Abstract
Background
Requests from researchers for olfactory mucosal biopsies are increasing as a result of advances in the fields of neuroscience and stem cell biology. Published studies report variable rates of success in obtaining true olfactory tissue, often below 50%. In cases where biopsies are not obtained carefully and confirmed through histological techniques, erroneous conclusions are made. Attention to the epithelium alone without submucosal analysis may add to the confusion. A consistent biopsy technique can help rhinologists obtain higher yields of olfactory mucosa. Confirmatory tissue staining analysis assures olfactory mucosa has been obtained thereby strengthening clinical correlations and scientific conclusions.
Methods
Biopsies of the septum within the anterior olfactory cleft were obtained under endoscopic guidance in an office procedure room using topical local anesthetic (lidocaine). After mucosal incision, a small, cupped, biopsy forceps was used to obtain specimens approximately 2-3 mm in size. Specimens were sectioned and analyzed with immunohistochemistry for presence of olfactory epithelium and/or olfactory fascicles.
Results
A total of 14 subjects were biopsied in this analysis. Four subjects had biopsies in the operating room (OR). The remaining ten underwent biopsies in the clinic. All biopsies obtained in the OR revealed evidence of olfactory mucosa. A total of eight out of ten (80%) clinic biopsies revealed evidence of olfactory mucosa. No complications were encountered.
Conclusion
High yields of olfactory mucosa can be obtained safely in an office-based setting. Technique, including attention to the area of biopsy, and confirmatory analysis are important in assuring presence of olfactory tissue.
Keywords: olfactory epithelium, olfactory fila, humans, immunohistochemistry, S100, intermediate filaments, p75 neurotrophin receptor, olfactory ensheathing cells
Background
The olfactory epithelium (OE), including the olfactory receptor neurons (ORNs) that maintain first-order synapses with the olfactory bulb, is unique in its ability to regenerate throughout life.1 The basal cells of the epithelium have a major role in this capacity and respond with up-regulation of cell division after injury to the system.2,3 The olfactory mucosa of humans has been shown to be very similar to other mammals such as rodents in regards to cellular consistency and immunohistochemical staining characteristics.4 The regenerative capacity of the OE also appears to be maintained in humans based on biopsy culture results5 and the continued presence of both mature and immature OSNs regardless of age with expression of cell division markers in the basal cells.4
In humans, as opposed to rodents, it appears that the regenerative process in response to insult may be less successful in terms of function. Common causes for loss of olfaction have been consistently identified among smell and taste centers and include those related to head injury, upper respiratory tract infections, chronic rhinosinusitis, and age while a large proportion have no identifiable cause (idiopathic).6,7 However, we have a poor understanding of the pathophysiology underlying these most common causes. As the basic mechanisms of olfactory physiology and regenerative processes are discovered in mouse models, comparisons with biopsies of human olfactory mucosa will remain critical in identifying causality.
In addition, the basal cells of the OE--with their characteristic features that satisfy the criteria of bone fide stem cells and their accessibility for biopsy--are of interest to researches in the fields of neurodegenerative disorders and neural regeneration. There has been a surge of interest in culturing these basal stem cells for potential use in repair of various forms of central nervous system disorders.8 In addition, the olfactory ensheathing cells enveloping the olfactory axons and bundled together as fila olfactoria in the lamina propria, are thought to have special properties to allow for enhanced axonal guidance and regeneration after injury to motor neurons of the spinal cord and facial nerve.9-11
Obtaining biopsies of human olfactory mucosa is not new12-16, but the technique is often poorly described. Frequently, published results from research labs provide conclusions depending on biopsies from “olfactory areas” reported by a surgeon's general anatomic description. In certain cases the biopsies are cultured without histological confirmation that the tissue is indeed olfactory with conclusions made entirely on stained cells that have been grown and passaged in various culture conditions.17 For purposes of OE analysis for olfactory disorders, the pervasive presence of respiratory epithelium in a biopsy could be a result of sample selection error and result in erroneous conclusions of respiratory metaplasia within an olfactory region.13 For these reasons, consistency in the biopsy technique as well as care in sample evaluation is crucial for advancing the field.
We describe an endoscopic biopsy technique for consistently obtaining human olfactory mucosa in awake, non-sedated subjects in a clinic setting using widely available otolaryngology instruments. We also argue for the importance of confirming olfactory origin of the mucosa using described methods that can be used regardless of epithelial presence or respiratory status.
Methods
Subjects
Research subjects were recruited from patients visiting the Sinus Center at Massachusetts Eye and Ear Infirmary (MEEI) for reasons of smell disorders. Subjects with an identifiable cause of smell loss either related to upper respiratory infection (URI) or head trauma were enrolled. Olfactory function was assessed using a 40-item, scratch-and-sniff, forced-choice, smell-identification test (SIT, Sensonics, Inc., Haddon Heights, NJ). A group of subjects undergoing septoplasty (one subject with URI-related anosmia) were also chosen for operating room (OR) based biopsies as a comparison. All subjects provided informed consent for participation in this study that was approved by the Human Subjects Committee at MEEI.
OR-based Olfactory Biopsies
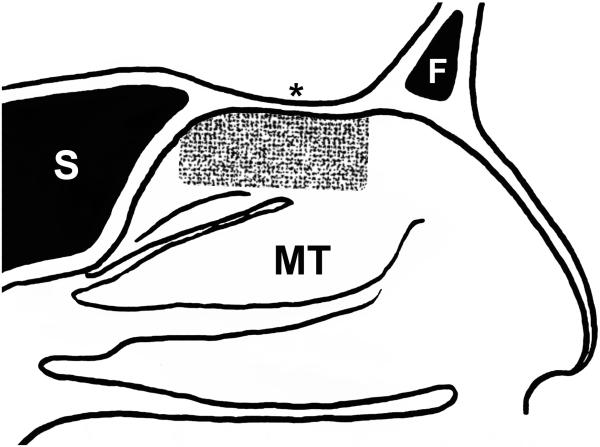
Biopsies performed in the OR were obtained under general anesthesia after the septoplasty procedure using injections of 1% lidocaine with 1:100,000 parts epinephrine. Three specimens were obtained from one side only to reduce the risk of smell loss. Specimens were obtained from the superior septum within the olfactory cleft and adjacent to the middle turbinate using a sickle knife and pediatric blakesley forceps or cupped forceps. The area considered high-yield for OE (Figure 1) was defined using averaged areas calculated from immunostaining for neurons over multiple whole-mount specimens (data not shown).18
Figure 1.
Cartoon representation of a sagittal section through the human nasal cavity showing the high-yield area of olfactory epithelium (OE). The peppered pattern demonstrates the area of the nasal septum most often associated with OE based on averaging from multiple autopsy specimens and shows the positional relationship among easily identifiable nasal structures. Notice that the OE typically does not reach the anterior edge of the middle turbinate in adults. F = frontal sinus, MT = middle turbinate, S = sphenoid sinus, asterisk = cribriform plate.
Clinic-based Olfactory Biopsies
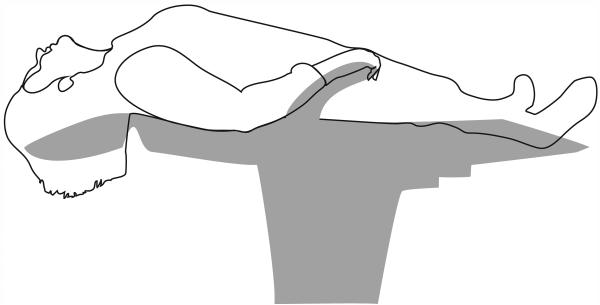
Biopsies performed in the clinic were obtained from subjects without the use of sedation in an upright sitting position. The nasal cavity was sprayed with a mixture of lidocaine (2%) and oxymetazoline (0.025%), and after several minutes rigid endoscopy was performed using a 2.7 mm, zero-degree endoscope (Hopkins II, Karl Storz) to identify the side with the greatest olfactory cleft space. In initial biopsies, a small cotton wisp soaked in the lidocaine/oxymetazoline mixture was inserted into the olfactory cleft for further anesthesia (subjects HB5 and HB6); however, concerns with exfoliation of the epithelium with this technique resulted a change in procedure. For the remaining biopsies, further anesthetic was applied by placing the subject supine and hanging the head backwards off the edge of the chair with the vertex of the head parallel to the floor (Figure 2). A flexible angiocath was then used to direct 0.5-1.0 cc of anesthetic solution into the superior nasal cavity and olfactory cleft. The subjects remained in this position for two to three minutes and then returned to the upright sitting position. A sickle knife was then used to create a 5-7 mm long, posterior-to-anterior, diagonal incision along the septum approximately 5 to 8 mm below the cribriform plate and greater than 5 mm posterior to the anterior attachment of the middle turbinate (video). Given the variability of OE anterior extent and regression of OE area with age, attempts at accessing the more posterior regions while still allowing for insertion of the forceps with minimal discomfort to the subject were made. The sickle knife was then used to carefully elevate a superior flap of mucosa away from the bone. A 3 mm cupped biopsy forceps or pediatric blakesley forceps was used to pinch off a portion of the superiorly raised flap. The cupped biopsy forceps became the preferred instrument due to its small size and ability to fit within the olfactory cleft. Two biopsies were routinely obtained from each subject. Minimal bleeding was encountered and controlled with a lidocaine/oxymetazoline soaked cotton wisp.
Figure 2.
Subject position used during topical anesthesia of the nose. Illustration by Olivia Schwob.
Tissue Processing
Biopsy specimens were placed immediately in standard 10% neutral buffered formalin solution and placed on ice for transport. The tissue was then rinsed in phosphate buffered saline (PBS) after two hours and then submerged in 30% sucrose overnight. Orientation has been found to be critical in the histological assessment of biopsy specimens. To help identify the epithelial surface of the specimens during processing, the area designated for biopsy on the septum was initially inked with a standard surgical marking pen early in the study (subjects HB3, HB4, HB5). This practice was then rejected for fear of causing epithelial loss or decrease in cellular viability. Instead, a dissecting microscope was used to identify the epithelial and lamina propria surfaces. The biopsy was then placed in OCT mounting medium (Miles Inc., Elkhart, IN) taking care to orient the biopsy in the cassette for sectioning in the proper cross-sectional plane through the epithelium. The tissue was snap frozen in liquid nitrogen, cryosectioned at 10 μm (Leica CM3050S, Bannockburn, IL), and mounted on Plus slides (Fisher Scientific, Pittsburg, PA) and stored at −20°C for future use.
Immunohistochemistry
Slides were immersed in PBS for removal of OCT for 5 minutes and then placed in 3% hydrogen peroxide for 10 minutes to quench inherent peroxidase activity. Sections were then puddled with 0.01 M citric acid buffer (pH 6.0) and placed in a commercial food steamer containing water in its reservoir for 10 minutes for antigen retrieval. Slides were rinsed in PBS and then incubated in blocking solution (10% donkey serum + 5% nonfat dry milk + 4% bovine serum albumin [BSA] + 0.1% Triton X-100 in PBS) for ten minutes. Incubation with primary antibodies occurred overnight at 4°C in a humid chamber. The dilutions and antigen specificity for each primary antibody are listed in Table 1. Bound primary antibodies were visualized by incubating with Alexa 488 or Alexa 594-conjugated secondary antibody (Invitrogen, Carlsbad, CA) or through a biotinylated secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and Alexa 488 or Alexa 594-conjugated strep-avidin system (Invitrogen, Carlsbad, CA) incubating for one hour each at room temperature. Immunofluorescent stained sections were additionally labeled for nuclei with 4’,6-diamidino-2-phenylindole (DAPI) and coverslipped with glycerol/n-propyl gallate (NPG) mounting medium.
Table 1.
Primary Antibody Details
| Antibody | Species | Concentration | Company | Catalogue # | Antigen | Staining Characteristic |
|---|---|---|---|---|---|---|
| Tuj1 | Mouse | 1:300 | Covance Emeryville, CA | MMS-435P | rat brain beta-tubulin III | all neurons |
| Beta-Tubulin IV | Mouse | 1:1000 | Sigma St. Louis, MO | T7941 | c-terminal synthetic peptide beta-tubulin IV | respiratory cilia |
| hNF | Mouse | 1:50 | Dako North America, Inc. Carpinteria, CA | 2F11 | neurofilament from adult human brain (70KD) | trigeminal axons |
| OMP | Goat | 1:100 | Santa Cruz Biotechnology, Inc. Dallas, TX | SC-49070 | rodent olfactory marker protein | mature olfactory neurons |
| p75 (NTR) | Mouse | 1:50 | Millipore Billerica, MA | MAB365 | protein isolated from plasma membrane of PC-12 cells | perineurium and ensheathing cells |
| PGP9.5 | Rabbit | 1:1000 | Cedarlane Burlington, NC | RA95101 | human brain protein gene product 9.5 | all neurons |
| S100 | Rabbit | 1:100 | Dako North America, Inc. Carpinteria, CA | Z0311 | S100 protein from cow extracts | neurons, ensheathing cells, some gland tissue |
Photography
Sections were imaged with a Spot RT color digital camera (Spot, Sterling Heights, MI) attached to a Nikon 800 E microscope (Nikon Instruments Inc., Melville, NY). Image preparation, assembly, and analysis were performed in Photoshop CS6 (Adobe Systems, San Jose, CA). In all cases, only balance, contrast, brightness, and evenness of illumination were altered.
Results
Subject profiles
A total of 14 subjects (7 females) had biopsies of the septal olfactory epithelium (Table 2). The average age was 53.4 (range 22-68). Seven subjects (4 female) had smell loss related to upper respiratory infection (URI), three subjects (1 female) had smell loss related to head trauma, two subjects had no subjective loss and tested normal, and two subjects had no subjective loss and tested hyposmic. Of the seven URI related smell loss subjects, six were anosmic and one was hyposmic on testing. All traumatic smell loss subjects were anosmic. Four of the subjects were biopsied in the OR (3 subjective normal and 1 URI-related anosmia). No complications relating to bleeding, infection, CSF leak, or changes in smell function were experienced with any subject as noted previously.19,20 Any reports of discomfort were related to pressure during access to the olfactory cleft region, and all subjects entering the protocol tolerated the procedure without requests to abort.
Table 2.
Subject Demographics and Biopsy Results
| Specimen | Age | Sex | Olfactory Function | PMH | Location | Marker | Pledget | Bx Forcep | Epithelium | Fascicles |
|---|---|---|---|---|---|---|---|---|---|---|
| HB5 | 57 | F | Anosmia | URI | Clinic | X | X | Blakesley | Mix | |
| HB6 | 24 | F | Normal | -- | Clinic | X | Cup | Absent | Trigeminal | |
| HB7 | 61 | M | Anosmia | Trauma | Clinic | Cup | Respiratory | None | ||
| HB8 | 64 | M | Anosmia | URI | Clinic | Cup | Respiratory | Empty | ||
| HB9 | 47 | F | Anosmia | URI | Clinic | Cup | Mix | |||
| HB10 | 62 | F | Anosmia | URI | Clinic | Cup | Mix | |||
| HB11 | 59 | F | Anosmia | Trauma | Clinic | Cup | Mix | |||
| HB12 | 60 | M | Anosmia | Trauma | Clinic | Cup | Olfactory | |||
| HB13 | 58 | M | Anosmia | URI | Clinic | Cup | Respiratory | Empty | ||
| HB14 | 41 | M | Hyposmia | URI | Clinic | Cup | Respiratory | Empty |
Immunohistochemistry of the epithelium
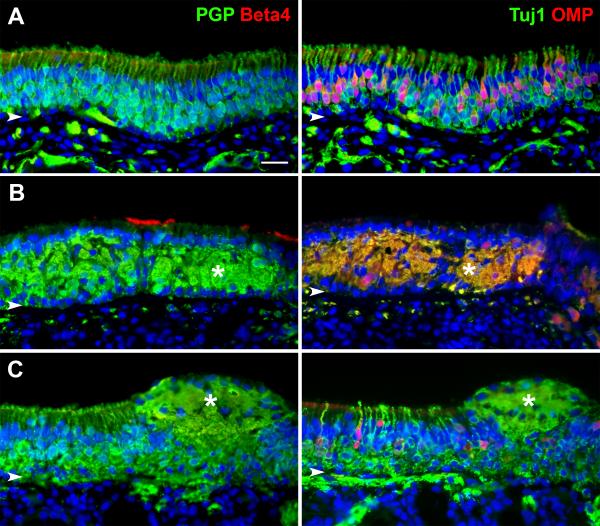
In most cases (13/14 subjects) an epithelium was observed in the biopsy specimen. To confirm biopsies as true OE, an immunohistochemical staining analysis using four different antibodies provided information on the presence of olfactory neurons, the relative overall maturity of the neurons, and the presence of respiratory epithelium. We used antibodies against olfactory marker protein (OMP) to identify mature olfactory sensory neurons (OSN's); and to identify all OSN's of both mature and immature age, we used antibodies against the protein gene product 9.5 (PGP) or antibodies against beta-tubulin III (Tuj1). Respiratory cells were labeled at the ciliated surface with antibodies against beta-tubulin IV (beta4).4 As shown in Figure 3A stained adjacent sections of a biopsy from a control subject (HB4) reveal the typical multi-cell layered epithelium comprised of many PGP(+)/Tuj1(+) neurons and absent beta4(+) respiratory cells. Many of the OSN's are mature with OMP(+) staining in the more apically positioned neurons as expected.
Figure 3.
Immunohistochemistry demonstrates the presence of OE. Adjacent section from three separate biopsies are double labeled with antibodies against PGP9.5 (PGP) and beta-tubulin IV (Beta4) or beta-tubulin III (Tuj1) and olfactory marker protein (OMP) with DAPI nuclear staining in blue. A. A biopsy from subject HB4 provides an example of normal OE with multiple layers of PGP(+)/Tuj1(+) neurons. The epithelium is without any respiratory cells as confirmed with absent Beta4 staining. A healthy mix of immature Tuj1(+)/OMP(−) and mature Tuj1(+)/OMP(+) OSN's are appreciated. B. In comparison, a biopsy from subject HB10 with URI-related anosmia reveals a disordered epithelium with less defined OSN cell bodies. OSN staining with PGP, Tuj1, and OMP is present confirming the epithelium as OE, but patches of Beta4(+) respiratory cilia can also be seen. An asterisk shows a large intraepithelial neuroma. Notice how the neuroma and disorganized OSN's remain compartmentalized between the row of supporting cell nuclei apically and the basal cell nuclei below. C. A biopsy from subject HB12 with trauma-related anosmia has an overall immature OE. The OSN's are confirmed with PGP(+)/Tuj1(+) labeling but mature OMP(+) cells are limited. Once again, an intraepithelial neuroma is seen (asterisks), but in this example there is disruption of the supporting cell layer. Arrowheads = basal lamina, scale bar = 25 μm.
In contrast, biopsies from subjects with olfactory loss often revealed deviation from the normal pattern of staining. Stained sections from a subject with URI-related anosmia (HB10) demonstrates a more disorganized presence of PGP(+)/Tuj1(+) neurons within the epithelium and less distinct apically positioned mature OMP(+) cells (Figure 3B). In addition, there is evidence of respiratory cell metaplasia with patches of beta4(+) apical staining and intraepithelial neuromas which in this case appears to be comprised of mature and immature axons by double-labeling.
The epithelium from a subject with trauma-related anosmia (HB12) has a more ordered appearance to the OE similar to the control subject, but the OSN's are largely immature with infrequent OMP(+) mature cells (Figure 3C). In this case, respiratory epithelium is absent; but another intraepithelial neuroma is present, although comprised mostly of Tuj1(+)/OMP(−) immature axons.
Immunohistochemistry of the submucosa
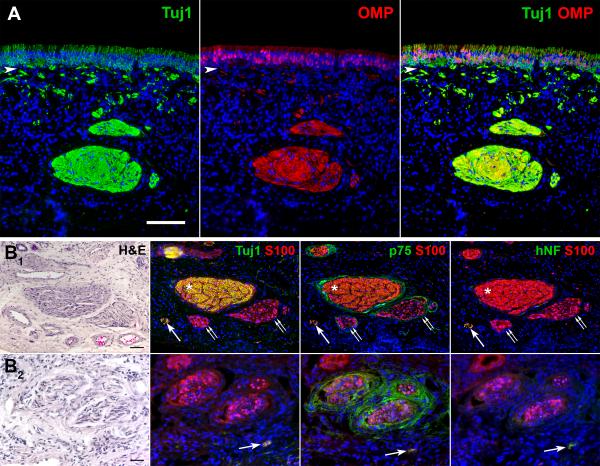
Loss of the epithelial layer may occur while obtaining the biopsy or during tissue processing. In addition, respiratory cell replacement is common during normal aging and increases in various pathologic states.15,21-23 Biopsies may also be mistakenly obtained outside the region of OE. For this reason, an analysis of the epithelium alone may not be sufficient to determine the biopsy as olfactory in origin. In cases where OE was not identified in the biopsy, immunohistochemical characterization of the axon bundles within the submucosa was performed. Similar antibodies used in the epithelial analysis (Tuj1, OMP) identified axon bundles in a control biopsy (HB4) with presence of mature Tuj1(+)/OMP(+) OSN axons (Figure 4A). In cases where the epithelium is missing or has been identified as respiratory, nerve fascicle-type structures can be found on routine hematoxylin and eosin (H&E) staining with elongated nuclei of the olfactory ensheathing cells (OEC's) and connective tissue elements. These fascicles can be further identified as olfactory fascicles, empty olfactory fascicles, or non-olfactory fascicles when stained with specific antibodies (Figure 4B). Antibodies against S100 (S100) label OEC's surrounding olfactory axons within the fila olfactoria.24-26 When OSN axons are present, double labeling of fascicles with Tuj1 and S100 is seen (figure 4B1). However, fascicles empty of OSN axons remain S100(+) but are absent of Tuj1(+) staining (Figure 4B1, 4B2). The S100 staining in these fascicles appear more dispersed with distinct rounded ensheathing cell bodies with their associated DAPI(+) nucleus as apposed to the more evenly stained axon-full fascicles (comparison in Figure 4B1). Antibodies against p75 neurotrophin receptor (p75) label perineural fibroblasts.25 Staining with p75 surrounds both intact and empty olfactory fascicles (Figure 4B1, 4B2), which help to confirm S100(+)/Tuj1(−) structures as empty nerve fascicles as apposed to other submucosal structures (i.e. glands).
Figure 4.
Immunohistochemistry helps to confirm olfactory nerve fascicles. A. Adjacent sections through a biopsy from subject HB4 reveals fila olfactoria that contains abundant mature Tuj1(+)/OMP(+) axons and is reflective of the health of the overlying OE. Arrowhead = basal lamina, scale bar = 100 μm. B. Sections through biopsies stained with H&E reveal structures resembling nerve fascicles. Immunohistochemical double labeling with antibodies against S100 and Tuj1, p75 neurotrophic receptor (p75), or human neurofilament protein (hNF) further define the fascicles. B1. H&E and immunofluorescent staining of sections through a biopsy from subject HB1 shows a typical large olfactory fascicle (asterisk) stained with S100 and Tuj1. The absence of hNF staining confirms the axons as olfactory and not trigeminal. Two smaller fascicles (double arrows) are S100(+) but lack Tuj1(+) axon staining. The p75(+) staining of perineural tissue surrounding the S100(+) olfactory ensheathing cells (OEC's) confirms these structures as empty olfactory fascicles. A smaller trigeminal nerve fascicle (single arrow) stained with S100, Tuj1, and hNF is also present. Scale bar = 50 μm. B2. H&E and immunofluorescent staining of adjacent sections through a biopsy from a subject with URI-related hyposmia (HB14) at higher power magnification demonstrates a group of three empty fascicles stained with S100 and absent of Tuj1(+) axons. Perineural staining with p75 confirms the structures as nerve fascicles. Note the wider spaced S100(+) OEC's in the empty fascicles as opposed to the larger Tuj1(+) olfactory nerve fascicle in B1. Again, a small trigeminal nerve branch is seen (arrow). Scale bar = 25 μm.
In our analysis of the submucosa, small S100(+)/Tuj1(+) bundles were frequently identified. With absence of OMP(+) labeling, these small fascicles could be small immature olfactory fascicles or trigeminal nerve branches. We used antibodies to human neurofilament proteins (hNF) known to label trigeminal nerve fibers.27 Adjacent sections stained with hNF antibodies labeled these small Tuj1(+) fascicles and supports their identification as trigeminal (Figure 4B).
Office-based biopsy efficiency
Immunohistochemical staining was performed on all the biopsies to identify them as olfactory. Of our ten biopsies performed in the clinic, nine contained epithelium for immunohistochemical analysis (Table 2). Of these nine samples, five contained either a mix of OE and respiratory epithelium or OE alone. When only respiratory epithelium was encountered (4 out of 10) or in the absence of epithelium, further staining of the submucosa was performed. Evidence for empty olfactory fascicles was present in three samples (3 out of 5). Interestingly, the one normal subject biopsied in the clinic (HB6) had no epithelium present and only trigeminal nerve branches. When taken together, an evaluation of both the epithelium and submucosa in our population of office-based biopsies revealed successful sampling from olfactory regions in 80% of the subjects.
Discussion
Olfactory mucosal biopsies have been reported in the literature with variable rate of success, often less than 50%.5,28,29 Intraoperative sampling of olfactory tissue would be expected to result in higher success given the ability to access more confined regions and the creation of working space during planned surgical procedures under general anesthesia. However, requests for olfactory biopsies on subjects without planned nasal surgery are becoming more frequent either for the study of olfactory loss, for diseases with related olfactory pathology, or for harvesting OE stem cells in normal subjects. In these cases, a technique is needed with high-yield and relatively low discomfort.
We present a technique of olfactory mucosal biopsies that can be performed under endoscopic visualization using routine clinic instruments in an office-based setting with a success rate of 80%. Our samples were obtained from anterior septal mucosa in a region known to contain olfactory neurons based on prior autopsy analysis. In this manner, we avoided problems with patient discomfort and restrictions in instrumentation typically encountered with attempts at accessing more posterior regions, such as the superior turbinate. Although procedure discomfort was not a major focus of this study and specific measurements of pain were not included, we found the technique to be well tolerated by the subjects. Our technique also minimizes introduction of anesthetic materials including injections and cotton pledgets that may disrupt the histology and viability of the cells. We did not receive reports of olfactory loss from subjects after biopsies were performed, and further investigations on this potential complication would be beneficial. However, previously published papers with human OE biopsies were also without complications of smell loss.
Our analysis of the success rate in obtaining olfactory mucosal biopsies takes into account both the epithelium as well as the submucosa. The presence of Tuj1(+)/PGP(+) neurons within the epithelium either mature OMP(+) or immature OMP(−) confirms the mucosa as olfactory. However, replacement of OE with respiratory epithelium is known to occur in humans and appears to increase with age.21,23 In addition, conditions resulting in smell loss such as URI-related anosmia may result in increased respiratory metaplasia.13,30,31 Therefore, finding only respiratory epithelium in a biopsy specimen does not preclude it from being olfactory in nature. Conversely, one cannot assume a biopsy containing only respiratory epithelium obtained from an area thought to be olfactory reflects respiratory metaplasia when in reality a sampling error has occurred. This is not trivial given previously published reports of neurospheres cultured from olfactory epithelium when the source tissue was never confirmed as olfactory prior to cell dissociation.17 This possibility becomes more problematic as reports of nasal cell cultures from areas far outside those known to be olfactory result in neurosphere formation, perhaps due to the presence of neural crest-derived cells.32 Furthermore, the act of the biopsy or tissue processing can result in the loss of epithelium for analysis. For these reasons, a deeper analysis of the submucosa is needed for establishing olfactory origin of biopsies.
Immunohistochemistry using antibodies important for epithelial analysis can help identify the presence of olfactory nerve fascicles below the surface. Positive OMP staining of fascicles confirms the presence of fila olfactoria and the epithelial surface as olfactory in origin. In humans, the olfactory axon bundles travel directly to the cribriform plate and do not span across regions known to be originally respiratory in nature. This is supported by our observations and others of whole complete sheets of OE in fetal tissue without intervening respiratory zones.21,33 Therefore, positive identification of OMP(+) axons within nerve fascicles would occur only deep to epithelium that was originally olfactory.
Further characterization of the underlying fascicles is helpful when OMP staining is absent (Table 3) and has proven useful for other investigators interested in harvesting OEC's.34 Neuronal staining with antibodies Tuj1 and PGP in the absence of OMP denotes the presence of axons that may reflect dominance of immature OSN's. Additional positive staining of these fascicles with hNF identifies them as trigeminal in nature. In our experience, the hNF(+) bundles are much smaller than the fila olfactoria. Empty olfactory nerve fascicles, absent of axons and with collagen infiltration, have been previously reported and associated with olfactory disorders.13 In these cases, staining with neuronal markers including OMP, Tuj1, and PGP is lacking. Immunostaining with antibodies to S100 can identify the OEC's remaining within the axon-deficient fascicles. S100 is not specific to just OEC's and the additional staining with antibodies against p75 neurotrophic receptor will identify perineuronal fibroblasts surrounding the S100 labeled empty fascicles. Identification of these fascicles not only helps to confirm the olfactory nature of the biopsy but also lends additional description to the overall status of the OE.13
Table 3.
Staining Characteristics of Nerve Fascicles Within Olfactory Mucosa.
| S100 | Tuj1/PGP | OMP | p75 | hNF | |
|---|---|---|---|---|---|
| Olfactory | X | X | X | X | O |
| Immature Olfactory | X | X | O | X | O |
| Empty Olfactory | X | O | O | X | O |
| Trigeminal | X | X | O | X or O | X |
Confirming mucosal biopsy samples as olfactory is important in making statements regarding origin of cells grown in culture. Although we believe adjacent biopsies reduce sampling error in obtaining true olfactory mucosa, it is possible that they may be taken from two different regions—olfactory or respiratory. However, if one sample demonstrates olfactory mucosa regardless of the overlying epithelium displaying respiratory or olfactory cells, the second adjacent sample will have a high likelihood of originating from the same. To increase confirmation of samples as either olfactory or respiratory, biopsy samples should be divided in two pieces with one portion dedicated to immunohistochemical confirmation.
We recognize the low numbers of office-based biopsies used in this report, however our technique has been successfully used previously for obtaining OE samples.35 The overall purpose of this manuscript is twofold. First, it provides rhinologists with a reproducible technique for obtaining olfactory mucosa biopsies in an office setting under local anesthesia with minimal patient discomfort and with simple office instruments. Our limitations relate to the space restrictions of the olfactory cleft, and we recognize better results could be obtained with specially designed thinner cutting instruments. We recognize the value of preserving as much of the mucosa as possible--especially the epithelial surface. Therefore, our technique evolved to avoid any insertion of anesthetic soaked cotton wisps or pledgets for concern of possible epithelial exfoliation. This certainly reduced our ability to reach more posterior anatomy which has been successfully biopsied by others.34 Secondly, this manuscript stresses the importance of analyzing the tissue below the epithelium with focus on the nerve fascicles contained within this compartment, and we provide an immunohistochemical staining protocol helpful for characterizing these structures.
Summary
Olfactory biopsies can be reproducibly obtained with high yields in an office-based setting using common clinic instruments with low patient discomfort under local anesthetic. It is the responsibility of both the surgeon and the research team to ensure the biopsy material being studied is in fact olfactory in nature before conclusions are made. This can be done with a high level of certainty using routine immunohistochemistry and a small battery of specific antibodies.
Supplementary Material
Acknowledgments
Funding: NIH R01 DC010242
Footnotes
Disclosures: The authors have no financial disclosures or conflicts of interest related to the subject of this manuscript.
This manuscript was presented at the American Rhinologic Society 61st Annual Meeting in Dallas, TX, September 26th, 2015.
References
- 1.Graziadei PP, Monti Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979;8(1):1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- 2.Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol. 1998;400(4):469–486. [PubMed] [Google Scholar]
- 3.Schnittke N, Herrick DB, Lin B, et al. Transcription factor p63 controls the reserve status but not the stemness of horizontal basal cells in the olfactory epithelium. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1512272112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holbrook EH, Wu E, Curry WT, Lin DT, Schwob JE. Immunohistochemical characterization of human olfactory tissue. Laryngoscope. 2011;121(8):1687–1701. doi: 10.1002/lary.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feron F, Perry C, McGrath JJ, Mackay-Sim A. New techniques for biopsy and culture of human olfactory epithelial neurons. Arch Otolaryngol Head Neck Surg. 1998;124(8):861–866. doi: 10.1001/archotol.124.8.861. [DOI] [PubMed] [Google Scholar]
- 6.Deems DA, Doty RL, Settle RG, et al. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 1991;117(5):519–528. doi: 10.1001/archotol.1991.01870170065015. [DOI] [PubMed] [Google Scholar]
- 7.Landis BN, Konnerth CG, Hummel T. A study on the frequency of olfactory dysfunction. Laryngoscope. 2004;114(10):1764–1769. doi: 10.1097/00005537-200410000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Duan D, Lu M. Olfactory mucosa: a rich source of cell therapy for central nervous system repair. Rev Neurosci. 2015;26(3):281–293. doi: 10.1515/revneuro-2014-0065. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Huang H, Xi H, et al. A prospective randomized double-blind clinical trial using a combination of olfactory ensheathing cells and Schwann cells for the treatment of chronic complete spinal cord injuries. Cell Transplant. 2014;23(Suppl 1):S35–44. doi: 10.3727/096368914X685014. [DOI] [PubMed] [Google Scholar]
- 10.Tabakow P, Raisman G, Fortuna W, et al. Functional regeneration of supraspinal connections in a patient with transected spinal cord following transplantation of bulbar olfactory ensheathing cells with peripheral nerve bridging. Cell Transplant. 2014;23(12):1631–1655. doi: 10.3727/096368914X685131. [DOI] [PubMed] [Google Scholar]
- 11.Guntinas-Lichius O, Wewetzer K, Tomov TL, et al. Transplantation of olfactory mucosa minimizes axonal branching and promotes the recovery of vibrissae motor performance after facial nerve repair in rats. J Neurosci. 2002;22(16):7121–7131. doi: 10.1523/JNEUROSCI.22-16-07121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rawson NE, Gomez G, Cowart B, et al. Selectivity and response characteristics of human olfactory neurons. J Neurophysiol. 1997;77(3):1606–1613. doi: 10.1152/jn.1997.77.3.1606. [DOI] [PubMed] [Google Scholar]
- 13.Holbrook EH, Leopold DA, Schwob JE. Abnormalities of axon growth in human olfactory mucosa. Laryngoscope. 2005;115(12):2144–2154. doi: 10.1097/01.MLG.0000181493.83661.CE. [DOI] [PubMed] [Google Scholar]
- 14.Borgmann-Winter KE, Rawson NE, Wang HY, et al. Human olfactory epithelial cells generated in vitro express diverse neuronal characteristics. Neuroscience. 2009;158(2):642–653. doi: 10.1016/j.neuroscience.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yee KK, Pribitkin EA, Cowart BJ, et al. Neuropathology of the olfactory mucosa in chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24(2):110–120. doi: 10.2500/ajra.2010.24.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feron F, Perry C, Girard SD, Mackay-Sim A. Isolation of adult stem cells from the human olfactory mucosa. Methods Mol Biol. 2013;1059:107–114. doi: 10.1007/978-1-62703-574-3_10. [DOI] [PubMed] [Google Scholar]
- 17.Wrobel BB, Mazza JM, Evgrafov OV, Knowles JA. Assessing the efficacy of endoscopic office olfactory biopsy sites to produce neural progenitor cell cultures for the study of neuropsychiatric disorders. Int Forum Allergy Rhinol. 2013;3(2):133–138. doi: 10.1002/alr.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holbrook EH, Schwob JE. An integrated immunohistochemical analysis of human whole-mount and cryosectioned olfactory tissue. Chem Senses. 2008;33:S78. [Google Scholar]
- 19.Lanza DC, Deems DA, Doty RL, et al. The effect of human olfactory biopsy on olfaction: a preliminary report. Laryngoscope. 1994;104(7):837–840. doi: 10.1288/00005537-199407000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Feron F, Mackay-Sim A, Andrieu JL, Matthaei KI, Holley A, Sicard G. Stress induces neurogenesis in non-neuronal cell cultures of adult olfactory epithelium. Neuroscience. 1999;88(2):571–583. doi: 10.1016/s0306-4522(98)00233-4. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima T, Kimmelman CP, Snow JB., Jr. Structure of human fetal and adult olfactory neuroepithelium. Arch Otolaryngol. 1984;110(10):641–646. doi: 10.1001/archotol.1984.00800360013003. [DOI] [PubMed] [Google Scholar]
- 22.Hasegawa S, Yamagishi M, Nakano Y. Microscopic studies of human olfactory epithelia following traumatic anosmia. Arch Otorhinolaryngol. 1986;243(2):112–116. doi: 10.1007/BF00453761. [DOI] [PubMed] [Google Scholar]
- 23.Paik SI, Lehman MN, Seiden AM, Duncan HJ, Smith DV. Human olfactory biopsy. The influence of age and receptor distribution. Arch Otolaryngol Head Neck Surg. 1992;118(7):731–738. doi: 10.1001/archotol.1992.01880070061012. [DOI] [PubMed] [Google Scholar]
- 24.Vincent AJ, West AK, Chuah MI. Morphological and functional plasticity of olfactory ensheathing cells. J Neurocytol. 2005;34(1-2):65–80. doi: 10.1007/s11068-005-5048-6. [DOI] [PubMed] [Google Scholar]
- 25.Smithson LJ, Kawaja MD. A comparative examination of biomarkers for olfactory ensheathing cells in cats and guinea pigs. Brain Res. 2009;1284:41–53. doi: 10.1016/j.brainres.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Rawji KS, Zhang SX, Tsai YY, Smithson LJ, Kawaja MD. Olfactory ensheathing cells of hamsters, rabbits, monkeys, and mice express alpha-smooth muscle actin. Brain Res. 2013;1521:31–50. doi: 10.1016/j.brainres.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Bae JY, Kim JH, Cho YS, Mah W, Bae YC. Quantitative analysis of afferents expressing substance P, calcitonin gene-related peptide, isolectin B4, neurofilament 200, and Peripherin in the sensory root of the rat trigeminal ganglion. J Comp Neurol. 2015;523(1):126–138. doi: 10.1002/cne.23672. [DOI] [PubMed] [Google Scholar]
- 28.Winstead W, Marshall CT, Lu CL, Klueber KM, Roisen FJ. Endoscopic biopsy of human olfactory epithelium as a source of progenitor cells. Am J Rhinol. 2005;19(1):83–90. [PubMed] [Google Scholar]
- 29.Rawson NE, Gomez G, Cowart BJ, Kriete A, Pribitkin E, Restrepo D. Age-associated loss of selectivity in human olfactory sensory neurons. Neurobiol Aging. 2012;33(9):1913–1919. doi: 10.1016/j.neurobiolaging.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jafek BW, Eller PM, Esses BA, Moran DT. Post-traumatic anosmia. Ultrastructural correlates. Arch Neurol. 1989;46(3):300–304. doi: 10.1001/archneur.1989.00520390066018. [DOI] [PubMed] [Google Scholar]
- 31.Yamagishi M, Fujiwara M, Nakamura H. Olfactory mucosal findings and clinical course in patients with olfactory disorders following upper respiratory viral infection. Rhinology. 1994;32(3):113–118. [PubMed] [Google Scholar]
- 32.Hauser S, Widera D, Qunneis F, et al. Isolation of novel multipotent neural crest-derived stem cells from adult human inferior turbinate. Stem Cells Dev. 2012;21(5):742–756. doi: 10.1089/scd.2011.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naessen R. The identification and topographical localisation of the olfactory epithelium in man and other mammals. Acta Otolaryngol. 1970;70(1):51–57. doi: 10.3109/00016487009181858. [DOI] [PubMed] [Google Scholar]
- 34.Kachramanoglou C, Law S, Andrews P, Li D, Choi D. Culture of olfactory ensheathing cells for central nerve repair: the limitations and potential of endoscopic olfactory mucosal biopsy. Neurosurgery. 2013;72(2):170–178. doi: 10.1227/NEU.0b013e31827b99be. discussion 178-179. [DOI] [PubMed] [Google Scholar]
- 35.Pantazopoulos H, Boyer-Boiteau A, Holbrook EH, et al. Proteoglycan abnormalities in olfactory epithelium tissue from subjects diagnosed with schizophrenia. Schizophr Res. 2013;150(2-3):366–372. doi: 10.1016/j.schres.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.