Abstract
The Urea Cycle Disorders Consortium (UCDC) has conducted, beginning in 2006, a longitudinal study (LS) of 8 enzyme deficiencies/transporter defects associated with the urea cycle. These include N-acetylglutamate synthase deficiency (NAGSD); Carbamyl phosphate synthetase 1 deficiency (CPS1D); Ornithine transcarbamylase deficiency (OTCD); Argininosuccinate synthetase deficiency (ASSD) (Citrullinemia); Argininosuccinate lyase deficiency (ASLD) (Argininosuccinic aciduria); Arginase deficiency (ARGD, Argininemia); Hyperornithinemia, hyperammonemia, homocitrullinuria (HHH) syndrome (or mitochondrial ornithine transporter 1 deficiency [ORNT1D]); and Citrullinemia type II (mitochondrial aspartate/glutamate carrier deficiency [CITRIN]). There were 678 UCD patients enrolled in 14 sites in the U.S., Canada and Europe at the writing of this paper. This review summarizes findings of the consortium related to outcome, focusing primarily on neuroimaging findings and neurocognitive function. Neuroimaging studies in late onset OTCD offered evidence that brain injury caused by biochemical dysregulation may impact functional neuroanatomy serving working memory processes, an important component of executive function and regulation. Additionally, there were alteration in white mater microstructure and functional connectivity at rest. Intellectual manifestations in OTCD and other urea cycle disorders (UCD) vary. However, when neuropsychological deficits occur, they tend to be more prominent in motor/performance areas on both intelligence tests and other measures. In some disorders, adults performed significantly less well than younger patients. Further longitudinal follow-up will reveal whether this is due to declines throughout life or to improvements in diagnostics (especially newborn screening) and treatments in the younger generation of patients.
Introduction
In 2002 the U.S. Congress authorized funding for the National Institutes of Health to support the development of a Rare Diseases Clinical Research Network (RDCRN). The goals of this program were to perform natural history studies of rare diseases and to hasten the bringing to market of orphan products to treat rare disorders. A total of 23 RDCRN Centers currently are funded to study a total of 213 rare diseases (http://www.rarediseasesnetwork.org). One of the first Centers funded was the UCDC which started its LS in 2006 (Seminara, et al 2010, Batshaw, et al 2014).
Urea cycle disorders (UCD) are inherited as autosomal recessive disorders, except for ornithine transcarbamylase deficiency (OTCD) which is X-linked. Our LS and data from referral centers have allowed us to estimate a collective incidence of about 1:35,000 for all UCD (Summar et al, 2013). The urea cycle and the 8 specific disorders being studied by the UCDC are illustrated in Figure 1 and listed below:
N-acetylglutamate synthase deficiency (NAGSD)
Carbamyl phosphate synthetase 1 deficiency (CPS1D)
Ornithine transcarbamylase deficiency (OTCD)
Argininosuccinate synthetase deficiency (ASSD) (Citrullinemia)
Argininosuccinate lyase deficiency (ASLD) (Argininosuccinic aciduria)
Arginase deficiency (ARGD, Argininemia)
Hyperornithinemia, hyperammonemia, homocitrullinuria (HHH) syndrome (or mitochondrial ornithine transporter 1 deficiency (ORNT1D)
Citrullinemia type II (mitochondrial aspartate/glutamate carrier deficiency (CITRIN)
Figure 1.
The Urea Cycle: N-acetylglutamate synthase (NAGS), Carbamyl phosphate synthetase 1 (CPS1), Ornithine transcarbamylase (OTC), Argininosuccinate synthetase (ASS), Argininosuccinate lyase (ASL), Arginase deficiency (ARG), mitochondrial ornithine transporter 1 (ORNT 1), and mitochondrial aspartate/glutamate carrier (CITRIN).
Infants with a complete block in a urea cycle enzyme (other than ARG) commonly present in the newborn period with hyperammonemic (HA) coma. In our initial (1980s) study of these babies we found that virtually all survivors had developmental disabilities that correlated with the number, severity and duration of HA episodes (Msall, et al, 1984, Msall, et al, 1988). We also found that patients with partial defects of the urea cycle can manifest HA at any age and have a significant risk for developmental disabilities and even death (Msall, et al, 1988, Batshaw, et al, 1986). We found that even asymptomatic OTCD heterozygotes have mild cognitive deficits (Gyato, et al, 2004).
For the last few decades there have been three key components to conventional therapy of UCD: (a) pharmacological intervention (Brusilow, et al, 1979, Batshaw, et al, 1982, Batshaw et al, 2001), so-called Nitrogen (N) scavenger therapy; (b) supplementation with the amino acids L-citrulline or L-arginine; and (c) a low-protein diet that balances N restriction with growth requirements. The only known “cure” for UCD is liver transplantation which carries not insignificant morbidity and mortality (Perito, et al, 2014, Campeau, et al, 2010).
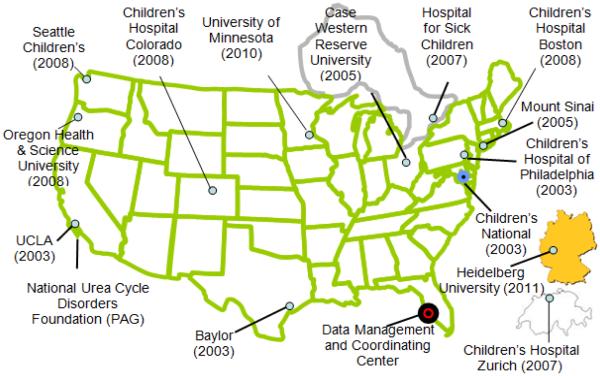
This review summarizes the neuroimaging and neuropsychological findings of the UCDC (Seminara et al., 2010; Batshaw et al, 2014). There are presently 14 UCDC sites: 11 in the U.S., 1 in Canada and 2 in Europe (Figure 2). Accrual of patients in the LS by diagnosis as of July 31, 2015 is shown in Table 1; 678 subjects have been enrolled to date and there has been a 91% retention rate over the 9 years the study has been open.
Figure 2.
The Urea Cycle Disorders Consortium (UCDC): Map of the 14 sites and the date the institution joined the consortium.
Table 1.
Accrual Summary by Disorder – Data current as of July 31, 2015
| Neonatal | Late Onset | Total | |
|---|---|---|---|
| NAGSD | 1 | 3 | 4 |
| CPSID | 14 | 8 | 22 |
| OTCD | 48 | 349 | 397 |
| ASSD | 64 | 34 | 98 |
| ASLD | 50 | 59 | 109 |
| ARGD | 1 | 25 | 26 |
| HHH/ORNT1D | 1 | 10 | 11 |
| CITRIN | 1 | 1 | 2 |
| Diagnosis Pending | 2 | 7 | 9 |
| 182 | 496 | 678 |
What are the neuroimaging abnormalities in UCD and how can neuroimaging help to identify differences in brain structure, biochemistry and cognitive function?
Neuroimaging detects subtle abnormalities that can be correlated with neurocognitive abnormalities even in asymptomatic OTCD heterozygotes. The neuroimaging/neurocognitive studies we performed as part of the UCDC focused on adolescents and adults with OTCD. Our collective studies demonstrated that OTCD heterozygotes have changes in function of the prefrontal cortex (PFC) in association with an altered neurocognitive profile in working memory, executive functioning and attention.
This correlates with our fMRI studies that showed deficits in performance of working memory tasks of increasing difficulty in this group of patients (Gropman, et al, 2013). The finding that “asymptomatic” female carriers of OTCD demonstrate cognitive deficits compared to the normative population has helped to elucidate the areas of the brain that are potentially sensitive to HA even in the absence of a clinically recognizable metabolic event.
1H Magnetic Resonance Spectroscopy (MRS) is a technique that provides biomarkers of HA in OTCD
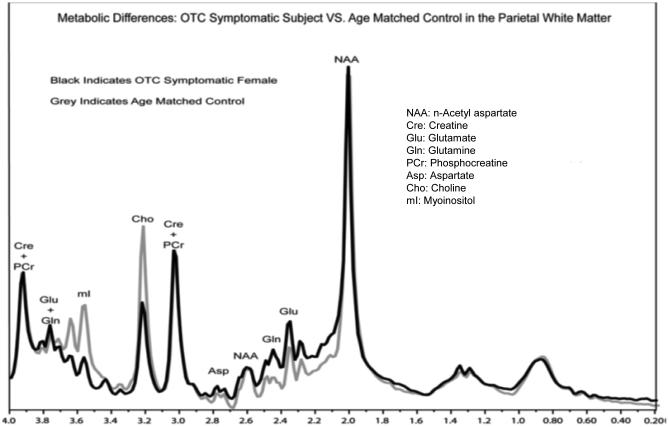
1H MRS is particularly useful in OTCD since the key metabolites of interest are present in sufficient concentrations in brain to be measured (Gropman, et al, 2008). These include glutamine (Gln), myoinosital (mI) and choline (Cho). Since Gln accumulation is considered neurotoxic and is implicated in the neuropathology of OTCD, we investigated whether brain Gln is also elevated in patients who were not experiencing clinical HA. We hypothesized that metabolic perturbations would occur in subjects with partial OTCD and account for the underlying, previously described cognitive deficits. We used single voxel 1H MRS to investigate parietal white matter (PWM) and posterior cerebral gray matter (PCGM). A total of 16 subjects and 22 age matched controls were studied. We detected significant increases of Gln levels (p< 0.007) in the PCGM and PWM, in subjects with OTCD (symptomatic and asymptomatic subjects) (Figure 3a). The patients had normal plasma Gln levels while their brain Gln was elevated. We also found significant decreases in mI concentrations in PWM and PCGM, and the degree of mI depletion inversely correlated with increased Gln levels. This leads us to propose the Gln/mI ratio as a brain biomarker of HA. In fact, although 10 patients were treated with nitrogen scavenger drugs, the inverse relationship between Gln and mI was maintained. The reduction of mI also correlated with cognitive impairments in working memory (digit span backwards and performance IQ), a pattern suggesting a white matter (WM) injury model (Figure 3b, c). Thus, we suggest that low mI and/or high Gln/mI ratio is a biomarker of a prior HA episode and that mI may represent an osmotic compensatory mechanism to correct astrocytic swelling due to high Gln. Choline, which is a marker of membrane integrity, was found to be reduced in the WM of the patients and may also be a biomarker. Our ongoing studies will address biochemical changes in other UCDs as we suspect that additional important biochemical markers such as creatine, guanidinoacetate, and arginine deficiency play a role in the downstream cognitive effects in these disorders, and creatine and guanidinoacetate are measurable by MRS.
Figure 3a.
1H MRS metabolite differences in OTCD versus age matched control Reprinted with permission from Gropman AL, Fricke ST, Seltzer RR, Hailu A, et al (2008). Mol Genet Metab. 95(1-2):21-30. NAA=N acetylaspartate; Glu=glutamate; Gln= glutamine; Asp=aspartate; Cre and PCr=creatine and phosphocreatine;Cho=choline; mI=myoinosital
Figure 3b.
This shows the inverse relationship of Brain Gln levels and Performance IQ. From: Pacheco-Colón I, Fricke S, VanMeter J, Gropman AL. Advances in urea cycle neuroimaging: Proceedings from the 4th International Symposium on urea cycle disorders, Barcelona, Spain, September 2013. Mol Genet Metab. 2014 113(1-2):118-126. Reprinted with permission. Gln=glutamine; PIQ=performance IQ
Figure 3c.
this shows the relationship between Fractional anisotropy (FA), a measure of white matter integrity with brain glutamine levels in patients with symptomatic OTCD.
Diffusion Tensor Imaging (DTI) allows examination of white matter changes in brain
We utilized DTI to determine whether there are WM microstructural abnormalities in symptomatic OTCD that could underlie the cognitive phenotype. We focused on WM alterations because prior neuropathology studies in UCD patients showed WM to be primarily affected, and there is a reported relationship between Gln toxicity and WM damage (Albrecht, et al, 2010). We hypothesized that changes in WM microstructure would be found in pathways that correlate with the neurocognitive profile of OTCD. We found that the fractional anisotropy (FA; a reflection of fiber density, axonal diameter, and myelination in WM) of the frontal lobe, cingulum, and supplemental motor WM was significantly lower in patients than in controls, indicating changes in WM microstructure in these regions (Gropman, et al, 2010). Since fiber tracts connecting these regions underlie executive function and working memory pathways, DTI provides an objective means for determining the relationship to cognitive deficits in OTCD, even in cases where the injury was sustained years prior to the imaging study (Figure 4).
Figure 4.
Diffusion tensor imaging shows evidence of white matter injury in motor tracts that connect parts of the brain important in attention and memory
Reproduced with permission from Gropman et al., Metab Brain Dis. 2013 Jun; 28(2): 269–275.
Functional MRI (fMRI) examines networks involved in specific cognitive tasks and processes
FMRI provides detailed maps of brain areas participating in human mental activities (cognitive and motor), including the effect of HA on brain activation during performance of working memory tasks under conditions of high memory load. We hypothesized that brain activation maps would differ in subjects with late-onset OTCD in regions that underlie the cognitive deficits in executive function and working memory. We used the N-back task that probes association cortex function (dorsal lateral prefrontal lobe), areas subserving working memory. These results combined with those from DTI and MRS suggest there is impaired frontal lobe processing in subjects with OTCD that is compensated for as a result of recruitment of additional cortical and subcortical areas, suggestive of cognitive inefficiency (Gropman, et al, 2013).
Overall, these neuroimaging findings offer evidence that brain injury caused by biochemical dysregulation, i.e. HA in OTCD, may impact the integrity of the underlying neural networks involved in working memory processes which are important components of executive function and regulation. These imaging studies performed on clinically stable individuals with OTCD expand upon this concept by applying advanced imaging techniques to investigate the underlying neural networks that differ between individuals with OTCD and an age matched normative population. OTCD patients show relatively higher dorsolateral prefrontal cortex (DLPFC) activity, suggesting a pattern of prefrontal inefficiency, possibly related to decreased white matter integrity between hemispheres.
Additionally, the brains of individuals with OTCD show alterations in functional connectivity at rest. Internodal functional connectivity in the default mode network (DMN) and set-maintenance network (SMN) is reduced in patients with partial OTCD compared to controls, most likely due to HA-related white matter damage (Pacheco-Colón , et al, 2015). Because several of the affected areas are involved in executive functioning, it is postulated that this reduced connectivity may be an underlying cause of the deficits OTCD patients display in this cognitive domain.
What are the neuropsychological testing results in the different UCDs?
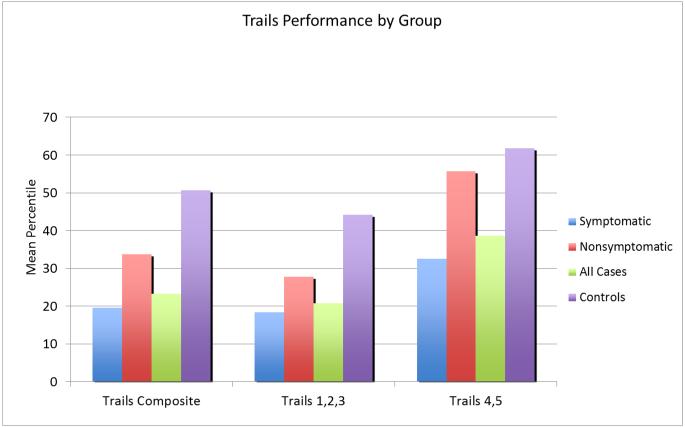
In addition to studies focused on executive functioning and memory in conjunction with the MRI evaluations, previous work we performed in the 1990’s (Gyato, et al, 2004), demonstrated that visual motor tasks elicited significant differences in performance between control participants, asymptomatic OTCD carriers and symptomatic late onset (hemizygous male and heterozygous female) OTCD patients. As noted in Figure 5, individuals with OTCD performed less well on the Comprehensive Trail Making Test. Processing speed and selective attention also appeared to be affected, especially with higher cognitive loading, as indicated by poorer performance on the Color Stroop task (Figure 6) (Sprouse, et al, 2014).
Figure 5.
Trails Test Performance in patients with OTCD by group.
From: Pacheco-Colon I, Fricke S., VanMeter J, Gropman AL. Advances in urea cycle neuroimaging: Proceedings from the 4th International Symposium on urea cycle disorders, Barcelona, Spain, September 2013. Mol Genet Metab. 2014 113(1-2): 118-126. Reprinted with permission.
Figure 6.
Stroop Performance group means. Reprinted with permission from Gropman A. Brain imaging in UCD. Mol Genet Metab. 2010;100 Suppl 1:S20-30.
In addition to these previous reports on individuals with OTCD, we conducted neuropsychological evaluations in cohorts of individuals with other UCD’s, as part of the LS. The LS included neuropsychological testing data from 528 different individuals, with 8 different UCD/transporter defects. The number of subjects in each group varied, ranging from 4 with CITRIN deficiency to 320 with OTCD. The testing battery screened for delays or deficits in a range of domains and included age-appropriate measures of behavior, intelligence, memory, attention/executive functioning, visual motor skills, motor strength and dexterity, behavior and quality of life (Table 2). While the intent was to obtain longitudinal data during infancy and at ages 4, 8, 14 and 18 years, only 55 children were evaluated more than once after 3 years of age. Below is a summary of the main neuropsychological outcomes in each UCD. Table 3 summarizes these data.
Table 2.
Neuropsychological Testing Battery
| Age Group | Tests Administered |
|---|---|
|
Infants
(6 mo-3 yrs) |
Bayley Scales of Infant & Toddler Development, |
| Adaptive Behavior Assessment System, Second Edition (ABAS-II) | |
| Child Behavior Checklist (CBCL) | |
|
| |
|
Preschool
(3-5 yrs) |
CBCL, ABAS-II |
| Behavior Rating Inventory of Executive Function (BRIEF)-Preschool | |
| Wechsler Preschool and Primary Scale of Intelligence, Fourth Edition (WPPSI-IV) | |
| California Verbal Learning Test (CVLT) | |
| NEPSY-II (Word Generation), | |
| Beery Buktenika Developmental Test of Visual-Motor Integration (VMI) | |
| Grip strength and Lafayette Pegboard | |
| Pediatric Quality of Life Inventory (Peds- QL) | |
|
| |
|
School-aged
(6-16 yrs) |
CBCL, ABAS-II, BRIEF |
| Wechsler Abbreviated Scale of Intelligence (WASI) | |
| CVLT | |
| NEPSY-II (Word Generation) | |
| Rey-Osterreith Complex Figure | |
| VMI | |
| Grip strength and Pegboard | |
| PedsQL | |
|
| |
|
Adults (≥ 17
yrs) |
ABAS-II, BRIEF |
| WASI | |
| CVLT | |
| D-KEFS (Verbal Fluency) | |
| Rey-Osterreith Complex Figure | |
| VMI | |
| Grip strength and Pegboard | |
ABAS-II = Adaptive Behavior Assessment System, Second Edition
CBCL = Child Behavior Checklist
WPPSI-IV=Wechsler Preschool and Primary Scales of Intelligence, Fourth Edition
BRIEF=Behavior Rating Inventory of Executive Function
VMI = Visual Motor Integration
Peds QL=Pediatric Quality of Life
WASI-II=Wechsler Abbreviated Scale of Intelligence, Second Edition
TEAch = Test of Everyday Attention for Children
D-KEFS = Delis-Kaplan Executive Function System
NIH PROMIS = National Institutes of Health, Patient Reported Outcomes Measurement Information System
Table 3.
Summary of Neuropsychological Test Results: Mean Scores on tests of development, intelligence and adaptive behavior
| Early Development 6 mo-3 yrs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Condition | Number of evaluations |
DQ - Bayley Cognitive Composite |
Bayley Language Composite |
Bayley Motor Composite |
% DQ < 86 |
%DQ <71 |
ABAS GAC |
Developmental Issues noted |
| CPS1D | 11 | 77 | 78 | 76 | 64 | 27 | 85 | None noted |
| OTCD FEMALES |
25 | 96 | 92 | 92 | 21 | 13 | 90 | Gross motor delays |
| OTCD MALES |
30 | 74 | 73 | 68 | 67 | 52 | 75 | Gross motor delays |
| ASSD | 44 | 94 | 93 | 88 | 30 | 18 | 93 | Gross motor delays |
| ASLD | 64 | 87 | 86 | 84 | 45 | 23 | 83 | Fine & gross motor delays |
| ARGD | 3 | 88 | 94 | 88 | 67 | 0 | 105 | None noted |
| ORNT1D/ HHH |
6 | 89 | 95 | 83 | 60 | 20 | 84 | Gross motor delays |
| CTRIN | 4 | 101 | 93 | 97 | 0 | 0 | 77 | Gross motor delays |
| Pre-School 4-5 yrs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Number of evaluations |
FS IQ |
VIQ | PIQ | % IQ < 86 |
% DQ < 71 |
ABAS GAC |
Developmental Issues noted |
|
| CPS1D | 5 | 74 | 80 | 74 | 50 | 0 | 70 | 4/5 children with PDD |
| OTCD FEMALES |
31 | 105 | 104 | 99 | 13 | 0 | 92 | Attention, fine motor delay |
| OTCD MALES |
15 | 91 | 94 | 88 | 21 | 13 | 82 | Withdrawn, motor delay, slow processing speed |
| ASSD | 23 | 88 | 92 | 88 | 41 | 18 | 84 | Emotionally reactive, ADHD, 2 with PDD |
| ASLD | 21 | 86 | 90 | 84 | 53 | 24 | 79 | Attention, social, fine motor delay |
| ARGD | 3 | 65 | 74 | 62 | 100 | 50 | 46 | 1 child with PDD |
| ORNT1D/ HHH |
4 | 89 | 92 | 83 | 50 | 50 | 73 | 2 with anxiety, acting out behaviors |
| CTRIN | 1 | 100 | 93 | 101 | 0 | 0 | 80 | relative weakness in verbal skills |
| School age 6-16 yrs | ||||||||
| Number of evaluations |
FS IQ |
VIQ | PIQ | %IQ < 86 |
% IQ < 71 |
ABAS GAC |
Cognitive and Behavioral Issues noted |
|
| NAGSD | 1 | 110 | 110 | 107 | 0 | 0 | NA | None noted |
| CPS1D | 4 | 105 | 113 | 98 | 0 | 0 | 84 | None noted |
| OTCD FEMALES |
97 | 95 | 99 | 93 | 21 | 8 | 86 | Impulsive, poor behavioral regulation, memory, fine motor |
| OTCD MALES |
34 | 100 | 101 | 98 | 18 | 9 | 83 | Depression and withdrawn |
| ASSD | 42 | 87 | 88 | 86 | 31 | 17 | 83 | Memory, attention, social, impulsive |
| ASLD | 27 | 77 | 81 | 77 | 69 | 38 | 74 | memory, attention, social, thought disorder |
| ARGD | 6 | 77 | 77 | 70 | 33 | 33 | 76 | Memory |
| CITRIN | 1 | 103 | 97 | 109 | 0 | 0 | 95 | None noted |
| Adults ≥17 yrs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Number of evaluations |
FS IQ |
VIQ | PIQ | %IQ < 86 |
% IQ < 71 |
ABAS GAC Self/Parent |
Cognitive and Behavioral Issues noted |
|
| NAGSD | 3 | 106 | 106 | 104 | 0 | 0 | 106/105 | None noted |
| CPS1D | 4 | 80 | 81 | 82 | 50 | 50 | 102/63 | None noted |
| OTCD FEMALES |
156 | 102 | 102 | 99 | 14 | 5 | 106/91 | Visual motor, Memory (self- report only) |
| OTCD MALES |
25 | 101 | 100 | 101 | 20 | 15 | 98/97 | None noted |
| ASSD | 19 | 68 | 70 | 69 | 42 | 26 | NA/83 | Memory and fine motor |
| ASLD | 23 | 65 | 68 | 69 | 74 | 52 | 82/81 | Memory and fine motor |
| ARGD | 9 | 76 | 79 | 74 | 56 | 33 | NA/84 | Memory and fine motor |
| ORNT1D/ HHH |
2 | 92 | 88 | 97 | 50 | 0 | NA/NA | None noted |
DQ = Developmental Quotient obtained from the Bayley Scales of Infant and Toddler Development, Third Edition, Cognitive Composite Score; ABAS GAC =Adaptive Behavior Assessment System, Second Edition, General Adaptive Composite; FS IQ = Full Scale IQ, VIQ=Verbal IQ, PIQ=Performance IQ; PDD =Pervasive Developmental Delay and refers elevated scores on this scale from the Child Behavior Checklist.
N-acetyl glutamate synthase (NAGS) deficiency (n=4)
The 1 child and 3 adults with NAGS deficiency performed within the average intellectual range (IQ range 87 to 116). However, all 4 individuals appeared to have weaknesses in auditory memory, and one adult had experienced serious psychiatric and cognitive problems prior to her correct diagnosis and treatment. None of these participants received follow-up evaluations.
Carbamyl phosphate synthetase 1 (CPS1) deficiency (n=10)
CPS 1 deficiency was associated with global early developmental delays in 7 children (one tested twice). Mean Bayley Cognitive Composite score was 77 ± 17, ranging from 60 to 85. (The child receiving repeat testing was evaluated at age 4 months and received a score of 110 but by 2 years attained a score of 85). Preschool and school-aged children also experienced a range of cognitive skills, with IQs ranging from 50 to 141. Behaviors common in children with autism spectrum disorder, as evidenced by elevated scores on the Pervasive Developmental Delay (PDD) subscale of the Child Behavior Checklist, were noted in 4 of 5 preschool children but not in school-aged children. Among the 3 children over age 3 years receiving follow-up testing, none experienced significant declines in cognitive functioning over time. Two adults with CPS1 deficiency performed in the range of intellectual disability (IQ = 66 and 62) and two others performed in the average range (IQ = 105, 86). The adults with higher IQs rated themselves as doing well in terms of day-to-day functioning as evidenced by their self-reports on the ABAS-II, while the two adults with intellectual disability received lower scores on the ABAS-II as reported by their parents.
Ornithine transcarbamylase deficiency-OTCD (n=320)
Since OTCD is an X-linked disorder, results from males and females were considered separately. Infant girls (n=25) had a mean Bayley Cognitive Composite score of 96 ± 16, while infant boys (n=27) had a mean score of 74 ± 19. In both boys and girls gross motor skills were not as well developed as fine motor skills (t=2.84, p = 0.01). Early language development, while still somewhat delayed in the boys, was a relative strength in both boys and girls. Our preschool cohort of 31 girls with OTCD continued to perform well with a mean full scale IQ of 105 ± 15. Among the 15 boys in this cohort, the full scale IQ was 91 ± 22, with evidence of solid verbal skills but relatively delayed motor skills and slow processing speed. Memory appeared intact in both boys and girls. Behavior problems were rarely noted on the Child Behavior Checklist, although 40% of the boys were rated as “withdrawn” by their parents.
At school-age, 97 evaluations were conducted in girls with OTCD, and 34 evaluations were conducted in boys. Boys now performed, on average, well within the average range and no longer performed less well than the girls. The full scale IQ was 95 ± 19 for girls and 103 ± 18 for boys. In this age-group, 23% of the girls performed more than a standard deviation below the normative mean on nonverbal tasks of the WASI, with a Performance IQ below 70. Working memory and other executive functioning skills were problematic for girls but not boys, as indicated by parents’ responses on the BRIEF. On the Beery Visual Motor Integration test, in which children are asked to copy increasingly complex geometric figures, girls and boys performed in the low average range, although their scores on the perceptual task, where they were asked to simply identify figures that matched a target figure, were consistently higher. This suggests difficulties in fine motor control. Emotionally, half of boys and girls were rated by their parents as having “internalizing problems” on the Child Behavior Checklist. Nearly 2/3 of the girls were rated as having significant somatic complaints, and 43% received scores above 60 (the cut-off for indicating risk) on the Child Behavior Checklist Thought Disorder subscale. Boys were rated as having fewer of these kinds of issues. Both groups were rated as well-behaved overall, although about a third in each group was rated as having difficulties in social relationships. Among the girls over 3 years of age, 25 received more than one evaluation. The mean change in IQ was plus 0.4 points, with only 3 girls experiencing declines of 10 points or more. Among boys over age 3 years, only 4 were evaluated twice, and the mean change in IQ was plus 4 points.
The adult OTCD cohort was tested only once and consisted of 156 women and 25 men. Nearly all the women were considered asymptomatic females, identified because of an affected person in the family of origin or the birth of a symptomatic child. These women attained a full scale IQ of 102 ± 16. The men attained a full scale IQ of 101 ± 21. No differences were noted between verbal and performance scores. Self-reports on the BRIEF did not indicate global problems in executive functioning; however, 27% (23/85) of female and 33% (6/18) of male respondents received scores suggesting deficits in working memory. Direct measurement of auditory memory on Trial 1 of the California Verbal Learning Test – Adult Version (CVLT-A) suggested that this is an area of difficulty for 24% (25/105) of women but only 9% (2/22) of men. Self-reports and informant reports (parents or others) from the ABAS-II did not highlight specific areas of difficulties.
Argininosuccinate synthetase deficiency (ASSD) or citrullinemia (n=75)
Although the Cognitive Composite score on the Bayley was 94± 19 and well within the average range, gross motor skills were significantly less well developed than expressive language skills (t=2.19, p = 0.035). The preschool cohort (n=23) had a full scale IQ of 88 ± 21. They exhibited strengths in reasoning abilities and weaknesses in visual-spatial understanding and fine motor skills. Parents perceived these children as “emotionally reactive”, with about 30% rated as hyperactive with attention issues and 2 children at risk for autism spectrum disorder. Children who had reached school age varied considerably in intellectual abilities, with IQs ranging from 55 to 133 (mean=87 ± 21), with little difference between verbal and nonverbal skills noted. Seventeen percent had IQ scores in the range of intellectual disability. On the ABAS-II, adaptive behavior as rated by parents was in the range of intellectual disability in 39% of children. More than half the children were rated by their parents as having attention and social problems. Forty percent of parents also rated their children as having significant rule-breaking behaviors and aggression. Eight children ages 5 years and older received two evaluations and only one declined in IQ by 10 points over time. The average IQ for the group was 6 points higher at the second evaluation. The adult cohort included 19 individuals with citrullinemia. Among the 10 who received IQ testing, the mean full scale IQ was 68 ± 20, ranging from 54 to 106, with 50% in the range of intellectual disability. Parents rated 70% of the adults in this group, however, as communicating well despite cognitive challenges.
A mean Bayley Cognitive Composite Score of 101 ± 12 was found for 22 newborn screened cases with ASSD and 82 ± 20 for 13 clinically identified cases (p<.001).
Argininosuccinate lyase deficiency/argininosuccinic aciduria ASLD (or ALD or ASA) (n=71):
Data from 64 evaluations during infancy resulted in a Bayley Cognitive Composite Score of 87 ± 18, in the low average range, and a Global Adaptive Composite (GAC) Score of the ABAS-II only a few points lower (GAC=83 ± 16). These infants experienced relative delays in the gross motor area, with scores in this area significantly lower than scores in receptive language (t=2.04, p=0.046). During preschool (n=21 evaluations), children with ASLD attained a full scale IQ still in the low average range, but parents’ responses on the ABAS-II suggested delays in adaptive behavior, especially self-help, motor and communication skills (GAC=79 ± 18). Symptoms of attention deficits were also noted. By school age (n=27 evaluations), full scale IQ was 77 ± 20 in the cohort of children tested at this age. Verbal skills were significantly stronger than performance skills (t=2.15, p = 0.04). Deficits were noted in memory, verbal fluency, attention, visual motor skills, motor strength and dexterity. Behavioral issues were not noted. Two of the 14 children receiving two evaluations experienced declines in IQ of more than 10 points. The average change in IQ for this group was 0.79. The adult cohort (n=23 cases) performed in the range of intellectual disability with a full scale IQ of 65 ± 12, and scores on most other tests within this range. Parent and self-reports of adaptive behavior suggest that these adults managed fairly well, with GAC = 81± 19 for parent reports and 82 ± 9 for self-reports. From these cross-sectional analyses, a general picture emerges of well-behaved, motivated children and adults with primary motor issues.
The 27 children with ASLD who were identified by newborn screening attained a mean Bayley Cognitive Composite score of 93 ± 18, while the 16 children with ASLD who were identified as a result of clinical HA episodes attained a score of 69 ± 12 (p<.0001).
Arginase deficiency ARGD (n=17)
Among children with arginase deficiency there was considerable variability during infancy, with Cognitive Composite scores ranging between 80 to100 on the Bayley Scales. Three children evaluated during preschool had intellectual disability, one of whom also exhibited behaviors consistent with autism spectrum disorder Five of 6 school-aged children performed in the range of intellectual disability, two with significant attention deficits and aggressive behaviors. One child was tested twice during school age and performed both times in the range of intellectual disability. Another child was doing well in all areas and continued to do well when tested at age 18 years. The 8 adults with ARGD received scores on intelligence tests indicating intellectual disability. Although half the adults lived independently, they experienced significant memory and fine motor deficits.
HHH (Hyperornithinemia-Hyperammonemia-Homocitrullinuria) Syndrome (n=7)
One infant appeared to be developing normally and three had developmental delays and, when re-tested, continued to experience developmental delays. The infant who had been developing normally continued to do so with a Verbal IQ of 104 and Performance IQ of 90. IQs in the school-aged children ranged from 70 to 107, with two children performing within the average range and two performing in the borderline range. The one adult with HHH Syndrome had an IQ of 100 at age 21 years and 84 at age 26, with significant declines in scores on all neuropsychological tests administered.
CITRIN deficiency
The 2 children with CITRIN deficiency were each evaluated several times and appeared to be developing normally with DQ scores well within the average range on all occasions, although both had lower verbal than performance scores. Development continued to be normal at preschool, although at this time verbal skills were relatively less strong than nonverbal abilities. In the one child evaluated at school age (9 years) there were no indications of cognitive or behavioral consequences of this condition.
SUMMARY AND CONCLUSIONS
The last two decades have brought advances in the early identification and comprehensive management of UCD. The data accumulated by the UCDC reflects these changes and provides the basis for selection of outcome variables in clinical trials and directions for future research.
Our neuroimaging studies, performed in OTCD, show predominant WM injury, metabolic changes and alterations of neural networks that are important in executive function. This was anticipated in patients who have experienced HA, however, it was also seen in our “asymptomatic carriers”, raising question and concern for lesser but present degrees of injury. MRI spectroscopy revealed significant increases of glutamine levels and decreases in myoinosital (mI) in the PWM and posterior cerebral gray matter (PCGM) in both symptomatic and asymptomatic OTCD, despite normal plasma Gln levels. These biomarkers correlated with neuropsychological test results, suggesting that perturbations in brain metabolites impact functioning, even when the individual experiences no clinical symptoms associated with OTCD. As a result, subclinical injury may go unrecognized and untreated because routine clinical assessments underestimate the degree of injury (Sprouse et al., 2014). This finding was consistent with our previously published neurocognitive studies (Krivitzky, et al, 2009).
Given the non-invasive method of studying brain metabolism with MRS, use of this technology clinically to follow disease progression and treatment is feasible. Diffusion Tensor Imaging (DTI) showed poorer fiber density, axonal diameter and myelination of the frontal lobe, cingulum and supplemental white matter. These findings may explain the relative weaknesses in working memory and executive functioning found in OTCD adults. Functional MRI provided further evidence for a relationship between reduced connectivity in the brain and inferior neuropsychological performance in adults with OTCD compared to controls. Given these findings, neuroimaging studies in the other UCD may reveal information about the impact of metabolic dysregulation even in the absence of clinically recognizable HA.
Neuropsychological evaluations in the LS suggest that the disorders should be studied separately, since each appeared to be associated with a particular phenotype of cognitive and behavioral characteristics that varied within the subpopulations and over time. Despite this variability in outcomes, when neuropsychological deficits occurred, they tended to be more prominent in motor/performance and memory areas on both intelligence tests and other measures. When behavioral difficulties occurred, they were primarily noted in the areas of attention, hyperactivity, and aggression. Boys with OTCD were the exception and tended to be withdrawn. Autism spectrum disorders were occasionally reported, but standardized screening tests were not administered for this condition. In cross-sectional analyses and in some cases longitudinal analyses, individuals with OTCD, CPS1D, NAGSD and HHH syndrome tended to have increased scores over time on intelligence tests or to stay about the same. In contrast, when studied cross-sectionally, ARGD, ASLD and ASSD were associated with typical early development followed by declines in functioning. Although cross-sectional data indicated that in general older children performed less well than younger children, longitudinal data in the 19 children (ages 4-16 years) with ASLD and ASSD who received more than one evaluation did not suggest declines in functioning over the course of the study. The discrepancy between the cross-sectional and longitudinal data may reflect earlier detection through newborn screening, better treatments now than in earlier years, or sample bias since a low percentage of children were evaluated more than once.
Large collaborative, longitudinal studies involving disease groups present challenges due to the diversity in the populations studied, treatments prescribed, and clinics providing services. In the UCDC longitudinal study, 14 centers provided data, 8 UCD/transporter defects were studied, age at treatment initiation ranged from 1 day to 63 years, and over 100 different medications were prescribed to subjects. Over the course of the study, three new UCD medications became available and newborn screening was introduced for 3 conditions. While possibly confounding the results, this variability provides a unique opportunity for identifying sensitive outcome measures and the impact of diverse treatments. Moreover, data gleaned from the UCDC can be incorporated into evidence-based guidelines for the treatment of UCD in the future.
To date, this study cannot answer very important questions about the long-term impact of liver transplantation in UCD. Earlier results from the UCDC (Campeau et al, 2010) reported no deaths among 44 patients. There did not appear to be a significant difference in IQ of transplanted vs. non-transplanted patients. However, the study could not answer the question of whether post-liver transplant patients are protected from further brain injury that might affect medically treated patients who sustain subsequent HA episodes.
Additional questions remain about the long-term outcome of children who receive newborn screening and/or novel therapies. While adults did less well than younger patients, we do not yet know if this was due to more HA events and exposure to toxic metabolites throughout life in the older individuals or to improvements in diagnostics and treatments in the younger generation.
In the most common disorder, OTCD, HA episodes and resultant alterations in cellular volume and neurotransmitters are believed to be at the core of the neurological injury and neuropsychological dysfunction. In contrast, for the distal disorders (ASSD and ASLD) cognitive impairment can be evident even in the absence of recurrent acute HA crises. As a consequence, both citrulline and argininosuccinate or their metabolic products are suspected to be neurotoxic agents. Taken together, the data suggest that the pathology in CPS1D and OTCD may be a consequence of ammonia and Gln accumulation while in the more distal defects arginine, argininosuccinate and citrulline or their products additionally may be neurotoxic.
Our data suggest that the age at initial onset of symptoms and the number of subsequent HA events fail to explain the variability noted between and within UCD groups. We plan in future analyses to examine the impact of variability in metabolic status (for example calculating the standard deviation rather than the mean of lifetime measures of glutamine, arginine or citrulline) and relate this to neuropsychological outcomes. We will also assess threshold effects in the absence of HA episodes.
Despite the challenges inherent in large collaborative studies, we have amassed a comprehensive set of data about UCD. Analyses to date provide insights into the neuropathology and neuropsychological impact of these conditions. With continued collection and analyses of laboratory, neuroimaging and neuropsychological data, the UCDC provides the opportunity for establishing evidence-based treatment guidelines and subsequently improving the lives of affected individuals.
Acknowledgements
The Urea Cycle Disorders Consortium (UCDC; U54HD061221) is a part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported through collaboration between the Office of Rare Diseases Research (ORDR), the National Center for Advancing Translational Science (NCATS and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The UCDC is also supported by the O’Malley Foundation, the Rotenberg Family Fund, the Dietmar-Hopp Foundation, and the Kettering Fund.
Footnotes
Compliance with Ethical Guidelines
Conflict of Interest
Mark Batshaw declares that he has no conflict of interest.
Andrea Gropman declares that she has a grant under the UCD from which the data were generated.
Susan Waisbren declares that she served as the Coordinating Psychologist and Principal Investigator for the New England Region for the LS of Urea Cycle Disorders that was funded by a subcontract to Boston’s Children’s Hospital from Children’s National Medical Center through an NIH grant with additional support from the O’Malley Foundation (a philanthropic organization).
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Animal Rights
This article does not contain any studies with animal subjects performed by the any of the authors.
Details of Contributions of Individual Authors
Mark Batshaw was the speaker at the symposium and conceived the article. He developed the initial draft of the paper and critically reviewed the final draft.
Andrea Gropman wrote the sections on neuroimaging and critically reviewed the final draft.
Susan Waisbren wrote the sections on neuropsychological findings, drafted the introduction and discussion, and performed final editing. .
References
- Albrecht J, Zielińska M, Norenberg MD. Glutamine as a mediator of ammonia neurotoxicity: A critical appraisal. Biochem Pharmacol. 2010;80:1303–1308. doi: 10.1016/j.bcp.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batshaw ML, Brusilow SW, Waber L, et al. Treatment of inborn errors of urea synthesis: activation of alternate pathways of waste nitrogen synthesis and excretion. N Engl J Med. 1982;306:1387–1392. doi: 10.1056/NEJM198206103062303. [DOI] [PubMed] [Google Scholar]
- Batshaw ML, MacArthur RB, Tuchman M. Alternative pathway therapy for urea cycle disorders: Twenty years later. J Pediatr. 2001;138:S46–S55. doi: 10.1067/mpd.2001.111836. [DOI] [PubMed] [Google Scholar]
- Batshaw ML, Msall M, Beaudet AL, Trojak J. Risk of serious illness in heterozygotes for ornithine transcarbamylase deficiency. J Pediatr. 1986;108(2):236–241. doi: 10.1016/s0022-3476(86)80989-1. PubMed PMID: 3944708. [DOI] [PubMed] [Google Scholar]
- Batshaw ML, Tuchman M, Summar M, Seminara J, Members of the Urea Cycle Disorders Consortium A longitudinal study of urea cycle disorders. Mol Genet Metab. 2014;113(1-2):127–130. doi: 10.1016/j.ymgme.2014.08.001. PMID: 25135652; PMCID: PMC4178008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusilow SW, Batshaw ML, Valle D. New pathways of waste nitrogen excretion in inborn errors of urea synthesis. Lancet. 1979;2:452–454. doi: 10.1016/s0140-6736(79)91503-4. [DOI] [PubMed] [Google Scholar]
- Campeau PM, Pivalizza PJ, Miller G, McBride K, Karpen S, Goss J, Lee BH. Early orthotopic liver transplantation in urea cycle defects: follow up of a developmental outcome study. Mol Genet Metab. 2010;100(Suppl 1):S84–7. doi: 10.1016/j.ymgme.2010.02.012. PMID: 20223690; PMC2867349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropman AL, Gertz B, Shattuck K, Kahn IL, Seltzer R, Krivitsky L, Van Meter J. Diffusion tensor imaging detects areas of abnormal white matter microstructure in patients with partial ornithine transcarbamylase deficiency. AJNR. 2010;31:1719–1723. doi: 10.3174/ajnr.A2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropman AL, Fricke ST, Seltzer RR, et al. 1H MRS identifies symptomatic and asymptomatic subjects with partial ornithine transcarbamylase deficiency. Mol Genet Metab. 2008;95:21–30. doi: 10.1016/j.ymgme.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropman AL, Shattuck K, Prust MJ, et al. Altered neural activation in ornithine transcarbamylase deficiency during executive cognition: an fMRI study. Hum Brain Mapp. 2013;34:753–761. doi: 10.1002/hbm.21470. [PubMed: 22110002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyato K, Wray J, Huang ZJ, Yudkoff M, Batshaw ML. Metabolic and neuropsychological phenotype in women heterozygous for ornithine transcarbamylase deficiency. Ann Neurol. 2004;55:80–86. doi: 10.1002/ana.10794. [DOI] [PubMed] [Google Scholar]
- Krivitzky L, Babikian T, Lee HS, Thomas NH, Burk-Paull KL, Batshaw ML. Intellectual, adaptive, and behavioral functioning in children with urea cycle disorders. Pediatr Res. 2009 Jul;66(1):96–101. doi: 10.1203/PDR.0b013e3181a27a16. doi: 10.1203/PDR.0b013e3181a27a16. PubMed PMID: 19287347; PubMed Central PMCID: PMC2746951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msall M, Batshaw ML, Suss R, Brusilow SW, Mellits ED. Neurologic outcome in children with inborn errors of urea synthesis. N Engl J Med. 1984;310:1500–1505. doi: 10.1056/NEJM198406073102304. [DOI] [PubMed] [Google Scholar]
- Msall M, Monahan PS, Chapanis N, Batshaw ML. Cognitive development in children with inborn errors of urea synthesis. Acta Paediatr Jpn. 1988;30:435–441. doi: 10.1111/j.1442-200x.1988.tb02534.x. [DOI] [PubMed] [Google Scholar]
- Pacheco-Colón I, Washington SD, Sprouse C, Helman G, Gropman AL, VanMeter JW. Reduced Functional Connectivity of Default Mode and Set-Maintenance Networks in Ornithine Transcarbamylase Deficiency. PLoS One. 2015;10(6):e0129595. doi: 10.1371/journal.pone.0129595. doi: 10.1371/journal.pone.0129595. PMID: 26067829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perito ER, Rhee S, Roberts JP, Rosenthal P. Pediatric liver transplantation for urea cycle disorders and organic acidemias: United Network for Organ Sharing data for 2002-2012. Liver Transpl. 2014;20(1):89–99. doi: 10.1002/lt.23765. PubMed PMID: 24136671, PMC3877181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara J, Tuchman M, Krivitzky L, et al. Establishing a Consortium for the Study of Rare Diseases: The Urea Cycle Disorders Consortium. Mol Genet Metab. 2010;100(Suppl 1):S97–S105. doi: 10.1016/j.ymgme.2010.01.014. PMCID: PMC285879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprouse C, King J, Helman G, et al. Investigating neurological deficits in carriers and affected patients with ornithine transcarbamylase deficiency. Mol Genet Metab. 2014;113(1-2):136–141. doi: 10.1016/j.ymgme.2014.05.007. PMID: 24881970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summar ML, Koelker S, Freedenberg D, Le Mons C, Haberle J, Lee HS, Kirmse B. European Registry and Network for Intoxication Type Metabolic Diseases (E-IMD) Mol Genet Metab. 2013;110(1-2):179–180. doi: 10.1016/j.ymgme.2013.07.008. Electronic address: http://www.e-imd.org/en/index.phtml; Members of the Urea Cycle Disorders Consortium (UCDC). Electronic address: http://rarediseasesnetwork.epi.usf.edu/ucdc/. The incidence of urea cycle disorders. PubMed PMID: 23972786. [DOI] [PMC free article] [PubMed] [Google Scholar]