Abstract
Background
The Renal Physicians Association’s clinical practice guideline recommends that physicians address advance care planning with dialysis patients. Data are lacking, however, about how best to implement this recommendation.
Study Design
Quality improvement project in two Tufts-affiliated dialysis facilities in Boston, Massachusetts.
Settings and Participants
Nephrologists caring for patients treated with maintenance hemodialysis at two facilities identified patients who might benefit most from advance care planning using the “surprise” question (“Would I be surprised if this patient died in the next year?”).
Quality Improvement Plan
Patients identified with a ‘no’ response to the surprise question were invited to participate in nephrologist-facilitated advance care planning including the use of a medical Orders for Life-Sustaining Treatment (MOLST) form.
Outcomes
Change in MOLST completion rate and identification of preferences for limits on life-sustaining treatment.
Measurements
Pre- and post-intervention cardiopulmonary resuscitation status, MOLST completion rate, and vital status at one year were measured.
Results
Nephrologists answered ‘no’ to the surprise question for 50 of 201 hemodialysis patients (25%). Of these, 41 (82%) patients had a ‘full-code’ status and 9 (18%) had a ‘do not resuscitate’ (DNR) status. Encounters lasted 15–60 minutes. Following the encounter, 21 (42%) patients expressed preference for a ‘DNR’ status and 29 (58%) maintained full-code status (p = 0.001). The MOLST completion rate increased from 10% to 90%. One-year survival for patients whose nephrologists answered ‘no’ to the surprise question was 58% compared with 92% for those with a ‘yes’ answer (p < 0.001).
Limitations
Sample size and possible non-representative dialysis population.
Conclusions
Nephrologist-facilitated advance care planning targeting hemodialysis patients with limited life expectancy led to significant changes in documented patient preferences for cardiopulmonary resuscitation and limits on life-sustaining treatment. These changes demonstrate the benefit of advance care planning with dialysis patients and likely reflect better understanding of end-of-life treatment options.
Index Words: advance care planning, end-of-life care, hemodialysis, end-stage renal disease (ESRD), code status, medical orders for life-sustaining treatment (MOLST), physician orders for life-sustaining treatment (POLST), do not resuscitate (DNR), cardiopulmonary resuscitation, life expectancy, shared decision making, quality improvement
At any age, persons with end-stage renal disease (ESRD) treated with dialysis have approximately one third the life expectancy observed in the general population. Indeed, a 67 year-old person receiving dialysis has a life expectancy of 4.6 years compared to 15.5 years for the general population(1). Patients older than 75 years are the fastest growing sector of the dialysis population, and for a 77 year old person receiving dialysis, life expectancy drops from 9.1 to 3.3 years (1). These alarming figures call for the establishment of strategies to systematically address goals of care in this vulnerable and high-risk population (2).
Establishing strategies to provide end-of-life care is particularly important due to the underutilization of advance care planning in patients with ESRD (3–5). Advance care planning is the process of clarifying the patient’s current health status, eliciting goals of care, and designating a health care agent to implement these goals. In the United States, the national physician orders for life-sustaining treatment (POLST; also referred to as MOLST [medical orders for life-sustaining treatment]) paradigm is a method of end-of-life planning that emphasizes advance care planning discussions between patients, physicians and loved ones; shared decision-making between patients and their physicians about the end-of-life care they would like to receive; and ensuring that patient wishes are honored (4, 6–8). The POLST paradigm is a transferable validated medical order form designed to ensure that patients’ treatment preferences are honored throughout the healthcare system. Engaging patients with terminal illnesses in advance care planning increases knowledge and is perceived favorably without inducing anxiety, or decreased hope(9, 10). Furthermore, there is compelling evidence that advance care planning favorably affects the quality of life of patients with serious illnesses including those receiving dialysis (11–15).
The Renal Physicians Association has published a clinical practice guideline recommending that physicians address advance care planning with their patients (16). However, evidence-based recommendations evaluating strategies for discussing advance care planning with patients who have limited life expectancy and their families are lacking (5). In the United States, the majority of patients with ESRD treated by hemodialysis receive care in an integrated multidisciplinary setting. Hence, a structured approach to discussing goals of care that can be implemented in dialysis centers is a potentially effective strategy to educate selected patients regarding end-of-life care and facilitate informed decision-making about goals of care and desired future medical treatment.
In this quality improvement project, we utilized a dedicated clinical encounter to educate hemodialysis patients with physician-estimated limited life expectancy on advance care planning and end-of-life care. The goals of this project were to increase patient autonomy and informed decision-making as measured by the completion rate of the Massachusetts MOLST (a form legally authorized for medical orders by the Commonwealth of Massachusetts; provided in the supplementary material as Item S1), identifying patient preferences for life-sustaining treatment options, and clarifying the code status. We elected to use this MOLST form as a tool and marker of patient autonomy due to its simple language, wide availability, legal acceptance in Massachusetts, and ability to deal with a range of treatment options and limitations including dialysis. Additionally, it can be easily maintained and transferred between different institutes (17, 18). Further, literature suggests that POLST forms accurately convey and lead to honoring the treatment preferences and wishes of patients in most instances (19, 20). In this project we aimed to develop a method that is practical, widely applicable, and comprehensive with a focus on the core values of patient choice and autonomy.
METHODS
Setting
In June 2013–July 2014, we evaluated all adults receiving hemodialysis at two outpatient dialysis facilities (Dialysis Clinic, Inc. Boston and St. Elizabeth’s Medical Center, Boston, MA) affiliated with the Tufts nephrology fellowship training program. Institutional review board approval with waiver of consent was obtained for this quality improvement project at both participating sites.
Intervention
The primary nephrologists caring for the patients identified those with a predicted shorter life expectancy by answering the following ‘surprise’ question, which has previously been shown to be effective at identifying sicker patients who have a high risk for early mortality and who might benefit from palliative care interventions: “Would I be surprised if this patient died in the next year?”(21). Patients identified with a ‘no’ answer were invited to participate in a dedicated clinical encounter focusing on advance care planning. The dedicated face-to-face encounter was conducted during a routine hemodialysis session by a nephrology fellow (O.W.A. or M.R.). Each encounter lasted 15–60 minutes.
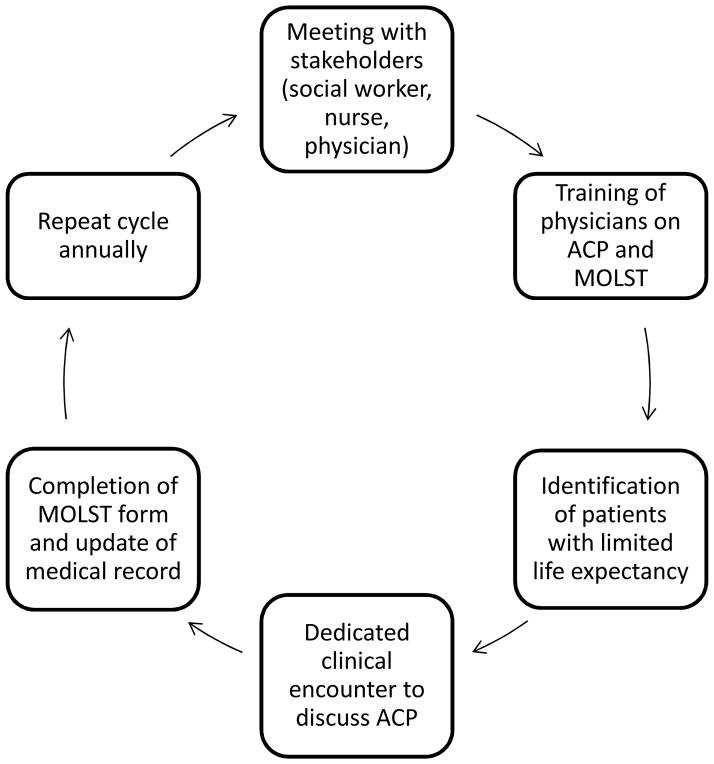
As part of the preparatory work (Figure 1), the planned quality improvement project was first introduced to all stakeholders at each dialysis facility, including the social worker, the nurse manager, the medical director, and the treating physicians. The two fellows championing this project who had expressed an interest in conducting the clinical encounters received training from a faculty member (Klemens B. Meyer, MD) with research and clinical experience in end-of-life care and patient-physician communication. The training included the conduct of mock interviews with patients and families addressing goals of care and end of life care issues. The fellows’ performance in the mock interviews was evaluated by the session’s moderator who provided suggestions to improve communication skills. A separate training session on the elements of the Massachusetts MOLST form and its use was conducted (moderated by B.L.J.). Intentionally, no written script was provided to the fellows conducting the dedicated encounter, and the discussion format was left to their discretion.
Figure 1.
Key elements of the advance care planning quality improvement program. The program started by meeting with stakeholders (social worker, nurse, physician), followed by training of physicians on advance care planning (ACP) and completion of the Massachusetts MOLST form who conducted the dedicated clinical encounters with selected patients who had with a limited life expectancy. This educational cycle is repeated annually.
Prior to the encounter, primary nephrologists were consulted to determine the decision-making capacity of their patients to participate in advance care planning and complete the MOLST form. Patients deemed to have capacity were informed of the purpose of the meeting and were offered the option of having a family member present. After obtaining the patient’s permission, family members were contacted to check for their availability for the meeting. Information on the Massachusetts MOLST form was provided during the patient encounter. Conveying the information regarding physician’s predicted life expectancy was left to the primary nephrologist’s discretion and was not part of the encounter. For patients who lacked capacity (mainly due to dementia), the encounters were conducted with their health care proxy after verifying the legal documents in the dialysis charts.
The teach-back technique was used to clarify what physicians and patients understood at the end of each encounter. This was achieved by asking patients to explain in their own words what had been discussed. The teach-back technique has been recommended to enhance communication with patients and to confirm understanding (22–24). Interpreter services were provided to patients who were not English proficient, based on the recommendation by the social worker, dialysis nurse, or primary nephrologist.
Following completion of the MOLST form (Item S1) by the patient and their physician, the original document was given to the patient, and a copy was placed in the dialysis facility’s medical record along with a written documentation of the discussion. Where applicable, a copy of the MOLST form was provided to the long-term care facility where the patient resided, and a brief discussion was carried out with the patient’s physician at that facility to communicate any wishes in the patient’s code status following the dedicated clinical encounter.
Measures
Prior to the intervention, the following patients’ demographic, clinical and laboratory characteristics were extracted from the medical records. These included age, sex, self-reported race/ethnicity, and need for language interpretation; history of hypertension, diabetes mellitus, ischemic heart disease, heart failure, peripheral artery disease, stroke, amputations, dementia, and active cancer; and serum creatinine and albumin levels.
The patient’s code status was ascertained before and after the intervention by reviewing the dialysis medical record. Patients were followed up for 12 months to ascertain their vital status, in accordance with the ‘surprise’ question classification at baseline. In addition, MOLST form completion rate prior to and following the intervention was ascertained.
Analyses
Continuous variables were described as mean ± standard deviation and categorical variables as count (percentage). Comparisons were conducted using the Chi-square and McNemar’s tests for independent and paired binary variables, respectively. The student t-test was used for continuous variables. A Kaplan-Meier survival curve was constructed according to the physician response to the ‘surprise’ question stratified according to predicted shorter or longer life expectancy over the ensuing 12 months. The log rank test was used to test survival time differences between the two groups. A two-sided P value of less than 0.05 was considered statistically significant. All analyses were performed using the R statistical package (25).
RESULTS
A total of 201 patients with ESRD receiving hemodialysis were enrolled from the two outpatient dialysis facilities (Figure 2). Mean age was 66 years, and 35% were Caucasian. All 9 staff nephrologists at the two dialysis facilities participated in the project. Eight (88%) of the physicians had been practicing for more than 10 years.
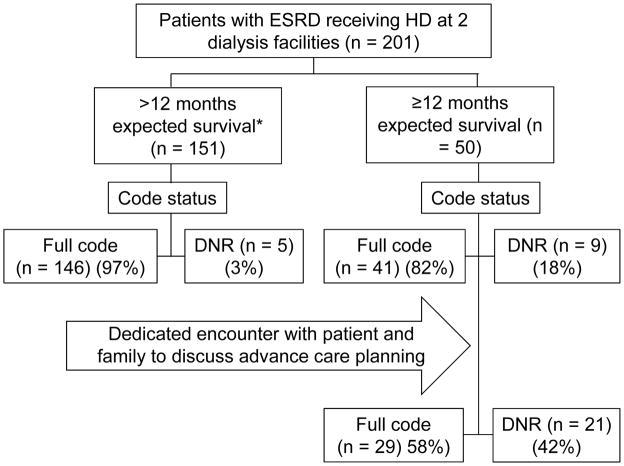
Figure 2.
Quality improvement project flow diagram. *The predicted survival expectation is based on the response of the nephrologist caring for the patient to the following ‘surprise’ question: “Would I be surprised if this patient died in the next year?” (21). A ‘yes’ answer to the question indicates a physician-predicted life expectancy of > 1 year; a ‘no’ answer to the question indicates a physician-predicted life expectancy ≤ 1 year. DNR denotes Do Not Resuscitate. The patient’s code status significantly changed following the dedicated clinical encounter on advance care planning, with an increase in the DNR order status from 18% to 42% (p = 0.001 by the McNemar’s test).
Nephrologists answered ‘no’ to the surprise question on 50 (25%) of their patients. As shown in Table 1, patients identified with a ‘no’ response, projected to have a shorter life expectancy, tended to be older and had a higher prevalence of comorbid conditions, including coronary artery disease, heart failure, cerebrovascular disease, dementia, peripheral vascular disease, amputation, and active cancer. Prior to the planned intervention, only 9 (18%) of the 50 patients with a physician-predicted shorter life expectancy had a written ‘do not resuscitate’ (DNR) physician order in the medical record. Of these 50 patients, 48 patients and/or their family members/proxies participated in the dedicated encounter. One patient declined to participate and one health care proxy could not be reached; 13 (26%) patients required interpreter services. Following the dedicated encounter to discuss advance care planning, an additional 12 patients opted for a DNR order, bringing the total to 21 (42%) of the 50 patients (p = 0.001; Figure 2). Among these 12 additional patients, 2 had previously discussed their preferences for life-sustaining treatment limitations with their primary care physician, but this information had not been communicated to the treating nephrologist. Following the intervention, the proportion of patients completing a Massachusetts MOLST form increased from 10% prior to the intervention to 90% following the intervention (p < 0.001). A 6-month reevaluation showed no significant change in completion of the MOLST form among patients who did not receive the dedicated encounter (data not shown). The elements of the MOLST form that were completed by 45 of the 48 patients who received the dedicated encounter are provided in Table 2. In brief, in case of cardiopulmonary arrest, 42% of patient did not wish to receive cardiopulmonary resuscitation and mechanical ventilation, 22% did not wish to receive artificial nutrition, and 2% did not wish to be transferred to a hospital. All patients who completed the MOLST form opted for artificial hydration if indicated. Three patients did not complete the forms citing no change in preference (1 patient) or the need for more time to reflect (2 patients).
Table 1.
Baseline Characteristics of the study population according to physician response to the ‘surprise’ question
| Variable | Physician response to the ‘surprise’ question* | P value | |
|---|---|---|---|
|
| |||
| No (Shorter predicted life expectancy) (n = 50) | Yes (Longer predicted life expectancy) (n = 151) | ||
|
| |||
| Age, years | 74 ± 13 | 63 ± 14 | < 0.001 |
|
| |||
| Men | 23 (46) | 82 (54) | 0.08 |
|
| |||
| Race/Ethnicity† | 0.07 | ||
| White | 25 (50) | 45 (30) | |
| African American | 7 (14) | 39 (26) | |
| Hispanic | 3 (6) | 14 (9) | |
| Asian | 14 (28) | 48 (32) | |
| Other | 1 (2) | 5 (3) | |
|
| |||
| Need for interpreter services | 13 (26) | 41 (27) | 0.8 |
|
| |||
| Comorbid conditions | |||
|
| |||
| Hypertension | 31 (62) | 109 (72) | 0.1 |
|
| |||
| Diabetes mellitus | 19 (38) | 59 (39) | 0.9 |
|
| |||
| Coronary artery disease | 20 (40) | 27 (18) | < 0.001 |
|
| |||
| Heart failure | 16 (32) | 27 (18) | 0.02 |
|
| |||
| Cerebrovascular accident | 9 (18) | 11 (7) | 0.02 |
|
| |||
| Dementia | 8 (16) | 5 (3) | 0.004 |
|
| |||
| Peripheral vascular disease | 10 (20) | 6 (4) | < 0.001 |
|
| |||
| Amputation | 7 (14) | 0 (0) | < 0.001 |
|
| |||
| Active cancer | 4 (8) | 3 (2) | 0.03 |
|
| |||
| Serum creatinine (mg/dL) | 7.6 ± 2.3 | 10.0 ± 3.0 | < 0.001 |
|
| |||
| Serum albumin (gm/dL) | 3.3 ± 0.5 | 4.0 ± 1.5 | < 0.001 |
Note: Values for categorical values are given as frequency (percentage); for continuous variables, as mean ± standard deviation.
The surprise question: “Would I be surprised if the patient died in the next year?” (21);
Race and ethnicity are self-reported.
Table 2.
Selected elements of Massachusetts MOLST form completed by patients who received advance care planning
| No. (%) | |
|---|---|
| Cardiopulmonary Resuscitation | |
| Do not resuscitate | 21 (47) |
| Attempt resuscitation | 24 (53) |
| Ventilation | |
| Do not intubate and ventilate | 21 (47) |
| Intubate and ventilate | 24 (53) |
| Transfer to Hospital | |
| Do not transfer to hospital | 1 (2) |
| Transfer to hospital | 44 (98) |
| Artificial Nutrition | |
| Do not use artificial nutrition | 11 (24) |
| Use artificial nutrition | 34 (76) |
| Artificial Hydration | |
| Do not use artificial hydration | 0 (0) |
| Use artificial hydration | 45 100) |
Note: n=45.
MOLST, Medical Orders for Life-Sustaining Treatment
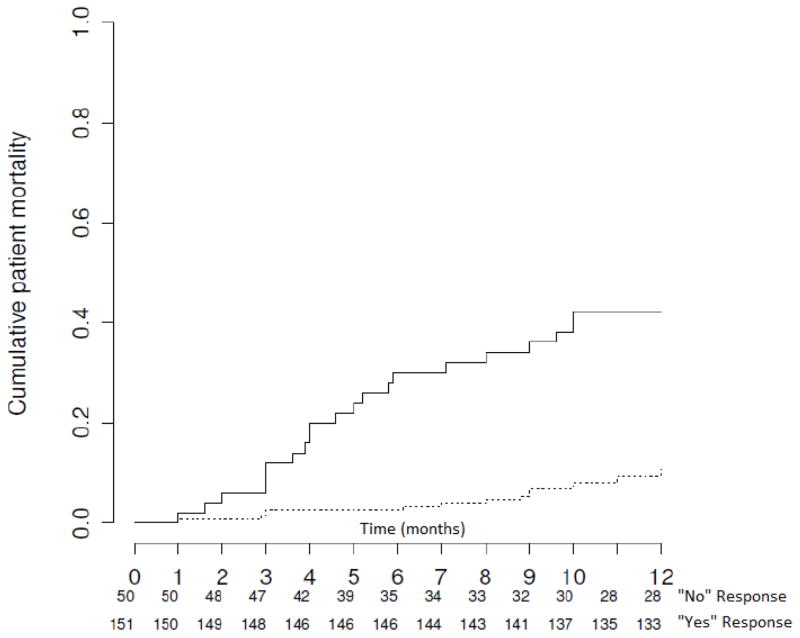
Vital status was ascertained on all 201 patients, and, by 12 months of follow-up, 39 (19%) had died. Patients with physician-predicted lower life expectancy had a cumulative lower 12-month survival rate compared to those with a longer predicted life expectancy (58% versus 92%; p < 0.001; Figure 3), supporting the prognostic validity of the ‘surprise’ question for targeting advance care planning efforts.
Figure 3.
Kaplan-Meier survival plot depicting cumulative mortality among patients with shorter (solid line) and longer (dashed line) physician-estimated life expectancy. The analysis is censored at 12 months. p < 0.001 by the log-rank test. The physician-estimated life expectancy is based on the physician response to the following ‘surprise’ question: “Would I be surprised if this patient died in the next year?” (21).
DISCUSSION
In this quality improvement project, using the POLST paradigm, we demonstrated that a clinical encounter dedicated to advance care planning that targets hemodialysis patients with limited life expectancy can lead to the proper identification of their preferences on life-sustaining treatments and wishes about code status. The use of these encounters resulted in a 90% completion rate of the Massachusetts MOLST form and a greater than 50% increase in the percentage of patients who opted for a DNR code status. The ‘surprise’ question was effective at identifying higher risk dialysis patients and was effectively used to prioritize our targeted intervention.
The significant increase in the percentage of patients with a documented DNR code status following our intervention was partly related to the fact that some patients who were classified as ‘full code’ in their medical records were under the impression that their wishes to the contrary were already known. The remainder of the code status change might reflect better patient understanding of end-of-life care, which was facilitated by the clinician who led the discussion. Patients were able to select the code status that was in line with their preferences and beliefs once they were able to better understand their treatment options. In a prior study, if seriously ill patients had talked about their care preferences with their physicians, they were more likely to receive care in accordance with their preferences (26). In our study, 24% percent of patients who received the dedicated encounter did not wish to rely on artificial nutrition. This preference is important to identify, especially among patients with advanced comorbidity and at high risk of cognitive decline, as there is no evidence to suggest that artificial nutrition prolongs survival, or improves quality of life in such patients (27, 28). The preference to avoid artificial nutrition is consistent with a previous cross sectional study where 39% of patients surveyed upon hospitalization for symptoms of advanced cancer expressed preference to forgo artificial nutrition (29).
The in-center hemodialysis setting carries particular barriers to advance care planning, including a lack of understanding among patients, caregivers, and providers (30, 31). The greatest asset for the successful completion of this quality improvement project was the unwavering support of the treating nephrologists, social workers and dialysis nurses as well as the presence of two dedicated champions, in this case nephrology fellows, to lead these discussions with patients and their families. A national survey on the quality of nephrology fellowship training and attitude toward end-of-life care recently found that while nearly all surveyed fellows believed that physicians had a responsibility to help patients at end of life, the quality of this teaching had not improved over the past decade, and this report called for integrating palliative care rotations into nephrology fellowship curricula (32).
Language barrier can be a significant obstacle to achieving these goals in patients with limited life expectancy and who are not conversant in the English language, and who may have different cultural values. In our project, we utilized interpreter services liberally to ensure that patients who did not speak English fluently could be full participants in the advance care planning discussions. We elected to use a patient-centered approach to discuss goals of care that took advantage of the established rapport between patients and their primary nephrologists. Too often, patients are approached during a hospitalization to discuss advance care planning by a provider they hardly know. In our approach, the discussions were assisted by the clinicians who knew the patients, and were carried out in a relaxed, non-urgent ambulatory setting. The encounters were dedicated entirely for the purpose of discussing advance care planning. Using the MOLST form, the initiating clinician stressed the desire to listen to, and not influence, the wishes of the patients, who appeared to be comfortable discussing this topic and sharing their wishes and grateful for the opportunity to do so.
Our quality improvement project has several strengths. Foremost, it addresses the critical importance of identifying goals of care and matching patients to their preferred treatments. Furthermore, end-of-life is associated both with substantial financial and emotional outlays. The Dartmouth Atlas Project has documented variation in care for Medicare beneficiaries for more than 20 years, forming the basis for many of the continuing attempts to improve health and health systems across the United States (33). The unnecessarily high levels of care in the last six months of life have been used as an indicator of the propensity to use life-saving technology. In 2012, total Medicare reimbursements per decedent in the last 6 months of life reached a national average of $35,931 (34). For the purpose of advance care planning, we developed a simple, practical, and effective approach to address goals of care in the unique setting of dialysis facilities.
There are important limitations to consider. The patient experience and comfort with the encounter were not systematically captured in this project. There is no control group for comparison, and the sample size was too small to examine the impact of cultural or religious beliefs on the completion of the MOLST form. A patient-specific estimate of prognosis was not calculated prior to the encounter with each patient. For patients who subsequently died, whether their wishes were honored or not at the time of death was not ascertained. The effect of our intervention on changing expressed preferences in other dialysis populations will presumably depend on the socio-demographic characteristics of patients and the burden of their chronic illnesses, and on whether they have previously been exposed to similar information. The approach is also likely to be variably successful depending on the clinicians conducting the advance care planning encounters. For example, in a physician survey, nephrologists older than 65 years are more likely to recommend dialysis to their patients (35). The success of the approach we adopted is highly dependent on the clinician’s communication skills. In this regard, incorporating a palliative care curriculum during nephrology fellowship training is essential (36).
In conclusion, this quality improvement project demonstrates the powerful impact of a dedicated encounter in addressing advance care planning in a selected cohort of patients receiving long-term hemodialysis who have a limited life expectancy. The findings of this report are consistent with other initiatives aimed at addressing goals of care with patients and their families (37). This simple, patient-centered and effective process is instructive and useful for dialysis facilities that have not incorporated dedicated advance care planning encounters in their routine practice. Future studies are needed to examine the efficacy of adopting such a program on a broader scale and in different patient populations and settings. For our dialysis facilities, we plan to implement this program annually with emphasis on physician training. Our report calls for the need to establish policies in dialysis facilities that address advance care planning in a systematic way among patients with ESRD who have a limited life expectancy. This critical period needs to be studied prospectively to identify interventions that can deescalate care, prevent hospital admissions and readmissions, provide compassionate end-of-life care including hospice care, and curtail unnecessary health care expenditures.
Supplementary Material
Item S1: Massachusetts Medical Orders for Life-Sustaining Treatment (MOLST) form.
Acknowledgments
The authors thank Dr Klemens B. Meyer for his thoughtful suggestions and support for this quality improvement project.
Support: Dr Amro was supported by a National Institutes of Health (NIH) institutional training grant (5T32DK007777). This project was supported by the National Center for Advancing Translational Sciences, NIH, grant UL1 TR001064. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: OWA, BLJ; data acquisition: OWA, MR, DEW, JAS, BLJ; data analysis/interpretation: OWA, DEW, BLJ; statistical analysis: OWA, DEW, BLJ; Supervision or mentorship: DEW, BLJ. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. BLJ takes full responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Peer Review: Evaluated by 3 external peer reviewers, a statistician, and an Acting Editor-in-Chief.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.United States Renal Data System. 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2014. [Google Scholar]
- 2.Davison SN, Levin A, Moss AH, et al. Executive summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: developing a roadmap to improving quality care. Kidney Int. 2015;88(3):447–59. doi: 10.1038/ki.2015.110. [DOI] [PubMed] [Google Scholar]
- 3.Perry E, Swartz R, Smith-Wheelock L, Westbrook J, Buck C. Why is it difficult for staff to discuss advance directives with chronic dialysis patients? Journal of the American Society of Nephrology : J Am Soc Nephrol. 1996;7(10):2160–8. doi: 10.1681/ASN.V7102160. [DOI] [PubMed] [Google Scholar]
- 4.Holley JL, Stackiewicz L, Dacko C, Rault R. Factors influencing dialysis patients’ completion of advance directives. Am J Kidney Dis. 1997;30(3):356–60. doi: 10.1016/s0272-6386(97)90279-1. [DOI] [PubMed] [Google Scholar]
- 5.Bristowe K, Horsley HL, Shepherd K, et al. Thinking ahead - the need for early Advance Care Planning for people on haemodialysis: A qualitative interview study. Palliat Med. 2015;29(5):443–50. doi: 10.1177/0269216314560209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillick MR. Reversing the code status of advance directives? N Engl J Med. 2010;362(13):1239–40. doi: 10.1056/NEJMe1000136. [DOI] [PubMed] [Google Scholar]
- 7.Lo B, Steinbrook R. Resuscitating advance directives. Arch Intern Med. 2004;164(14):1501–6. doi: 10.1001/archinte.164.14.1501. [DOI] [PubMed] [Google Scholar]
- 8.Messinger-Rapport BJ, Baum EE, Smith ML. Advance care planning: Beyond the living will. Cleveland Clin J Med. 2009;76(5):276–85. doi: 10.3949/ccjm.76a.07002. [DOI] [PubMed] [Google Scholar]
- 9.Green MJ, Schubart JR, Whitehead MM, Farace E, Lehman E, Levi BH. Advance Care Planning Does Not Adversely Affect Hope or Anxiety Among Patients With Advanced Cancer. J Pain Symptom Manage. 2015 Jun;49(6):1088–96. doi: 10.1016/j.jpainsymman.2014.11.293. [DOI] [PubMed] [Google Scholar]
- 10.Weisbord SD, Carmody SS, Bruns FJ, et al. Symptom burden, quality of life, advance care planning and the potential value of palliative care in severely ill haemodialysis patients. Nephrol Dial Transplant. 2003;18(7):1345–52. doi: 10.1093/ndt/gfg105. [DOI] [PubMed] [Google Scholar]
- 11.Brinkman-Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end-of-life care: A systematic review. Palliat Med. 2014;28(8):1000–25. doi: 10.1177/0269216314526272. [DOI] [PubMed] [Google Scholar]
- 12.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. doi: 10.1136/bmj.c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt RJ, Weaner BB, Long D. The Power of Advance Care Planning in Promoting Hospice and Out-of-Hospital Death in a Dialysis Unit. J Palliat Med. 2015;18(1):62–6. doi: 10.1089/jpm.2014.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown MA, Collett GK, Josland EA, Foote C, Li Q, Brennan FP. CKD in elderly patients managed without dialysis: survival, symptoms, and quality of life. Clin J Am Soc Nephrol. 2015;10(2):260–8. doi: 10.2215/CJN.03330414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davison SN, Simpson C. Hope and advance care planning in patients with end stage renal disease: qualitative interview study. BMJ. 2006;333(7574):886. doi: 10.1136/bmj.38965.626250.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrington K, Warwick G. Renal Association Clinical Practice Guideline on planning, initiating and withdrawal of renal replacement therapy. Nephron Clin Pract. 2011;118(Suppl 1):c189–208. doi: 10.1159/000328069. [DOI] [PubMed] [Google Scholar]
- 17.Hickman SE, Tolle SW, Brummel-Smith K, Carley MM. Use of the Physician Orders for Life-Sustaining Treatment program in Oregon nursing facilities: beyond resuscitation status. J Am Geriatr Soc. 2004;52(9):1424–9. doi: 10.1111/j.1532-5415.2004.52402.x. [DOI] [PubMed] [Google Scholar]
- 18.Hickman SE, Nelson CA, Moss AH, et al. Use of the Physician Orders for Life-Sustaining Treatment (POLST) paradigm program in the hospice setting. J Palliat Med. 2009;12(2):133–41. doi: 10.1089/jpm.2008.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyers JL, Moore C, McGrory A, Sparr J, Ahern M. Physician orders for life-sustaining treatment form: honoring end-of-life directives for nursing home residents. J Gerontol Nurs. 2004;30(9):37–46. doi: 10.3928/0098-9134-20040901-08. [DOI] [PubMed] [Google Scholar]
- 20.Lee MA, Brummel-Smith K, Meyer J, Drew N, London MR. Physician orders for life-sustaining treatment (POLST): outcomes in a PACE program. Program of All-Inclusive Care for the Elderly. J Am Geriatr Soc. 2000;48(10):1219–25. doi: 10.1111/j.1532-5415.2000.tb02594.x. [DOI] [PubMed] [Google Scholar]
- 21.Moss AH, Ganjoo J, Sharma S, et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3(5):1379–84. doi: 10.2215/CJN.00940208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamura-Lis W. Teach-Back for quality education and patient safety. Urol Nurs. 2013;33(6):267–71. 98. [PubMed] [Google Scholar]
- 23.Schwartz DB, Barrocas A, Wesley JR, Kliger G, Pontes-Arruda A, Marquez HA, et al. Gastrostomy tube placement in patients with advanced dementia or near end of life. Nutr Clin Pract. 2014;29(6):829–40. doi: 10.1177/0884533614546890. [DOI] [PubMed] [Google Scholar]
- 24.Kripalani S, Weiss BD. Teaching about health literacy and clear communication. J Gen Intern Med. 2006;21(8):888–90. doi: 10.1111/j.1525-1497.2006.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 26.Teno JM, Fisher E, Hamel MB, Wu AW, Murphy DJ, Wenger NS, et al. Decision-making and outcomes of prolonged ICU stays in seriously ill patients. J Am Geriatr Soc. 2000;48(5 Suppl):S70–4. doi: 10.1111/j.1532-5415.2000.tb03144.x. [DOI] [PubMed] [Google Scholar]
- 27.Harwood RH. Feeding decisions in advanced dementia. J R Coll Physicians Edinb. 2014;44(3):232–7. doi: 10.4997/JRCPE.2014.310. [DOI] [PubMed] [Google Scholar]
- 28.Good P, Cavenagh J, Mather M, Ravenscroft P. Medically assisted nutrition for palliative care in adult patients. The Cochrane database of systematic reviews. 2008;(4):CD006274. doi: 10.1002/14651858.CD006274.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Bukki J, Unterpaul T, Nubling G, Jox RJ, Lorenzl S. Decision making at the end of life--cancer patients’ and their caregivers’ views on artificial nutrition and hydration. Support Care Cancer. 2014;22(12):3287–99. doi: 10.1007/s00520-014-2337-6. [DOI] [PubMed] [Google Scholar]
- 30.Grubbs V, Moss AH, Cohen LM, et al. A palliative approach to dialysis care: a patient-centered transition to the end of life. Clin J Am Soc Nephrol. 2014;9(12):2203–9. doi: 10.2215/CJN.00650114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luckett T, Sellars M, Tieman J, et al. Advance care planning for adults with CKD: a systematic integrative review. Am J Kidney Dis. 2014;63(5):761–70. doi: 10.1053/j.ajkd.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Combs SA, Culp S, Matlock DD, Kutner JS, Holley JL, Moss AH. Update on end-of-life care training during nephrology fellowship: a cross-sectional national survey of fellows. Am J Kidney Dis. 2015;65(2):233–9. doi: 10.1053/j.ajkd.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasternak S. End-of-Life Care Constitutes Third Rail of US Health Care Policy Debate. The Medicare News Group; [Accessed December 25, 2015]. Available from: https://web.archive.org/web/20150917010545/http://www.medicarenewsgroup.com/context/understanding-medicare-blog/understanding-medicare-blog/2013/06/03/end-of-life-careconstitutes-third-rail-of-u.s.-health-care-policy-debate. [Google Scholar]
- 34.The Dartmouth Atlas of Health Care. cited 2015 May 23 Available from: http://www.dartmouthatlas.org.
- 35.Foote C, Morton RL, Jardine M, et al. COnsiderations of Nephrologists when SuggestIng Dialysis in Elderly patients with Renal failure (CONSIDER): a discrete choice experiment. Nephrol Dial Transplant. 2014;29(12):2302–9. doi: 10.1093/ndt/gfu257. [DOI] [PubMed] [Google Scholar]
- 36.Schell JO, Green JA, Tulsky JA, Arnold RM. Communication skills training for dialysis decision-making and end-of-life care in nephrology. Clin J Am Soc Nephrol. 2013;8(4):675–80. doi: 10.2215/CJN.05220512. [DOI] [PubMed] [Google Scholar]
- 37.Poppel DM, Cohen LM, Germain MJ. The Renal Palliative Care Initiative. J Palliat Med. 2003;6(2):321–6. doi: 10.1089/109662103764978650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1: Massachusetts Medical Orders for Life-Sustaining Treatment (MOLST) form.