Abstract
Background
Serum β-trace protein (BTP) and β2-microglobulin (B2M) are independently associated with end-stage renal disease (ESRD) and mortality in the general population and high-risk groups with diabetes or advanced chronic kidney disease (CKD). Less is known about their associations with outcomes and predictive ability in adults with moderate CKD.
Study Design
Prospective cohort study.
Setting & Participants
3613 adults from the Chronic Renal Insufficiency Cohort (CRIC) Study (45% women; mean age, 57.9 years; 41.0% non-Hispanic black; 51.9% with diabetes).
Predictors
BTP and B2M with a reciprocal transformation to reflect their associations with filtration, creatinine-based estimated glomerular filtration rate (eGFRcr), measured GFR (mGFR) and a 4-marker composite score combining BTP, B2M, creatinine, and cystatin C. Predictors were standardized as z scores for comparisons across filtration markers.
Outcomes
ESRD, all-cause mortality, and new-onset cardiovascular disease. Results: Over a six-year median follow-up, 755 (21%) participants developed ESRD, 653 died, and 292 developed new-onset cardiovascular disease. BTP, B2M, and the 4-marker composite were independent predictors of ESRD and all-cause mortality, and B2M and the 4-marker composite of cardiovascular events, after multivariable adjustment. These associations were stronger than those observed for eGFRcr (p vs. eGFRcr ≤0.02). The 4-marker composite led to improvements in the C statistic and 2.5 year risk reclassification beyond eGFRcr for all outcomes.
Limitations
Filtration markers measured at one time point; mGFR available in subset of cohort.
Conclusions
BTP and B2M may contribute additional risk information beyond eGFRcr and the use of multiple-markers may improve risk prediction beyond this well-established marker of kidney function among persons with moderate CKD.
Keywords: Beta-trace protein (BTP), β2-microglobulin (B2M), CKD Biomarkers Consortium, filtration markers, renal function, estimated glomerular filtration rate (eGFR), chronic kidney disease (CKD), end-stage renal disease (ESRD), mortality, cardiovascular events, Chronic Renal Insufficiency Cohort (CRIC)
Chronic kidney disease (CKD) is common in the United States and is associated with progression to end-stage renal disease (ESRD), cardiovascular disease (CVD) and increased risk of CVD and death.1–4 Current established filtration markers for prediction of outcomes in persons with CKD include serum creatinine and cystatin C; identification and validation of additional biomarkers can lead to improvements in prediction. Both β-trace protein (BTP) and β2-microglobulin (B2M) are serum proteins of interest as novel filtration markers. In prior work BTP and B2M were shown to be independently associated with increased risk of all-cause and CVD mortality, and with CVD events in US adults, and, for BTP, with CKD progression and mortality in adults with hypertensive kidney diseases and non-diabetic kidney disease and all-cause and CVD mortality in adults on hemodialysis.5–9 However, less is known about associations with outcomes and the utility of these markers together compared to measured GFR (mGFR) in adults with mild-to-moderate CKD because the prior work in CKD did not evaluate B2M and is limited to selected CKD patient populations that may not be generalizable to broader CKD populations.
The Chronic Renal Insufficiency Cohort (CRIC) Study is a representative prospective cohort of adults with CKD in the United States. We compared associations of BTP, B2M, and established filtration markers cystatin C and creatinine―alone and in combination―with ESRD, all-cause mortality, and new onset CVD and evaluated whether using a combination of filtration markers can improve overall risk prediction beyond creatinine-based eGFR alone in the CRIC Study. We hypothesize that BTP and B2M are associated with these outcomes independent of known risk factors and established markers of kidney function including eGFRcr.
Methods
Study Population
The CRIC Study is a multicenter observational cohort study of adults aged 21–74 years with mild-to-moderate CKD recruited from seven clinical centers in the United States in 2003–2008; the design of the study and enrollment has been described previously.10,11 Of the 3939 participants at baseline, we excluded participants with missing data on serum BTP, B2M, and multivariable model covariates resulting in a study sample of 3613 participants, of whom 2405 were free of baseline CVD. Analyses that include mGFR are limited to participants who underwent GFR assessment (n=1324). The CRIC Study was approved by institutional review boards at participating institutions. All participants provided written informed consent.
Exposure Assessment
In 2012, BTP, B2M, and creatinine were measured at the University of Minnesota in stored serum samples from the baseline visit; samples were stored at −80° C prior to performing measurements. Both BTP and B2M were measured using the Siemens Dade Behring ProSpec nephelometer (Siemens Healthcare Diagnostics) and creatinine was measured using the Roche enzymatic method on a Roche Modular P Analyzer (Roche Diagnostics). Inter-assay laboratory coefficients of variation for BTP, B2M, and creatinine at the University of Minnesota laboratory were 6.0%, 2.7%, and 2.3%, respectively. Cystatin C was previously measured in stored samples using a particle-enhanced immunonephelometric assay on the Siemens BN II system with standardization to account for laboratory drift over time12 and standardized to the international reference standard ERM-DA47/IFCC (International Federacy of Clinical Chemistry and Laboratory Medicine). Creatinine-based eGFR (eGFRcr) was determined using the 2009 CKD-EPI (CKD Epidemiology Collaboration) creatinine equation and cystatin C—based eGFR (eGFRcys) was determined using the 2012 CKD-EPI cystatin C equation.13,14 The GFR was measured (mGFR) in a sub-sample of participants using a standardized urinary 125I-iothalamate clearance protocol, originally selected with sampling stratified by age, sex, race, and diabetes status.12
Outcome Assessment
Outcomes were ESRD, all-cause mortality, and incident cardiovascular events. Participants were asked every 6 months about hospitalizations, ESRD events, or possible cardiovascular events. The ESRD events were defined as initiation of maintenance dialysis or receiving a kidney transplant during follow-up, with additional events identified through US Renal Data System (USRDS) linkage. Hospitalizations with diagnostic or procedural discharge codes for cardiovascular events underwent further review by at least two physicians for possible heart failure, myocardial infarction, or cerebrovascular events. Incident cardiovascular events were defined as the first in-patient hospitalization for definite or probable myocardial infarction, heart failure, or cerebrovascular event after baseline; analyses for incident CVD events were limited to participants free of baseline CVD. All-cause mortality events were identified by reporting through next of kin, death certificates, hospital records, and Social Security Death Master File Linkage.
Covariate Assessment
Demographic and clinical characteristics were assessed at the baseline examination. Demographic characteristics of interest included age, sex, and race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Hispanic/Other). Smoking status was defined as current, former, or never smoker based on self-report. Prevalent CVD at baseline was defined as a self-reported history of coronary artery disease or prior revascularization of blood vessels. Hypertension was defined as a systolic blood pressure ≥140mmHg, a diastolic blood pressure ≥90mmHg, or use of antihypertensive medication. High density lipoprotein cholesterol, high-sensitivity C-reactive protein, glucose, and hemoglobin A1c were measured in baseline blood samples. Diabetes was defined as a fasting blood glucose ≥126 mg/dL, a random blood glucose ≥200 mg/dL, hemoglobin A1c≥6.5, or self-reported use of insulin or oral diabetes medication. Urinary albumin-creatinine ratio (UACR, expressed as milligrams per gram) was estimated based on a 24-hour urine collection.
Statistical Analyses
Descriptive statistics are presented as mean ± standard deviation for continuous variables (median [interquartile range] for skewed variables) and as number (percentage) for categorical variables. We applied a reciprocal transformation to BTP (1/BTP) and B2M (1/B2M) to reflect the association of these markers with filtration. For comparisons across filtration markers, we modeled each marker (1/BTP, 1/B2M, eGFRcr, mGFR, four-marker combination score) as a standardized z score (see Figure S1, available as online supplementary material). To evaluate a combined marker approach, we used Cox proportional hazards regression models to create a composite score based on the weighted linear combination of four serum filtration markers (eGFRcr, eGFRcys, BTP [1/BTP], B2M [1/B2M]) and their respective β coefficients:
Composite=−(βeGFRcr*eGFRcr+βeGFRcys*eGFRcys+β1/BTP*1/BTP+ β1/B2M*1/B2M)
Further details are in Item S1 and Figure S2.
We used Cox Proportional Hazards models to estimate the association of each blood biomarker and composite score with incident ESRD, all-cause mortality, and the cardiovascular events, first using restricted cubic splines with knots at z score quintiles and multivariable adjustment for potential confounders (age, sex, race, diabetes, hypertension, prevalent CVD, smoking status, high-density lipoprotein cholesterol, natural log (ln)-transformed C-reactive protein, ln-transformed UACR). Markers and the composite were also modeled continuously with hazard ratios (HRs) presented per 1-unit decrease in the z score (representing a standard deviation lower marker level at baseline). Models were adjusted for age, sex, and race with additional multivariable adjustment for the aforementioned potential confounders and further adjustment in separate models for eGFRcr and mGFR. We used seemingly unrelated regression, an approach that allows for correlated errors across a set of regression models, to statistically compare the strength of associations for BTP, B2M, and mGFR with the association observed for eGFRcr.15
We evaluated improvement in model discrimination when using the 4-marker composite compared to eGFRcr by comparing Harrell’s C statistics and likelihood ratio tests in multivariable Cox Proportional Hazards models. We evaluated improvement in 2.5-year risk prediction using the continuous net reclassification improvement (NRI) and relative integrated discrimination improvement (RIDI) for each outcome beyond eGFRcr when using the 4-marker composite in multivariable Poisson regression models.16 We selected 2.5-year follow-up for risk prediction models due to short follow-up time for participants who entered the study at the end of the baseline examination period. Statistical analyses were performed in Stata, Version 12.1 (StataCorp LP).
Results
Baseline Characteristics
Overall, participants were on average 57.9 years old, 44.7% were women, 41.0% were non-Hispanic black, and 51.9% had diabetes (Table 1). Hypertension and CVD were common, found in 86.0% and 33.4% of participants, respectively. Average baseline mGFR, eGFRcr, BTP, and B2M were 49 mL/min/1.73m2, 44 mL/min/1.73m2, 1.21 mg/L, and 4.4 mg/L, respectively. Some differences in characteristics were observed in the subsample with mGFR compared to those without mGFR; participants with mGFR were younger with a different race/ethnicity and baseline smoking distribution, lower high-density lipoprotein cholesterol and high-sensitivity CRP, higher UACR and B2M, and lower eGFRcr (Table S1). Moderate to strong correlations were observed among filtration markers and mGFR (Table 2; Table S2); correlations of 1/B2M with eGFRcr and mGFR tended to be stronger than those observed with 1/BTP.
Table 1.
Baseline characteristics of the study sample.
| Overall | |
|---|---|
| No. of participants | 3613 |
| Age (y) | 57.9 ±10.9 |
| Female Sex | 1615 (44.7%) |
| Race/ethnicity | |
| Non-Hispanic White | 1542 (42.7%) |
| Non-Hispanic Black | 1482 (41.0%) |
| Hispanic | 443 (12.3%) |
| Other | 146 (4.0%) |
| Smoking Status | |
| Never | 1624 (45.0%) |
| Former | 1520 (42.1%) |
| Current | 469 (13.0%) |
| Prevalent CVD | 1208 (33.4%) |
| Hypertension | 3107 (86.0%) |
| Diabetes | 1875 (51.9%) |
| Total cholesterol (mg/dL) | 183 ± 45 |
| HDL Cholesterol (mg/dL) | 48 ± 15 |
| High-sensitivity CRP (mg/L) | 2.6 [1.1–6.4] |
| Kidney Measures | |
| UACR (mg/g) | 51 [8–450] |
| mGFR (n=1324), mL/min/1.73m2 | 49 ± 21 |
| eGFRcr (mL/min/1.73m2) | 44 ± 15 |
| BTP (mg/L) | 1.21 ± 0.60 |
| B2M (mg/L) | 4.4 ± 2.2 |
Other includes Asian/Pacific Islander and Native Americans
Note: Values for categorical variables are given as percentages; values for continuous variables, as mean ± standard deviation or median [interquartile range]. Conversion factor for cholesterol in mg/dL to mmol/L, ×0.02586.
CRP, C-reactive protein; CVD, cardiovascular disease; HDL. high-density lipoprotein; mGFR, measured glomerular filtration rate; eGFRcr, creatinine-based estimated glomerular filtration rate; BTP, β-trace protein; B2M, β2-microglobulin; UACR, urinary albumin-creatinine ratio
Table 2.
Pearson correlations for the filtration markers
| mGFR* | eGFRcr | 1/BTP | 1/B2M | |
|---|---|---|---|---|
| mGFR | 1.00 | |||
| eGFRcr | 0.83 | 1.00 | ||
| 1/BTP | 0.68 | 0.67 | 1.00 | |
| 1/B2M | 0.83 | 0.78 | 0.74 | 1.00 |
Note: N=3613. All p<0.001.
mGFR, measured glomerular filtration rate; eGFRcr, creatinine-based estimated glomerular filtration rate; BTP, β-trace protein; B2M, β2-microglobulin
Correlations with mGFR in in subsample of N=1324 with mGFR assessment.
ESRD
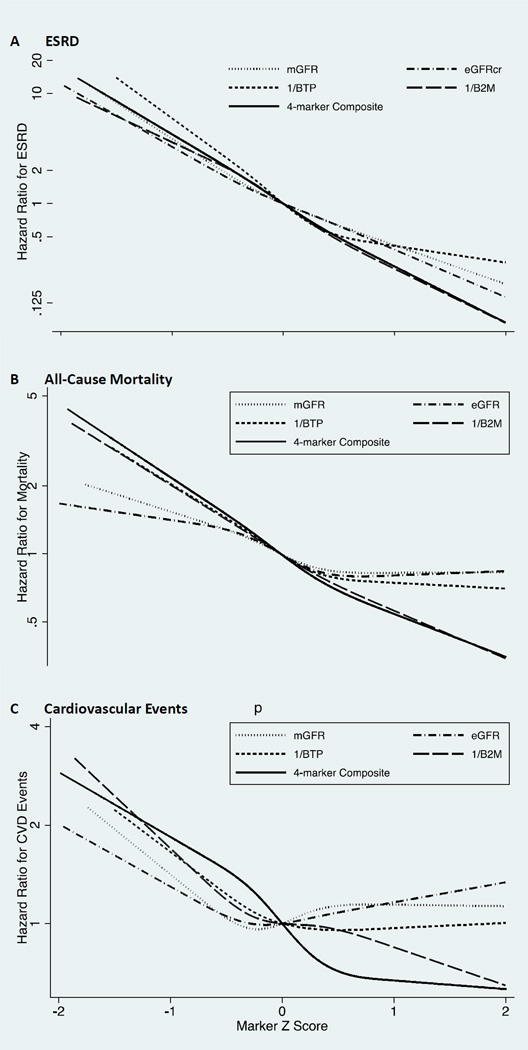
Over a median follow-up of 6.1 years, 755 participants (21%) developed ESRD. Multivariable-adjusted risk associations of each marker and the 4-marker composite with ESRD were similar and linear on the log-scale, with higher HRs at lower marker levels (Figure 1a; 95% confidence intervals [CIs] in Figure S3). In models adjusted for age, sex, and race (Table 3) lower baseline levels of individual filtration markers and the 4-marker composite were all significantly associated with increased ESRD risk, with associations stronger than observed for eGFRcr. Associations were attenuated but remained significant with multivariable adjustment, with 1/BTP, 1/B2M, and the 4-marker composite but not mGFR more strongly associated with ESRD than eGFRcr (Table 3). Associations with eGFRcr, 1/BTP, 1/B2M, and the 4-marker composite persisted with additional adjustment for mGFR (Table 3, all p<0.001), however, only the 4-biomarker composite was more strongly associated with ESRD than eGFRcr (p versus eGFRcr <0.001). Similar results were observed when analyses were limited to participants with mGFR (Table S3). The 4-marker composite led to modest, statistically significant improvement to the C statistic when compared to a multivariable Cox regression model with eGFRcr (Table 4). In multivariable models for 2.5 year risk prediction, the 4-marker composite modestly improved the RIDI and risk classification beyond eGFRcr alone for ESRD (Table 4), with statistically significant improvements observed in those who did not develop ESRD (NRInon-event, 0.13; 95% CI, 0.10–0.17) and in those who developed ESRD (NRIevent, 0.12; 95% CI, 0.001–0.24).
Figure 1.
Hazard Ratios for (A) end-stage renal disease (ESRD), (B) all-cause mortality, and (C) cardiovascular events modeled as functions of filtration markers. Filtration markers are modeled as restricted cubic splines on the z score scale (knots at quintiles) for comparisons across markers. Abbreviations: mGFR, measured GFR; eGFR, creatinine-based eGFR; BTP, beta trace protein; B2M, beta-2 microglobulin.
Table 3.
Adjusted Hazard Ratios of incident events Note: HRs are per unit decrease in z score, representing a standard deviation decrease in the marker.
| Age-sex-race Adjusted | Multivariable adjusted§ | Multivariable§+mGFR§§ | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p vs. eGFRcr** |
HR (95% CI) | p vs. eGFRcr** |
HR (95% CI) | p vs. eGFRcr** |
|
| ESRD (755 events/3613 participants) | ||||||
| mGFR, n=1324 | 4.67 (3.86, 5.64)* | 0.1 | 3.51 (2.85, 4.31)* | 0.3 | - | - |
| eGFRcr | 4.03 (3.63, 4.48)* | - | 3.18 (2.84, 3.55)* | - | 2.55 (1.96, 3.32)* | - |
| 1/BTP | 8.55 (7.28, 10.04)* | <0.001 | 4.59 (3.86, 5.45)* | <0.001 | 2.84 (1.92, 4.18)* | 0.6 |
| 1/B2M | 4.81 (4.29, 5.41)* | <0.001 | 3.56 (3.13, 4.04)* | 0.01 | 2.52 (1.89, 3.36)* | 0.9 |
| Combination | 5.52 (4.90, 6.22)* | <0.001 | 4.10 (3.61, 4.66)* | <0.001 | 4.80 (3.31, 6.94)* | <0.001 |
| All-Cause Mortality (653 events/3613 participants) | ||||||
| mGFR, n=1324 | 1.90 (1.54, 2.35)* | 0.1 | 1.36 (1.08, 1.71)^ | 0.7 | - | - |
| eGFRcr | 1.59 (1.44, 1.74)* | - | 1.30 (1.18, 1.44)* | - | 0.73 (0.55, 0.97)^ | - |
| 1/BTP | 1.96 (1.75, 2.20)* | <0.001 | 1.52 (1.34, 1.74)* | 0.02 | 1.13 (0.84, 1.53) | 0.03 |
| 1/B2M | 2.32 (2.09, 2.58)* | <0.001 | 1.93 (1.71, 2.18)* | <0.001 | 1.59 (1.13, 2.25)^ | <0.001 |
| Combination | 2.46 (2.21, 2.74)* | <0.001 | 2.03 (1.80, 2.29)* | <0.001 | 1.77 (1.31, 2.38)* | <0.001 |
| Cardiovascular Events (292 events/2405 participants free of CVD at baseline) | ||||||
| mGFR, n=978 | 1.58 (1.26, 1.98)* | 0.4 | 1.10 (0.86, 1.42) | 0.8 | - | - |
| eGFRcr | 1.46 (1.28, 1.66)* | - | 1.07 (0.93, 1.23) | - | 0.86 (0.62, 1.20) | - |
| 1/BTP | 1.87 (1.58, 2.20)* | <0.001 | 1.16 (0.98, 1.39) | 0.2 | 1.03 (0.73, 1.46) | 0.4 |
| 1/B2M | 2.05 (1.78, 2.37)* | <0.001 | 1.45 (1.22, 1.72)* | <0.001 | 1.69 (1.14, 2.51)^ | 0.002 |
| Combination | 2.16 (1.86, 2.50)* | <0.001 | 1.64 (1.38, 1.96)* | <0.001 | 1.75 (1.28, 2.41)* | 0.01 |
CI, confidence interval; HR, hazard ratio; mGFR, measured glomerular filtration rate; eGFRcr, creatinine-based estimated glomerular filtration rate; BTP, β-trace protein; B2M, β2-microglobulin
p<0.05,
p<0.001 for 1-unit decline in z score in Cox proportional hazards regression models.
Multivariable model adjusted for age, sex, race, diabetes, hypertension, prevalent cardiovascular disease, smoking status, high density lipoprotein cholesterol, natural log-transformed C-reactive protein, natural log transformed urinary albumin-creatinine ratio.
Comparison to eGFRcr performed in seemingly unrelated regression models with same adjustment variables as appropriate for column.
HRs from multivariable model additionally adjusted for mGFR and performed in the subset of participants with mGFR.
Table 4.
C statistics, Likelihood Ratio Test P-Values, and the 2.5-Year RIDI and Continuous NRI for Multivariable Models with eGFRcr and with the 4-marker Composite Score
| Outcome | C statistic With eGFR |
C statistic With 4-marker Composite |
Difference in C- statistic (95% CI) |
Likelihood Ratio Test P-Value |
RIDI (95% CI) | Continuous NRI (95% CI) |
|---|---|---|---|---|---|---|
| ESRD | 0.888 | 0.895 | 0.007 (0.004–0.010) | <0.001 | 0.026 (0.006–0.047) | 0.252 (0.130–0.374) |
| All-cause Mortality | 0.744 | 0.770 | 0.026 (0.017–0.036) | <0.001 | 0.233 (0.162–0.304) | 0.446 (0.304–0.588) |
| Cardiovascular Events | 0.765 | 0.780 | 0.015 (0.004–0.025) | <0.001 | 0.185 (0.094–0.276) | 0.392 (0.216–0.567) |
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HR, hazard ratio; RIDI, relative integrated discrimination improvement; mGFR, measured glomerular filtration rate; NRI, net reclassification improvement.
Note: Multivariable models adjusted for age, sex, race/ethnicity, diabetes, hypertension, prevalent cardiovascular disease, smoking status, high density lipoprotein cholesterol, natural log-transformed C-reactive protein, natural log transformed urinary albumin-creatinine ratio
All-Cause Mortality
Over a median follow-up of 6.7 years, 653 participants (18%) died. The multivariable-adjusted restricted cubic spline models (Figure 1b; 95% CI in Figure S4) indicated weaker associations and flatter HR functions for mGFR and eGFRcr. In contrast, 1/B2M and the 4-marker composite had stronger, linear relationships with mortality across their range of values. At lower values 1/BTP showed a similar relationship as 1/B2M but at higher values flattens and was similar to mGFR and eGFRcr.
In models adjusted for age, sex, and race (Table 3) lower baseline levels of individual filtration markers and the 4-marker composite were each significantly associated with increased mortality risk (all p<0.001). These associations remained after multivariable adjustment, with 1/BTP, 1/B2M, and the 4-marker composite (Ps versus eGFRcr of 0.02, <0.001, and <0.001, respectively) but not mGFR (p versus eGFRcr = 0.7) more strongly associated with mortality than eGFRcr (Table 3). Lower 1/B2M and the 4-marker composite remained independently associated with increased mortality risk after additional adjustment for mGFR with significantly stronger associations than observed for eGFRcr (p versus eGFRcr <0.001); eGFRcr was associated with decreased mortality risk (HR, 0.73; 95% CI, 0.55–0.97). Similar results were observed when analyses were limited to participants with mGFR (Table S4).
The 4-marker composite had modest, statistically significant improvements in the C statistic when compared to a multivariable Cox regression model with eGFRcr (Table 4). The 4-marker composite also improved the RIDI and mortality risk for overall risk classification beyond eGFR alone over a period of 2.5-years (Table 4), with statistically significant, but modest, improvements observed in both those who did (NRIevent, 0.25; 95% CI, 0.11–0.39) and those who did not die (NRInon-event, 0.20; 95% CI, 0.17–0.23).
Cardiovascular Events
Compared to those with CVD at baseline, participants free of baseline CVD were more likely to be younger, female, and non-Hispanic white, and with a better cardiovascular risk factor profile and higher kidney function (Table S5) Of the 2405 participants free of CVD at baseline, 292 (12%) had a cardiovascular event. The shape of cardiovascular event HR functions based on restricted cubic splines varied across filtration markers (Figure 1c; 95% CI in Figure S5). Curves for mGFR and eGFR suggested slight U-shaped relationships, with HRs at lower values smaller in magnitude than those for 1/BTP, 1/B2M, and the 4-marker composite. Risk associated with 1/B2M tended to be higher with lower 1/B2M z scores. Curves for 1/BTP and the 4-marker composite were flat at higher values and increased at lower values, suggesting a threshold effect.
With adjustment for age, sex, and race (Table 3) lower baseline levels of individual filtration markers and the 4-marker composite were all significantly associated with increased risk for an incident cardiovascular event; associations of 1/BTP, 1/B2M, and the 4-marker composite (Ps versus eGFRcr of <0.001 for all three) but not mGFR (p versus eGFRcr = 0.4) were stronger than observed for eGFRcr (Table 3). Associations for 1/B2M and the 4-marker composite remained significantly associated with cardiovascular event risk both with multivariable and additional adjustment for mGFR, with stronger associations than observed for eGFRcr (multivariable: p versus eGFR both <0.001; multivariable + mGFR: p versus eGFR of 0.002 and 0.01, respectively; Table 3). Similar results for the composite cardiovascular outcome were observed when analyses were limited to participants with mGFR (Table S3). Similar trends in HRs were observed for individual components of our cardiovascular composite outcome, although with wider 95% CI due to a smaller number of events (Table S4). The 4-marker composite led to modest, statistically significant improvements in the C statistic when compared to a multivariable Cox regression model with eGFRcr (Table 4). In multivariable models, the 4-marker composite improved overall risk classification beyond eGFR alone for cardiovascular event risk over a period of 2.5 years (Table 4), with significant, but modest improvements observed in both those who did (NRIevent, 0.23; 95% CI, 0.06–0.40) and those who did not have an incident cardiovascular event (NRInon-event, 0.16; 95% CI, 0.12–0.20).
Discussion
In a large, diverse cohort of adults with mild-to-moderate CKD with prospective follow-up, BTP and B2M, and a composite of the 2 markers with eGFRcr and eGFRcys, are independently associated with risk of ESRD and all-cause mortality beyond established risk factors. Similar findings were observed for the association of B2M and the 4-marker composite for cardiovascular events. These associations were greater in magnitude than observed for eGFRcr. In addition, associations of BTP, B2M, and the composite score were independent on mGFR, indicating that non-GFR determinants contribute to the risk associated with these filtration markers.
Our findings extend the current literature by describing risk associations for BTP and B2M in a large, well-characterized, multi-ethnic, nationally representative cohort of adults with mild-to-moderate CKD. Associations of BTP alone with ESRD and mortality were previously described in patients with hypertensive and non-diabetic CKD from AASK and the MDRD Study―cohorts derived from highly selected CKD patient populations in randomized controlled trials that may not be generalizable to broader CKD populations. Increased risk for ESRD and mortality events persisted in these cohorts after accounting for potential confounders.7,9 Similarly, in hemodialysis patients higher BTP was reported to be associated with increased mortality risk over a median follow-up of 3.3 years.8 Three prior studies measured both BTP and B2M in addition to the established filtration markers creatinine and cystatin C, allowing for comparisons of ESRD,5,17 mortality,5,6,17 or cardiovascular risk5 across all markers, as well as for comparisons to mGFR in Southwestern American Indians with type 2 diabetes.17 Two prior studies reported on ESRD. In the Atherosclerosis Risk in Communities (ARIC) Study higher BTP and B2M levels were associated with increased ESRD risk and associations were both stronger than observed with eGFRcr.5 In Pima Indians with type 2 diabetes, higher BTP and B2M were associated with increased ESRD risk and were of similar magnitude as eGFRcr.17 Three prior studies reported on mortality.5,6,17 In ARIC and the Third National Health and Nutrition Examination Survey (NHANES III), BTP and B2M were independent predictors of all-cause mortality with associations of greater magnitude than observed for eGFRcr and with stronger associations observed for B2M than BTP.5,6 Consistent with this observation, B2M, but not BTP, was associated with all-cause mortality risk in Pima Indians with type 2 diabetes. For CVD, in ARIC, higher BTP and B2M were associated with increased coronary heart disease and heart failure risk with B2M associations stronger than those observed for eGFRcr.5 In NHANES III, BTP and B2M were both associated with cardiovascular and coronary heart disease mortality but were not significantly stronger than observed for eGFRcr.6 Reports from ARIC and NHANES III explored the utility of multiple markers by evaluating risk reclassification with the continuous NRI and observed significant improvements in risk reclassification when BTP, B2M, and cystatin C were added to models that included established risk factors and eGFRcr.5,6 Risk reclassification results were less consistent in the Pima Indians but suggested that B2M may improve model discrimination beyond eGFRcr and eGFRcys.17
Comparing the relative strengths of associations with BTP and B2M, alone and in combination across studies provides information on marker performance in different study populations. First, BTP and B2M are consistently associated with ESRD, indicating that these markers have potential to contribute to ESRD risk prediction beyond established risk factors and eGFRcr. Second, B2M tends to be a stronger predictor of all-cause mortality risk than BTP; however, the association of BTP with mortality differs across studies, suggesting that its utility for predicting mortality outcomes may vary based on CKD status and severity at baseline. Combining markers may provide a method for quantifying prognostic information that is more robust across population differences and across study outcomes, although future work is needed to evaluate this approach.
Both B2M and the 4-marker composite remained significantly associated with all-cause mortality and cardiovascular events after adjustment for mGFR, potentially indicating that other factors, besides reduced GFR, affect all-cause mortality and cardiovascular event risk. Accounting for mGFR in the evaluation of the association between marker and outcomes allows for the evaluation of whether increased risk associated with these markers reflects their associations with GFR or other non-GFR determinants. In NHANES III prior work found that creatinine, cystatin C, BTP, and B2M have differential associations with demographic and clinical characteristics,18 providing support for the hypothesis that factors other than GFR vary across these four markers and could have differential effects on prognosis. In AASK and the MDRD Study, increased ESRD and mortality risk for BTP persisted after accounting for mGFR.7,9 Only in the Pima Indian study did we measure both BTP and B2M in a cohort in which mGFR was assessed, with results suggesting that higher BTP and B2M were associated with increased ESRD risk and higher B2M was associated with increased mortality risk independent of mGFR.17 The ESRD results were of borderline significance, which could be attributed to limited power given the small sample size (n=250). In the CRIC Study, we measured these filtration markers in a cohort with mGFR that was five times greater in size than the Pima Indian cohort with many more events, allowing for more precise risk estimates in replication. Our findings are consistent with those in the Pima Indians―that BTP and B2M are associated with ESRD and B2M is associated with mortality beyond established risk factors and mGFR, suggesting that non-GFR determinants contribute to risk estimates beyond kidney function.
Strengths of our study include a large, well-characterized multi-ethnic sample of adults with mild-to-moderate CKD and a reasonably large number of varied events during follow-up. In addition, GFR was measured in a sub-cohort of participants, allowing us to determine whether associations were independent of measured kidney function. Our study has limitations that should also be considered. Filtration markers were measured at a single time point using previously frozen samples from the baseline examination. We combined markers using regression coefficients derived for each outcome within this study sample; thus, performance of the 4-marker composite score was likely enhanced among CRIC Study participants and may not be generalizable to other populations or outcomes and may be of limited applicability in current clinical settings because serum BTP is not currently available for clinical use in the United States. The influence of non-GFR determinants on serum levels of BTP and B2M may reduce their accuracy as pure filtration markers; future studies should examine the performance of a single equation incorporating multiple filtration markers into one estimating equation or obtain an average estimate for multiple markers from sets of GFR estimating equations (eGFRcr, eGFRcys, eGFRBTP, eGFRB2M) that can be used across populations in research. However, our current method represents an internally valid approach that provides evidence for proof of concept. Given that the baseline CRIC visit occurred between 2005 and 2008, we have a relatively short follow-up period (median, 6.1 years), which limits our ability to evaluate long-term risk associated with these filtration markers in adults with mild-to-moderate CKD. Prevalent CVD at baseline was also based on self-report.
In summary, we showed that BTP and B2M were independent predictors of ESRD and all-cause mortality and B2M was an independent predictor of cardiovascular events in a large group of adults with mild-to-moderate kidney disease. Associations were of stronger magnitude than that for eGFRcr, suggesting that these markers contribute additional risk information beyond this well-established marker of kidney function. The use of a multiple-marker approach may improve risk prediction beyond eGFRcr in this population.
Supplementary Material
Acknowledgments
The CRIC Study Investigators are Lawrence J. Appel, MD, MPH, Alan S. Go, MD, Jiang He, MD, PhD, James P. Lash, MD, Mahboob Rahman, MD, and Raymond R. Townsend, MD. The CKD Biomarkers Consortium Investigators are listed at www.ckdbiomarkersconsortium.org.
Support: The CKD Biomarkers Consortium is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; U01DK85649, U01DK085673, U01DK085660, U01DK085688, U01DK085651, and U01DK085689) and by the Intramural Research Program of the NIDDK. Dr Foster was supported in part by National Heart, Lung and Blood Institute grant T32 HL007024. Funding for the CRIC Study was obtained under a cooperative agreement from the NIDDK (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the following: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the NCATS component of the NIH and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research UL1TR000433, University of Illinois at Chicago Clinical and Translational Science Award UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente NIH/National Center for Research Resources University of California San Francisco—Clinical and Translational Science Institute UL1 RR-024131. Institutional Review Board approval numbers for the CRIC Study include University of Pennsylvania (707819 and 807882), Johns Hopkins University (NA_00044034), University of Maryland (HCR-HP-00041233-6), University Hospitals of Cleveland (02-03-04), MetroHealth Medical Center (IRB03-00052), Cleveland Clinic Foundation (5969), University of Michigan (HUM00073515), St. John Health System (SJ 0403-04), Wayne State University (071803MP2F), University of Illinois (2003-0149), Tulane University (H0340), Kaiser Permanente/UCSF (CN-01AGo-02-H). The funders of this study did not have a role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Parts of the study were presented as an oral abstract at the American Society of Nephrology’s Kidney Week 2014, November 11–16, 2014, Philadelphia, Pennsylvania.
Financial Disclosure: The authors declare that the have no other relevant financial interests.
Contributions: Research idea and study design: JC, C-yH, ASL, RGN, JHE, HIF, JWK, AOO, LAI; data acquisition: JC, C-yH, ASL, RGN, JHE, HIF, JWK, AOO, LAI; data analysis/interpretation: MCF, JC, C-yH, DX, ASL, RGN, JHE, RSV, PLK, JS, MS, JHS, AHA, SA, HIF, JWK, AOO, LAI; statistical analysis: MCF, JC, LAI; supervision or mentorship: JC, ASL, LAI. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. LAI takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Table S1: Baseline characteristics of participants with and without mGFR.
Table S2: Pearson correlations for filtration markers in mGFR sample.
Table S3: Adjusted HRs of incident events in subgroup of participants with mGFR.
Table S4: HRs of components of CV composite outcome.
Table S5: Baseline characteristics of participants with and without CVD at baseline.
Figure S1: Distribution of z scores, means, and SDs used to calculate z scores for mGFR, eGFRcr, 1/BTP, and 1/B2M.
Figure S2: Distribution of z scores for the ESRD, all-cause mortality, and incident CV events composite marker scores.
Figure S3: HRs for ESRD associated with mGFR, eGFRcr,1/BTP, 1/B2M, and 4-marker composite score.
Figure S4: HRs for all-cause mortality associated with mGFR, eGFRcr,1/BTP, 1/B2M, and 4-marker composite score.
Figure S5: HRs for CV events associated with mGFR, eGFRcr,1/BTP, 1/B2M, and 4-marker composite score.
Item S1: Supplemental methods.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Supplementary Material Descriptive Text for Online Delivery
Supplementary Table S1 (PDF). Baseline characteristics of participants with and without mGFR.
Supplementary Table S2 (PDF). Pearson correlations for filtration markers in mGFR sample.
Supplementary Table S3 (PDF). Adjusted HRs of incident events in subgroup of participants with mGFR.
Supplementary Table S4 (PDF). HRs of components of CV composite outcome.
Supplementary Table S5 (PDF). Baseline characteristics of participants with and without CVD at baseline.
Supplementary Figure S1 (PDF). Distribution of z scores, means, and SDs used to calculate z scores for mGFR, eGFRcr, 1/BTP, and 1/B2M.
Supplementary Figure S2 (PDF). Distribution of z scores for the ESRD, all-cause mortality, and incident CV events composite marker scores.
Supplementary Figure S3 (PDF). HRs for ESRD associated with mGFR, eGFRcr,1/BTP, 1/B2M, and 4-marker composite score.
Supplementary Figure S4 (PDF). HRs for all-cause mortality associated with mGFR, eGFRcr,1/BTP, 1/B2M, and 4-marker composite score.
Supplementary Figure S5 (PDF). HRs for CV events associated with mGFR, eGFRcr,1/BTP, 1/B2M, and 4-marker composite score.
Supplementary Item S1 (PDF). Supplemental methods.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van LF, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010 Jun 12;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, Jong PE, Coresh J, Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, de Jong PE, Coresh J, El-Nahas M, Eckardt KU, Kasiske BL, Wright J, Appel L, Greene T, Levin A, Djurdjev O, Wheeler DC, Landray MJ, Townend JN, Emberson J, Clark LE, Macleod A, Marks A, Ali T, Fluck N, Prescott G, Smith DH, Weinstein JR, Johnson ES, Thorp ML, Wetzels JF, Blankestijn PJ, van Zuilen AD, Menon V, Sarnak M, Beck G, Kronenberg F, Kollerits B, Froissart M, Stengel B, Metzger M, Remuzzi G, Ruggenenti P, Perna A, Heerspink HJ, Brenner B, de Zeeuw D, Rossing P, Parving HH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011 Jun;79(12):1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011 Jul;80(1):93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Astor BC, Shafi T, Hoogeveen RC, Matsushita K, Ballantyne CM, Inker LA, Coresh J. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis. 2012 May;59(5):653–662. doi: 10.1053/j.ajkd.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster MC, Inker LA, Levey AS, Selvin E, Eckfeldt J, Juraschek SP, Coresh J. Novel filtration markers as predictors of all-cause and cardiovascular mortality in US adults. Am J Kidney Dis. 2013 Jul;62(1):42–51. doi: 10.1053/j.ajkd.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhavsar NA, Appel LJ, Kusek JW, Contreras G, Bakris G, Coresh J, Astor BC, Group AS. Comparison of Measured GFR, Serum Creatinine, Cystatin C, and Beta-Trace Protein to Predict ESRD in African Americans With Hypertensive CKD. Am J Kidney Dis. 2011;58(6):886–893. doi: 10.1053/j.ajkd.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shafi T, Parekh RS, Jaar BG, Plantinga LC, Oberai PC, Eckfeldt JH, Levey AS, Powe NR, Coresh J. Serum beta-trace protein and risk of mortality in incident hemodialysis patients. Clin J Am Soc Nephrol. 2012 Sep;7(9):1435–1445. doi: 10.2215/CJN.02240312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tangri N, Inker LA, Tighiouart H, Sorensen E, Menon V, Beck G, Shlipak M, Coresh J, Levey AS, Sarnak MJ. Filtration markers may have prognostic value independent of glomerular filtration rate. J Am Soc Nephrol. 2012 Feb;23(2):351–359. doi: 10.1681/ASN.2011070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003 Jul;14(7 Suppl 2):S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 11.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009 Aug;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012 Aug;60(2):250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van LF, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012 Jul 5;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zellner A. An Efficient Method of Estimating Seemingly Unrelated Regressions and Tests for Aggregation Bias. J Am Stat Assoc. 1962;57(298):348–368. [Google Scholar]
- 16.Chambless LE, Cummiskey CP, Cui G. Several methods to assess improvement in risk prediction models: extension to survival analysis. Stat Med. 2011 Jan 15;30(1):22–38. doi: 10.1002/sim.4026. [DOI] [PubMed] [Google Scholar]
- 17.Foster MC, Inker LA, Hsu CY, Eckfeldt JH, Levey AS, Pavkov ME, Myers BD, Bennett PH, Kimmel PL, Vasan RS, Coresh J, Nelson RG. Filtration Markers as Predictors of End-Stage Renal Disease and Mortality in Southwestern American Indians. Am J Kidney Dis. 2015 Jul 66;(1):75–83. doi: 10.1053/j.ajkd.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juraschek SP, Coresh J, Inker LA, Levey AS, Kottgen A, Foster MC, Astor BC, Eckfeldt JH, Selvin E. Comparison of serum concentrations of beta-trace protein, beta2-microglobulin, cystatin C, and creatinine in the US population. Clin J Am Soc Nephrol. 2013 Apr;8(4):584–592. doi: 10.2215/CJN.08700812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.