Abstract
Background
Minimally invasive mitral valve surgery (mini-MVR) has grown in popularity. Although single centers have reported excellent outcomes, there is limited data on real world outcomes and costs of mini-MVR. Moreover, mini-MVR has been criticized as adding additional cost without clear benefit. We hypothesized that mini-MVR provides superior outcomes with incremental increased costs in a multi-institutional cohort.
Methods
Records for patients undergoing mitral valve surgery with or without atrial ablation from 2011-2014 were extracted from a multi-institutional, regional Society of Thoracic Surgeons database and stratified according to right chest approach/ minimally invasive or conventional sternotomy. Patients undergoing CABG or other concomitant procedures were excluded. Patients undergoing isolated mitral surgery were propensity matched based on factors including age, comorbidities, and preoperative laboratory values; clinical outcomes and cost differences were assessed by approach.
Results
A total of 1304 patients underwent mitral surgery, including 425 (32.6%) via minimally invasive approach. In the propensity-matched analysis (n=355 per group), mini-MVR patients had similar rates of mortality, stroke, and other complications compared to conventional MVR. Meanwhile, mini-MVR patients experienced shorter ICU and hospital lengths of stay, as well as fewer transfusions. Importantly, total hospital costs were no different between the two matched groups.
Conclusions
Compared to conventional sternotomy, mini-MVR in the “real world” demonstrated no differences in major morbidity, but was associated with shorter length of stay and fewer transfusions. Contrary to our hypothesis, mini-MVR can be performed with similar total hospital costs as conventional sternotomy. In summary, minimally invasive mitral surgery in select patients can provide superior outcomes without increased cost.
Mitral valve surgery has evolved substantially in recent years, with several operative approaches now available to patients. Many patients request the least invasive approach to allow the most rapid recovery and return to function. Adoption of minimally invasive mitral surgical techniques has, however, experienced relatively slow adoption by surgeons despite single center experiences suggesting that minimally invasive mitral surgery (mini-MVR) can be performed with excellent outcomes compared to conventional sternotomy [1–3]. The cautious adoption of minimally invasive approaches is likely due to multiple concerns including the learning curve associated with these techniques for surgeon and team, the need for specialized equipment, as well as the potential for increased costs associated with minimally invasive procedures. The majority of studies examining outcomes and costs in minimally invasive mitral surgery have been single-center retrospective analyses published by high-volume centers. We sought to compare mini-MVR with conventional sternotomy approach in the real-world setting of a regional, multi-institutional patient population. Our hypothesis was that mini-MVR in the modern era has similar operative outcomes compared with conventional sternotomy approach, albeit with incremental increased hospital costs.
Patients and Methods
Data was obtained from the Virginia Cardiac Surgery Quality Initiative (VCSQI), a consortium of 18 hospitals and 14 cardiac surgery practices which captures >99% of open heart procedures in the Commonwealth of Virginia. The VCSQI database is certified by the Society of Thoracic Surgeons (STS, version 2.73). Formal institutional review board approval has been exempted at each participating center as this data is collected for quality analysis and all Health Insurance Portability and Accountability Act patient identifiers are removed.
Operative approach for mitral valve procedures was recorded in the VCSQI database beginning in January 2011. The study population encompassed all patients who underwent mitral valve surgery from January 1st 2011 through June 30th 2014. Patients were included if concomitant atrial fibrillation ablation was performed. Patients undergoing non-ablation concomitant procedures and emergent procedures were excluded. Patients were grouped by operative approach, with minimally invasive and right thoracotomy procedures comprising the mini-MVR cohort and the remaining patients in the conventional sternotomy cohort. Partial sternotomy approach was not included in either group and comprised a small fraction of overall cases for the timeframe. Preoperative demographics and risk factors, operative characteristics, and postoperative events were evaluated. Additionally, total cost data was obtained, using raw charge data from the UB-04 database with application of the Ratios of Cost to Charge supplied by the Centers for Medicare and Medicaid Services.
Primary outcomes included operative mortality, postoperative morbidity, length of stay, and hospital costs. Postoperative morbidity was defined according to STS definitions for complications and postoperative events, including stroke, prolonged ventilation, renal failure, deep sternal wound infection, new-onset atrial fibrillation, and blood product use/administration.
Statistical Analyses
The two study cohorts were analyzed using Pearson's chi-squared or Fisher's exact tests for categorical variables, and Student's t-test or the Wilcoxon rank-sum test for continuous variables as appropriate based on variable distribution. Subsequently, propensity matched groups were generated to facilitate adjusted comparisons between the two cohorts. Using the psmatch2 package, propensity scores were generated using a logistic model, and all model variables were selected a priori[4]. These included demographics (e.g. age), comorbidities (e.g. diabetes) and preoperative laboratory and hemodynamic values (e.g. creatinine, ejection fraction). Patients were matched using a 1:1 nearest-neighbor algorithm with replacement and imposition of common support. Standardized difference in means, or standardized bias, of included covariates as well as the STS predicted risk of mortality and morbidity (PROM, PROMM) scores was used to determine adequacy of the model. The baseline characteristics of the unmatched and matched groups are presented in Tables 1 and 2a, respectively. The matched groups’ postoperative outcomes were then compared using unpaired univariate statistical tests [5]. All calculations were performed using Stata/IC 13 (StataCorp LP, College Station, TX).
Table 1.
Baseline characteristics, unmatched cohorts
| Conventional sternotomy (n=879) | Mini-MVR (n=425) | P value | |
|---|---|---|---|
| Age | 64 [54,73] | 59 [51,68] | <0.001 |
| Female gender | 446 (50.7%) | 164 (39%) | <0.001 |
| Body mass index | 26.9 [24,31] | 25.6 [23,29] | <0.001 |
| Hypertension | 572 (65%) | 238 (56%) | 0.002 |
| Diabetes | 184 (21%) | 33 (8%) | <0.001 |
| Peripheral vascular disease | 50 (6%) | 16 (4%) | 0.14 |
| End-stage renal disease | 30 (3%) | 4 (1%) | 0.009 |
| Chronic lung disease | <0.001 | ||
| Mild | 124 (14%) | 42 (10%) | |
| Moderate | 82 (9%) | 17 (4%) | |
| Severe | 63 (7%) | 9 (2%) | |
| Congestive heart failure | <0.001 | ||
| NYHA class I | 22 (4%) | 15 (9%) | |
| NYHA class II | 155 (32%) | 73 (45%) | |
| NYHA class III | 209 (43%) | 61 (38%) | |
| NYHA class IV | 106 (22%) | 12 (7%) | |
| Left ventricular ejection fraction (%) | 57 [50,60] | 60 [55,65] | <0.001 |
| STS PROMM (%) | 18.4 [11, 30] | 8.9 [6.5,14.2] | <0.001 |
| STS PROM (%) | 1.8 [0.8,4] | 0.5 [0.3,1.2] | <0.001 |
Continuous variables are expressed as median [IQR]. Categorical variables are expressed as n (%).
Table 2a.
Baseline characteristics, propensity-matched cohorts
| Conventional sternotomy (n=355) | Mini-MVR (n=355) | P value | |
|---|---|---|---|
| Age | 57.1 ± 15.1 | 59 ± 11.6 | 0.03 |
| Female gender | 145 (41%) | 135 (38%) | 0.44 |
| Body mass index | 25.3 ± 5.1 | 26.3 ± 4.7 | 0.1 |
| Hypertension | 207 (58%) | 196 (55%) | 0.41 |
| Diabetes | 22 (6%) | 26 (7%) | 0.55 |
| Peripheral vascular disease | 25 (7%) | 14 (4%) | 0.07 |
| End-stage renal disease | 7 (2%) | 2 (0.6%) | 0.09 |
| Chronic lung disease | 0.006 | ||
| Mild | 65 (18%) | 39 (11%) | |
| Moderate | 8 (2%) | 16 (5%) | |
| Severe | 15 (4%) | 8 (2%) | |
| Congestive heart failure | 0.016 | ||
| NYHA class I | 3 (0.8%) | 9 (2.5%) | |
| NYHA class II | 53 (15%) | 59 (17%) | |
| NYHA class III | 50 (14%) | 55 (15%) | |
| NYHA class IV | 24 (7%) | 9 (2.5%) | |
| Left ventricular ejection fraction (%) | 58.1 ± 10 | 57.7 ± 9.1 | 0.57 |
| STS PROMM (%) | 15.8 ± 13 | 12.8 ± 10.6 | 0.002 |
| STS PROM (%) | 2.1 ± 4.8 | 1.4 ± 2.9 | 0.047 |
Continuous variables are expressed as mean ± SD. Categorical variables are expressed as n (%).
Results
Patient Characteristics and Operative Strategy
A total of 1304 patients underwent mitral surgery with or without ablation procedure during the study timeframe. Characteristics for the full study population are listed in Table 1. Patients in the mini-MVR group were less likely to be female (38.6% vs. 50.7%) and more likely to be of younger age (median 59 vs. 64 years) and with fewer comorbidities, reflected in lower STS predicted risk of mortality (median 0.5% vs. 1.8%). With respect to operative strategy, 425 cases (32.6%) were performed via a minimally invasive right chest approach, while the remaining 879 (67.4%) were performed via conventional full sternotomy. The number of robotic cases was not captured accurately in our data set and this parameter was not separately analyzed. Arterial cannulation strategy differed in the two incisional groups, with femoral arterial cannulation utilized in 93% of mini-MVR patients and 5.8% of sternotomy patients. Aortic occlusion strategy information was incomplete in our data set and could not be fully analyzed.
Improved Operative Outcomes for Mini-MVR in Unmatched Cohorts
Patients demonstrated overall superior outcomes in the mini-MVR group when analyzing the unmatched full study population. Raw mortality was lower for mini-MVR (1.2% vs 2.7%, p=0.07, Table 3), although likely the result of selection bias. Rates of prolonged ventilation, atrial fibrillation, and transfusion were likewise lower for mini-MVR. Total postoperative ventilator time was also significantly shorter in the mini-MVR group (4.2 vs. 6.7 hours, p<0.001). Rates of stroke were similar for the unmatched groups, at 0.9% for mini-MVR and 1.3% for conventional sternotomy (p=0.62).
Table 3.
Operative details and outcomes for entire (unmatched) cohort
| Conventional sternotomy (n=879) | Mini-MVR (n=425) | P value | |
|---|---|---|---|
| Concomitant atrial fibrillation procedure | 312 (35.5%) | 82 (19.3%) | <0.001 |
| Mitral repair | 457 (52%) | 356 (84%) | <0.001 |
| Femoral arterial cannulation | 29 (3.3%) | 394 (93%) | <0.001 |
| Operative mortality | 24 (2.7%) | 5 (1.2%) | 0.07 |
| Stroke | 11 (1.3%) | 4 (0.9%) | 0.62 |
| Renal failure | 24 (2.7%) | 5 (1.2%) | 0.07 |
| Prolonged ventilation | 116 (13.2%) | 30 (7.1%) | 0.001 |
| Total postoperative ventilation time (hours) | 6.7 [4.4,15.9] | 4.2 [3.2,7.4] | <0.001 |
| Deep Sternal Wound Infection | 0 (0%) | 0 (0%) | 1.0 |
| Reoperation | 56 (6.4%) | 17 (4%) | 0.08 |
| Atrial fibrillation | 190 (22%) | 63 (15%) | 0.004 |
| Any postoperative transfusion | 340 (39%) | 49 (12%) | <0.001 |
Continuous variables are expressed as median [IQR]. Categorical variables are expressed as n (%).
Similar Mortality and Morbidity in Propensity Matched Cohorts
Patients were matched by propensity scores as described above, resulting in two groups comprised of 355 patients each. The full list of matching variables is provided in Table 2b. For key variables including PROM the standardized bias was less than 15%, and the mean bias of the entire model was 13%, demonstrating well-matched groups. While the groups did differ in the proportion of patients undergoing concomitant atrial fibrillation ablation, there is evidence that concomitant ablation procedures does not increase morbidity or mortality in mitral valve surgery [6] and we elected to keep these patients in the matched cohorts to ensure adequate population size for analysis. Compared to conventional sternotomy, mini-MVR required longer operative times (mean cross clamp time: 108 vs. 85 minutes [p<0.001] and mean cardiopulmonary bypass time: 149 vs. 127 minutes [p<0.001]). Patients in the mini-MVR group were more likely to receive a mitral valve repair (83.1% vs. 72.7%, p=0.001).
Table 2b.
Variables utilized to generate propensity-matched groups
| Demographics |
| Age |
| Body Mass Index |
| Body Surface Area |
| Comorbidities |
| Diabetes mellitus |
| Dialysis dependent status |
| Dyslipidemia |
| Endocarditis |
| Hypertension |
| Illicit drug use |
| Peripheral arterial disease |
| Prior congestive heart failure |
| Prior myocardial infarction |
| Pulmonary disease |
| Smoking status |
| Hemodynamic and laboratory data |
| Left ventricular ejection fraction |
| Creatinine |
| Hematocrit |
| Hemoglobin A1c |
| Model for End-stage Liver Disease (MELD) score |
| Platelet count |
| White blood cell count |
| Surgeon characteristics |
| Total surgeon volume over study timeframe (<25 cases, 25-75 cases, >75 cases) |
Operative mortality was identical between the two approaches (1.1% in both groups, p=1.0). There were no significant differences noted in the rates of postoperative stroke (0.9% in both groups, p=1.0) or other major postoperative events (Table 4).
Table 4.
Operative details and outcomes for propensity-matched groups
| Conventional sternotomy (n=355) | Mini-MVR (n=355) | P value | |
|---|---|---|---|
| Concomitant atrial fibrillation procedure | 128 (36%) | 66 (18.6%) | <0.001 |
| Mitral repair | 258 (72.7%) | 295 (83.1%) | <0.001 |
| Femoral arterial cannulation | 39 (11%) | 331 (93%) | <0.001 |
| Cardiopulmonary bypass time (min)* | 112 [90,153] | 137 [116,168] | <0.001 |
| Crossclamp time (min)* | 75 [63,104] | 107.5 [83,125] | <0.001 |
| Operative mortality | 4 (1.1%) | 4 (1.1%) | 1.0 |
| Stroke | 3 (0.9%) | 3 (0.9%) | 1.0 |
| Renal failure | 2 (0.6%) | 4 (1.1%) | 0.41 |
| Prolonged ventilation | 15 (4.2%) | 25 (7%) | 0.1 |
| Total postoperative ventilation time (hours)* | 5.2 [3.6,6.7] | 4.2 [3.2,6.4] | 0.003 |
| Deep Sternal Wound Infection | 0 (0%) | 0 (0%) | 1.0 |
| Reoperation | 12 (3.4%) | 15 (4.2%) | 0.56 |
| Atrial fibrillation | 65 (18.3%) | 55 (15.5%) | 0.32 |
| Any postoperative transfusion | 99 (28%) | 41 (11.6%) | <0.001 |
| RBC units** | 0.69 ± 2.4 | 0.27 ± 1.2 | 0.004 |
| Blood product units (total)** | 1.41 ± 5.4 | 0.55 ± 2.3 | 0.006 |
| ICU length of stay (hours)* | 29.3 [23,70] | 24 [10.9,47] | 0.006 |
| Postoperative length of stay (days)* | 5 [4,8] | 4 [3,6] | <0.001 |
Expressed as median [Interquartile range]
Expressed as mean ± SD.
Improved Resource Utilization in Minimally Invasive Mitral Surgery Patients
ICU duration (24 vs. 29.3 hours, p=0.006) and hospital length of stay (4 vs. 5 days, p<0.001) were significantly shorter in mini-MVR patients compared to patients undergoing sternotomy. Mini-MVR patients were also less likely to require any postoperative blood products (11.6% vs. 27.9%, p<0.001), and consequently on average received significantly fewer units of packed red blood cells (mean 0.27 vs. 0.69 units per patient, p=0.004) and fewer blood products overall (mean 0.55 vs. 1.41 units for conventional sternotomy patients, p=0.006). Table 4 lists additional outcomes related to resource utilization in the propensity-matched groups.
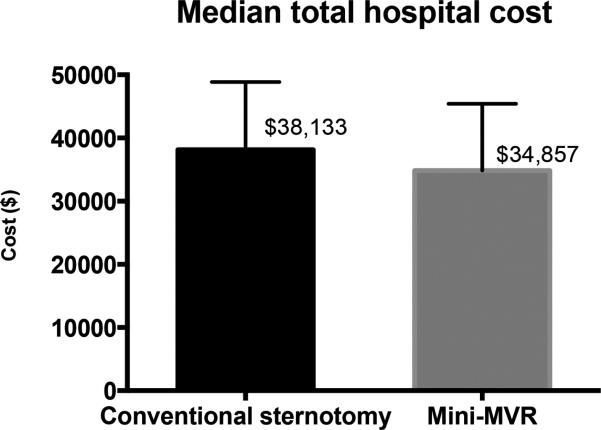
Median hospital costs were similar for the two matched groups, at $34,857 for mini-MVR patients and $38,133 for conventional sternotomy patients (p=0.17, see Figure 1). In the unmatched groups, median costs were significantly lower in the mini-MVR group ($34,857 vs. $43,255, p<0.001).
Figure 1.
Median total hospital costs for mini-MVR
Comment
This real world evaluation of minimally invasive MVR across multiple institutions demonstrates that mini-MVR can be performed with no difference in mortality or morbidity compared to conventional sternotomy approach. This includes similar rates of stroke in the propensity-matched groups, an important finding given concern regarding the potential increased risk of stroke with mini-MVR raised in prior studies. Patients in the mini-MVR group demonstrated evidence of simpler postoperative recovery with fewer transfusions and shorter ICU and hospital length of stay. Despite perceived higher costs with minimally invasive approaches, we found no difference in total hospital costs between matched groups. Our findings confirm single-institution reports regarding the excellent outcomes achievable with mini-MVR approach, and add the perspective that cost is not increased with a minimally invasive approach.
Despite the appeal of alternative approaches to sternotomy, mini-MVR has experienced slow adoption by surgeons. An analysis of STS data from 2004-2008 by Gammie et al suggested the frequency of less-invasive mitral surgery increased from 11% to 20%, with the vast majority of procedures still performed via median sternotomy in 2008[3]. The reasons for this incremental acceptance of minimally invasive surgery are varied, and likely include such factors as 1) desire to ensure that operative outcomes are equivalent to sternotomy approach, 2) concern for increased risk of stroke, 3) increased costs associated with this approach, and 4) the need for specialized training for the surgeon and surgical team. It should be noted that not all patients with isolated mitral valve disease are ideal candidates for minimally invasive surgery, including those with severe mitral annular calcification (MAC), right ventricular dysfunction, or significant aortic calcification. Moreover, careful evaluation including body habitus (chest wall depth), peripheral vessel suitability for cannulation must be carefully examined in the decision regarding operative approach. In our experience, the minimally invasive approach is suitable for many patients and is particularly useful in both in elderly patients with mobility issues who may difficulty maintaining sternal precautions as well as in young, healthy patients desiring quicker recovery and return to work.
Minimally invasive mitral valve surgery has evolved significantly over the past two decades and offers patients an alternative to a full sternotomy. Operative approaches include parasternal incision, partial sternotomy, right port-access/ mini-thoractomy approach, and robotic approaches [7]. Our study focuses on port-access and right thoracotomy approaches. Minimally invasive incisions are desirable to patients for producing improved cosmesis and faster recovery. Moreover, particular benefits may be seen in obese patients who are at highest risk for sternal complications[8]. Previous single-institution studies have reported equivalent operative mortality and morbidity between mini-MVR and conventional sternotomy approaches [2,3,7,9–11]. Our confirmation of equivalent outcomes between approaches in a real world setting (as opposed to limited high-volume experiences) should provide reassurance to surgeons considering adding this technique to their armamentarium.
Despite a lack of differences in mortality and most postoperative outcomes, Gammie et al found an increased risk of stroke in less-invasive mitral surgery compared to conventional sternotomy [3]. This concern may inhibit some surgeons from pursuing mini-MVR. The increased stroke risk was mainly found in patients undergoing fibrillatory arrest or beating heart procedures. A meta-analysis by Cheng et al similarly found increased stroke risk, mainly associated with use of an endoaortic balloon occlusion device (Edwards Lifesciences, LLC, Irvine, CA) as opposed to the transthoracic aortic crossclamp[12]. By contrast, propensity-matched comparisons using large single-institution series have shown similar stroke rates with mini-MVR and conventional sternotomy approach; in that series, endoaortic occlusion is used with low frequency (30% of mini-MVR cases) or not at all, and fibrillatory arrest is not used[2,11]. While our data set is missing comprehensive information regarding myocardial protection strategy (certainly a limitation), our analysis demonstrates no significant difference in stroke rate between mini-MVR and conventional sternotomy patients in propensity-matched groups. Additionally, data has been mixed regarding the relationship between retrograde arterial perfusion using femoral cannulation and stroke risk in these patients[13]. Our mini-MVR group underwent femoral cannulation in more than 90% of cases, and still did not demonstrate an increase in stroke risk. This is the first multi-institutional study to document similar stroke rates with a mini-MVR approach in the modern era after the concerns raised by previous reports from the STS and is likely the result of careful patient selection and screening.
Minimally invasive approaches are often considered more expensive than conventional approaches. The need for specialized equipment, ranging from thoracoscopic instruments to cannulas and catheters, poses additional costs to hospitals considering implementing a mini-MVR program. Though not used everywhere, the endoaortic balloon can account for significant added operating room cost [9]. Additionally, early publications indicated that endoaortic occlusion may be technically difficult to achieve. The potential for aortic dissection related to endoaortic occlusion was also raised early in the minimally invasive port-access experience, but more recent studies and large single center reviews demonstrate that this technique is quite safe in the hands of experienced surgeons [7,14,15]. Operating room times may also be a feared source of increased costs, and minimally invasive approaches consistently report significantly longer cardiopulmonary bypass and cross-clamp times. However, with excellent operative outcomes seen in minimally invasive cases, no clinically significant impact from longer operating room times has been noted[3,9,11].
There is limited published data beyond single center reports analyzing the hospital costs for mini-MVR surgery. Iribarne et al. report a single-institution cost analysis with propensity matched groups, showing lower costs associated with minimally invasive mitral valve surgery driven by reductions in both direct and indirect costs [16]. Our data with respect to cost is limited to total hospital costs which are well-validated, but we are unable to assess itemized costs for categories such as equipment, operating room time, or postoperative care. We found that despite the need for specialized equipment and longer operative times, there were no differences in cost between mini-MVR and sternotomy approaches. This is likely the result of 1) fewer blood transfusions in the mini-MVR group, 2) shorter ICU and hospital length of stay, and 3) overall excellent clinical outcomes with no increase in postoperative complications in the mini-MVR group. These three factors each contribute to reduction of hospital costs, and likely compensate for any additional costs incurred by employing a minimally invasive approach.
Our study has several limitations to note. First, data with respect to mitral pathology was not complete enough to incorporate this into the matching process. We did find that the matched mini-MVR group was more likely to undergo valve repair, but without comprehensive and complete mitral etiology information we cannot assess the implications of differing repair rates in this population. We likewise do not have complete information regarding the crossclamp/myocardial protection strategy utilized in each case, and there is likely a diverse range of preferred techniques given the 18 hospital centers represented in our data. We are also unable to capture conversions from mini-MVR to sternotomy approach with the data available, and this is an important operative complication to document for minimally invasive cases. As with any retrospective analysis, there is inherent selection bias in surgeons’ decision to perform a given operative approach. Patients may have seemingly equal preoperative risks but those with MAC or right ventricular dysfunction are not typically selected for a minimally invasive approach. A randomized trial of minimally invasive approach versus sternotomy would be the only way to analyze these outcomes without inherent selection bias, but this type of study could only assess patients who have suitable anatomy and physiology to undergo either operative approach. Our conclusions regarding the advantages of minimally invasive mitral surgery reflect the average outcomes of our regional database and are encouraging in that centers with a variety of volumes and experience levels are included, but we cannot categorically state that every institution performing minimally invasive mitral surgery will observe the same outcome and cost benefits. Finally, there are many different methods and techniques comprising minimally invasive mitral surgery, from the specific incision type to the cannulation strategy used and other operative methods. Our regional database likely captures several different mini-MVR strategies employed by various surgeons, so while our results are encouraging and supportive of this technology in general, we cannot draw any conclusions about a specific mini-MVR technique.
Conclusion
This study utilizing a multi-institutional regional database demonstrated that minimally invasive MVR in the “real world” can be performed with excellent outcomes and without increased costs in a real-world setting. Minimally invasive mitral surgery produced reductions in blood transfusion and improved resource utilization with shorter ICU and hospital lengths of stay. In summary, mini-MVR should be the preferred approach for isolated mitral surgery in appropriately selected patients at centers demonstrating excellence in minimally invasive outcomes.
DISCUSSION
11. Minimally Invasive Mitral Valve Surgery Has Superior Outcomes to Conventional Sternotomy Without Increased Costs. Paper presented by Emily A. Downs, MD, Charlottesville, VA. ead6m@virginia.edu
Discussion by Vinay Badhwar, MD, Pennsylvania badhwarv@upmc.edu Dr. V. Badhwar (Pittsburgh, PA): Congratulations on an elegant presentation.
Downs and colleagues from the Virginia Cardiac Surgery Quality Initiative provide us with an interesting analysis from 14 practices in 18 hospitals to examine the impact of primary minimally invasive mitral operations on outcome and costs in 369 propensity matched groups compared to sternotomy. As we have heard, their clinical registry data comes from version 2.73, for clarify, of the STS database from January 2011 to June 2014. I see the important take-aways from this study are that it joins the growing body of contemporary era global institutional evidence that a minimally invasive mitral operation, and perhaps particularly retrograde femoral perfusion, can be performed cost effectively and without increased cerebrovascular complications as previously noted in remote studies.
An important potential bias in the analysis that the authors justly disclose but needs specific mention, is the inability to define mitral pathoanatomy, such as, mitral annular calcification, that may impact the selection of a patient for a minimally invasive approach and, for that matter, mitral valve repair. This is not necessarily a deficiency in the study but in the available data. For all future analyses and national reporting purposes, this highlights the major importance of documenting the mitral pathoanatomic details by all users of the STS database.
So I have two questions for the authors as we attempt to maximize understanding of this important study. The first pertains to technical operative and surgeon factors and the second pertains to the cost analysis.
First, we know from other registry analyses that surgeon experience has a significant impact in the safe application of retrograde femoral perfusion as well as in the performance of durable mitral valve repair with good outcomes. The current study cohort is defined in the manuscript by “right chest approach” or “minimally invasive” terms. Can the authors clarify what proportion of these patients were done with retrograde femoral perfusion and why could they not account for surgeon experience in propensity matching?
Second, the UB-04 database CMS ratio of cost-to-charge methodology used to assess cost in this study really is a general estimate of Medicare beneficiaries in order to assess claims. As a broad statement, younger patients often have less comorbid disease and thus less costs in healthcare. The median age of this study was 59, well below the Medicare age, but as one would expect for a study on isolated mitral operations. This questions the applicability of the UB-04 to this younger population. Finally, certain fixed costs of equipment, such as robotics and video equipment specific to minimally invasive surgery that hospitals amortize often in the budgeting process and cost analysis of such programs, may not necessarily be accounted for with this UB-04 method. Could you comment on what proportion of the 369 minimally invasive patients were performed with robotic assistance in the 14 practices and what current or future provisions you might consider to aid the optimal interpretations of costs given some of these stated limitations?
I thank the Association for the opportunity to discuss this excellent paper.
11. Minimally Invasive Mitral Valve Surgery Has Superior Outcomes to Conventional Sternotomy Without Increased Costs. Response by Emily A. Downs, MD, Charlottesville, VA.
DR. DOWNS: Thank you so much for those questions, and those are some excellent points that really raise some of the concepts that we need to look at going forward using these large databases, especially when we are using multicenter data from different institutions that we need uniform data capture and as much information as possible with factors such as mitral pathology, as you mentioned.
I will start in reverse with those questions if you don't mind. With respect to the cost data, what we have available estimates total hospital costs, and while some of the expenses of equipment may be amortized over time, some of the more expensive components of these cases include one-time use devices such as the balloon occlusion when used, and specialized cannulas used for peripheral cannulation or LV vents (when used) with the minimally invasive approach. So those costs should be included in the total hospital costs. But you are correct that it is difficult to assess the cost of additional equipment that the hospital procures, either video equipment or robotic. A fairly small percentage of the patients in this entire group were listed as having had robot used in that slightly unspecific STS field, and we have a hard time interpreting that, because a few of those patients were listed as being full sternotomy approach. So it is unclear that that field was accurately captured as well. Once again, the accuracy of data is paramount to being able to properly assess these patients.
Going back to the question about surgeon experience, while we do have de-identified surgeon ID's and we could potentially assess surgeon volume over the time period studied, that really doesn't give us a good starting point for when a given surgeon began practicing or began practicing in minimally invasive surgery. So we still only have a snapshot of during this time frame without knowing what the surgeon's original start date was either of cardiac surgery in general or of a minimally invasive practice.
And then going back to your first question regarding femoral arterial cannulation, interestingly, the vast majority of the patients in this study with the minimally invasive approach were femorally arterially cannulated, over 90%, and we had very few in the axillary or other category. Less than 10%, more like 5%, were femorally cannulated in the sternotomy group.
11. Minimally Invasive Mitral Valve Surgery Has Superior Outcomes to Conventional Sternotomy Without Increased Costs. Paper presented by Emily A. Downs, MD, Charlottesville, VA. ead6m@virginia.edu
Discussion by W. Randolph Chitwood, Jr., MD, North Carolina chitwoodw@ecu.edu Dr. W. R. Chitwood (Greenville, NC):
Emily, congratulations on a great talk, and clearly there are positives and there are negatives in any talk like this, not your presentation but in the data. I want to congratulate the VCSQI team in the state of Virginia for amassing these kinds of data. We need this in all of our states, and I know that Alan Speir and his colleagues have done a great job with that initiative.
In many minimally invasive series, comparators are not comparing the same patient cohort. I congratulate you on doing propensity matched studies, suggesting that there is no difference in mortality for minimally invasive mitral surgery compared with the traditional approach Moreover, there are definite advantages to minimally invasive surgery. I have been speaking this mantra since 1995-1996 when surgeons thought we were completely insane to do operate through tiny incisions.
Several meta-analyses, especially the one one from the minimally invasive society - ISMICS, have suggested that retroperfusion causes more strokes. Other meta-analyses, including the one we did the one that was done by Sunderman and Falk, did not show an increase in strokes. I have a couple of questions.
You didn't give any ventilator data. We all are trying to get asymptotic to no ventilator time and less ICU stays. The Mayo Clinic group has probably done the best job. Yesterday, Joe Dearani from there told me that they were extubating their robotic and minimally invasive patients in the operating room. Do you have any data on ventilator times?
11. Minimally Invasive Mitral Valve Surgery Has Superior Outcomes to Conventional Sternotomy Without Increased Costs. Response by Emily A. Downs, MD, Charlottesville, VA.
DR. DOWNS: We do have total ventilator hours and I think in the slides we had the percentage of prolonged ventilation time, which in the overall unmatched cohort trended towards less common to have prolonged ventilation in the minimally invasive unmatched group but it was comparable in the matched groups.
DR. CHITWOOD: It has become a mission to get ventilator times down but I think that this goal requires too many people in the process chain to gain the best times. The process includes surgeons, anesthesiologists, ICU nurses, and intensivists - too many people in charge at different times.
And lastly complete follow-up is often impossible, because most groups don't have long-term echo data. Did you have any short-term follow-up on repair failures? Clearly, not everybody can repair a mitral valve. I know that in Virginia some do a lot of repairs and some do a few yearly. Do you have any follow-up, at least the short term, for repair failures?
DR. DOWNS: We have the reoperation field for valve indication, which would be within 30 days or within the same hospitalization, and I don't have the specific numbers on that, but I would be curious as well to see whether or not there is any difference.
DR. CHITWOOD: If you could get some of that information, it would be great. I think Tirone David is probably the only one and his group that has followed people long term with echos. Excellent presentation with excellent data.
DR. DOWNS: Thank you so much, Dr. Chitwood.
11. Minimally Invasive Mitral Valve Surgery Has Superior Outcomes to Conventional Sternotomy Without Increased Costs. Paper presented by Emily A. Downs, MD, Charlottesville, VA. ead6m@virginia.edu
Discussion by Kevin D. Accola, MD, Florida kaccola@cvsorlando.com Dr. K. Accola (Orlando, FL):
Emily, a nice presentation. You mentioned your limitations, but I think it really is important to distinguish -- and I certainly think you could probably do this again in your database -- if they were replacements, repairs and whether they were complex repairs versus simple repairs, because I think that would impact the cost analysis going on forward into a manuscript. Have you considered that, or what are your circumstances or your capabilities to do that?
11. Minimally Invasive Mitral Valve Surgery Has Superior Outcomes to Conventional Sternotomy Without Increased Costs. Response by Emily A. Downs, MD, Charlottesville, VA.
DR. DOWNS: We can certainly document repair versus replacement and, with more and more data fields available, the complexity of the mitral valve repair. However, what we don't have necessarily is the detailed information on the initial valve pathology and how that affects the subsequent decision to repair or replace.
DR. ACCOLA: Congratulations again on a nice presentation.
DR. DOWNS: Thank you so much.
NOTES
Article Type = Original Article/STSA Oral Presentation
Run footnote: Presented at the Southern Thoracic Surgical Association 62nd Annual Meeting; Orlando, FL, November 4-7, 2015
Set conflict box: Dr Ailawadi discloses a financial relationship with Mitralign, Abbott Vascular, Edwards LifeSciences, St. Jude Medical.
Discussion included in text file.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dogan S, Aybek T, Risteski PS, et al. Minimally Invasive Port Access Versus Conventional Mitral Valve Surgery: Prospective Randomized Study. Ann Thorac Surg. 2005;79:492–8. doi: 10.1016/j.athoracsur.2004.08.066. [DOI] [PubMed] [Google Scholar]
- 2.Svensson LG, Atik F a., Cosgrove DM, et al. Minimally invasive versus conventional mitral valve surgery: A propensity-matched comparison. J Thorac Cardiovasc Surg. 2010;139:926–32.e2. doi: 10.1016/j.jtcvs.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 3.Gammie JS, Zhao Y, Peterson ED, et al. Less-Invasive Mitral Valve Operations: Trends and Outcomes From The Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg. 2010;90:1401–10.e1. doi: 10.1016/j.athoracsur.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 4.Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. 2003 Vers. 4.0. [Google Scholar]
- 5.Stuart E. Developing practical recommendations for the use of propensity scores: Discussion of “A critical appraisal of propensity score matching in the medical literature between 1996 and 2003” by Peter Austin, Statistics in Medicine. Stat Med. 2008;27:2062–5. doi: 10.1002/sim.3207. [DOI] [PubMed] [Google Scholar]
- 6.Gillinov a M, Gelijns AC, Parides MK, et al. Surgical Ablation of Atrial Fibrillation during Mitral-Valve Surgery. N Engl J Med. 2015:1–11. doi: 10.1056/NEJMoa1500528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Algarni KD, Suri RM, Schaff H. Minimally invasive mitral valve surgery: Does it make a difference? Trends Cardiovasc Med. 2015;25:456–65. doi: 10.1016/j.tcm.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Santana O, Reyna J, Grana R, et al. Outcomes of Minimally Invasive Valve Surgery Versus Standard Sternotomy in Obese Patients Undergoing Isolated Valve Surgery. Ann Thorac Surg. 2011;91:406–10. doi: 10.1016/j.athoracsur.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 9.Atluri P, Stetson RL, Hung G, et al. Minimally invasive mitral valve surgery is associated with equivalent cost and shorter hospital stay when compared with traditional sternotomy. J Thorac Cardiovasc Surg. 2015 doi: 10.1016/j.jtcvs.2015.08.106. In press. [DOI] [PubMed] [Google Scholar]
- 10.McClure RS, Cohn LH, Wiegerinck E, et al. Early and late outcomes in minimally invasive mitral valve repair: An eleven-year experience in 707 patients. J Thorac Cardiovasc Surg. 2009;137:70–5. doi: 10.1016/j.jtcvs.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 11.Goldstone AB, Atluri P, Szeto WY, et al. Minimally invasive approach provides at least equivalent results for surgical correction of mitral regurgitation: A propensity-matched comparison. J Thorac Cardiovasc Surg. 2013;145:748–56. doi: 10.1016/j.jtcvs.2012.09.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng DCH, Martin J, Lal A, et al. Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations (Phila) 2011;6:84–103. doi: 10.1097/IMI.0b013e3182167feb. [DOI] [PubMed] [Google Scholar]
- 13.Modi P, Chitwood WR. Retrograde femoral arterial perfusion and stroke risk during minimally invasive mitral valve surgery: is there cause for concern? Ann Cardiothorac Surg. 2013;2:E1. doi: 10.3978/j.issn.2225-319X.2013.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atluri P, Goldstone AB, Fox J, Szeto WY, Hargrove WC. Port Access Cardiac Operations Can Be Safely Performed With Either Endoaortic Balloon or Chitwood Clamp. Ann Thorac Surg. 2014;98:1579–84. doi: 10.1016/j.athoracsur.2014.06.051. [DOI] [PubMed] [Google Scholar]
- 15.Bentala M, Heuts S, Vos R, et al. Comparing the endo-aortic balloon and the external aortic clamp in minimally invasive mitral valve surgery. Interact Cardiovasc Thorac Surg. 2015;21:359–65. doi: 10.1093/icvts/ivv160. [DOI] [PubMed] [Google Scholar]
- 16.Iribarne A, Easterwood R, Russo MJ, et al. A minimally invasive approach is more cost-effective than a traditional sternotomy approach for mitral valve surgery. J Thorac Cardiovasc Surg. 2011;142:1507–14. doi: 10.1016/j.jtcvs.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]