Abstract
Purpose
Current culture schema for human intestinal stem cells (hISCs) frequently rely on a 3D culture system using Matrigel™, a laminin-rich matrix derived from murine sarcoma that is not suitable for clinical use. We have developed a novel 2D culture system for the in vitro expansion of hISCs as an intestinal epithelial monolayer without the use of Matrigel.
Methods
Cadaveric duodenal samples were processed to isolate intestinal crypts from the mucosa. Crypts were cultured on a thin coat of type I collagen or laminin. Intestinal epithelial monolayers were supported with growth factors to promote self-renewal or differentiation of the hISCs. Proliferating monolayers were sub-cultured every 4–5 days.
Results
Intestinal epithelial monolayers were capable of long-term cell renewal. Less differentiated monolayers expressed high levels of gene marker LGR5, while more differentiated monolayers had higher expressions of CDX2, MUC2, LYZ, DEF5, and CHGA. Furthermore, monolayers were capable of passaging into a 3D culture system to generate spheroids and enteroids.
Conclusion
This 2D system is an important step to expand hISCs for further experimental studies and for clinical cell transplantation.
Keywords: Human intestinal epithelial stem cells, Monolayers, Differentiation, Type I collagen, Human laminin, Spheroids, Enteroids, In vitro culture
Introduction
In vitro intestinal epithelial cell culture can be used to increase our understanding of the basic biology of the intestinal epithelium and is an important tool for researching various intestinal disorders. In addition, this methodology holds the promise of expanding treatment options for various intestinal epithelial disorders [1, 2]. Intestinal epithelial stem cells (ISCs) are crypt-based cells that continuously undergo self-renewal and differentiation to populate the various epithelial lineages of the gut [3]. Specifically, goblet cells, enteroendocrine cells, Paneth cells, enterocytes, tuft and M cells are six differentiated epithelial lineages that arise from ISCs [3]. Recent years have seen significant advancement in murine and human intestinal epithelial cell culture [2, 4–18].
Spheroids and enteroids generated from dissociated crypts and ISCs are three-dimensional (3D) cell clusters [2, 5, 15, 17]. Successful culture of dissociated crypts/ISCs requires several components, including appropriate matrices and Wnt agonists [11, 12, 19–28]. Most commonly, Matrigel™ is used as a 3D support matrix for in vitro ISCs cultures [2, 15, 18]. However, as this field advances towards clinical application, we must move beyond utilizing Matrigel as a matrix for in vitro cell expansion. Matrigel is a gelatinous protein mixture derived from Engelbreth-Holm-Swarm (EHS) mouse sarcoma [2, 15] and is not FDA approved for clinical use. The protein mixture contains laminin-111, type IV collagen, perlecan, nidogen and small amounts of growth factors [29]. We sought to develop a system utilizing FDA approved matrices to rapidly generate and expand human intestinal stem cells (hISCs). Herein we describe a two dimensional (2D) in vitro culture system using thin coatings of type I collagen and recombinant human laminin to generate proliferating monolayers of hISCs. Furthermore, we demonstrate that such monolayers of cells can be induced to differentiate into mature intestinal epithelium.
1. Methods
1.1 Intestinal Crypt Isolation
Human cadaveric intestinal samples were obtained from Texas Organ Sharing Alliance (San Antonio, Texas) and were received en-bloc with the pancreas and spleen. The duodenum was carefully dissected from the head of the pancreas and then opened and washed with 1% chlorhexidine. A 4-cm segment of duodenum was used to isolate intestinal crypts as previously described [5, 15–17]. In brief, the seromuscular layers were dissected and discarded, and the remaining mucosa was washed with ice-cold 10 % phosphate buffered saline (PBS) (Fisher Scientific, Pittsburgh, PA). Diced mucosal segments were then treated incubated with 4 mM ethylenediaminetetraacetic acid (EDTA, Sigma, St. Louis, MO) and 1 mM dithiothreitol (DTT, Sigma, St. Louis, MO) at 4°C for 30 minutes with gentle shaking to facilitate crypts dissociation. The remaining washing and centrifugation steps were identical to the previously published protocols [5, 15–17]. Supernatant was discarded and the pellet containing isolated crypts was re-suspended in basic medium [Advanced Dulbecco’s Modified Eagle Medium (ADMEM)/Ham’s F12 (Invitrogen) with 2 mM GlutaMAX (Invitrogen), 10 mM HEPES (Invitrogen), and 1×Antibiotic-Antimycotic (Invitrogen)]. Crypt yield was determined by counting the number of crypts in 10 μl using an inverted light microscope.
1.2 Generating and Passaging Monolayers of Epithelial Cells
The culture methods are summarized in Figure 1. Thin coats of bovine type I collagen (Purecol, Advance BioMatrix, Inc. San Diego, Ca) and recombinant human laminin isotypes 111, 211, 332, and 511 (Biolamina, Sundbyberg, Sweden) were used as support matrix for monolayer growth. Coats were prepared on 48-well Nunclon Delta-treated cell culture plates (Thermo Scientific, Waltham, MA). A 120 μl coating solution of type I collagen at a concentration of 100 μg/ml was pipetted into each well and incubated at 37°C for one hour. After incubation, the coating solution was aspirated and crypts suspended in culture medium were added to each well. Laminin isotype coating solutions (111, 211, 332, and 511) were prepared as previously described [29]. After two hours incubation, the laminin coating solution was aspirated and crypts suspended in culture medium were added to each well.
Figure 1.
Flow chart describing culturing methodology.
Approximately 200–250 crypts per well were seeded to generate monolayers. In order to promote growth of undifferentiated monolayers, cultures were supported with 50% complete medium [basic medium, 1 mM N-acetylcysteine (Sigma), 1x N2 supplement (Invitrogen), 1x B27 supplement (Invitrogen)], 100 ng/ml recombinant murine Noggin (PeproTech), 50 ng/ml recombinant murine EGF (PeproTech), 1 μg/mL recombinant human (rh) R-spondin 1 (R&D Systems, Minneapolis, MN), 10 μM ROCK-inhibitor (Sigma)] and 50% intestinal subepithelial myofibroblast (ISEMF) conditioned medium (CM), which we denote as ENRY-ISEMF-CM. ISEMF-CM was prepared using pediatric human ISEMFs. ISEMFs were cultured for 5–7 days in Dulbecco’s Modified Eagle Medium (DMEM)/Low Glucose/GlutaMAX (Invitrogen), 10% FBS (Invitrogen), 1×Antibiotic-Antimycotic (Invitrogen), 0.25 U/mL insulin (Sigma), 20 ng/mL recombinant murine EGF (PeproTech, Rocky Hill, NJ), and 10 μg/mL transferrin (Sigma).
After 3 to 5 days of growth, monolayers reached 90–100% confluency and were sub-cultured as follows. TrypLE (Life Technologies) was pre-warmed at 37°C and 150 μl of TrypLE containing 10 μM ROCK-inhibitor was added to each well containing a confluent monolayer. Monolayers were incubated for 5 to 7 minutes at 37 °C to facilitate detachment of monolayers into single cells. TrypLE was then quenched using 10% FBS in ADMEM/F12. Cells were split 1:2 or 1:3 and re-seeded on a new thin coat of type I collagen, laminin-111, laminin-211, laminin-332, or laminin-511. Doubling-time was measured at time of sub-culture by using a hemocytometer.
1.3 Generating and Passaging More Differentiated Monolayers
Thin coats of type I collagen and laminin isotypes (111, 211, 332, and 511) were prepared and crypts were seeded to generate monolayers as described above. In order to promote growth of differentiated monolayers, cultures were supported with 50% complete medium, 5 μM GSK-inhibitor (Stemgent, Cambridge, MA), 10 μM SB 431542 (Tocris Bioscience, R&D Systems), and 50% L-Wnt3A conditioned medium (CM), which we denote as ENRYG-50Wnt3A. The 50% L-Wnt3A conditioned medium was prepared as previously described [30]. After 3 to 5 days of growth, monolayers reached 90–100% confluency and were sub-cultured and counted similar to undifferentiated monolayers as noted above.
1.4 Transforming Monolayers into More Differentiated Cells
At the time of passage, cells from undifferentiated monolayers were seeded onto new thin coatings and initially supported with ENRYG-50Wnt3A. After 3 days of culture, monolayers were supported with the following medium: 50% complete medium, 5 μM DAPT (Millipore) and 5% L-Wnt3A conditioned medium, which we denote as ENRY-DAPT-5Wnt3A.
1.5 Generating 3D Structures from Monolayers
Single cells from undifferentiated monolayers were re-suspended in Matrigel at the time of passage to generate spheroids and enteroids. Cells were then plated into 48-well Nunclon Delta-treated cell culture plates. Spheroid generation was promoted by supporting suspended cells with ENRY-ISEMF-CM. Generated spheroids were passaged every 5 days. Enteroids were generated by treating 5 days old spheroids with ENRYG-50Wnt3A for another 5 days. Thereafter, the newly forming enteroids were supported with ENRY-DAPT-5Wnt3A for another 3–5 days prior to passaging.
1.6 RNA Analysis
Messenger RNA (mRNA) was isolated from monolayers, spheroids, and enteroids using RNeasy Mini Kit (Qiagen, Valencia, CA). Reverse transcriptase polymerase chain reaction (RT-PCR) was performed to determine the expression levels of genes of interest, using Taqman Gene Expression Assays (Applied Biosystems, Carlsbad, CA) caudal type homeobox 2 (CDX2), chromogranin A (CHGA), lysozyme (LYZ) defensin (DEFA5), mucin 2 (MUC2), Lgr5 (LGR5), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), as previously described [1]. RT-PCR reactions were performed on a Prism 7900 HT Sequence Detection System (Applied Biosystems). Cycle numbers were analyzed according to the comparative CT method using GAPDH as the internal calibrator and human intestinal crypts as the reference tissue [1].
1.7 Statistical analysis
Data are expressed as mean ± standard deviation. Differences between groups were determined using paired t test and associated p-values are reported.
2. Results
2.1 Generating Monolayers of Epithelial Cells
We assessed the ability to generate monolayers of cells using intestinal crypts seeded on thin films of support matrices (Figure 2A). We were able to generate monolayers of intestinal epithelial cells on thin films of type I collagen and on thin films of laminin isotypes (111, 211, 332, and 511). By supporting dissociated crypts with ENRY-ISEMF-CM, we were able to generate proliferative and confluent monolayers containing hISCs. These cells expressed very high levels of the stem cell marker LGR5 compare to isolated crypts (p<0.05, Figure 3). The phenotype of cultures grown on collagen and the various laminin isotypes as assessed by light microscopy and RT-PCR were similar.
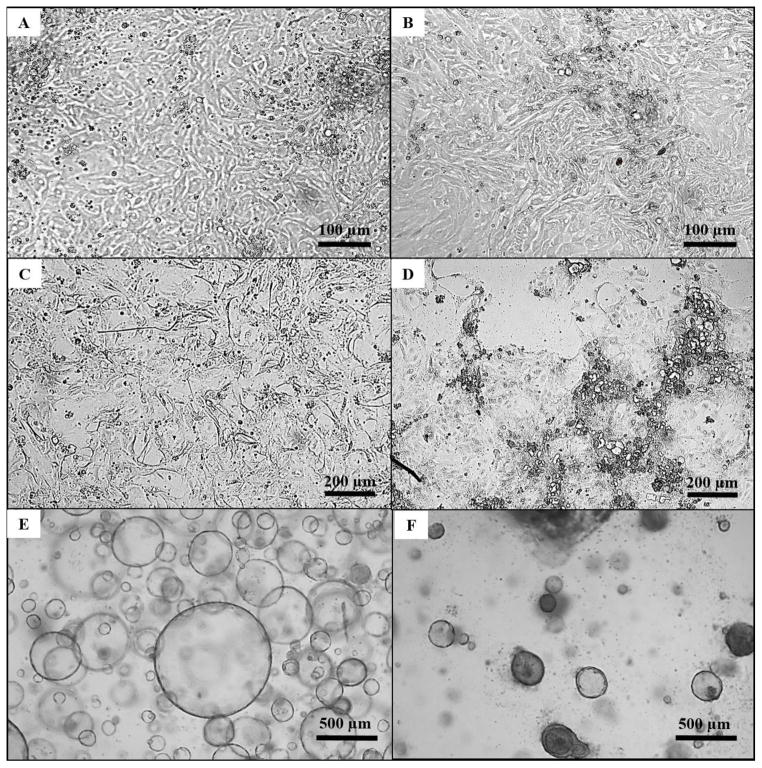
Figure 2. Light microscopy of human intestinal epithelial cultures.
A: A less differentiated monolayer of epithelial cells (Passage 4, Day 3). B: A more differentiated monolayer of epithelial cells (Passage 4, Day 3). C: A less differentiated monolayer of epithelial cells prior to transforming cells into a more differentiated monolayer of cells. D: A more differentiated monolayer generated from supporting monolayers with ENRYG-50Wnt3A and ENRY-DAPT-5Wnt3A. E: Spheroids (Passage 8, Day 5) generated from monolayers cells under light microscopy at 25x magnification. F: Enteroids (Passage 9, Day 16) generated by supporting spheroids with ENRYG-50Wnt3A and ENRY-DAPT-5Wnt3A under light microscopy at 25x magnification.
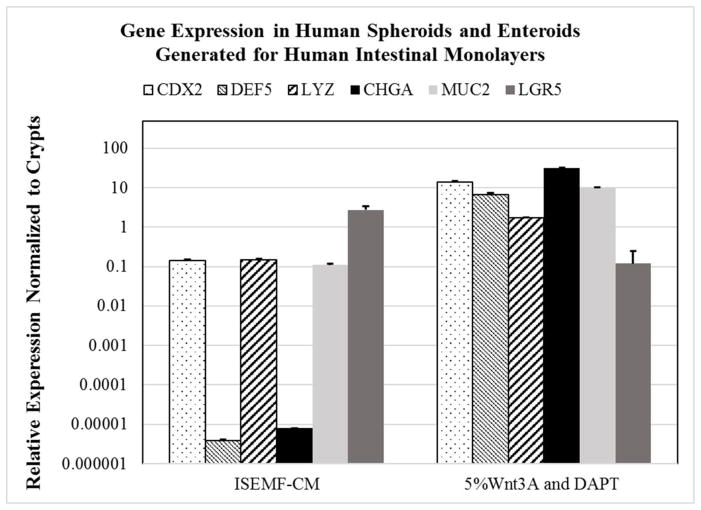
Figure 3. RT-PCR relative gene expression in monolayers.
RT-PCR was performed using GAPDH as a housekeeping gene, and relative mRNA expression levels are plotted on a logarithmic scale. Error bars, standard deviation. CDX2, DEF5, LYZ, CHGA, and MUC2 represent genes of mature intestinal epithelium/cells. LGR5 gene expression depicts intestinal stem cells.
2.2 Generating a More Differentiated Monolayer of Epithelial Cells
We assessed the ability to generate a more differentiated monolayer of epithelial cells by changing the culture medium (Figure 2B). By supporting dissociated crypts with ENRYG-50Wnt3A, we were able to generate proliferative and confluent monolayers which expressed lower levels of LGR5 and higher levels of mature intestinal markers (Figure 3). Upon passaging monolayers that were previously cultured in ENRY-ISEMF-CM, cells seeded on new thin films of type I collagen and laminin isotypes (111, 211, 332, and 511) were cultured with ENRYG-50Wnt3A for 2 days. After 2 days of culture, cells were then supported with ENRY-DAPT-5Wnt3A (Figure 2C). Gene expression of such cells showed a significant decrease in LGR5 and an increase CDX2, CHGA, LYZ, and DEF5 (p<0.05, Figure 3).
2.3 Monolayers of Cells can Expand Rapidly
We found that at earlier passage points (i.e. passage 0–5), monolayers had similar doubling-time in the two media formulations (Figure 4). However, at later passages (5 and greater), cells cultured in ENRYG-50Wnt3A ceased to divide. In contrast, cells cultured in ENRY-ISEMF-CM continued to grow and generated a large number of hISCs (Figure 4).
Figure 4. Monolayer cell amplification at each passage point.
Nine passage points are shown above. Graph is plotted on a logarithmic scale. Error bars denote standard deviation.
2.4 Generating Spheroids and Enteroids from Monolayers
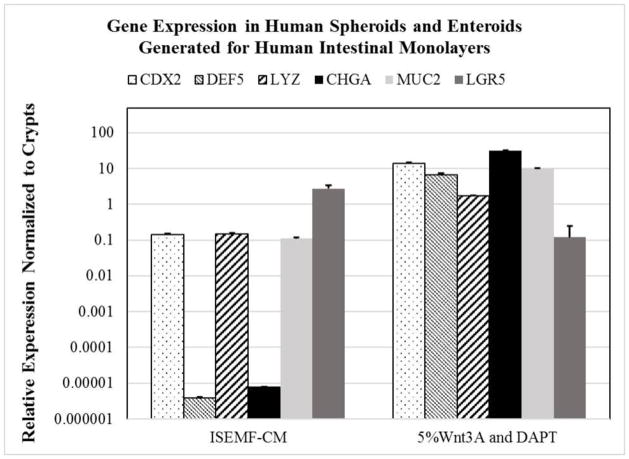
Cells from monolayers at the time of passage were re-suspended in Matrigel. Spheroids formed within Matrigel when cells were cultured with ENRY-ISEMF-CM (Figure 2E). Gene expression of cells in spheroids had increased expression of LGR5 when compared to enteroids (Figure 5). Enteroids were generated from spheroids using ENRYG-50Wnt3A and ENRY-DAPT-5Wnt3A (Figure 2F). Enteroids converted from such spheroids had increased expression of CDX2, CHGA, LYZ, DEF5, and MUC2 (Figure 5).
Figure 5. RT-PCR relative gene expression in spheroids and enteroids generated from monolayers.
RT-PCR was performed using GAPDH as a housekeeping gene, and relative mRNA expression levels are plotted on a logarithmic scale. Error bars denote standard deviation.
Discussion
Here we have demonstrated the ability to rapidly expand the population of hISCs by culturing and passaging intestinal cells as monolayers (Figure 2A). We have shown that dissociated crypts can be seeded onto thin films of type I collagen and various laminin isotypes (111, 211, 332, and 511), thereby generating proliferating monolayers of cells. Furthermore, cells in monolayers can be converted to not only more differentiated monolayers but also can form spheroids and enteroids in 3D cultures. The key benefit of this approach is the ability to generate large quantities of hISCs in the absence of Matrigel, a frequently used product derived from mouse sarcoma and not FDA approved substance for human use [2, 15]. Developing culture methods that utilize FDA approved substances is critical in moving this technology toward clinical application.
The use of ISEMF-CM has been shown to promote spheroids generation from cultured crypts/hISCs [2, 5, 14, 15, 17]. Spheroids are 3D structures that are comprised of mostly undifferentiated cells type [2, 14]. LGR5+ cells are stems cells that are actively cycling in the crypt-base [14, 17] and are responsible for intestinal epithelial self-renewal. Crypts containing hISCs seeded on thin films of type I collagen and laminin isotypes (111, 211, 332, and 511) and supported with ENRY-ISEMF-CM resulted in proliferating monolayers of cells. We found that only such monolayers resulted in long-term passage and self-renewal of hISCs (Figure 4), reflecting their “stemness”. When compared to more differentiated monolayers, LGR5 gene expression was on average greater than 80-fold higher (Figure 3). Low levels of CDX2, CHGA, LYZ, DEF5, and MUC2 expression in less differentiated monolayers illustrate the complexity of Wnt and Notch signaling, such that both Wnt and Notch signaling pathways can promote self-renewal of hISCs and epithelial differentiation [14].
The use of GSK-inhibitor (CHIR99021), DAPT, and Wnt3A-CM to support hISCs seeded on thin films of type I collagen and laminin isotypes promoted the generation of monolayers that expressed more differentiated cell lineages. We have demonstrated that not only can differentiated monolayers be generated from initially seeded crypts but they can also be generated from undifferentiated monolayers of cells (Figure 1A and 2A). GSK-inhibitor (CHIR99021), a glycogen synthase kinase 3β (GSK3β) inhibitor, has been shown to promote the proliferation of crypts cells and can synergisze with Wnt3A to activate the β-catenin signaling pathway, which plays a role in downstream hISCs differentiation [14]. DAPT promotes hISCs differentiation through Notch inhibition [14]. Differentiated monolayers showed increased expression of gene markers indicative of different mature cell lineages (Figure 4) [5, 15–17].
Currently most in vitro intestinal epithelial cell culture systems involve the use of Matrigel to generate 3D structures, i.e. spheroids and enteroids [2, 4, 5, 11, 14, 17]. We have previously shown that type I collagen can be used as a 3D support matrix to generate spheroids and enteroids [15, 16]. In the present work, we have demonstrated that hISCs grown as monolayers can be used to generate spheroids and enteroids (Figure 2E–F and Figure 5). Similar to previous studies, generated spheroids showed high expression of LGR5 when compared to enteroids [5, 14, 17], whereas enteroids showed higher expression of different intestinal cell lineage markers (Figure 3C).
Intestinal stem cell in vitro culturing has been used to study cellular mechanisms behind many pathologic states [31]. Intestinal diseases such as congenital tufting enteropathy, celiac disease, Crohn’s disease, or ulcerative colitis can potentially be treated with intestinal stem cell therapy [31, 32]. Stem cells and stem cell derived organoids can be transfected with specific vectors and transplanted into patients with known mutations, achieving integration of proper genetic material [31, 32].
Conclusion
Human small intestinal crypts can be cultured in a 2D system on thin films of type I collagen and various human laminin isotypes. The use of ENRY-ISEMF-CM to support cells resulted in the self-renewal of hISCs that express high levels of LGR5. Furthermore, monolayers can be passaged long-term without the use of Matrigel to generate large quantities of hISCs. Differentiated monolayers of cells can be generated directly from dissociated crypts and also from undifferentiated monolayers of cells. This 2D intestinal stem cell culture system which utilizes FDA approved substances to support hISCs provides a critical step toward clinical stem cell transplantation.
Acknowledgments
This work was performed as a project of the Intestinal Stem Cell Consortium (https://iscc.coh.org), a collaborative research project funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of Allergy and Infectious Diseases (DK085535-01 and DK085535-02S2, DK083762, and DK083319), and the California Institute for Regenerative Medicine (RT2-01985).
Footnotes
Level of Evidence: 1 Experimental
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jabaji Z, Brinkley GJ, Khalil HA, et al. Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium. PLoS One. 2014;9:e107814. doi: 10.1371/journal.pone.0107814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyoshi H, Stappenbeck TS. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat Protoc. 2013;8:2471–2482. doi: 10.1038/nprot.2013.153. doi:2410.1038/nprot.2013.2153. Epub 2013 Nov 2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–60. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 4.Cao L, Kuratnik A, Xu W, et al. Development of intestinal organoids as tissue surrogates: cell composition and the epigenetic control of differentiation. Mol Carcinog. 2015;54:189–202. doi: 10.1002/mc.22089. doi:110.1002/mc.22089. Epub 22013 Sep 22021. [DOI] [PubMed] [Google Scholar]
- 5.Lahar N, Lei NY, Wang J, et al. Intestinal subepithelial myofibroblasts support in vitro and in vivo growth of human small intestinal epithelium. PLoS One. 2011;6:e26898. doi: 10.1371/journal.pone.0026898. doi:26810.21371/journal.pone.0026898. Epub 0022011 Nov 0026817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCracken KW, Howell JC, Wells JM, et al. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat Protoc. 2011;6:1920–1928. doi: 10.1038/nprot.2011.410. doi:1910.1038/nprot.2011.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyoshi H, Ajima R, Luo CT, et al. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338:108–113. doi: 10.1126/science.1223821. doi:110.1126/science.1223821. Epub 1222012 Sep 1223826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mustata RC, Vasile G, Fernandez-Vallone V, et al. Identification of Lgr5-independent spheroid-generating progenitors of the mouse fetal intestinal epithelium. Cell Rep. 2013;5:421–432. doi: 10.1016/j.celrep.2013.09.005. doi:410.1016/j.celrep.2013.1009.1005. Epub 2013 Oct 1017. [DOI] [PubMed] [Google Scholar]
- 9.Ootani A, Li X, Sangiorgi E, et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15:701–706. doi: 10.1038/nm.1951. doi:710.1038/nm.1951. Epub 2009 Apr 1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasetyanti PR, Zimberlin C, De Sousa EMF, et al. Isolation and propagation of colon cancer stem cells. Methods Mol Biol. 2013;1035:247–59. doi: 10.1007/1978-1001-62703-62508-62708_62721. [DOI] [PubMed] [Google Scholar]
- 11.Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. doi:1710.1053/j.gastro.2011.1707.1050. Epub 2011 Sep 1762. [DOI] [PubMed] [Google Scholar]
- 12.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. doi:210.1038/nature07935. Epub 02009 Mar 07929. [DOI] [PubMed] [Google Scholar]
- 13.Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. doi:110.1038/nature09691. Epub 02010 Dec 09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin X, Farin HF, van Es JH, et al. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods. 2014;11:106–112. doi: 10.1038/nmeth.2737. doi:110.1038/nmeth.2737. Epub 2013 Dec 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jabaji Z, Brinkley GJ, Khalil HA, et al. Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium. PLoS One. 2014;9:e107814. doi: 10.1371/journal.pone.0107814. doi:107810.101371/journal.pone.0107814. eCollection 0102014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jabaji Z, Sears CM, Brinkley GJ, et al. Use of collagen gel as an alternative extracellular matrix for the in vitro and in vivo growth of murine small intestinal epithelium. Tissue Eng Part C Methods. 2013;19:961–969. doi: 10.1089/ten.tec.2012.0710. doi:910.1089/ten.TEC.2012.0710. Epub 2013 May 1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei NY, Jabaji Z, Wang J, et al. Intestinal subepithelial myofibroblasts support the growth of intestinal epithelial stem cells. PLoS One. 2014;9:e84651. doi: 10.1371/journal.pone.0084651. doi:84610.81371/journal.pone.0084651. eCollection 0082014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VanDussen KL, Marinshaw JM, Shaikh N, et al. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut. 2015;64:911–920. doi: 10.1136/gutjnl-2013-306651. doi:910.1136/gutjnl-2013-306651. Epub 302014 Jul 306659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman S, Liu X, Meyers C, et al. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest. 2010;120:2619–2626. doi: 10.1172/JCI42297. doi:2610.1172/JCI42297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dignass AU, Sturm A. Peptide growth factors in the intestine. Eur J Gastroenterol Hepatol. 2001;13:763–770. doi: 10.1097/00042737-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009;174:715–721. doi: 10.2353/ajpath.2009.080758. doi:710.2353/ajpath.2009.080758. Epub 082009 Feb 080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann C, Obermeier F, Artinger M, et al. Cell-cell contacts prevent anoikis in primary human colonic epithelial cells. Gastroenterology. 2007;132:587–600. doi: 10.1053/j.gastro.2006.11.017. Epub 2006 Nov 2016. [DOI] [PubMed] [Google Scholar]
- 23.Kim KA, Kakitani M, Zhao J, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- 24.Korinek V, Barker N, Moerer P, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 25.Kuhnert F, Davis CR, Wang HT, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A. 2004;101:266–271. doi: 10.1073/pnas.2536800100. Epub 2003 Dec 2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon C, VanDussen KL, Miyoshi H, et al. Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol. 2014;7:818–828. doi: 10.1038/mi.2013.98. doi:810.1038/mi.2013.1098. Epub 2013 Nov 1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. doi:510.1038/cr.2008.1047. [DOI] [PubMed] [Google Scholar]
- 28.Pinto D, Gregorieff A, Begthel H, et al. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodin S, Domogatskaya A, Strom S, et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol. 2010;28:611–615. doi: 10.1038/nbt.1620. doi:610.1038/nbt.1620. Epub 2010 May 1030. [DOI] [PubMed] [Google Scholar]
- 30.Willert K, Brown JD, Danenberg E, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. Epub 2003 Apr 2027. [DOI] [PubMed] [Google Scholar]
- 31.Mohamed MS, Chen Y, Yao CL. Intestinal stem cells and stem cell-based therapy for intestinal diseases. Cytotechnology. 2015;67:177–189. doi: 10.1007/s10616-014-9753-9. doi:110.1007/s10616-10014-19753-10619. Epub 12014 Jul 10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grabinger T, Luks L, Kostadinova F, et al. Ex vivo culture of intestinal crypt organoids as a model system for assessing cell death induction in intestinal epithelial cells and enteropathy. Cell Death Dis. 2014;5:e1228. doi: 10.1038/cddis.2014.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]