Abstract
The adolescent transition from childhood to adulthood requires both reproductive and behavioral maturation as individuals acquire the ability to procreate. Gonadal steroid hormones are key players in the maturation of behaviors required for reproductive success. Beyond activating behavior in adulthood, testicular and ovarian hormones organize the adolescent brain and program adult- and sex-typical expression of sociosexual behaviors. Testicular hormones organize sexual and agonistic behaviors, including social proficiency—the ability to adapt behavior as a function of social experience. Ovarian hormones organize behaviors related to energy balance and maternal care. These sex differences in the behaviors that are programmed by gonadal hormones during adolescence suggest that evolution has selected for hormone-dependent sex-specific organization of behaviors that optimize reproductive fitness.
Introduction
The adolescent transition from childhood to adulthood requires a metamorphosis of brain and behavior as individuals acquire the ability to procreate and provide for themselves and their offspring. The beginning of adolescence is marked by the onset of puberty, when activation of the hypothalamic-pituitary-gonadal axis results in the maturation of gametes, elevated levels of sex steroid hormones, the appearance of secondary sex characteristics, and fertility. But merely being fertile isn’t sufficient for reproductive fitness in most mammals. Sexual reproduction usually requires complex social behaviors for bringing sperm and egg together, and additional behavioral capacities are needed to successfully rear young. Thus, full attainment of adulthood encompasses not only pubertal maturation of the reproductive system, but also adolescent maturation of the social and behavioral skills necessary for successful reproduction. The scope of this review is therefore not limited to adolescent maturation of male and female sexual behavior per se; it includes adolescent maturation of the range of behaviors that together lead to passing along one’s genes. The central thesis of this review is that testicular and ovarian hormones, when elevated at the onset of puberty, program behavioral responses to hormones and social experiences in adulthood. The particular behaviors that are organized by gonadal hormones are different in males and females, but in each sex, hormones program adult behaviors that are important for reproductive success.
Organizational vs activational effects of gonadal steroid hormones
Steroid hormone action in the nervous system can be dichotomized as activational or organizational. Activational effects refer to the ability of steroids to modify the activity of target cells in ways that facilitate expression of particular behaviors in specific contexts. Activational effects are transient; they come and go with the presence and absence of hormone and are typically associated with steroid action in the adult brain. In contrast, organizational effects refer to the ability of steroids to sculpt nervous system structure and function during development. Organizational effects are long-lasting, persist beyond the period of developmental exposure to hormone, and program activational responses to hormones in adulthood.
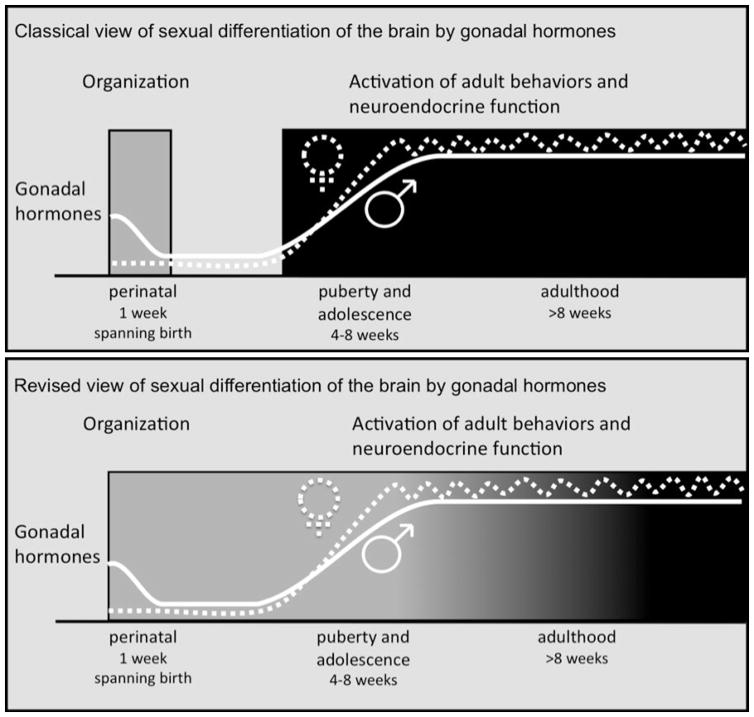
Conceptualization of the relationship between organizational and activational effects of steroid hormones has evolved over the past fifty years. In order to explain sex differences in behavioral responses to hormones in adulthood, Phoenix and colleagues [1] first proposed that sex-typical adult behavioral (activational) responses to steroid hormones are programmed (organized) by steroid hormones acting on the nervous system during early development. Subsequently, scores of experiments led to the identification of a sensitive period for hormone-dependent sexual differentiation (organization) of the brain during prenatal and early neonatal development in non-human primates and rodents (reviewed in [2–5]). Research over the past twenty years has revealed that in addition to the perinatal period of hormone-dependent organization of behavioral neural circuits, adolescence is another period of development during which gonadal hormones organize the nervous system [6–8]. The current conceptual framework of organizational and activational effects of gonadal steroid hormones is a two-stage model of development in which the perinatal period of hormone-dependent organization is followed by a second wave of organization during puberty and adolescence (Figure 1). During this second wave, pubertal hormones first organize neural circuits in the developing adolescent brain, and then facilitate the expression of adult sex-typical behaviors in specific social contexts by activating those circuits. Thus, adolescent organization reinforces and refines the sexual differentiation that occurred during perinatal neural development by inducing long-lasting structural changes in the nervous system that determine adult behavioral responses to hormones and socially-relevant sensory stimuli. One important distinction between the perinatal and pubertal periods of organization is the contribution of ovarian hormones. Perinatal organization is accomplished primarily through the masculinizing and defeminizing effects of testicular hormones; ovarian hormones, which are not elevated perinatally, do not play a major role, and the developing brain is not actively feminized. In contrast, both testicular and ovarian hormones are actively involved in the pubertal organization of behavior in males and females.
Figure 1.
Schematic representation of the classical and revised views of sexual differentiation of the rodent brain and behavior. In the classical view, brain architecture is permanently modified by exposure of the male brain to testicular hormones during a brief perinatal period. In the revised view, the period of organization/structural differentiation is extended, continuing well through puberty and adolescence, during which both testicular and ovarian hormones impact the structure of the male and female brain, respectively. Adolescence encompasses puberty, which refers specifically to reproductive maturation. Although fertility is attained within 2–3 wks of the onset of puberty, additional maturation occurs during later adolescence before adult brain and behavioral phenotypes are achieved. From [43].
Adolescent organization of sociosexual behaviors in males
The general experimental strategy used to determine whether behavioral circuits are organized during adolescence is to manipulate gonadal hormones during that time and assess behavior in adulthood. Typically, animals are gonadectomized prior to the onset of puberty to allow adolescent development in the absence of testicular hormones, and then testosterone is replaced in adulthood prior to behavioral tests. The behavior of males treated in this way is compared with that of males that were similarly treated, except that gonadectomy, washout period, and testosterone replacement all occurred in adulthood. With this experimental design, the observed deficits in adult behavior of males that did not experience testicular hormones during adolescence can be attributed to organizational effects of gonadal hormones if testosterone replacement in adulthood does not reverse these deficits.
When testosterone is absent during adolescence, a wide range of male-typical adult sociosexual behaviors is compromised (Table 1). For example, prepubertally gonadectomized male Syrian hamsters display lower levels of mounts, intromissions, and ejaculations compared with male hamsters gonadectomized in adulthood. The deficits in sexual behavior seen after prepubertal gonadectomy are not reversed either by prolonged testosterone replacement therapy or sexual experience in adulthood [9]. Similarly, prepubertal gonadectomy results in a reduction in adult male-male aggressive behavior and flank-marking, the deposition of flank gland secretions used to maintain dominant/subordinate status [10,11]. Other studies provide evidence for organizational effects of testicular hormones during puberty on territorial scent marking in male tree shrews [12], aggression in male mice and gerbils [13,14], play fighting in male rats [15], and social interactions in a novel environment in male rats [16,17]. Thus, the absence of testicular hormones during adolescence results in long-lasting impairments in of sociosexual behaviors, and conversely the presence of testicular hormones during adolescence masculinizes neural circuits underlying sociosexual behaviors and programs enhanced activational responses to testosterone in adulthood.
Table 1.
Behaviors organized by gonadal hormones during puberty and adolescence.
| Behavior | Species | Reference |
|---|---|---|
| Sexual behavior | male hamster | Schulz et al., 2004 |
| Agonistic behavior | male hamster male mouse male gerbil |
Schulz & Sisk, 2006 Shrenker et al., 1985 Lumia et al., 1977 |
| Scent marking | male tree shrew male hamster |
Eichmann & Holst, 1999 Schulz et al., 2006 |
| Play fighting | male rat | Pellis, 2002 |
| Social interaction in novel environment | male rat | Brand & Slob, 1988 Primus & Kellogg, 1990 |
| Psychosexual development | male human | Dwyer et al., 2015 |
| Food guarding behavior | female rat | Field et al., 2004 |
| Food intake in response to metabolic challenge | female rat | Swithers et al., 2008 |
| Maternal behavior | female mouse | Kercmar et al., 2014 |
What features of sociosexual behaviors are organized by pubertal testosterone? It doesn’t seem to be the performance or motor execution of the behaviors per se, because males deprived of testosterone during adolescence do display the consummatory components of sexual behavior, aggression, and scent marking, albeit at lower levels compared with males that did experience testosterone during adolescence. This suggests that some aspect of social cognition associated with these complex social behaviors is organized by pubertal testosterone. Social cognition refers to the mental processes by which an individual encodes, interprets, and responds to sensory information from a conspecific [18]. Interpretation of a social sensory stimulus involves assignment of valence and an associated emotional state, leading to motivation to approach or avoid the perceived stimulus, and ultimately, selection of a behavioral response within a particular social context. The outcome of the social interaction (positive or negative) influences the probability that the behavioral response will be selected again in the future. In other words, individuals learn to modify their behavior based on their social experiences. How quickly and successfully these behavioral adaptations are learned is an index of social proficiency or social competence. Successful adolescent acquisition of social proficiency plays a key role in ensuring the ability of an individual to become independent and appropriately interact with her or his peers. Research in hamsters suggests that testosterone organizes social proficiency—the ability to make behavioral adaptations as a function of social experience (reviewed in [19]).
Expression of sexual behavior in hamsters requires the neural integration of an internal signal, testosterone, and an external signal, female pheromones present in vaginal secretions [20]. How the male hamster interprets this external chemosensory signal changes during pubertal development. For example, juvenile hamsters are not attracted to female pheromones, nor do they form a conditioned place preference (CPP) to pheromones, whereas sexually naïve adult male hamsters prefer and readily form a CPP to female pheromones [21–23]. Thus, as a result of pubertal development, female pheromones become an unconditioned reward for male hamsters. Furthermore, both testosterone-treated juvenile hamsters and adult hamsters deprived of testosterone during adolescence form a CPP to female pheromones [23,24]. Thus, activational effects of testosterone are both necessary and sufficient for pheromone reward in juvenile and adult male hamsters, and the perception of female pheromones as a rewarding social stimulus does not require organizational effects of testosterone.
In contrast, adolescent maturation of social proficiency does depend on organizing effects of pubertal testosterone. Sexual experience normally leads to proficiency in male hamsters. During their first encounter with a receptive female, sexually naïve adult hamsters display high levels of ectopic (mis-directed) mounts; over the course of additional sexual encounters, ectopic mounts decrease to low levels and are only occasionally observed. However, male hamsters deprived of testosterone during adolescence do not show this behavioral adaptation to experience [11]. Male hamsters also gain social proficiency over the course of repeated encounters with another male in a neutral arena. During the first social encounter between two unfamiliar males, an aggressive interaction initially occurs, and a dominant-subordinate relationship is established within a few minutes. In subsequent encounters, there is little aggression per se, but the dominant-subordinate relationship is maintained through flank marking by both males. This experience-dependent pattern of behavior is disrupted in males deprived of testosterone during adolescence: these males display low overall levels of flank marking, even if they are dominant, and the dominant/subordinate relationship is not maintained by flank-marking, but instead is re-established via aggression in subsequent encounters [25]. Thus, during adolescent development, testosterone programs the ability to make behavioral adaptations to social experience that enhance reproductive efficiency and avert aggression, both of which serve to enhance reproductive fitness.
To what extent do these findings in rodent models extend to humans? Anecdotally, Kinsey reported that late onset of puberty in boys, which results in low levels of testosterone during the early stages of adolescent development, is associated with lower levels of sexual activity both initially and throughout the rest of adulthood [26]. Of course, experimental manipulation of hormones during adolescence is not possible, but cases of delayed puberty are experiments of nature that provide insight into the effects of hormones on human behavior. Idiopathic hypogonadotropic hypogonadism (IHH) is a disorder primarily affecting males in which puberty is delayed. IHH is typically treated by testosterone replacement therapy, which stimulates appearance of secondary sex characteristics and libido, but hormone therapy may be initiated late relative to adolescent development because patients often don’t present until well after the typical age of onset of puberty. In these cases, the levels of testosterone are low or undetectable during adolescent development, similar to prepubertally gonadectomized rodents. In an early study of psychosocial development in men with IHH, normal development occurred when hormone therapy was begun during the normal age range for puberty, but psychological disturbances occurred if therapy began after the age of 20 (reviewed in [27]). A more recent study of IHH men on testosterone therapy found that these men were less likely to be sexually active, and suffered from a variety of long-lasting psychosexual problems, including difficulty with intimate relationships and body image concerns [28]. Thus, although clinical treatment precludes the experimental study of hormones and behavioral development in humans, the information that is available provides evidence for an organizational effect of testicular hormones during adolescence on sociosexual behaviors.
Adolescent organization of sociosexual behaviors in females
Research on the role of ovarian hormones in the adolescent organization of female sociosexual behaviors lags behind that in males. One likely reason is the presumption (based on research of sexual differentiation during the perinatal period) that a feminine phenotype (whether brain or behavior) is the default developmental trajectory, and does not require active feminization by ovarian hormones. However, researchers have recently begun to question that presumption, and there is now solid evidence that ovarian hormones, at least estradiol, does indeed organize the adolescent female brain, but in a surprise twist, affects behaviors that might not have been predicted from work in males.
For females, reproductive success depends not only on being fertile and finding a mate, but also on being physiologically prepared to sustain a pregnancy and provide nutrition for her young. It appears that elevated levels of ovarian hormones during puberty organize behaviors related to fertility, which is contingent on metabolic signals that predict sufficient energy availability to sustain pregnancy, lactation, and maternal care. In rats, defense of food is a sexually dimorphic behavior, with males and females displaying different postural strategies for guarding their food source [29]. Prepubertal ovariectomy alters the defense strategy to be more phenotypically male, whereas adult ovariectomy has no effect on this behavior, indicating that ovarian hormones during adolescence actively feminize postural strategies for food defense. Ingestive responses to metabolic challenge are also sexually dimorphic in rats, and research indicates that pubertal estradiol feminizes these responses [30]. Treatment with mercaptoacetate, a drug that interferes with fatty acid oxidation, causes an increase in food intake in male, but not female, rats. Ovariectomy in adulthood does not affect this sex difference. However, females that are ovariectomized prior to puberty show a male-like response to mercaptoacetate (i.e., increase food intake). This effect of prepubertal ovariectomy can be prevented by treatment with estradiol during the time of puberty, indicating that estradiol (and not progesterone) is the organizing hormone.
Maternal care of offspring is essential for reproductive fitness in mammals, and there is evidence for pubertal programming of maternal behavior by ovarian hormones. If female mice are ovariectomized before puberty and then examined for maternal behavior in adulthood, they spend less time with foster pups, take longer to retrieve them, and retrieve fewer of them, as compared to either females that are ovariectomized after puberty in adulthood, or females that are ovariectomized before puberty and given estradiol replacement during the time of puberty [31]. Thus, the presence of estradiol during puberty organizes the female mouse brain to facilitate maternal care.
Mechanisms of adolescent organization of sociosexual behaviors
Research on the neural mechanisms by which sociosexual behaviors are organized by pubertal hormones is just beginning, and to date, much of it is correlational in nature. That is, pubertal hormones induce long-lasting structural changes in brain regions known to mediate the behaviors that are organized, but these structural changes have not been causally linked to the behavioral changes. For example, the lateral septum is a site of action for vasopressin activation of flank marking and aggressive behaviors in male hamsters [32,33]. If testosterone is absent during adolescence, flank marking and aggression are compromised in adulthood, and concomitantly, vasopressin receptor 1a binding in the lateral septum is increased [10], and lateral septum neurons are less activated after an aggressive encounter [25]. The posterodorsal medial amygdala (MePD) is associated with expression of male sexual behavior, and its volume increases during pubertal development [34,35]. Synaptic reorganization of the MePD also occurs during puberty, including synapse formation and elimination [36,37] and changes in morphological complexity of astrocytes [38]. Testosterone deprivation during adolescence results in a smaller MePD volume in adulthood, even after hormone replacement [39]. Together, these results suggest that testosterone, acting during the time of puberty, alters neural transmission within the lateral septum and MePD, but the relationship of these changes to the alterations in behavior is not known at this time.
Studies using the cell birthdate marker BrdU find that new cells, including both neurons and glial cells, are added to several brain regions associated with male and female sociosexual behaviors in rodents, including the MePD and the anteroventral periventricular nucleus (AVPV), which has recently been linked to aggressive behavior in males and maternal behavior in females [40]. There are sex differences in the number of pubertally born cells in these brain regions, with more cells added to the MePD in males than in females, and more cells added to the AVPV in females than in males [41]. Furthermore, prepubertal gonadectomy abolishes these sex differences, indicating that gonadal hormones drive differential cell addition to these sexually dimorphic nuclei [41]. In male hamsters, some pubertally born MePD cells express fos after a sexual encounter with a female, indicating that they are functionally incorporated into behavioral circuits [42]. Thus, the pubertal addition of new neurons and glial cells to brain regions associated with both male and female sociosexual behaviors is a potential mechanism for hormone-dependent organization of the adolescent brain.
Summary
It is clear that both testicular hormones and ovarian hormones organize the mammalian adolescent brain and program adult behaviors, but the types of behaviors that are programmed are different in males and females. Testicular hormones exert organizational effects on male social behaviors such as sex behavior, aggression, and scent marking, whereas ovarian hormones exert organizational effects on behaviors related to energy balance and maternal care. This apparent dichotomy may be explained by considering that, from an evolutionary perspective, the ultimate goal of puberty and adolescence is to become an adult with the capability of procreating and passing along ones genes. The biological and behavioral demands of procreation are different for females and males. For females, reproductive success depends on having sufficient energy reserves built up to sustain a pregnancy, and then once pregnant, maintaining those reserves to nourish and care for young. For males, reproductive success depends on the ability to outdo competitors in attracting and impregnating a mate, which for many male mammals involves defending a territory. This leads to the proposition that evolution has selected for organizational effects of gonadal hormones during puberty that serve to optimize reproductive fitness, and that selection pressures are different for females and males. If so, then the particulars of what neural circuits and behaviors are organized by gonadal hormones during adolescence will be a function of both sex and species.
Highlights.
Gonadal hormones organize sociosexual behaviors during adolescence
Sexual behavior and aggression are organized in males
Behaviors related to energy balance and maternal care are organized in females
Mechanisms include synaptic rearrangement and the addition of new neurons and glia
Acknowledgments
The author was supported by NIH R01 MH090091 and R01 MH068764.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of special interest
** of outstanding interest
- 1.Phoenix C, Goy R, Gerall A, Young W. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 2.Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front Neuroendocrinol. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Wallen K, Baum MJ. Masculinization and defeminization in altricial and precocial mammals: comparative aspects of steroid hormone action. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 4 Elsevier; 2002. pp. 385–423. [Google Scholar]
- 4.Ward IL, Ward BK. Sexual behavior differentiation. In: Adler N, Pfaff DW, Goy RW, editors. Handbook of Behavioral Neurobiology. Vol. 7 Plenum Press; 1985. pp. 77–98. [Google Scholar]
- 5.Baum MJ. Differentiation of coital behavior in mammals: a comparative analysis. Neurosci Biobehav Rev. 1979;3:265–284. doi: 10.1016/0149-7634(79)90013-7. [DOI] [PubMed] [Google Scholar]
- 6.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 7.Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 8**.Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. A comprehensive review of organizational effects of gonadal hormones on the adolescent brain and behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulz KM, Richardson HM, Zehr JL, Osetek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav. 2004;45:242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Schulz KM, Menard TA, Smith DA, Albers HE, Sisk CL. Testicular hormone exposure during adolescence organizes flank-marking behavior and vasopressin receptor binding in the lateral septum. Horm Behav. 2006;50:477–483. doi: 10.1016/j.yhbeh.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol Cell Endocrinol. 2006;254–255:120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 12.Eichmann F, Holst DV. Organization of territorial marking behavior by testosterone during puberty in male tree shrews. Physiol Behav. 1999;65:785–791. doi: 10.1016/s0031-9384(98)00230-3. [DOI] [PubMed] [Google Scholar]
- 13.Shrenker P, Maxson SC, Ginsburg BE. The role of postnatal testosterone in the development of sexually dimorphic behaviors in DBA/1Bg mice. Physiol Behav. 1985;35:757–762. doi: 10.1016/0031-9384(85)90408-1. [DOI] [PubMed] [Google Scholar]
- 14.Lumia A, Raskin L, Eckhert S. Effects of androgen on marking and aggressive behavior of neonatally and prepubertally bulbectomized and castrated male gerbils. J Comp Physiol Psychol. 1977;91:1377–1389. [Google Scholar]
- 15.Pellis SM. Sex differences in play fighting revisited: traditional and nontraditional mechanisms of sexual differentiation in rats. Arch Sex Behav. 2002;31:17–26. doi: 10.1023/a:1014070916047. [DOI] [PubMed] [Google Scholar]
- 16.Brand T, Slob A. Peripubertal castration of male rats, adult open field ambulation and partner preference behavior. Behavioural Brain Research. 1988;30:111–117. doi: 10.1016/0166-4328(88)90141-6. [DOI] [PubMed] [Google Scholar]
- 17.Primus R, Kellogg C. Gonadal hormones during puberty organize environment-related social interaction in the male rat. Hormones and Behavior. 1990;24:311–323. doi: 10.1016/0018-506x(90)90012-m. [DOI] [PubMed] [Google Scholar]
- 18.Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 19*.De Lorme K, Bell MR, Sisk CL. The Teenage Brain: Social Reorientation and the Adolescent Brain-The Role of Gonadal Hormones in the Male Syrian Hamster. Curr Dir Psychol Sci. 2013;22:128–133. doi: 10.1177/0963721413479607. This paper reviews the evidence that during puberty, testosterone activates social reward but organizes social proficiency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood RI. Integration of chemosensory and hormonal input in the male Syrian hamster brain. Ann N Y Acad Sci. 1998;855:362–372. doi: 10.1111/j.1749-6632.1998.tb10594.x. [DOI] [PubMed] [Google Scholar]
- 21.Johnston RE, Coplin B. Development of responses to vaginal secretion and other substances in golden hamsters. Behav Neural Biol. 1979;25:473–489. doi: 10.1016/s0163-1047(79)90242-5. [DOI] [PubMed] [Google Scholar]
- 22.Bell MR, Meerts SH, Sisk CL. Male Syrian hamsters demonstrate a conditioned place preference for sexual behavior and female chemosensory stimuli. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell MR, De Lorme KC, Figueira RJ, Kashy DA, Sisk CL. Adolescent gain in positive valence of a socially relevant stimulus: engagement of the mesocorticolimbic reward circuitry. Eur J Neurosci. 2013;37:457–468. doi: 10.1111/ejn.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Lorme KC, Bell MR, Sisk CL. Maturation of social reward in adult male Syrian hamsters does not depend on organizational effects of pubertal testosterone. Horm Behav. 2012;62:180–185. doi: 10.1016/j.yhbeh.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Lorme KC, Sisk CL. Pubertal testosterone programs context-appropriate agonistic behavior and associated neural activation patterns in male Syrian hamsters. Physiol Behav. 2013;112–113:1–7. doi: 10.1016/j.physbeh.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinsey AC. Sexual Behavior in the Human Male. Bloomington: Indiana University Press; 1948. [Google Scholar]
- 27.Meyer-Bahlburg HF. Sexuality in early adolescence. In: Wolman B, Money J, editors. Handbook of Human Sexuality. Prentice-Hall; 1980. pp. 62–82. [Google Scholar]
- 28*.Dwyer AA, Quinton R, Pitteloud N, Morin D. Psychosexual development in men with congenital hypogonadotropic hypogonadism on long-term treatment: a mixed methods study. Sex Med. 2015;3:32–41. doi: 10.1002/sm2.50. A recent paper examining the influence of delayed puberty on sexual behavior and psychological function in men. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Field EF, Whishaw IQ, Forgie ML, Pellis SM. Neonatal and pubertal, but not adult, ovarian steroids are necessary for the development of female-typical patterns of dodging to protect a food item. Behav Neurosci. 2004;118:1293–1304. doi: 10.1037/0735-7044.118.6.1293. [DOI] [PubMed] [Google Scholar]
- 30**.Swithers SE, McCurley M, Hamilton E, Doerflinger A. Influence of ovarian hormones on development of ingestive responding to alterations in fatty acid oxidation in female rats. Horm Behav. 2008;54:471–477. doi: 10.1016/j.yhbeh.2008.05.009. This empirical paper is one of the best examples of estradiol-dependent pubertal organization of female behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kercmar J, Snoj T, Tobet SA, Majdic G. Gonadectomy prior to puberty decreases normal parental behavior in adult mice. Horm Behav. 2014;66:667–673. doi: 10.1016/j.yhbeh.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albers HE, Cooper TT. Effects of testosterone on the behavioral response to arginine vasopressin microinjected into the central gray and septum. Peptides. 1995;16:269–273. doi: 10.1016/0196-9781(94)00188-x. [DOI] [PubMed] [Google Scholar]
- 33.Irvin RW, Szot P, Dorsa DM, Potegal M, Ferris CF. Vasopressin in the septal area of the golden hamster controls scent marking and grooming. Physiol Behav. 1990;48:693–699. doi: 10.1016/0031-9384(90)90213-n. [DOI] [PubMed] [Google Scholar]
- 34.Schulz KM, Zehr JL, Salas-Ramirez KY, Sisk CL. Testosterone programs adult social behavior before and during, but not after, adolescence. Endocrinology. 2009;150:3690–3698. doi: 10.1210/en.2008-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooke BM, Jordan CL, Breedlove SM. Pubertal growth of the medial amygdala delayed by short photoperiods in the Siberian hamster, Phodopus sungorus. Horm Behav. 2007;52:283–288. doi: 10.1016/j.yhbeh.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooke BM. Synaptic reorganisation of the medial amygdala during puberty. J Neuroendocrinol. 2011;23:65–73. doi: 10.1111/j.1365-2826.2010.02075.x. [DOI] [PubMed] [Google Scholar]
- 37.Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol. 2006;66:578–590. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]
- 38.Johnson RT, Breedlove SM, Jordan CL. Androgen receptors mediate masculinization of astrocytes in the rat posterodorsal medial amygdala during puberty. J Comp Neurol. 2012;521:2298–2309. doi: 10.1002/cne.23286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Lorme KC, Schulz KM, Salas-Ramirez KY, Sisk CL. Pubertal testosterone organizes regional volume and neuronal number within the medial amygdala of adult male Syrian hamsters. Brain Res. 2012;1460:33–40. doi: 10.1016/j.brainres.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott N, Prigge M, Yizhar O, Kimchi T. A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature. 2015;525:519–522. doi: 10.1038/nature15378. [DOI] [PubMed] [Google Scholar]
- 41*.Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. This paper provides evidence for gonadal hormone-dependent sex differences in the pubertal addition of new neurons and glial cells to sexually dimorphic cell hypothalamic and amygdalar cell groups. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohr MA, Sisk CL. Pubertally born neurons and glia are functionally integrated into limbic and hypothalamic circuits of the male Syrian hamster. Proc Natl Acad Sci U S A. 2013;110:4792–4797. doi: 10.1073/pnas.1219443110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43**.Juraska JM, Sisk CL, DonCarlos LL. Sexual differentiation of the adolescent rodent brain: hormonal influences and developmental mechanisms. Horm Behav. 2013;64:203–210. doi: 10.1016/j.yhbeh.2013.05.010. A review of research providing evidence that many sexually dimorphic cell groups, including hypothalamic, limbic, and cortical regions, are further sexually differentiated by testicular and ovarian hormones during puberty and adolescence. [DOI] [PubMed] [Google Scholar]