Abstract
Background
For patients with chronic rhinosinusitis (CRS), the decision to elect continued medical management vs. surgery is complex and involves tradeoffs between benefits, risks, and overall effectiveness of each therapy. The purpose of this study is to investigate whether baseline disease-specific quality of life (QOL) can assist in predicting outcomes in patients with refractory CRS who elect continued medical management.
Methods
CRS patients electing medical management were enrolled in a prospective, multi-institutional cohort study. Patients were stratified into pre-treatment Sino-nasal Outcome Test (SNOT-22) subgroups based on 10-point score increments (e.g. 10-19,20-29,30-39,etc.) to capture potential outcome differences by baseline SNOT-22 disease burden. The proportion of patients achieving minimal clinically important difference (MCID≥9 points) and relative improvement (%) for each score category were calculated.
Results
Seventy-five CRS patients with a mean pre-treatment SNOT-22 score of 45.2[16.6] were followed for a mean of 14.9 months. The majority of participants electing medical therapy failed to improve one MCID (57%) with a mean relative score improvement of 16%. Overall, 37% of patients maintained baseline SNOT-22 QOL status, while 20% of patients deteriorated >1 MCID. When treatment crossover patients (to ESS) were included (n=117), approximately 1 in 4 (27%) patients achieved a MCID.
Conclusions
Results from this study suggest that the majority of CRS patients electing ongoing medical management with low baseline disease-specific QOL impairment maintain stable QOL with continued medical management. Furthermore, of CRS patients electing ongoing medical therapy, approximately 1 in 4 patients achieved MCID while 1 in 5 experienced deterioration by >1 MCID.
MeSH Key Words: Sinusitis, outcome assessment, patient outcome assessment, case-control studies, medical therapy management
Introduction
Many patients with chronic rhinosinusitis (CRS) will remain symptomatic despite medical management and be faced with a treatment decision to either continue with medical management alone or undergo endoscopic sinus surgery (ESS). 1-3 This decision is often multifactorial and includes both the provider and patient's analysis of a sum of factors including associated risks, benefits, and financial costs. Further, and possibly more important to patients making these decisions, is the impact CRS has on physical and mental well-being, lost quality of life (QOL), impaired productivity, and concern over the financial impact associated with the chosen treatment.
Previous investigation has demonstrated that CRS specific QOL as measured by the Sino-Nasal Outcomes Test-22 (SNOT-22) heavily influences a patient's decision to undergo endoscopic sinus surgery.4 The stratification of SNOT-22 scores (e.g 0-10, 10-20, 20-30, etc) prior to ESS provides additional information for the patient-centered decision making process. Rudmik et al. demonstrated that patients with a SNOT-22 score >30 had an 80% chance of achieving the minimal clinically important difference (MCID) following ESS in a US-Canadian based cohort, while Hopkins et al. demonstrated that patients with SNOT-22 scores >30 had a 66% chance of achieving the MCID in a large cohort from the United Kingdom.5,6 The ability to utilize a patient-reported outcome, such as the SNOT-22, advances the understanding of expected patient outcomes, improves the patient-physician shared decision making process, and may help to reduce the risk of unwarranted practice variation in the future.7 Given that CRS specific QOL is a major factor in a patient's decision to continue medical therapy or elect ESS, knowledge regarding expected outcomes from medical management is necessary to accurately counsel patients in regards to their treatment choices.
The primary objective of this study was to evaluate whether baseline disease-specific quality of life as measured by the SNOT-22 can assist in predicting outcomes in patients with refractory CRS who elect continued medical management. Outcomes from this study may improve the patient-provider shared-decision making process in patients with refractory CRS who elect continued medical management.
Materials and Methods
Study Population
Investigational data were obtained from a non-randomized, prospectively collected database designed to evaluate various treatment outcomes in adult (≥18 years) patients with CRS for a multi-institutional trial funded by the National Institutes of Health (clinicaltrials.gov: #NCT01332136). Patients were diagnosed with persistent symptoms of CRS defined by criteria described by the American Academy of Otolaryngology.8,9 All study participants had completed previous unstandardized medical therapy including, but not limited to, at least one course (≥14 days) of broad spectrum or culture-directed antibiotics and at least one course of either topical corticosteroid application (≥21 days) or a 5 day course of systemic corticosteroids. Patients met inclusion criteria if they elected to continue therapeutic treatment applicable to individual disease progression and patient tolerance under the discretion of the enrolling physician. Preliminary findings from this clinical trial have been previously published.10-13
The Institutional Review Board (IRB) at each enrollment site governed all investigational protocols and informed consent procedures. Enrollment sites were comprised of sinus and skull base surgery clinics within academic hospital systems including Oregon Health & Science University (OHSU; Portland, OR., IRB#7198), Stanford University (Palo Alto, CA., IRB#4947), the Medical University of South Carolina (Charleston, SC., IRB#12409), and the University of Calgary (Calgary, Alberta, Canada, IRB#E-24208), while central coordinating services were conducted at OHSU. Study participants were assured that study consent was voluntary and standard of care was not altered due to study protocols.
During each baseline enrollment meeting, participants were asked to provide detailed demographic information, as well as social and medical history cofactors including, but not limited to: age, gender, asthma, nasal polyposis, depression, allergy, and history of previous sinus surgery. Participants were followed for up to 18 months post-treatment and interim evaluations during regular 6 month intervals, either during physician-directed clinical appointments or via follow-up mailings using postal service and self-addressed return envelopes.
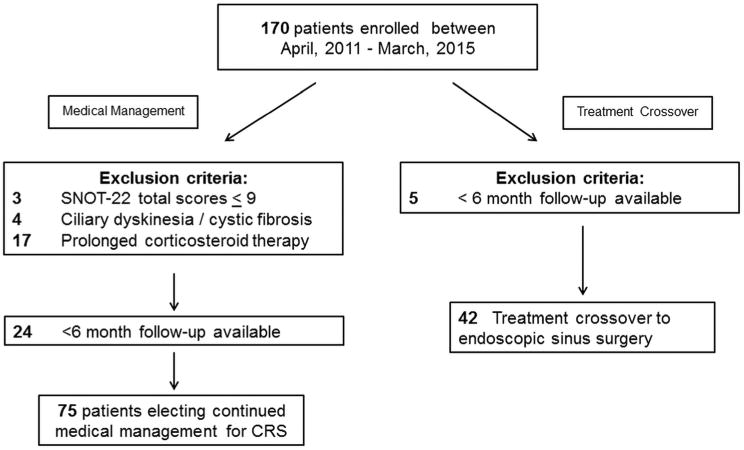
Study participants with comorbid ciliary dyskinesia, cystic fibrosis or any condition dependent on prolonged corticosteroid therapy were excluded from final analyses due to the heterogeneity of these diseases and the potential variation in treatment algorithms. Additional participants were excluded if they had not yet entered the primary post-treatment follow-up period (< 6 months). Patients with baseline SNOT-22 total scores between 0-9 were excluded due to the inability to achieve an MCID on reported scores (Figure 1).
Figure 1. Final cohort selection following exclusion criteria.
Treatment Modality
Prior to any study enrollment meeting, and following physician directed counseling, patients self-selected subsequent treatment for symptoms related to CRS. For the purposes of this study, patients electing continued medical management met primary inclusion criteria. Patients could also be categorized into a treatment crossover cohort of patients initially electing medical therapy who elected to change treatment modality to include endoscopic sinus surgery (ESS) at some point during the study duration. This treatment crossover cohort was included in the analysis to evaluate the treatment outcomes of all patients who initially elected medical management.
Measures of Medication Use
Preoperative and postoperative outcome evaluations also included questions of past days of medication use (days out of the previous 90) including: antibiotics, systemic corticosteroids, topical corticosteroid drops, topical corticosteroid sprays, antihistamines, decongestants, leukotriene modifiers, and saline irrigations.
Primary Outcome Measure
Study participants were asked to complete the SNOT-22 to evaluate the severity of symptoms related to CRS (©2006, Washington University, St. Louis, MO) during the baseline enrollment period and during each follow-up evaluation. The SNOT-22 is a validated, treatment outcome measure applicable to chronic sinonasal conditions.14 Individual item scores are measured using patient selected responses on a Likert scale where higher scores indicate worse symptom severity as follows: 0= “No problem”; 1=”Very mild problem”; 2=”Mild or slight problem”; 3=”Moderate problem”; 4=”Severe problem”; 5=”Problem as bad as it can be”. Higher total scores on the SNOT-22 suggest worse patient functioning or symptom severity (score range: 0-110). A MCID on the SNOT-22 has been previously described as an improvement of at least 8.9 points following ESS.14 The percentage of participants who reported achieving a MCID during continued medical management for CRS was evaluated across 10 baseline SNOT-22 score categories using 10-point increments from 10 to 110. Ten point increment score categories were chosen to improve the provider's ability to compare outcomes across studies, as results examining the predictive value of the SNOT-22 for surgically managed patients have previously been published using 10 point increments.5,6,14 Due to low sample sizes for participants with scores >70, these participants were grouped together and reported as SNOT-22 score >70. A total of 7 groups were analyzed.
Data Management
Study data were coded using unique study identification numbers and stripped of all protected health information to ensure confidentiality before transfer to OHSU from each enrollment site. All study data were manually entered into a relational database (Microsoft Access, Microsoft Corp., Redmond, WA.) and statistical analyses were conducted using commercially available software (SPSS v.22, IBM Corp., Armonk, NY). All data were evaluated descriptively while data normality was verified for all continuous measures using distributive analysis. Baseline characteristics and comorbid conditions were compared across baseline SNOT-22 score categories using Pearson's chi-square omnibus (χ2) testing with 2×7 contingency tables and the Kruskall-Wallis omnibus test for continuous variables. McNemar's (χ2) testing was used to compare the prevalence of medication use between baseline and follow-up evaluations. Reported p-values represent findings from ‘global’ omnibus tests which indicate a significant difference between at least two SNOT-22 scoring categories. The prevalence of participants reporting at least a 9-point improvement in SNOT-22 scores is described for each baseline SNOT-22 score category. Relative improvement across baseline categories of SNOT-22 total scores was determined for each participant with the formula: [(postoperative score) – (preoperative score)/(preoperative score)] × 100. Greater negative percentage values of mean relative improvements represent greater postoperative improvements in relation to a patient's baseline symptom status.
Results
After initial applied exclusion criteria, a total of 99 participants with refractory CRS who opted to continue with medical management alone were included for preliminary analysis while 75/99 (78%) subjects met the >6 month follow-up inclusion criteria and were followed for an average of 14.9 ± 4.6 months. The number of participants in each of the 7 final baseline SNOT-22 score categories followed an approximate standard normal distribution with reduced sample sizes (Figure 2).
Figure 2.
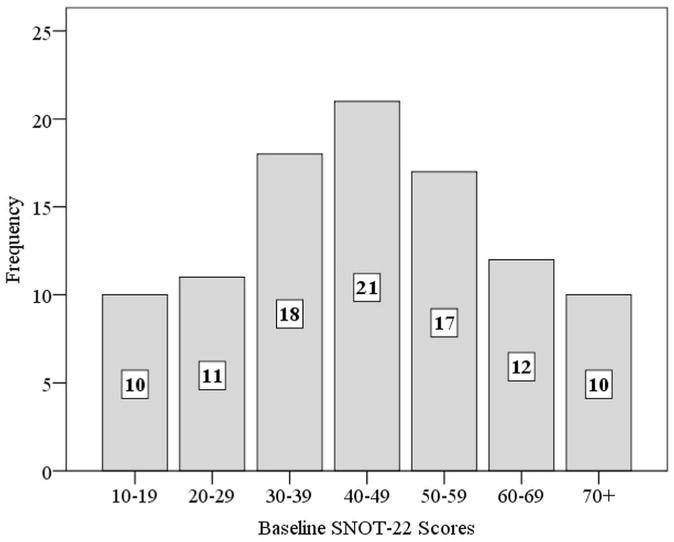
Distribution of baseline SNOT-22 score categories. SNOT-22, 22-item SinoNasal Outcome Test (n=99)
Overall cohort characteristics and comorbid conditions were compared across baseline SNOT-22 score categories (Table 1). No significant differences between any two SNOT-22 baseline score categories were found across any baseline measure using omnibus testing (p>0.050).
Table 1. Final cohort characteristics across baseline SNOT-22 score categories.
| Baseline characteristics: | Baseline SNOT-22 Score Category | |||||||
|---|---|---|---|---|---|---|---|---|
| 10-19 (n=10) | 20-29 (n=11) | 30-39 (n=18) | 40-49 (n=21) | 50-59 (n=17) | 60-69 (n=12) | 70+ (n=10) | p-value | |
| Age (years)- mean[SD] | 47.3 [14.5] | 55.7 [12.3] | 56.6 [17.1] | 53.6 [14.7] | 50.4 [14.3] | 48.1 [12.6] | 47.7 [7.1] | 0.311 |
| Males-n (%) | 4 (40%) | 6 (55%) | 9 (50%) | 11 (53%) | 8(47%) | 2(17%) | 3 (30%) | 0.432 |
| Asthma-n(%) | 5 (50%) | 2 (18%) | 6 (33%) | 6 (29%) | 6(35%) | 8 (67%) | 5 (50%) | 0.223 |
| Allergy-n (%) | 5 (50%) | 4 (36%) | 6 (33%) | 7 (33%) | 9(53%) | 5 (42%) | 5 (50%) | 0.845 |
| Depression-n(%) | 0 (0%) | 1(9%) | 2 (11%) | 2 (10%) | 1(6%) | 3 (25%) | 2 (20%) | 0.549 |
| Nasal polyps- n(%) | 3 (30%) | 5 (46%) | 6 (33%) | 6 (29%) | 9(53%) | 4 (33%) | 4 (40%) | 0.779 |
| Prior sinus surgery-n(%) | 6 (60%) | 4 (36%) | 7 (39%) | 12 (57%) | 10 (59%) | 10 (83%) | 5 (50%) | 0.265 |
| Endoscopy score-mean[SD] | 4.7 [2.9] | 6.0 [5.0] | 6.1 [3.3] | 4.9 [3.4] | 8.0[4.3] | 6.6 [3.9] | 5.4 [4.9] | 0.290 |
| CT score- mean[SD] | 10.4 [2.9] | 14.7 [5.4] | 12.2 [5.9] | 12.3 [7.5] | 13.7 [6.3] | 11.3 [5.4] | 13.6 [6.8] | 0.562 |
SNOT-22, 22-item SinoNasal Outcome Test; SD, standard deviation; N, sample size number; CT, computed tomography
Overall Improvement Across Baseline SNOT-22 Scores
Significant improvement in SNOT-22 total score in patients undergoing continued medical management for CRS was reported (n=75; 45.2[16.6] to 37.5[23.3]; Δ= 7.6; 95% CI: 3.1 – 12.2; p=0.001). A total of 32/75 participants (43%) reported improving at least one MCID after continued medical management and the average relative mean improvement in SNOT-22 total scores was 16%. The remainder of the cohort was comprised of 28/75 participants (37%) who reported no change greater than one MCID and 15/75 participants (20%) who reported deterioration/worsening of SNOT-22 scores of at least one MCID value. The proportion of patients reporting an improvement of at least one MCID, along with the relative mean improvement in SNOT-22 total scores for each of the 7 SNOT-22 score categories is reported in Table 2.
Table 2. Probability of achieving a MCID in SNOT-22 total scores across baseline SNOT-22 score categories in patients electing continued medical management for CRS (n=75).
| Baseline SNOT-22 Score Category | Probability of Achieving MCID n (%) | Relative Mean Improvement in SNOT-22 Scores (%) | Proportion of Patients Changing < 1 MCID n (%) | Proportion of Patients Worsening ≥1 MCID n (%) | |
|---|---|---|---|---|---|
| 10-19 | n=6 | 0 (0%) | 0% | 5 (83%) | 1 (17%) |
| 20-29 | n=8 | 3 (38%) | -21% | 5 (63%) | 0 (0%) |
| 30-39 | n=14 | 7 (50%) | -12% | 4 (29%) | 3 (21%) |
| 40-49 | n=19 | 6 (32%) | -6% | 6 (32%) | 7 (37%) |
| 50-59 | n=14 | 9 (64%) | -40% | 3 (21%) | 2 (14%) |
| 60-69 | n=7 | 2 (29%) | -5% | 3 (43%) | 2 (29%) |
| 70+ | n=7 | 5 (71%) | -20% | 2 (29%) | 0 (0%) |
CRS, chronic rhinosinusitis; SNOT-22, 22-item SinoNasal Outcome Test; MCID, minimal clinically important difference; positive relative mean improvement scores indicate average relative worsening quality of life scores.
Overall Improvement Across Baseline SNOT-22 Scores Including Treatment Crossovers
To capture all patients who elected medical management during initial study enrollment, participants meeting inclusion criteria who elected to switch treatment paradigms to undergo ESS during the study period (n=42) were combined with the medical management cohort and analyzed together (n=117). Within the treatment crossover cohort, initial SNOT-22 scores were compiled for analysis; however, SNOT-22 surveys were not re-administered prior to treatment crossover thereby precluding the ability to calculate relative improvement scores prior to ESS. Under the assumption that a change in treatment modality to ESS is a reflection of failure to achieve a MCID in those who initially elect medical management, the probability of achieving a MCID for the total cohort was calculated for those participants with follow-up (n=117). In total, 32/117 patients were found to achieve a MCID (27.3%). The prevalence of all participants reporting improvement of at least one MCID across baseline SNOT-22 scores is reported in Table 3.
Table 3. Probability of achieving a MCID in SNOT-22 total scores across baseline SNOT-22 score categories including subjects with follow-up electing to cross-over to ESS (n=117).
| Baseline SNOT-22 Score Category | Probability of Achieving MCID n (%) | |
|---|---|---|
| 10-19 | n=9 | 0 (0%) |
| 20-29 | n=14 | 3 (21%) |
| 30-39 | n=21 | 7 (33%) |
| 40-49 | n=23 | 6 (26%) |
| 50-59 | n=25 | 9 (36%) |
| 60-69 | n=11 | 2 (18%) |
| 70+ | n=14 | 5 (36%) |
SNOT-22, 22-item SinoNasal Outcome Test; MCID, minimal clinically important difference; ESS, endoscopic sinus surgery
Prevalence of patient-reported medication use for sinusitis at baseline and follow-up evaluations
Differences between initial enrollment prevalence of medication use were compared to prevalence of medication use at last follow-up evaluation. Participants were found to report significantly lower prevalence of oral antibiotic use (p≤0.001), oral/systemic corticosteroid use (p<0.001), and antihistamine use (p≤0.008). The prevalence of patient reported medication use is reported in Table 4.
Table 4. Prevalence of patient-reported medication use (in the past 90 days) for sinusitis at baseline and follow-up study evaluations (n=75).
| Baseline | Last Available Follow-up (≥6 months) | ||
|---|---|---|---|
| Medication regimen: | n (%) | n (%) | p-value |
| Topical nasal steroid sprays | 56 (75%) | 45 (60%) | 0.035 |
| Topical nasal steroid drops/irrigations | 22 (29%) | 28 (37%) | 0.286 |
| Decongestants | 35 (47%) | 28 (37%) | 0.230 |
| Oral antibiotics | 41 (55%) | 20 (27%) | <0.001 |
| Oral/systemic corticosteroid | 38 (51%) | 15 (20%) | <0.001 |
| Antihistamines | 39 (52%) | 27 (36%) | 0.008 |
| Leukotriene modifiers | 19 (25%) | 18 (24%) | >0.999 |
| Saline irrigations rinse | 63 (84%) | 57 (76%) | 0.210 |
p-value represents significance using McNemar's chi-square test statistics
Discussion
Patient-centered outcomes and comparative effectiveness studies are designed to improve health outcomes by providing evidence-based information to clinicians, patients, and decision-makers about which interventions are most effective for patients under individual circumstances.15-17 For patients with CRS, this information is of particular importance as they weigh the decision to continue medical therapy or pursue endoscopic sinus surgery.
Both medical and surgical therapies for CRS are directed at reducing mucosal inflammation, preventing disease recurrence or progression, and controlling sinonasal symptoms with the ultimate goal of improving both disease specific and general quality of life. We sought to improve patient centered decision making by examining the proportion of patients who achieve a minimal clinically important difference following continued medical management for CRS. The MCID for the SNOT-22 is defined as 8.9 points, or the minimal change in symptoms or QOL after a given intervention (e.g. medical management or ESS) that is perceptible and pertinent to the individual patient.14,18
Within SNOT-22 categories, there were no differences in baseline characteristics for participants enrolled in the current study. Clinical measures of disease severity such as CT scores and endoscopy scores did not increase across worsening baseline QOL measures, a finding which highlights the difficulty in stratifying CRS disease severity based on objective measures only.
Of those participants who did not change treatment course during the study, thirty two participants (43%) in the ongoing medical management cohort reported improving at least one MCID after ongoing medical management. The percentage of patients achieving MCID in this study is considerably lower than those who undergo ESS as reported by Rudmik et al.5 and Hopkins et al.6 In Rudmik's 2014 study, patients meeting similar enrollment criteria reported an average overall relative improvement of 46.4% following ESS, while 70-80% of participants achieved an MCID improvement of 9 points.5 Hopkins et al. reported similar findings in a large UK cohort in which 66% of patients achieved the MCID following ESS, with an overall relative improvement of 40%.6 Participants undergoing medical management in the current study reported an overall relative improvement of 16%, which is also considerably lower than values reported by Hopkins et al. and Rudmik et al. for patients undergoing ESS. The diminished relative improvement and percentage of patients who achieve MCID reported in the current study reflects recent literature in which patients with recalcitrant CRS undergoing medical management failed to gain significant improvement in disease specific QOL measures.19,20
Participants with baseline SNOT-22 scores less than 20 were found to report no improvement with continued medical management (0% MCID, 0% relative mean improvement). This finding is consistent with previous investigations in which patients with SNOT-22 scores <20 typically did not achieve meaningful clinical improvement following ESS.5,6 While failure to achieve MCID in this particular group is noteworthy, only one patient in the lowest baseline SNOT-22 category undergoing medical management reported worsening of symptoms at subsequent follow up time points. Additionally, patients with baseline SNOT-22 scores <30 did not exhibit progression of disease severity as measured by MCID. These data suggest that continued medical management may help to maintain or stabilize symptoms in those patients with relatively lower/better baseline SNOT-22 scores and highlight the added advantage of measuring pre-treatment quality of life to improve patient counseling regarding outcomes prior to selection of treatment modality.
While Hopkins et al. demonstrated that the probability of achieving MCID following surgery increased with increasing preoperative QOL severity, results from the current study are less consistent. Participants with a baseline SNOT-22 score >70 were most likely to achieve MCID (71%), whereas participants with baseline scores between 20 and 70 were found to have more variable results (achieving MCID: range 29%-64%). The differences seen across baseline SNOT-22 groups in % achieving MCID and relative improvement are likely a reflection of several factors, including the possibility of variations in medical management practices, lower overall enrollment, limitations of the MCID designation, and the possibility of statistical trends such as regression to the mean.
In a secondary intention-to- treat type analysis, participants initially enrolled in the medical management cohort who elected to crossover to surgery were also included (Table 3). While SNOT-22 scores were not re-tabulated prior to switching treatment modality in the current study design, it is reasonable to define those patients who crossover to ESS as a “failure to achieve MCID.” It may also be a more accurate reflection of an individual patient's disease burden. This assumption is supported by Smith et al.'s prospective crossover study in which participants with recalcitrant CRS reported worsened disease specific QOL despite ongoing medical management prior to crossover to ESS.20 Further, recent data from Soler et al. suggest that CRS-specific QOL, as measured by the SNOT-22, is the major determinant of whether a patient choses medical management or surgical therapy. For patients electing ESS, greater impairment of QOL was found to be the most robust determinant of this elected treatment strategy, surpassing factors such as finances, patient personality profiles, social support structures, and a patient's trust in their physician.4 Under this definition, 27% of patients undergoing medical management in the current study achieved MCID. This has substantial implications for pre-treatment patient counseling, as approximately 1 in 4 patients whom elect continued medical therapy after initial treatment will note clinical improvement.
While this study is strengthened by its prospective, longitudinal, multi-institutional nature, several additional caveats must be considered when interpreting the presented results. Patients enrolled in this study had previously completed an initial trial of medical therapy and represent a unique subset of CRS patients. Nevertheless, baseline pre-treatment SNOT-22 scores in the current cohort follow an approximate standard distribution and are likely representative of the variety of patients that a clinician might encounter in day-to-day practice. Additionally, continued medical management may vary according to both prescribing clinician practices and patient needs and tight control over medical regimens was precluded by the observational study design. While data characterizing the prevalence of medical regimens is provided in Table 4 for those participants with follow-up, the results from this study must be interpreted with potential variations in medical therapy in mind. This was left unstandardized in the study design to reflect current clinical practice and patient outcomes. Due to limited sample size, subgroup analysis (e.g., CRSsNP vs.CRSwNP, primary vs.revision ESS) was not performed as meaningful conclusions would be inherently challenging to report. Patients within the crossover cohort elected to switch treatment paradigms at varying points throughout the course of the study and follow up SNOT-22 surveys were not repeated prior to ESS. Results from this study must be interpreted with these limitations in mind, as there are no doubt additional considerations that drive patients to elect ESS (e.g., finances, social support, risk taking, etc). Despite these factors, outcomes from this study will help to inform shared patient-provider decisions about the chance of achieving the MCID and percentage of relative improvement that may be expected following continued medical management for CRS.
Conclusion
CRS patients with low baseline QOL impairment (SNOT-22 scores <30) report stable disease-specific QOL with continued medical management. The overall relative improvement and percentage of patients achieving MCID with medical management is lower than previously reported improvement with ESS. Outcomes from this study suggest that of all patients electing continued medical management as a treatment option, between 27% and 43% of patients will achieve a MCID improvement in reported QOL. The information reported in this study may improve the shared patient-provider decision making process as patients weigh the decision to continue medical management or pursue endoscopic sinus surgery.
Acknowledgments
Timothy L. Smith, Jess C. Mace, and Jeremiah A. Alt are supported by a grant for this investigation from the National Institute on Deafness and Other Communication Disorders (NIDCD), one of the National Institutes of Health, Bethesda, MD., USA (R01 DC005805; PI/PD: TL Smith). Public clinical trial registration (www.clinicaltrials.gov) ID# NCT01332136. This funding organization did not contribute to the design or conduct of this study; collection, management, analysis, or interpretation of the data; preparation, review, approval or decision to submit this manuscript for publication. Timothy L. Smith and Adam S. DeConde are consultants for IntersectENT, (Menlo Park, CA) which is not affiliated with this investigation.
Footnotes
Conflicts of Interest: None
Financial Disclosures: There are no financial disclosures for Toby O. Steele or Luke Rudmik.
The abstract for this manuscript was accepted for podium presentation to the American Rhinologic Society during the American Academy of Otolaryngology-Head and Neck Surgery annual meeting in Dallas, Texas, September 25-26th, 2015.
References
- 1.Luk LJ, Steele TO, Mace JC, Soler ZM, Rudmik L, Smith TL. Health utility outcomes in patients undergoing medical management for chronic rhinosinusitis: a prospective multiinstitutional study. Int Forum Allergy Rhinol. 2015 doi: 10.1002/alr.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lal D, Scianna JM, Stankiewicz JA. Efficacy of targeted medical therapy in chronic rhinosinusitis, and predictors of failure. Am J Rhinol Allergy. 2009;23(4):396–400. doi: 10.2500/ajra.2009.23.3334. [DOI] [PubMed] [Google Scholar]
- 3.Young LC, Stow NW, Zhou L, Douglas RG. Efficacy of medical therapy in treatment of chronic rhinosinusitis. Allergy Rhinol (Providence) 2012;3(1):e8–e12. doi: 10.2500/ar.2012.3.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soler ZM, Rudmik L, Hwang PH, Mace JC, Schlosser RJ, Smith TL. Patient-centered decision making in the treatment of chronic rhinosinusitis. Laryngoscope. 2013;123(10):2341–2346. doi: 10.1002/lary.24027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudmik L, Soler ZM, Mace JC, DeConde AS, Schlosser RJ, Smith TL. Using preoperative SNOT-22 score to inform patient decision for Endoscopic sinus surgery. Laryngoscope. 2015;125(7):1517–1522. doi: 10.1002/lary.25108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hopkins C, Rudmik L, Lund VJ. The predictive value of the preoperative Sinonasal outcome test-22 score in patients undergoing endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope. 2015;125(8):1779–1784. doi: 10.1002/lary.25318. [DOI] [PubMed] [Google Scholar]
- 7.Wennberg JE. Time to tackle unwarranted variations in practice. 3422011 doi: 10.1136/bmj.d1513. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 Suppl):S1–31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 Suppl):S1–S39. doi: 10.1177/0194599815572097. [DOI] [PubMed] [Google Scholar]
- 10.DeConde AS, Mace JC, Bodner T, et al. SNOT-22 quality of life domains differentially predict treatment modality selection in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(12):972–979. doi: 10.1002/alr.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steele TO, Mace JC, Smith TL. Does comorbid anxiety predict quality of life outcomes in patients with chronic rhinosinusitis following endoscopic sinus surgery? Int Forum Allergy Rhinol. 2015 doi: 10.1002/alr.21543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alt JA, Smith TL, Schlosser RJ, Mace JC, Soler ZM. Sleep and quality of life improvements after endoscopic sinus surgery in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(9):693–701. doi: 10.1002/alr.21364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudmik L, Smith TL, Schlosser RJ, Hwang PH, Mace JC, Soler ZM. Productivity costs in patients with refractory chronic rhinosinusitis. Laryngoscope. 2014;124(9):2007–2012. doi: 10.1002/lary.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(5):447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 15.Jonas DE, Mansfield AJ, Curtis P, et al. Identifying priorities for patient-centered outcomes research for serious mental illness. Psychiatr Serv. 2012;63(11):1125–1130. doi: 10.1176/appi.ps.201100369. [DOI] [PubMed] [Google Scholar]
- 16.Selby JV, Beal AC, Frank L. The Patient-Centered Outcomes Research Institute (PCORI) national priorities for research and initial research agenda. Jama. 2012;307(15):1583–1584. doi: 10.1001/jama.2012.500. [DOI] [PubMed] [Google Scholar]
- 17.Piccirillo JF. Outcomes research and otolaryngology. Otolaryngol Head Neck Surg. 1994;111(6):764–769. doi: 10.1177/019459989411100611. [DOI] [PubMed] [Google Scholar]
- 18.Soler ZM, Smith TL. Quality of life outcomes after functional endoscopic sinus surgery. Otolaryngol Clin North Am. 2010;43(3):605–612, x. doi: 10.1016/j.otc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith KA, Rudmik L. Impact of continued medical therapy in patients with refractory chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(1):34–38. doi: 10.1002/alr.21238. [DOI] [PubMed] [Google Scholar]
- 20.Smith KA, Smith TL, Mace JC, Rudmik L. Endoscopic sinus surgery compared to continued medical therapy for patients with refractory chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(10):823–827. doi: 10.1002/alr.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]


