction of acute kidney injury: a narrative review on current status and future challenges. Clin Kidney J. 2012;5:102–108. doi: 10.1093/ckj/sfs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez F, Vincent F. Biomarkers for acute kidney injury in critically ill patients. Minerva Anestesiologica. 2012;78:1394–1403. [PubMed] [Google Scholar]
- 30.Herget-Rosenthal S, Metzger J, Albalat A, Bitsika V, Mischak H. Proteomic biomarkers for the early detection of acute kidney injury. Prilozi. 2012;33:27–48. [PubMed] [Google Scholar]
- 31.Hoste EA, McCullough PA, Kashani K, et al. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29:2054–2061. doi: 10.1093/ndt/gfu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.KDIGO. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group: Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;(Supp):1–138. [Google Scholar]
- 33.Xia J, Broadhurst DI, Wilson M, Wishart DS. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics : Official journal of the Metabolomic Society. 2013;9:280–299. doi: 10.1007/s11306-012-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pickering JW, James MT, Palmer SC. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;65:283–293. doi: 10.1053/j.ajkd.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Zarbock A, Schmidt C, Van Aken H, et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313:2133–2141. doi: 10.1001/jama.2015.4189. [DOI] [PubMed] [Google Scholar]
- 36.Meersch M, Schmidt C, Van Aken H, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9:e93460. doi: 10.1371/journal.pone.0093460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wetz AJ, Richardt EM, Wand S, et al. Quantification of urinary TIMP-2 and IGFBP-7: an adequate diagnostic test to predict acute kidney injury after cardiac surgery? Critical care. 2015;19:3. doi: 10.1186/s13054-014-0717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meersch M, Schmidt C, Van Aken H, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury after pediatric cardiac surgery. PLoS One. 2014;9:e110865. doi: 10.1371/journal.pone.0110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gocze I, Koch M, Renner P, et al. Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLoS One. 2015;10:e0120863. doi: 10.1371/journal.pone.0120863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chawla LS, Davison DL, Brasha-Mitchell E, et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Critical care. 2013;17:R207. doi: 10.1186/cc13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koyner JL, Davison DL, Brasha-Mitchell E, et al. Furosemide Stress Test and Biomarkers for the Prediction of AKI Severity. J Am Soc Nephrol. 2015;26:2023–2031. doi: 10.1681/ASN.2014060535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.US Food and Drug Administration letter to Astute Medical. 2014 Sep 5; http://www.accessdata.fda.gov/cdrh_docs/pdf13/den130031.pdf.
- 43.U.S. Food and Drug Administration. Evaluation of Automatic Class III Designation for NephroCheckSystem® Test System Decision Summary. http://www.accessdata.fda.gov/cdrh_docs/reviews/den130031.pdf.
- 44.Thiele RH, Isbell JM, Rosner MH. AKI associated with cardiac surgery. Clinical journal of the American Society of Nephrology : CJASN. 2015;10:500–514. doi: 10.2215/CJN.07830814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.NEPHROCHECK® Test Kit Package Insert. Astute Medical, Inc; 2014. [Accessed February 5, 2016]. PN 300152 Rev E 2014/09/05. http://www.astutemedical.com/content/documents/us/NephroCheck_Test_Package_Insert_US_IVD_(PN_300152)_RevE.pdf. [Google Scholar]
- 46.Lusky K. AKI risk biomarkers may be “as early as it gets”. CAP Today. 2015 Jun;:12–16. http://digital.olivesoftware.com/Olive/ODE/CAPTODAY/
- 47.Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, et al. Cost and Mortality Associated With Postoperative Acute Kidney Injury. Ann Surg. 2015;261:1207–1214. doi: 10.1097/SLA.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.KDIGO. Clinical Practice Guideline for Acute Kidney Injury: Prevention and Treatment of AKI. Kidney international supplements. 2012;2:37–68. doi: 10.1038/kisup.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flynn N, Dawnay A. A simple electronic alert for acute kidney injury. Ann Clin Biochem. 2015;52:206–212. doi: 10.1177/0004563214534832. [DOI] [PubMed] [Google Scholar]
- 50.Thomas ME, Blaine C, Dawnay A, et al. The definition of acute kidney injury and its use in practice. Kidney Int. 2015;87:62–73. doi: 10.1038/ki.2014.328. [DOI] [PubMed] [Google Scholar]
- 51.Wallace K, Mallard AS, Stratton JD, Johnston PA, Dickinson S, Parry RG. Use of an electronic alert to identify patients with acute kidney injury. Clin Med. 2014;14:22–26. doi: 10.7861/clinmedicine.14-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson FP, Shashaty M, Testani J, et al. Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. Lancet. 2015;385:1966–1974. doi: 10.1016/S0140-6736(15)60266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ponce D, de Zorzenon CP, dos Santos NY, Balbi AL. Early nephrology consultation can have an impact on outcome of acute kidney injury patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26:3202–3206. doi: 10.1093/ndt/gfr359. [DOI] [PubMed] [Google Scholar]
- 54.Mehta RL, McDonald B, Gabbai F, et al. Nephrology consultation in acute renal failure: does timing matter? Am J Med. 2002;113:456–461. doi: 10.1016/s0002-9343(02)01230-5. [DOI] [PubMed] [Google Scholar]
- 55.Meier P, Bonfils RM, Vogt B, Burnand B, Burnier M. Referral patterns and outcomes in noncritically ill patients with hospital-acquired acute kidney injury. Clinical journal of the American Society of Nephrology : CJASN. 2011;6:2215–2225. doi: 10.2215/CJN.01880211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balasubramanian G, Al-Aly Z, Moiz A, et al. Early nephrologist involvement in hospital-acquired acute kidney injury: a pilot study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;57:228–234. doi: 10.1053/j.ajkd.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 57.Perazella MA, Coca SG, Hall IE, Iyanam U, Koraishy M, Parikh CR. Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:402–408. doi: 10.2215/CJN.06960909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perazella MA. The Urine Sediment as a Biomarker of Kidney Disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;66:748–755. doi: 10.1053/j.ajkd.2015.02.342. [DOI] [PubMed] [Google Scholar]
- 59.Tiong HY, Huang P, Xiong S, Li Y, Vathsala A, Zink D. Drug-induced nephrotoxicity: clinical impact and preclinical in vitro models. Molecular pharmaceutics. 2014;11:1933–1948. doi: 10.1021/mp400720w. [DOI] [PubMed] [Google Scholar]
- 60.Perazella MA. Drug use and nephrotoxicity in the intensive care unit. Kidney Int. 2012;81:1172–1178. doi: 10.1038/ki.2010.475. [DOI] [PubMed] [Google Scholar]
- 61.Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven't worked and what is on the horizon. Clinical journal of the American Society of Nephrology : CJASN. 2007;2:356–365. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- 62.Bove T, Zangrillo A, Guarracino F, et al. Effect of fenoldopam on use of renal replacement therapy among patients with acute kidney injury after cardiac surgery: a randomized clinical trial. JAMA. 2014;312:2244–2253. doi: 10.1001/jama.2014.13573. [DOI] [PubMed] [Google Scholar]
- 63.Hirschberg R, Kopple J, Lipsett P, et al. Multicenter clinical trial of recombinant human insulin-like growth factor I in patients with acute renal failure. Kidney Int. 1999;55:2423–2432. doi: 10.1046/j.1523-1755.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 64.Lewis J, Salem MM, Chertow GM, et al. Atrial natriuretic factor in oliguric acute renal failure. Anaritide Acute Renal Failure Study Group. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2000;36:767–774. doi: 10.1053/ajkd.2000.17659. [DOI] [PubMed] [Google Scholar]
- 65.Bonventre JV, Basile D, Liu KD, et al. AKI: a path forward. Clinical journal of the American Society of Nephrology : CJASN. 2013;8:1606–1608. doi: 10.2215/CJN.06040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soni SS, Ronco C, Katz N, Cruz DN. Early diagnosis of acute kidney injury: the promise of novel biomarkers. Blood Purif. 2009;28:165–174. doi: 10.1159/000227785. [DOI] [PubMed] [Google Scholar]
- 67.Hall IE, Yarlagadda SG, Coca SG, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol. 2010;21:189–197. doi: 10.1681/ASN.2009030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koyner JL, Vaidya VS, Bennett MR, et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:2154–2165. doi: 10.2215/CJN.00740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parikh CR, Jani A, Mishra J, et al. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6:1639–1645. doi: 10.1111/j.1600-6143.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- 70.Parikh CR, Mishra J, Thiessen-Philbrook H, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 71.Portilla D, Dent C, Sugaya T, et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73:465–472. doi: 10.1038/sj.ki.5002721. [DOI] [PubMed] [Google Scholar]
- 72.Doi K, Noiri E, Maeda-Mamiya R, et al. Urinary L-type fatty acid-binding protein as a new biomarker of sepsis complicated with acute kidney injury. Crit Care Med. 2010;38:2037–2042. doi: 10.1097/CCM.0b013e3181eedac0. [DOI] [PubMed] [Google Scholar]
- 73.Susantitaphong P, Siribamrungwong M, Doi K, Noiri E, Terrin N, Jaber BL. Performance of urinary liver-type fatty acid-binding protein in acute kidney injury: a meta-analysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;61:430–439. doi: 10.1053/j.ajkd.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parr SK, Clark AJ, Bian A, et al. Urinary L-FABP predicts poor outcomes in critically ill patients with early acute kidney injury. Kidney Int. 2015;87:640–648. doi: 10.1038/ki.2014.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Torregrosa I, Montoliu C, Urios A, et al. Urinary KIM-1, NGAL and L-FABP for the diagnosis of AKI in patients with acute coronary syndrome or heart failure undergoing coronary angiography. Heart and vessels. 2014 doi: 10.1007/s00380-014-0538-z. [DOI] [PubMed] [Google Scholar]
- 76.Du Y, Zappitelli M, Mian A, et al. Urinary biomarkers to detect acute kidney injury in the pediatric emergency center. Pediatric nephrology. 2011;26:267–274. doi: 10.1007/s00467-010-1673-0. [DOI] [PubMed] [Google Scholar]
- 77.Liang XL, Liu SX, Chen YH, et al. Combination of urinary kidney injury molecule-1 and interleukin-18 as early biomarker for the diagnosis and progressive assessment of acute kidney injury following cardiopulmonary bypass surgery: a prospective nested case-control study. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2010;15:332–339. doi: 10.3109/13547501003706558. [DOI] [PubMed] [Google Scholar]
- 78.Liangos O, Tighiouart H, Perianayagam MC, et al. Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2009;14:423–431. doi: 10.1080/13547500903067744. [DOI] [PMC free article] [PubMed] [Google Scholar]