Abstract
Background
Vitamin D3 (VD3) is a steroid hormone with known anti-proliferative properties. Patients with chronic rhinosinusitis with nasal polyps (CRSwNP) have been shown to be VD3 deficient. Moreover, VD3 deficiency is associated with worse disease in patients with CRSwNP. One cell type thought to play a role in chronic rhinosinusitis (CRS) is the human sinonasal fibroblast (HSNF). The aim of this study was to investigate VD3 deficiency and HSNF proliferation in CRSwNP.
Methods
Blood and sinus tissue explants were collected at the time of surgery from patients with CRSwNP (n=15). Control subjects (n=12) were undergoing surgery for cerebrospinal fluid leak repair or to remove non-hormone-secreting pituitary tumors. Ex-vivo HSNF proliferation was analyzed with flow cytometry using expression of fibroblast specific protein (FSP) and the proliferation marker Ki67. Plasma levels of 25-hydroxyvitaminD3 (25VD3) were measured by enzyme-linked immunosorbent assay. In vitro analysis of HSNF proliferation after treatment with 1,25VD3 was performed using carboxyfluorescein succinimidyl ester (CFSE) and analyzed with flow cytometry.
Results
In CRSwNP patients there was an inverse correlation between 25VD3 and proliferating HSNF (p= 0.0135). This correlation was not seen for control patients (p=0.3869). In vitro analysis showed that HSNF from patients with CRSwNP had a higher proliferation index at baseline than HSNF from control patients (p<0.01). When treated with 1,25VD3, there was a significant decrease in HSNF proliferation index in patients with CRSwNP (p<0.01), but not control patients.
Conclusions
VD3 deficiency is associated with increased HSNF proliferation in CRSwNP. Further investigation into how HSNFs and VD3 impact CRSwNP pathophysiology is warranted.
Keywords: Chronic rhinosinusitis, endoscopic sinus surgery, fibroblast, vitamin D
Introduction
Chronic rhinosinusitis (CRS) is a burdensome inflammatory disease of the sinonasal mucosa that affects 4–5% of the United States population [1]. Two disease phenotypes exist based on the presence or absence of nasal polyps, and both have distinct immunologic profiles [2]. Chronic rhinosinusitis with nasal polyps (CRSwNP) in the United States and Europe is a Th2-driven process characterized by a predominately eosinophilic infiltrate, and is notoriously recalcitrant to aggressive medical and surgical therapy [3]. A distinct neutrophilic Th1-shifted type of CRSwNP is also found in some Asian populations [4]. Oral steroids are the most effective medical treatment for CRSwNP; however, their utility is often reduced given substantial systemic side effects. To fully understand the pathogenesis of CRSwNP and develop targeted therapies, further delineation of immunomodulating influences on the local sinonasal cell populations is required.
Vitamin D3 (VD3) is a known immunomodulator, anti-inflammatory and anti-proliferative steroid hormone [5]. In patients with CRSwNP, VD3 levels are reduced, which is associated with more severe disease [6, 7]. Biologically inactive VD3 is either ingested or formed photochemically in the skin via UV light exposure. Initial hydroxylation of VD3 occurs in the liver to form the prohormone calcidiol (25VD3). Calcidiol is then circulated to peripheral tissues where it is activated to calcitriol (1,25VD3) by 1-α-hydroxylase. After binding to the intracellular vitamin D receptor (VDR), a number of cell signaling pathways are activated. Vitamin D has been shown to inhibit the differentiation of monocytes to dendritic cells [8, 9] and decrease epithelial pro-inflammatory cytokine production [10]. The vitamin D receptor (VDR) has been found on numerous cell types including the fibroblast [11]; however, the specific downstream effects in the sinonasal microenvironement are unknown.
Fibroblasts are large, stellate cells that predominate in connective tissue, and are responsible for the production of extracellular matrix (ECM), which consists of collagens, proteoglycans, cell adhesion-molecules, and hyaluronic acid. Fibroblast ECM production has been well described in lower airway remodeling seen in asthma [12]. However, the fibroblast also has complex cytokinetic immune regulatory function in numerous pathologies. There is increasing evidence that the fibroblast is at least partially responsible for recruitment of inflammatory cells in CRS [13]. In CRSwNP, 1,25VD3 has been shown to decrease eosinophil attractant chemokine release from human sinonasal fibroblasts (HSNF) in vitro [14, 15]. Furthermore while HSNF can respond to VD3, they lack the ability to convert VD3 to its metabolically active form 1,25VD3. However, the relationship between VD3 deficiency and HSNF proliferation remains incompletely characterized in CRSwNP. We hypothesize that VD3 deficiency is associated with increased HSNF proliferation in CRSwNP as compared to control, and treatment with 1,25VD3 may be of benefit in quelling HSNF proliferation.
Methods
Patient Enrollment
The Institutional Review Board at the Medical University of South Carolina approved the study protocol and written informed consent was obtained for all patients. Sinus tissue was collected at time of surgery from control patients and patients with CRSwNP as defined by the European Position Paper on Rhinosinusitis and Nasal Polyps [16]. Control patients were undergoing endoscopic surgery for non-inflammatory conditions such as cerebrospinal fluid leak repair or for non-hormone secreting pituitary tumors. Patients were excluded for use of systemic steroids, antibiotics or other immune modulatory agents within 30 days preceding surgery. Patients were also excluded if they were suffering from an acute upper respiratory tract infection (based on patient reported clinical symptoms of exacerbation of symptoms). All patients were evaluated for acute exacerbation at their clinic visits and via phone call one week prior to surgery by our mid-level provider. Any patient with exacerbation received pre-operative antibiotics and steroids, thus excluding them from the study. In addition, patients with other renal, gastrointestinal, endocrine, skeletal disorders, idiopathic pulmonary fibrosis, cystic fibrosis, ciliary dyskinesia, immune disorders (multiple myeloma, rheumatoid arthritis, immunodeficiency, ASA triad, etc.), or pregnancy were excluded. No patients were on vitamin D supplementation prior to surgery. Patients requiring blood work for other clinical indications did not have additional blood drawn for research purposes and therefore did not have 25VD3 levels available.
Tissue Processing, Immunostaining & Flow Cytometric Analysis
Sinonasal tissue explants were taken from sinus tissue biopsies and mechanically dissociated, prepared and stored as previously described [10]. Tissue from a total of 27 patients was used for flow cytometric analysis. Immunostaining and flow cytometric analysis of HSNF was conducted as previously described [6, 17]. Antibodies were used according to manufacturer instructions, and included fibroblast specific protein (FSP) (Abcam, Cambridge, MA), mucin-1 (MUC1) (BD Biosciences), Vitamin D receptor (VDR) (EMD Millipore Billerica, MA) and Ki67 (Biolegend, San Diego CA). An anti-IgM PE-Cy7 secondary antibody (Biolegend, San Diego, CA) was added at a concentration of 1:250. Analysis was performed with a Guava easyCyte 8HT flow cytometer (EMD Millipore, Billerica, MA) and analysis was carried out with FCS Express 4 software (De Novo Software, Los Angeles, CA). Only viable cells, as defined by negative staining for 7-Aminoactinomycin D (7AAD), were included in the analysis. Antibody-matched isotype controls were used and subtracted from positive staining, with all isotypes staining <5% of total cells.
Measurement of systemic vitamin D (25VD3) levels
Plasma was taken at the time of surgery and was processed as previously described [7]. Plasma 25VD3 levels were measured using an enzyme immunoassay (Immunodiagnostic Systems, Fountain Hills, Az), according to the manufacturer’s instructions. The assay detection limits were 5 to 152 ng/mL.
Establishment of HSNF and epithelial cell lines
HSNF and human sinonasal epithelial (HSNEC) cell lines were created from sinonasal tissue explants and grown as previously described [10, 13, 18]. HSNF lines were established from a total of 11 controls and 9 CRSwNP patients. For assays examining metabolism of 25VD3 to 1,25VD3, HSNEC were included as a positive control as they have demonstrated the capacity to metabolize 25VD3 [10]. A total of 3 control patient-derived HSNEC were used. All cells were used at passage two.
In vitro analysis of HSNF conversion of 25VD3 to 1,25VD3
Subconfluent cells were then treated with inactive 25VD3 (20 ng/ml) for 24 hours after which time conditioned media was collected. 1,25VD3 was immediately assayed using an enzyme immunoassay (Immunodiagnostic Systems, Fountain Hills, Ariz.) according to the manufacturer’s instruction and as previously described [10].
In vitro measurement of HSNF proliferation using carboxyfluorescein succinimidyl ester CFSE
CellTrace™ CFSE proliferation assay was performed according to the manufacturer’s instructions. Briefly, a 5mM CellTrace™ CFSE stock solution was prepared by dissolving the contents of 1 vial of CellTrace™ CFSE into 18 μL of DMSO. Equal numbers of HSNF were then treated with the 5mM CellTrace™ CFSE stock solution for a final working concentration of 10 μM. Cells were then incubated at 37°C with 5% CO2 for 15 minutes. The cells were then centrifuged, the supernatant was discarded and a second incubation in pre-warmed media was performed for 30 minutes. Equal numbers of cells were then divided into treatment groups on a 46 well plate. Treatment groups included negative control (no treatment), CFSE alone, and CFSE with 1,25VD3 at a dose of 50 pg/mL. HSNF were then incubated for 3 days at 37°C with 5% CO2 with daily media changes. Cells were then harvested with accutase and transferred to 96 well plate for flow cytometry. Flow cytometry analysis was then performed using a Guava easyCyte 8HT flow cytometer (EMD Millipore, Billerica, MA) set to capture 488-nm excitation. Proliferation analysis was carried out with FCS Express 4 software (De Novo Software, Los Angeles, CA). Values were reported as the proliferation index which is the average number of cells that one initial cell became.
Statistical Analysis
Data were analyzed using GraphPad Prism 6.0 software. Nominal data was analyzed using a Fisher’s exact test. For continuous variables, values were first determined to follow a normal distribution using a D’Agostino and Pearson omnibus normality test. For normally distributed data, comparing two groups, an unpaired t-test was utilized to determine significance. For normally distributed data, comparisons were made using one-way ANOVA followed by two-tailed t-test. For non-parametric data, Kruskal-Wallis followed by Mann-Whitney U test was used. Correlations for parametric and non-parametric data were examined using a Pearson or Spearman correlation analysis, respectively. P values <0.05 were considered significant.
Results
A total of 27 patients (12 Control and 15 CRSwNP) had sinonasal tissue explants available for ex vivo analysis of HSNF proliferation (patient demographics described in Table I). There were 3 Caucasian control patients, 7 Caucasian CRSwNP patients, 9 African American control patients and 8 African American CRSwNP patients. 25% of control patients were male and 47% of CRSwNP were male. The average age was 52 years for control patients and 50 years for CRSwNP patients. To determine HSNF proliferation, we prepared single-cell suspensions of the sinonasal tissue explants and analyzed them via flow cytometry analysis. Proliferating HSNFs were identified as cells that stained FSP+, Ki67+ and MUC1-. As shown in Figure 1, we observed that compared to controls, CRSwNP patients had a higher percentage of HSNF expressing the proliferation marker Ki67 (p=0.014). Next we compared the differences in HSNF proliferation as a function of 25VD3 (Fig 2). In CRSwNP patients, there was an inverse correlation between systemic 25VD3 and proliferating HSNFs (R= −0.6630;p= 0.0135; Figure 2). This correlation was not seen for control (p=0.3869) patients.
Table I.
| A. Demographics for Figure 1
| |||||
|---|---|---|---|---|---|
| # of Patients | Race | Gender (%male) | Age | % with 25VD3 Available | |
| Control | 12 | C 3; AA 9 | 25 | 52±14 | 50% |
| CRSwNP | 15 | C 7; AA 8 | 47 | 50±15 | 87% |
| B. Demographics for Figure 2
| |||||
|---|---|---|---|---|---|
| # of Patients | Race | Gender (%male) | Age | 25VD3 (ng/ml) | |
| Control | 6 | C 2; AA 4 | 33.3 | 45±16 | 20.8 ± 9.7 |
| CRSwNP | 13 | C 7; AA 6 | 53.8 | 52±12 | 34.5 ± 21.1 |
Values shown are Mean ± SD. C - Caucasian, AA - African American
Figure 1. Ex vivo analysis of sinonasal explant HSNF proliferation.
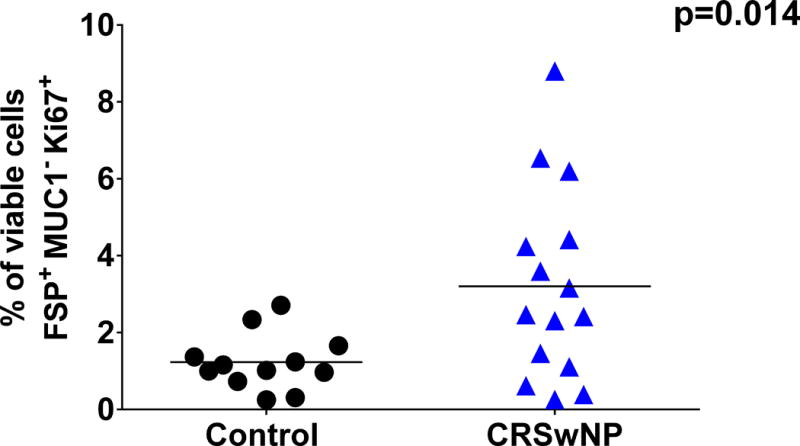
Compared to controls (n=12), patients with CRSwNP (n=15) have a higher percentage of sinonasal tissue cells that are proliferating HSNF as determined by positive staining for FSP and Ki67 and negative staining for MUC1. Dead cells (7-Aminoactinomycin D positive) were excluded from the analysis. Statistics shown are unpaired t test.
Figure 2. CRSwNP patients (n=13) with less systemic 25VD3 have increased proliferating HSNFs compared to control patients (n=6).
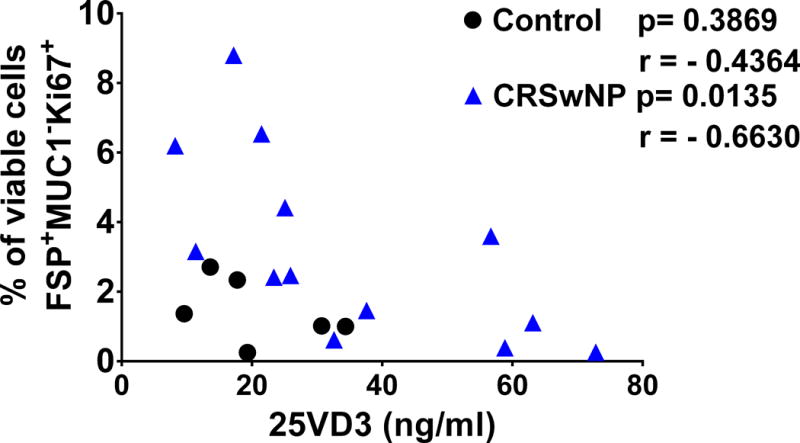
Proliferating HSNFs were identified ex vivo from sinonasal tissue biopsies via flow cytometry as cells that stained FSP+, Ki67+ and MUC1−. 25VD3 was measured in patient plasma at the time of surgery using commercially available ELISA. Each symbol represents an individual patient and statistics are the results of Pearson correlation. HSNF = human sinonasal fibroblast. CRSwNP = chronic rhinosinusitis with nasal polyps. 25VD3 = 25-hydroxy vitamin D3. ELISA = enzyme-linked immunosorbent assay.
Following our initial observation noting the relationship between systemic 25VD3 and HSNF proliferation, we next wanted to examine the ability of VD3 to regulate HSNF proliferation in vitro. Before doing this, we examined HSNF capacity to metabolize 25VD3, to its activate metabolite 1,25VD3. A shown in Figure 3, neither control nor CRSwNP-derived HSNF were able to convert 25VD3 to 1,25VD3. Furthermore, conditioned media from HSNF contained no more 1,25VD3 than media alone. As a positive control, we included control patient-derived HSNEC and observed that similar to previous studies they could metabolize 25VD3 to 1,25VD3. These results demonstrate the HSNF cannot independently convert 25VD3 to its active metabolite 1,25VD3.
Figure 3. HSNFs cannot convert 25VD3 to its biologically active form 1,25VD3. Cells were treated for 24 hours with 25VD3 (20 ng/ml) and 1,25VD3 was subsequently measured in conditioned media. Note that HSNF levels are the same as media alone.
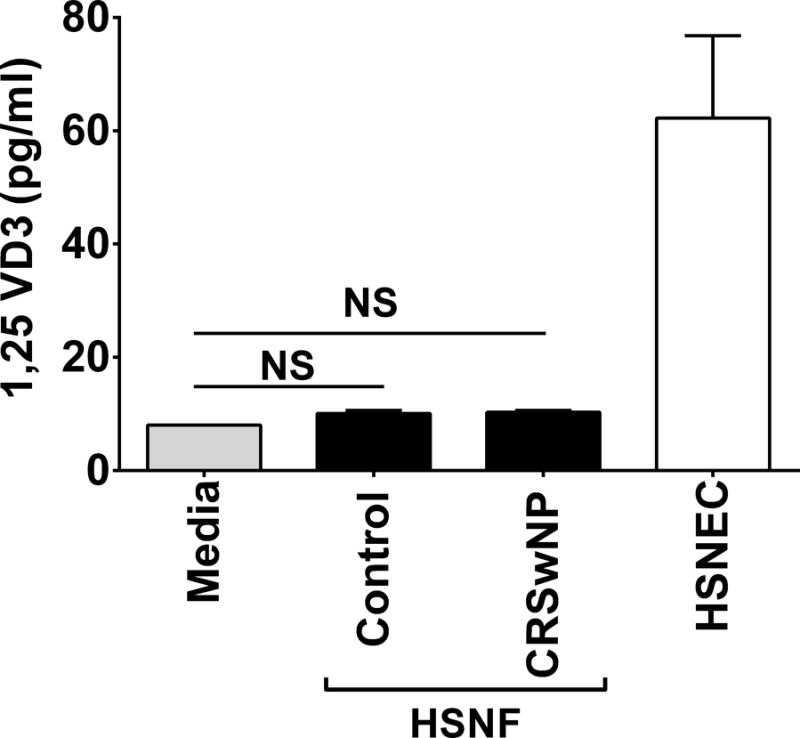
Human sinonasal epithelial cells (HSNEC) can activate 25VD3 to 1,25VD3 and have been included as a positive control. All cells were cultured in serum free media. Mean±SD. *p=0.01 control HSNEC conversion. n=3 patients/group. Statistics shown are result of post hoc analysis following one way ANOVA between indicated groups. NS = not significant.
Next we wanted to determine the ability of HSNF to respond to VD3 by examining their expression of the vitamin D receptor (VDR). We observed that HSNF derived from controls or patients with CRSwNP both expressed VDR (Fig 4). However, a significant decrease in the presence of VDR was seen in CRSwNP patients as compared to control patients (p=0.005).
Figure 4. HSNF from CRSwNP patients have reduced vitamin D receptor (VDR) expression.
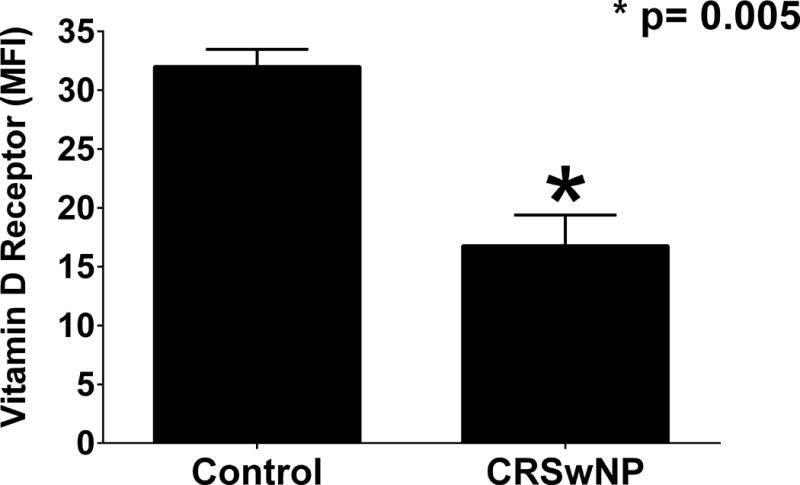
Immunostaining with flow cytometric analysis of VDR expression of cultures HSNFs. N=3 for each group. Statistics shown are results of an unpaired t-test. *p=0.005. (MFI – mean fluorescence intensity)
To corroborate the ex vivo flow cytometry, an in vitro proliferation assay was performed using CFSE to measure response to VD3. HSNFs from patients with CRSwNP had a higher proliferation index at baseline than HSNFs from control patients (p<0.01; Figure 5). When treated with physiological doses 1,25VD3, there was a significant decrease in HSNF proliferation index in patients with CRSwNP (P<0.01; Figure 4), but not control patients (P>0.05; Figure 4). Moreover, 1,25VD3 reduced CRSwNP HSNF proliferation to a level similar to that observed in control HSNF. In summary, the results of this study suggest that 1,25VD3 can suppress HSNF proliferation associated with CRSwNP.
Figure 5. HSNFs from patients with CRSwNP proliferate significantly more at baseline than HSNFs from control patients.
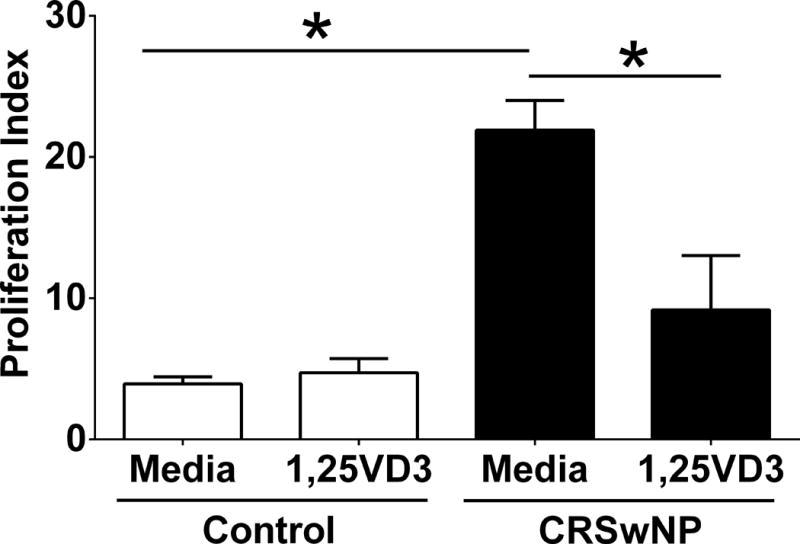
When treated with 1,25VD3 (50 pg/ml), there is a significant decrease in proliferation in patients with CRSwNP. Data shown is mean +/− SEM. Stats shown are results of post hoc analysis following one way ANOVA. N= 5 for control. N=3 for CRSwNP. Proliferation index is the average number of cells that one initial cell became. *p<0.01between indicated groups.
Discussion
Vitamin D deficiency is an increasing public health concern as obesity, time indoors and sunscreen usage all are increasing. VD3 deficiency is associated with multiple disorders of the upper and lower airway including CRSwNP [19, 20]. Adult and pediatric patients with CRSwNP are VD3 deficient and have more severe disease compared to control and CRSsNP [6, 7]. Oral supplementation with 25VD3 would provide a useful and simple solution; however, results are mixed from supplementation trials [21]. Moreover, there is evidence of impaired conversion of active 1,25VD3 from circulating 25VD3 in CRSwNP [10] and while systemic 1,25VD3 is comparable among groups, local 1,25VD3 is reduced in patients with CRSwNP (in press). Therefore, while systemic VD3 may be restored with supplementation, tissue levels will remain inadequate. A number of immune cells have been shown to carry the VDR receptor including the immunologically active fibroblast [11]. Fibroblasts are responsible for the recruitment of eosinophils, which is the dominant inflammatory cell in CRSwNP and drives symptomatology [13, 14]. We recently demonstrated that patients with CRSwNP have more HSNF than control and CRSsNP patients, and also feel worse as measured by SNOT-22 score (in press). Therefore, it is prudent to further investigate VD3 deficiency on the HSNF. We sought to determine whether VD3 deficiency is associated with increased HSNF proliferation.
We show here that HSNF proliferation in CRSwNP is inversely proportional to systemic VD3 levels, and in vitro HSNF proliferation is decreased significantly by treating with 1,25VD3. These results are similar to reports by Bostkowska-Nadolska et al, demonstrating that in conjunction with budesonide R, 1,25VD3 can reduce fibroblast proliferation [15]. We treated HSNF with 1,25VD3 after observing that HSNF could not metabolize 25VD3 themselves. This data suggest that HSNF may be dependent on other cell types present in the sinonasal mucosa, such as epithelial cells, to serve as a local source of 1,25VD3. We also found decreased expression of VDR in CRSwNP HSNF compared to control. A downregulation of VDR may be due to less substrate availability secondary to less 1,25VD3 production from 25VD3 by epithelial cells. Interestingly, total sinonasal VDR expression by PCR is not statistically different between control and CRSwNP [10]. This disparity between total VDR and HSNF VDR levels may be explained by the fact that CRSwNP has more immune infiltrate that express VDR (such as macrophages, B-cells and dendritic cells), which may account for the decreased expression by HSNF.
We have previously demonstrated that increased HSNF are associated with worse quality of life in CRSwNP, therefore the present study begs the question of how proliferating HSNF specifically impact the pathophysiology of CRSwNP. The HSNF’s role in chronic inflammation appears to be both immunologic and structural, as the HSNF is implicated in inflammatory cell recruitment as well as tissue remodeling.
Inflammatory cell recruitment by HSNF has been demonstrated in numerous studies to date. HSNF secrete RANTES which is a potent eosinophil chemotactic factor [22], and importantly, vitamin D has been shown to decrease in vitro secretion of RANTES from nasal polyp HSNF [14]. HSNF from nasal polyps have also been shown to release IL-6 which is implicated in tissue edema [23, 24], as well as thymic stromal lymphopoietin (TSLP), a potent promoter of TH2 inflammation [25]. Further study can help understand which cytokines released by HSNF are most affected by the presence of activated VD3, and guide further investigation into potential therapeutic targets.
HSNF are also involved in extracellular matrix production and resultant tissue remodeling. This dynamic process is well described in the asthma literature, and becoming more understood in CRS. The production of ECM in response to an insult is thought to be chiefly regulated by TGFbeta1, with subsequent ECM degradation tightly regulated by a balance of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs). A disturbance of this balance is thought to underly chronic airway remodeling of upper and lower airway pathology. MMP-9 is the most well described MMP that is elevated in CRSwNP [26]. This increase is associated with increased eosinophils [27]. An increase in MMP-9, without an increase in TIMP-1 is thought to drive the pseudocyst formation of nasal polyps [28]. VD3 has been implicated in regulation of MMP activity [29]. VD3 actions on HSNF proliferation may be a significant upstream factor in regulation of MMP activity and this warrants further examination.
Proliferation is just one method by which fibroblasts are increased in target tissue. Fibrocytes are blood-borne, bone marrow derived cells that are thought to represent an intermediate stage of mesenchymal differentiation, and may migrate into tissues to form fibroblasts [30, 31]. Fibroblasts may also form via epithelial to mesenchymal transition (EMT) in which an epithelial cell takes on fibroblast characteristics as described in tumor pathogenesis, embryogenesis and fibrosis [32]. Recent investigation shows the EMT may play a role in the pathogenesis of CRS as well [33]. Further study is needed to understand all of the mechanisms at work that increase fibroblast populations in CRS.
There are a number of limitations to the current study including small sample size, study design/level of evidence, and lack of confirmatory methods. Additionally, there may be unknown variations in HSNF levels based on anatomic site that was not accounted for. Biopsies were taken from the sphenoid sinus for control pituitary patients, while polyp tissue and other combined sinus tissue was collected from CRSwNP patients. Despite these limitations, we believe the current study provides compelling pilot data investigating the role of HSNF and VD3 in CRSwNP. Further study is needed to fully understand VD3 metabolism in the sinonasal microenvironment; and whether or not restoration of local activated VD3 levels is beneficial to patients with CRSwNP.
Conclusion
The goal of this investigation was to investigate the relationship between VD3 and HSNF proliferation. To our knowledge this is the first investigation to examine this with ex vivo and in vitro methods. We demonstrate an inverse relationship between HSNF proliferation and VD3 levels in CRSwNP. Furthermore, we provide evidence of impaired local production of activated 1,25VD3. Proliferation was significantly decreased upon stimulating HSNFs with 1,25 VD3. Further investigation into how HSNFs and VD3 impact CRSwNP pathophysiology is warranted.
Acknowledgments
These studies were funded by grants from the American Academy of Otolaryngology – Head and Neck Surgery to WWC, Flight Attendant Medical Research Institute to JKM (092401) and RJS (113039) and a Department of Veterans Affairs, Veterans Health Administration, Clinical Sciences Research and Development Merit Award (CSRD 1I01CX000377-01A2) to RJS. This material is the result of work supported with resources and the use of facilities at the Ralph H. Johnson VA Medical Center, Charleston, SC. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
The Medical University of South Carolina Institutional Review Board granted approval prior to initiation of the study and informed written consent was obtained from all participants.
Disclosures: Zachary M. Soler, consultant for Olympus; Rodney J. Schlosser, consultant for Olympus and Arrinex, grant support from Optinose, Intersect ENT and Entellus.
References
- 1.Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. Ann Otol Rhinol Laryngol. 2011;120(7):423–7. doi: 10.1177/000348941112000701. [DOI] [PubMed] [Google Scholar]
- 2.Van Zele T, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61(11):1280–9. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 3.Carney AS, et al. Th2 immunological inflammation in allergic fungal sinusitis, nonallergic eosinophilic fungal sinusitis, and chronic rhinosinusitis. Am J Rhinol. 2006;20(2):145–9. [PubMed] [Google Scholar]
- 4.Ikeda K, et al. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope. 2013;123(11):E1–9. doi: 10.1002/lary.24154. [DOI] [PubMed] [Google Scholar]
- 5.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15(14):2579–85. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 6.Mulligan JK, et al. Vitamin D3 correlates inversely with systemic dendritic cell numbers and bone erosion in chronic rhinosinusitis with nasal polyps and allergic fungal rhinosinusitis. Clin Exp Immunol. 2011;164(3):312–20. doi: 10.1111/j.1365-2249.2011.04325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulligan JK, et al. Vitamin D3 deficiency increases sinus mucosa dendritic cells in pediatric chronic rhinosinusitis with nasal polyps. Otolaryngol Head Neck Surg. 2012;147(4):773–81. doi: 10.1177/0194599812448852. [DOI] [PubMed] [Google Scholar]
- 8.Adorini L, Giarratana N, Penna G. Pharmacological induction of tolerogenic dendritic cells and regulatory T cells. Semin Immunol. 2004;16(2):127–34. doi: 10.1016/j.smim.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4(1):24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 10.Mulligan JK, et al. Cigarette smoke exposure is associated with vitamin D3 deficiencies in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2014;134(2):342–9. doi: 10.1016/j.jaci.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 11.Stio M, et al. Vitamin D receptor in IMR-90 human fibroblasts and antiproliferative effect of 1,25-dihydroxyvitamin D3. Biochem Mol Biol Int. 1997;43(6):1173–81. doi: 10.1080/15216549700205011. [DOI] [PubMed] [Google Scholar]
- 12.Benayoun L, et al. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167(10):1360–8. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 13.Oyer SL, Nagel W, Mulligan JK. Differential expression of adhesion molecules by sinonasal fibroblasts among control and chronic rhinosinusitis patients. Am J Rhinol Allergy. 2013;27(5):381–6. doi: 10.2500/ajra.2013.27.3934. [DOI] [PubMed] [Google Scholar]
- 14.Fraczek M, et al. Vitamin D analogs decrease in vitro secretion of RANTES and enhance the effect of budesonide. Adv Med Sci. 2012;57(2):290–5. doi: 10.2478/v10039-012-0043-5. [DOI] [PubMed] [Google Scholar]
- 15.Rostkowska-Nadolska B, et al. Influence of vitamin D(3) analogues in combination with budesonid R on proliferation of nasal polyp fibroblasts. Acta Biochim Pol. 2009;56(2):235–42. [PubMed] [Google Scholar]
- 16.Fokkens WJ, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;(23):3. p preceding table of contents, 1–298. [PubMed] [Google Scholar]
- 17.Mulligan JK, et al. Human sinonasal epithelial cells direct dendritic function and T-cell T helper 1/T helper 2 skewing following Aspergillus exposure. Int Forum Allergy Rhinol. 2011;1(4):268–74. doi: 10.1002/alr.20055. [DOI] [PubMed] [Google Scholar]
- 18.Psaltis AJ, et al. Characterization of B-cell subpopulations in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013;3(8):621–9. doi: 10.1002/alr.21173. [DOI] [PubMed] [Google Scholar]
- 19.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169(4):384–90. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brehm JM, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179(9):765–71. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yawn J, et al. Vitamin D for the treatment of respiratory diseases: is it the end or just the beginning? J Steroid Biochem Mol Biol. 2015;148:326–37. doi: 10.1016/j.jsbmb.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Maune S, et al. Fibroblasts but not epithelial cells obtained from human nasal mucosa produce the chemokine RANTES. Rhinology. 1996;34(4):210–4. [PubMed] [Google Scholar]
- 23.Peters AT, et al. Evidence for altered activity of the IL-6 pathway in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2010;125(2):397–403. e10. doi: 10.1016/j.jaci.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rostkowska-Nadolska B, et al. Vitamin D derivatives: calcitriol and tacalcitol inhibits interleukin-6 and interleukin-8 expression in human nasal polyp fibroblast cultures. Adv Med Sci. 2010;55(1):86–92. doi: 10.2478/v10039-010-0012-9. [DOI] [PubMed] [Google Scholar]
- 25.Nonaka M, et al. Synergistic induction of thymic stromal lymphopoietin by tumor necrosis factor alpha and Th2 cytokine in nasal polyp fibroblasts. Am J Rhinol Allergy. 2010;24(1):e14–8. doi: 10.2500/ajra.2010.24.3436. [DOI] [PubMed] [Google Scholar]
- 26.Lechapt-Zalcman E, et al. Increased expression of matrix metalloproteinase-9 in nasal polyps. J Pathol. 2001;193(2):233–41. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH771>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 27.Ohno I, et al. Eosinophils as a source of matrix metalloproteinase-9 in asthmatic airway inflammation. Am J Respir Cell Mol Biol. 1997;16(3):212–9. doi: 10.1165/ajrcmb.16.3.9070604. [DOI] [PubMed] [Google Scholar]
- 28.Watelet JB, et al. Matrix metalloproteinases MMP-7, MMP-9 and their tissue inhibitor TIMP-1: expression in chronic sinusitis vs nasal polyposis. Allergy. 2004;59(1):54–60. doi: 10.1046/j.1398-9995.2003.00364.x. [DOI] [PubMed] [Google Scholar]
- 29.Dean DD, et al. Vitamin D regulation of metalloproteinase activity in matrix vesicles. Connect Tissue Res. 1996;35(1–4):331–6. doi: 10.3109/03008209609029208. [DOI] [PubMed] [Google Scholar]
- 30.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87(9):858–70. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 31.Hong KM, et al. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282(31):22910–20. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- 32.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hupin C, et al. Features of mesenchymal transition in the airway epithelium from chronic rhinosinusitis. Allergy. 2014;69(11):1540–9. doi: 10.1111/all.12503. [DOI] [PubMed] [Google Scholar]


