Summary
A number of studies suggest that HIV-infected individuals have an elevated risk of cardiovascular disease (CVD), especially coronary heart disease, compared to the HIV-uninfected population. People living with HIV have an increased prevalence of traditional CVD risk factors but HIV-specific mechanisms such as immune activation and antiretroviral therapy also play critical roles. Although older, more metabolically harmful antiretroviral regimens likely contributed to the risk of cardiovascular disease, emerging data suggest that the overall effect of early and continuous use of modern regimens, which may have fewer metabolic consequences, minimizes the risk of myocardial infarction by maintaining viral suppression and decreasing immune activation. Even with antiretroviral therapy, however, immune activation persists in HIV-infected individuals and may contribute to accelerated atherosclerosis, especially of vulnerable coronary lesions that predispose to myocardial infarction. Thus, therapies that safely reduce inflammation in the HIV population may provide additional cardiovascular protection alongside treatment of both traditional and other, non-traditional risk factors.
Introduction
The estimated HIV-infected population totals 36·9 million people. In higher-income countries, up to one third of the HIV-infected population is 50 years or older, whereas this age group comprises roughly 10% of the HIV-infected population in low to middle-income areas. As access to combination antiretroviral therapy (cART) has improved throughout the world, mortality, including AIDS-related mortality, is declining.1 Several studies conducted in the era of cART suggest that the major causes of death are now non-AIDS related. Cardiovascular disease (CVD) has become one of the leading causes of non-AIDS related morbidity and mortality, and, as seen in the general population, CVD event rates increase with age.2 Thus, as cART use continues to expand and patients grow older, the incidence of CVD will likely rise unless effective management strategies are developed.3
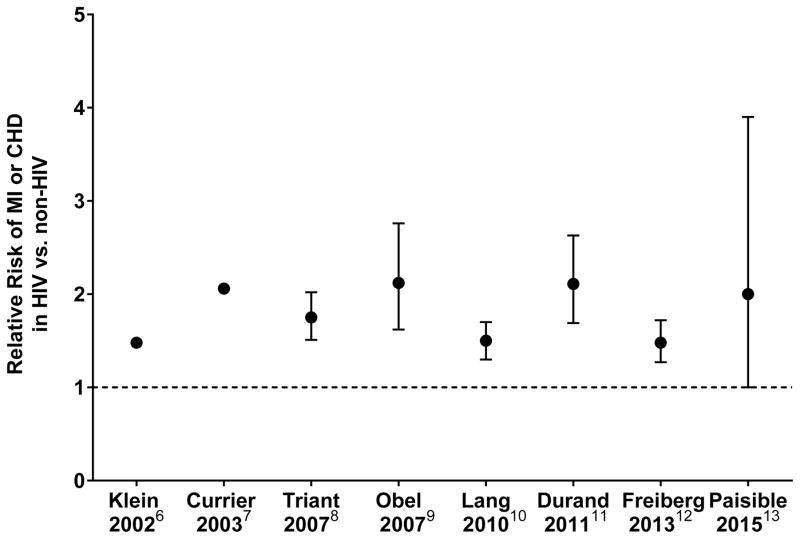
In areas with access to cART, the focus of care has changed from treating diseases related to immunodeficiency to managing chronic conditions like atherosclerosis. Coronary heart disease (CHD), for example, has become the leading cause of CVD in this patient population in developed countries.4, 5 Although traditional risk factors for CVD are more prevalent in patients with HIV, several epidemiologic studies have shown an increased risk of about 50 to 100% for CHD associated with HIV infection despite controlling for traditional risk factors (see Figure 1).6–13 This suggests that HIV-related mechanisms contribute to CVD risk as well. One limitation of several of these studies is that event rates were low, likely as a result of insufficient follow-up time for a relatively young HIV population.
Figure 1. Summary of epidemiology studies investigating relative risk of cardiovascular disease in HIV patients vs. control subjects.
Data are relative risk with 95% CI where available. Dotted line indicates relative risk of one.
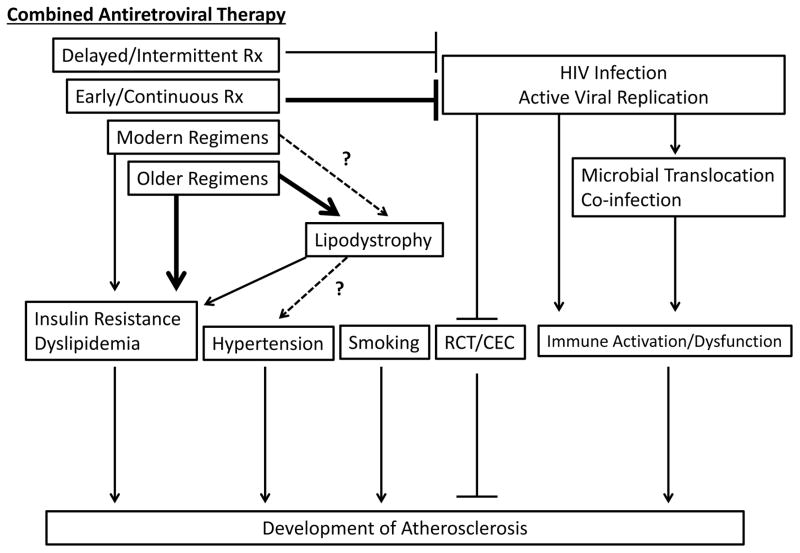
As a result, many investigators have utilized non-invasive imaging of the carotid and coronary arteries to evaluate subclinical disease.14 Techniques have included assessment of carotid intima-media thickness (CIMT), intra-luminal plaque visualization with coronary computed tomography angiography (cCTA), and coronary artery calcium (CAC) scoring, the latter of which has also gained some acceptance as a clinical screening tool to assess CVD risk.15 Most studies evaluating CIMT in asymptomatic individuals have shown an increase in subclinical atherosclerosis in HIV-infected patients compared to uninfected controls.16, 17 On the other hand, a meta-analysis of five studies assessing coronary artery calcium reported no significant difference between HIV-infected and uninfected individuals.16 Not all coronary lesions, however, are calcified. Thus, cCTA has provided additional insight, as it can detect both non-calcified and calcified plaque as well as visualize intra-luminal plaque morphology. Several studies utilizing cCTA have shown a higher prevalence of subclinical coronary atherosclerosis and an increased burden of coronary plaque, especially non-calcified plaque, in HIV-infected patients compared to uninfected controls, even after controlling for traditional CVD risk factors.18, 19 These data suggest that factors related to HIV infection may accelerate the development of coronary atherosclerosis. Moreover, in addition to accelerated disease, cCTA has also shown differences in plaque morphology between HIV-infected and uninfected populations, including an increased prevalence of high-risk morphologic features that have been associated with increased rates of MI in the general population.20, 21 Overall, data from non-invasive imaging suggest HIV-specific mechanisms likely play a significant role in accelerating a unique atherosclerotic phenotype with altered plaque morphology that is potentially more vulnerable to rupture. This review will discuss the roles of both traditional and non-traditional, HIV-specific risk factors in atherosclerotic development and illustrate the emerging paradigm regarding pathogenesis of plaque formation (see Figure 2). We will then review management strategies for CVD in this patient population.
Figure 2. Pathophysiology of atherosclerosis in HIV-infected individuals.
RCT = reverse cholesterol transport, CEC = cholesterol efflux capacity, Rx = treatment
Traditional Risk Factors
Modifiable traditional CVD risk factors such as smoking, hypertension, diabetes, and dyslipidemia are more prevalent in the HIV population. For example, most studies that have included uninfected controls have shown a higher prevalence of modifiable CVD risk factors in HIV-infected patients. In a large retrospective study of a U.S. healthcare system, Triant et al reported a higher prevalence of diabetes (11·5 vs. 6·6%), dyslipidemia (23·3 vs. 17·6%), and hypertension (21·2 vs. 15·9%) in patients with HIV compared to patients without HIV (p<0·0001 for each comparison).8 Similarly, the prevalence of smoking is higher in the HIV-infected population and is estimated to be about 32–60%.22, 23
Despite an emerging pattern of increased CVD risk factors in HIV patients, actual prevalence estimates differ from study to study. This variation is likely due to differences in the patient populations evaluated and definitions used for risk factor cut-offs. For example, several studies of large cohorts within the U.S. estimated the prevalence of diabetes among HIV-infected individuals at about 11–15%.8, 24, 25 In contrast, data from primarily European cohorts, including the large Data Collection of Adverse Events of Anti-HIV Drugs (D:A:D) study, estimated the prevalence of diabetes among HIV-infected patients at about 3–5%.26–28 The difference between estimates of diabetes prevalence between the U.S. and Europe are likely a result of dissimilar definitions for diabetes among the various studies and specific social and demographic factors unique to each location, such as body mass index which tended to be higher in the U.S. based studies. In addition to diabetes, the Strategic Timing of Antiretroviral Treatment (START) trial, a study conducted in thirty-five countries, reported that the prevalence of other modifiable CVD risk factors including smoking, hypertension, and dyslipidemia also varied by regional location.22
The effects of older cART on dyslipidemia, lipodystrophy, and diabetes likely contributed to the increased prevalence of these risk factors in HIV-infected patients, but this effect may be declining as newer medications appear to have less metabolic consequences. In general, older classes of cART such as non-nucleoside reverse transcriptase inhibitors (NNRTIs), nucleoside reverse transcriptase inhibitors (NRTIs), and protease inhibitors (PIs) have been extensively studied for their impact on metabolic function. As a result, the utilization of specific medications that have been associated with adverse metabolic effects has declined with time. Newer agents within these classes and newer classes of drugs such as entry inhibitors, fusion inhibitors, and integrase inhibitors have not been studied as thoroughly but major metabolic consequences have not yet been identified.
The increased prevalence of traditional risk factors in HIV-infected individuals has been associated with an increased risk of CHD. In the D:A:D cohort, dyslipidemia and diabetes were significantly associated with an increased risk of myocardial infarction (MI),29, 30 and in the study by Triant et al., hypertension, dyslipidemia, and diabetes were estimated to account for about 25% of the excess MI risk in HIV-infected patients.8 Furthermore, a Danish cohort study reported an increased risk of MI in HIV-infected individuals who were currently smoking.31 In the Veterans Aging Cohort Study Virtual Cohort, Paisible et al. investigated the effect of four traditional CVD risk factors—current smoking, diabetes, blood pressure/anti-hypertensive medication use, total cholesterol/statin use—on CHD and showed that patients with HIV had a step-wise increase in the risk of MI with an adjusted hazard ratio of 2·0 (95% CI 1·2–3·1) for those with 1 major CVD risk factor and 3·6 (95% CI 2·0 to 6·2) for those with 3+ major risk factors. In addition, they reported that HIV-infected veterans had higher rates of MI than uninfected veterans with the same CVD risk factor profile,13 implicating an effect of HIV-related mechanisms on CHD.
Effect of cART on CVD Risk
The D:A:D study prospectively investigated 23,468 HIV-infected patients living in Europe, Australia, and the United States for incident MI. They reported an adjusted relative rate of MI per year of PI exposure of 1·10 (95% CI 1·04–1·18) despite controlling for traditional risk factors.32 This association, however, does not appear to be a class effect. Older cART regimens containing the PIs, lopinavir-ritonavir or indinavir, for example, have been linked to an increased rate of MI33 while use of other, more contemporary PIs such as atazanavir have shown no increased risk,34 or as with darunavir, are still under investigation. The observation that some newer PIs appear to have no association with CVD may be a result of fewer associated cardiometabolic side effects, more aggressive treatment of traditional CVD risk factors in the modern era, or insufficient observational time in current studies for complications to have developed. Additionally, some newer PIs may also have cardioprotective effects that minimize harm such as atazanavir-ritonavir, which was shown to slow the rate of CIMT progression compared with darunavir-ritonavir, possibly as a result of increased bilirubin levels.35
The D:A:D study also reported an increased risk of MI with recent use of the NRTIs, didanosine and abacavir, but not other NRTIs such as tenofovir, zidovudine, stavudine, or lamivudine.33 In a secondary analysis of the SMART study, abacavir, but not didanosine, was associated with an increased risk of MI.36 Some studies have also reported increased rates of CVD related to abacavir use, including two recent publications from the large Swiss HIV Cohort and U.S. Veterans Health Administration Clinical Case registry,37–39 while others, including two meta-analyses, have shown no such relationship.40, 41 Thus, whether abacavir increases the risk of CVD remains unclear.
Despite the concerns that older cART regimens may exacerbate CVD, there is a growing body of literature suggesting continuous use, and possibly early initiation, of modern cART regimens could actually decrease CVD in patients with HIV. The SMART trial randomized HIV-infected patients with a CD4 T-cell count > 350 cells/uL to either continuous cART or intermittent therapy based on CD4 T-cell counts. Sensitivity analysis revealed that the intermittent cART group had an elevated risk of CVD events compared to the continuous cART group with a hazard ratio of 1·57 (95% CI 1·00–2·46, p=0.05).42 This suggests that despite greater exposure to cART, continuous viral suppression may be more beneficial in preventing CVD, possibly due to reductions in proinflammatory cytokines that are related to atherosclerosis.43–45 Furthermore, evidence that earlier commencement of cART may decrease CVD risk comes from studies that have shown that lower CD4 counts, lower nadir CD4 counts, and increased viremia—which are all associated with delayed initiation of cART—result in higher rates of MI.46, 47 It is important to note, however, that not all studies have confirmed these findings, including analysis of the D:A:D cohort.48
The recently published START trial was a randomized controlled trial investigating effects of starting cART at CD4 T-cell counts > 500 cells/uL versus delayed therapy at CD4 T-cell counts < 350 cells/uL. The trial was ended early as an interim analysis showed a significant decrease in serious AIDS and non-AIDS related events and death in the early treatment group with a hazard ratio of 0·43 (95% CI 0·3–0·62).49 Although CVD events were not significantly different between the two groups, event rates were lower than predicted, possibly related to the younger age of the population (median age was 36 years-old), and the study was stopped early, resulting in insufficient statistical power to detect potential benefits in CVD at this time. As a result, further research is needed to determine whether earlier initiation of cART protects against CVD. Strategies employing newer cART may permit earlier and sustained virological control and reduction in immune activation, with less metabolic effects, suggesting a potentially greater net benefit on CVD. Nonetheless, as discussed below, immune activation may persist even with effective cART, suggesting the possible need for additional strategies that address ongoing inflammation and immune activation.
Immune activation and cardiovascular disease
The immune system plays a central role in the development of atherosclerosis, especially monocytes/macrophages and T-cells. Patients with HIV have increased markers of inflammation/immune activation, some of which have been related to measures of atherosclerosis (see Table 1). cART attenuates this heightened stimulation but does not completely resolve it. The source of continued immune activation despite cART is still under investigation but is likely multifactorial. Thus, in addition to cART, therapies targeting immune activation may provide benefit in reducing the risk of CVD.
Table 1.
Relationship between immune markers and cardiovascular disease in HIV-infected individuals
| Immune Marker | Subclinical Atherosclerosis | Cardiovascular Disease |
|---|---|---|
| High sensitivity CRP | CIMT progression50 | MI and major CVD event45, 51 |
| IL-6 | MI, stroke, and major CVD event44, 45 | |
| D-dimer | MI, stroke, and major CVD event44, 45, 51 | |
| sTNFR | CIMT52 | MI and stroke44 |
| Lipopolysaccharide | CIMT progression53 | |
| MCP-1 | CIMT, stenosis ≥ 50%, CAC, coronary segments with plaque18, 54, 55 | |
| Lp-PLA2 | CIMT56 | |
| Oxidized LDL | CIMT55 | |
| HDL Redox Activity | Non-calcified plaque57 | |
| Soluble CD163 | CAC; vulnerable, total, non-calcified, mixed, and calcified plaque; coronary stenosis ≥ 50%20, 54, 58 | |
| Soluble CD14 | Non-calcified plaque, CIMT and coronary stenosis ≥ 50%53, 54, 58, 59 | Highest quartile sCD14 w/CVD death,60 MI, and stroke44 |
| CD14+CD16+ monocytes | CAC progression61 | |
| CX3CR1/CD16+ monocytes | CIMT62 | |
| CD11b/total monocytes | CIMT62 | |
| CD4+CD38+HLADR+ | CIMT63 | |
| CD8+CD38+HLADR+ | CIMT52, 63 | |
| Low CD4 count | CIMT64 | MI12, 47 |
| Low nadir CD4 count | MI46 | |
| Viral Load | CIMT progression65 | MI12, 46 |
CRP = C-reactive protein, IL-6 = interleukin-6, sTNFR = soluble tumor necrosis factor receptor, MCP-1 = monocyte chemoattractant protein-1, Lp-PLA2 = lipoprotein phospholipase-A2, LDL = low density lipoprotein, HDL = high density lipoprotein, CIMT = carotid intima media thickness, CAC = coronary artery calcium, MI = myocardial infarction, CVD = cardiovascular
T-cells
In 2003, Hunt et al reported that cART treated subjects with viral suppression had a lower median percentage of activated T-cells compared to untreated patients, which remained higher when compared to uninfected controls.66 As discussed above, the SMART trial revealed that antiretroviral therapy interruption may increase the risk of CVD, and in a separate study, Tebas et al. showed that treatment interruption also results in increased levels of T-cell activation.67 However, whether T-cell activation leads to the increased risk of MI remains uncertain. In cross-sectional studies, activated T-cells have been associated with CIMT.52, 63 On the other hand, Tenorio et al reported no relationship between T-cell activation and non-AIDS defining events including myocardial infarction.44 Thus, further research is needed to determine whether T-cell activation leads to an increased risk of MI in HIV-infected patients.
Monocytes/Macrophages
In humans, monocyte subsets have been classified as classical, intermediate/inflammatory, and nonclassical/patrolling based on their expression of CD14 and CD16. Some of these subsets are thought to be “proatherogenic.” In the general population, intermediate monocytes independently predict cardiovascular events,68 and patients with acute coronary syndrome have increased percentages of intermediate and nonclassical monocytes with elevated tissue factor expression, a procoagulant molecule. This expression profile has also been shown to exist in patients with HIV,69 suggesting that HIV infection results in an environment with more proatherogenic monocytes, which in turn increases the risk of CHD. Furthermore, soluble CD14 (sCD14), a monocyte/macrophage activation marker that is elevated in HIV-infected patients, is associated with subclinical atherosclerosis and clinical CVD events and independently predicts all-cause mortality in HIV-infected subjects.53, 54, 59, 60 Additionally, soluble CD163 (sCD163), another marker of monocyte/macrophage activation that is elevated with HIV infection, has been associated with arterial inflammation on FDG-PET/CT and subclinical atherosclerosis on cCTA in HIV-infected subjects.20, 54, 58, 70 These studies implicate a role for monocyte/macrophage activation in the development of CVD in HIV-infected patients.
HIV infection may accelerate atherosclerotic development not only through monocyte/macrophage activation but through macrophage dysfunction as well. Removal of cholesterol from macrophages to high-density lipoprotein (HDL) is known as reverse cholesterol transport and is thought to protect against plaque development. One of the initial steps in this process involves the cholesterol efflux capacity (CEC) of macrophages. In vitro experiments have shown that the HIV protein, Nef, can impair CEC by down-regulating ATP-binding cassette transporter A1 (ABCA-1).71 HIV-infected patients have reduced ABCA-1 dependent plasma CEC compared to uninfected controls 72 and initiation of cART restores CEC.73
Continued Immune Activation in cART-treated HIV-infected patients
There are multiple possible explanations for the increased immune activation in HIV despite treatment with cART, including co-infection, low-levels of ongoing viral replication, and microbial translocation. For example, hepatitis C co-infection has been associated with elevated sCD163 levels and an increased risk of MI74, 75 whereas low-levels of ongoing HIV viral replication have been associated with increased levels of sCD163 and T-cell activation.66, 74 Furthermore, microbial translocation across the gastrointestinal system has become an active area of investigation as another source of continued inflammation in patients with HIV. Brenchley et al demonstrated that HIV-infected patients have elevated levels of lipopolysaccharide (LPS), a component of gram-negative bacteria, that likely originated from translocation across the gastrointestinal system. Initiation of cART resulted in a decline in LPS levels that remained elevated compared to uninfected controls.76 LPS levels in HIV-infected patients on cART have been associated with progression of cIMT,53 suggesting a potential relationship between microbial translocation and atherosclerosis. In addition, Srinivasa et al demonstrated a relationship between trimethylamine, a product of gastrointestinal bacterial metabolism of certain nutrients, and coronary plaque burden on cCTA in patients with HIV.77 Together, these data suggest that HIV-infected patients have ongoing translocation of microbial products across a “leaky” gastrointestinal system despite cART, resulting in chronic immune activation and promotion of atherosclerosis.
Lessons from Elite Controller Data
Studies from elite controllers, a rare subset of the HIV-infected population that maintains viral control without cART, have shed light on the role of cART and persistent immune activation with respect to CVD in HIV. Two studies evaluating elite controllers have reported a higher prevalence of CIMT and coronary plaque on cCTA compared to uninfected controls along with elevated levels of C-reactive protein, sCD14, and sCD163.78, 79 Collectively, these studies suggest that even HIV-infected individuals who have never been exposed to cART may have accelerated atherosclerosis, which is likely a result of heightened immune activation and cannot be attributed to any cardiometabolic effects of cART.
Immune Activation and Unique Plaque Characteristics in Patients with HIV
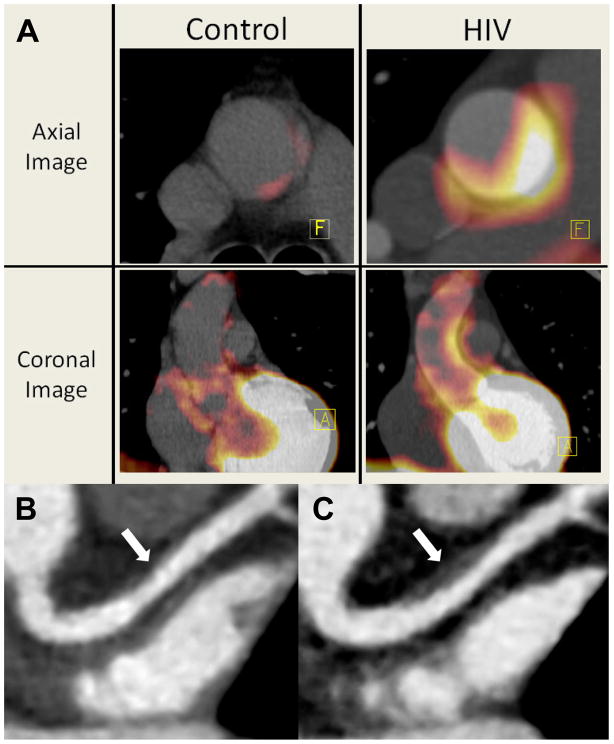
Cardiac imaging has provided a non-invasive tool to study the progression and morphology of atherosclerosis. Utilizing modalities such as cCTA and FDG-PET/CT, immune activation has been associated with coronary plaque that possesses characteristics suggesting vulnerability to rupture among HIV-infected patients. FDG-PET/CT can measure FDG-uptake in arterial walls as a tool to estimate arterial inflammation, which has been associated with risk of incident CVD in the general population.80 Distribution of FDG within coronary plaques has been shown to be predominantly in macrophages between the lipid core and fibrous cap and associated with areas of atherosclerosis.81 In a cross-sectional study of HIV-infected patients on cART without known CVD, Subramanian et al showed increased arterial inflammation measured by FDG uptake compared to uninfected controls matched by Framingham Risk score (see Figure 3A). They reported that the level of arterial inflammation equaled uninfected subjects with known atherosclerosis and was associated with sCD163.70 cCTA has also demonstrated increased rates of subclinical atherosclerosis among HIV-infected patients, especially prevalence of non-calcified plaques, which may be more prone to rupture (see Figure 3B and 3C).18, 19 In non-HIV studies, high risk plaques are often non-calcified and associated with four vulnerability features on cCTA—low attenuation, napkin ring sign, positive remodeling, and spotty calcification—and these characteristics along with plaque volume have been associated with culprit lesions in acute coronary syndrome.82 In HIV-infected patients, low attenuation plaques and positively remodeled plaques are increased when compared to HIV-negative controls matched for CVD risk factors.20 These vulnerability features in patients with HIV have been associated with increased arterial inflammation on FDG-PET/CT as well.83 These data taken together suggest that chronic immune activation among HIV-infected subjects may contribute to accelerated formation of high risk, inflamed plaque that is vulnerable to rupture.
Figure 3. FDG/PET and Coronary Computed Tomography Angiography Images of Arterial Inflammation and Non-Calcified Plaque Progression in HIV patients.
3A: Representative axial and coronal images of the aorta on FDG-PET. There is increased aortic PET-FDG uptake (red coloration) in an HIV-infected subject compared with a HIV-uninfected Framingham Risk score-matched control subject. A=Anterior-Posterior orientation, F=Foot-Head orientation. Figure is reproduced from Subramanian et al70 by permission of JAMA. 3B and 3C: Coronary computed tomography angiography images of the left anterior descending coronary artery of an HIV-infected individual at baseline (panel B) and at 12 months (panel C). Plaque volume increased from 11 to 124 mm3 (arrows) with high risk morphology features of low attenuation lipid core and positive remodeling at 12 months. Figure is reproduced from Lo et al120 by permission of Lancet HIV.
Management
Current Challenges
One of the primary issues concerning prevention of heart disease in HIV-infected individuals is first creating a method to adequately identify HIV-infected patients at risk for CVD who would benefit from primary prevention. Some studies suggest that traditional risk scores may underestimate the risk of CVD accurately in this patient population.84 Zanni et al reported that based on the 2013 ACC/AHA cholesterol guidelines, more HIV-infected patients with high risk morphology plaque on cCTA would receive statins compared to the 2004 ATP III guidelines, but 74% would still not be prescribed a statin.85 Furthermore, Metkus et al reported that even when the CAC score is zero, patients with HIV have more non-calcified plaque compared to uninfected controls independent of traditional risk factors, suggesting that the CAC score may underestimate plaque burden in this population.86 Traditional methods of screening for CVD may underestimate patients at risk because they do not take into account the non-traditional risk factors involved in the development of atherosclerosis. HIV-specific risk calculators have been created87 but require further corroboration with additional cohorts. Importantly, prospective studies are needed to assess the performance of the prediction calculators embedded in the new 2103 ACC/AHA guidelines, developed for the general population, to determine if they accurately predict CVD in HIV.
In light of the START trial mentioned above,49 an increased proportion of HIV-infected patients will likely be placed on cART regimens sooner in their clinical course. The impact of early initiation of cART on cardiovascular disease, however, remains uncertain and will require additional investigation, although continued follow-up of this cohort may provide an answer. Furthermore, the safety and efficacy of current treatment strategies used in the general population for prevention of CVD require validation in this distinct population as novel factors such as drug interactions and unique atherosclerotic mechanisms may play critical roles. As discussed above, therapies that target immune activation may provide additional benefit in treating CVD but additional research will be required to avoid detrimental immune suppression in this population at risk of immunodeficiency.
Managing Traditional CVD risk factors
The Infectious Disease Society of America and the European AIDS Clinical Society have produced guidelines for monitoring traditional CVD risk factors including modifiable risk factors such as smoking, hypertension, diabetes, and dyslipidemia.88, 89 Some evidence suggests that identification and treatment of traditional CVD risk factors may have a profound impact on the risk of MI in patients with HIV. In a large, retrospective study of HIV patients in the Kaiser Permanente health care system in California, Klein et al reported a declining risk of MI over time in HIV-infected patients in conjunction with increased prescriptions for lipid lowering and hypertensive treatment and better control of HIV disease with cART. Importantly, the risk of MI was equivalent between HIV-infected and uninfected individuals in the most recent year of the study (2010–2011), during which time HIV-infected patients had a lower Framingham Risk score compared with HIV-negative controls.90 These findings require validation in other cohorts but suggest implementation of current therapies could have a substantial benefit in reducing CHD in people living with HIV.
Smoking
Smoking cessation has a profound impact in reducing CVD in patients with HIV. Current estimates suggest that smoking cessation could lead to a 42% decrease in MI in HIV-infected patients.31 In the D:A:D study cohort, the incidence rate ratios for cardiovascular events declined from 2·32 (95% CI 1·69–3·18) within one year following smoking cessation to 1·49 (95% CI 0·99–2·44) after three or more years compared to those who never smoked.91 Furthermore, smoking cessation could also reduce mortality as in one study, HIV current smokers had a CVD-related mortality rate ratio (MRR) of 8·82 (95% CI 1·15–67.8) whereas previous smokers had an MRR of 4·55 (95%CI 0·55–37·6) compared to never smokers.92 Thus, developing successful management strategies to help HIV-infected patients to quit smoking is important in reducing CVD and mortality in this patient population. Studies are currently underway to identify effective strategies to help patients to quit smoking93 but additional, larger trials are needed to determine safety and efficacy of current therapies for smoking cessation in HIV-infected patients.
Lipodystrophy
Lipodystrophy refers to the development of central lipohypertrophy, especially of visceral adipose tissue and the dorsocervical fat pad, as well as peripheral lipoatrophy of subcutaneous fat of the face and extremities. Risk factors for developing lipodystrophy include increasing age and cumulative cART exposure, particular PIs and thymidine NRTIs such as zidovudine and stavudine.94, 95 Lipodystrophy may predispose patients to adverse metabolic consequences such as insulin resistance, dyslipidemia, and coronary artery disease, partly due to accumulation of harmful fat depots such as visceral and epicardial adipose tissue.96–99 Management of lipodystrophy primarily consists of avoiding offending cART regimens, especially as more recently developed anti-retroviral therapies appear to be less likely to promote lipodystrophy. In addition, tesamorelin, a synthetic growth-hormone releasing hormone analogue, may also be used as it has been shown to reduce visceral adiposity and liver fat and improve lipids and quality of life in patients with HIV,100, 101 but an effect on CVD has yet to be determined. Tesamorelin is currently approved in the United States and Canada for treatment of abdominal fat accumulation in HIV lipodystrophy.
Diabetes, Hypertension, and Dyslipidemia
Currently, there have been no large, randomized, prospective clinical trials in HIV-infected patients investigating potential benefit of specific therapies for traditional risk factors on CVD-related clinical events. As a result, there are no HIV-specific guidelines regarding treatment of these traditional risk factors, and general guidelines are often applied to the HIV-infected population while taking potential drug interactions with cART medications into account.
Nonetheless, important information has been gleaned from randomized controlled trials assessing treatments for insulin resistance in HIV. Metformin, for example, has been shown to improve insulin resistance in most studies of HIV-infected patients with positive effects on some related fibrinolytic inflammatory markers, including plasminogen activator inhibitor-1 and tissue type plasminogen activator, and may be useful for HIV-infected patients with increased glucose.102, 103 Glitazones have also been shown to improve glucose in HIV, but the use of this class is controversial with respect to CVD.104 Although management of hyperglycemia is largely the same as in the general population, it is important to note that hemoglobin A1c may underestimate fasting glucose in HIV-infected patients.105
Large randomized trials of antihypertensive therapy have not been performed among HIV-infected patients, but initial studies suggest increased activation of the renin-angiotensin-aldosterone system in HIV, which may be a target for therapy in this population with potential effects on blood pressure, insulin, and inflammation.106 Exercise and lifestyle modification may also have salutary effects on traditional metabolic parameters, such as glucose and blood pressure, in HIV and should be considered first when advising on CVD prevention.107
Numerous studies have shown effects of lipid lowering agents such as fibrates, niacin, and fish oil to reduce triglycerides,108, 109 but a link to reduction in CVD events has not been definitively established in HIV. Given the unique pathophysiology of CVD in patients with HIV involving heightened immune activation, strategies that affect traditional and non-traditional, inflammatory pathways may have a comparative advantage in decreasing CVD-risk in this patient population. Statins in particular are of great interest as they are considered to also have immunomodulatory effects that could provide additional benefit in managing CVD in patients with HIV. In the general population, newer guidelines also focus on the use of statins, as the most relevant strategy to reduce CVD events.110
Statins are well known for their ability to lower cholesterol levels, especially low density lipoprotein (LDL), and for their efficacy in primary and secondary prevention of CVD in the general population. Statins also possess anti-inflammatory properties that may contribute to their cardioprotective benefits. In 2008, the JUPITER trial reported results of a randomized placebo-controlled clinical trial of rosuvastatin in non-HIV-infected patients with LDL levels of less than 3·37 mmol/L but with hs-CRP levels of greater than 2 mg/L, indicating heightened inflammation. They found that statin therapy reduced cardiovascular events with a hazard ratio of 0·56 (95% CI 0·46–0·69) alongside reductions in hs-CRP and LDL.111 In comparison, a meta-analysis that primarily reviewed statin trials using LDL cholesterol criteria for enrollment reported about a 20% reduction in vascular events for every 1 mmol/L reduction in LDL,112 an effect that would have predicted a 25% lowering of events in the JUPITER trial. The observed effect, however, was almost doubled the predicted effect. Since the trial primarily enrolled patients based on elevated hs-CRP rather than elevated LDL, these data suggest a potential anti-inflammatory action of statins added to cardioprotection in a cohort with heightened inflammation. Nonetheless, the degree to which statins may decrease CVD through traditional and anti-inflammatory actions remains unknown and an important focus of current research.
Statin therapy may also be effective in reducing immune activation in the HIV population. Statins have been shown to lower sCD14 levels, decrease nonclassical monocytes and tissue factor expression, reduce macrophage derived phospholipase Lp-PLA2, and decrease T-cell activation in patients with HIV.113 Thus, statin therapy improves several markers of immune activation in HIV-infected patients, which in turn have been associated with subclinical atherosclerosis and CVD events, suggesting statins may also be efficacious in preventing CVD in this patient population.
Table 2 lists several trials that have investigated the effects of statins on mortality and atherosclerosis in HIV-infected patients. Statin therapy has been shown to decrease all-cause mortality in a non-randomized study of HIV-infected individuals.116 Lo et al also reported findings from a 12-month randomized controlled trial of atorvastatin in patients with HIV on cART. They showed significant reductions in the statin treated arm in total plaque volume, non-calcified plaque volume, low attenuation plaque, and positively remodeled plaque.120 Furthermore, in a non-randomized, observational study, 24 months of rosuvastatin therapy reduced cIMT,117 and a randomized, placebo-controlled trial with rosuvastatin for 96 weeks showed reduced progression in cIMT.121 Despite these promising results, however, all trials to date have either been small, retrospective, or performed with nonclinical endpoints (i.e., subclinical atherosclerosis). Thus, a large randomized trial is needed to definitely assess whether statins lower mortality and protect against CVD in HIV-infected patients. As a result, the multi-center, randomized, placebo-controlled REPRIEVE trial has been initiated to determine potential benefits of statin therapy in preventing cardiovascular disease in HIV-infected individuals.124 This large 6,500 person trial will assess effects on major adverse cardiovascular events (MACE) and will include a mechanistic study to also assess effects on plaque and immune function.
Table 2.
Studies investigating impact of statins on mortality and/or cardiovascular disease in HIV-infected individuals
| Study | Population | Design | N | Duration | Results |
|---|---|---|---|---|---|
| Stein et al114 | HIV pts on stable ART w a PI for 3 months, LDL > 3·36 mmol/L, and either TG > 3·38 mmol/L or HDL < 1·07 mmol/L | Placebo-controlled, double-blind, cross-over study of pravastatin 40mg | 20 | 8 weeks on treatment-placebo + 8 weeks on placebo-treatment | Flow mediated dilation tended to increase with pravastatin (0.7% ± 0.6%, p = 0.08) |
| Boccara et al115 | HIV pts on cART for 12 mos, LDL > 4·14 mmol/L, one additional vascular risk factor | Case-control study of pravastatin group versus group without lipid treatment | 84 (42 in each group) | Pravastatin group treated for at least 12 months | No difference in CCA-CIMT between groups |
| Moore et al116 | HIV pts initiating cART and achieving virologic suppression within 6 months with maintenance of virologic suppression | Analysis of the Johns Hopkins HIV Clinical Cohort for statin use and mortality | 1538 (238 with statin use) | Median time to censoring 570 days (IQR: 268–1286 days) | Statin use had relative hazard ratio of 0·33 [95% CI 0·14–0·76] for all-cause mortality. CVD-mortality not different between statin users and non-statin users but small number of deaths in statin users (7 of 85 total) and 15 deaths with unknown cause |
| Calza et al117 | HIV pts w/asymptomatic carotid atherosclerosis and hypercholesterolemia | Observational study of pts starting rosuvastatin 10mg | 36 | 24 months | Significant decreases of mean CIMT from baseline |
| Rasmussen et al118 | HIV pts initiating cART and achieving virologic suppression within 6 months | Danish nationwide population based cohort study of statin use and mortality | 1738 (169 with statin use) | 7,952 person-years of follow-up | With censoring for virologic failure, the adjusted mortality rate ratio for statin use was 0·75 [95%CI 0·33–1·68] and 0·34 [95%CI 0·11–1·04] after a diagnosis of CVD, CKD, or DM. |
| Overton et al119 | HIV pts from the ALLRT cohort not on a statin at baseline | Analysis of the ALLRT data for statin use and non-AIDS events | 3601 (484 initiated a statin during observation) | 15,135 person-years of follow-up | Statin use with adjusted hazard ratio of 0·81 [95%CI 0·53–1·24] for non-AIDS event and 0·89 [95%CI 0·32–2·44] for CVD event |
| Lo et al120 | HIV pts on cART w/arterial inflammation, subclinical atherosclerosis, and LDL < 3·36 mmol/L | Randomized to atorvastatin versus placebo | 40 (19 in atorvastatin arm) | 12 months | Significant reductions in total plaque volume, non-calcified plaque volume, low attenuation plaque, and positively remodeled plaque in atorvastatin group compared to increases in placebo |
| McComsey et al121 | HIV pts on cART for 6 months with LDL < 3·36 mmol/L and either hsCRP > 2 mg/L or CD8+CD38+HLADR+ > 19% | Randomized clinical trial of rosuvastatin 10mg versus placebo | 147 (72 in statin group) | 96 weeks | Significant increase in mean CCA CIMT in placebo group versus no change in treatment group with larger difference between groups in subset with baseline CAC |
| Lang et al122 | HIV pts from control group of the FHDH-ANRS CO4 Cohort | Case-control study for statin use and 7-year all-cause mortality | 1776 (138 on statin) | Median follow-up of 53 months | Statin use with estimated hazard ratio of 0·86 [95% CI 0·34–2·19] on all-cause mortality |
| Krsak et al123 | HIV pts on cART at anytime during follow-up from Nutrition for Healthy Living Cohort | Retrospective analysis for statin use and MI, stroke, and all-cause mortality composite | 438 (67 on statin) | Mean follow-up time 275 weeks in non-statin group and 411 weeks in statin group | Hazard ratio for composite 0·93 [95% CI 0·65–1·32] in statin duration model and 1·26 [95% CI 0·57–2·79] in statin history model |
cART = combined antiretroviral therapy, LDL = low density lipoprotein, CCA = common carotid artery, CIMT = carotid intima media thickness, CVD = cardiovascular disease, CKD = chronic kidney disease, DM = diabetes mellitus, hsCRP = high sensitivity C-reactive protein, CAC = coronary artery calcium
There are potential concerns regarding the safety of statins in this patient population given drug interactions with certain cART regimens. Fortunately, data thus far suggest statin use is generally safe. Statins have no significant effect on CD4 T-cell counts and viral load.125 In regards to drug-drug interactions with cART, some PIs inhibit while certain NNRTIs induce cytochrome P450 CYP3A4. Thus, these medications can interfere with specific statins such as simvastatin, which is contraindicated with PI use, and to a lesser extent, atorvastatin. Randomized trials in patients with HIV and a large retrospective study comparing lipid lowering therapy in HIV-infected versus HIV-uninfected patients showed that statins in general led to few, if any, serious adverse events.120, 126–128 Furthermore, some newer statins that are not metabolized by cytochrome P450 CYP3A4 appear to have minimal to no drug-drug interactions such as pitavastatin. In fact, pitavastatin is primarily metabolized by glucuronidation and pharmacokinetic studies suggest minimal interactions with PIs or NNRTIs.129 The safety of pitavastatin in this patient population will also be further evaluated in the REPRIEVE trial.
Immune Activation
Given the emerging role of immune activation in CVD in this patient population, several investigators are currently exploring therapies targeting inflammation or suspected mechanisms of continued immune activation such as microbial translocation. Sevelamer, a phosphate lowering medication, has been shown to reduce markers of inflammation and microbial translocation in non-HIV patients with renal insufficiency130 but did not do so in an 8-week trial in untreated HIV-patients.131 There were reductions in other factors related to CVD such as D-dimer, LDL, and oxidized LDL, and thus, further studies with sevelamer may show a cardioprotective effect. Additionally, in a 12-week randomized placebo-controlled trial in HIV-infected patients on cART, investigators found that probiotics led to a decrease in LPS-binding protein levels,132 suggesting that probiotics may decrease microbial translocation. Additional studies, however, are needed to further explore potential benefits of sevelamer, probiotics, and other therapeutic strategies in reducing atherosclerosis.
Investigators are also exploring a possible role of anti-inflammatory agents in HIV with the hope of controlling immune activation without losing viremic control. For example, methotrexate is currently being studied at low doses for safety and efficacy in reducing inflammation in cART treated HIV-infected patients.133 In addition, certain anti-viral medications such as CCR5 antagonists may possess therapeutic benefits specific for atherosclerosis in patients with HIV. CCR5 is a chemokine receptor on macrophages, T-cells, and other immune cells. A study comparing HIV-infected patients classified as atherosclerotic progressors versus non-progressors based on changes in CIMT over 2 years reported a significant association between progression and CCR5 mRNA expression in leukocytes.134 Administration of a CCR5 antagonist to ritonavir-treated, atherosclerotic-prone mice led to inhibition of atherosclerotic progression by preventing macrophage infiltration into plaques.135 Studies in humans involving CCR5 antagonists on cardiovascular endpoints, however, are still needed as randomized trials have reported differing results regarding the effects of maraviroc, a CCR5 antagonist, on reducing immune activation.136, 137
Conclusion
As HIV-infected patients live longer on cART, the impact of CVD on morbidity and mortality will likely worsen unless effective management strategies are developed. Currently, HIV-infected individuals have an increased risk of cardiovascular disease, especially CHD, compared to uninfected controls. An increased prevalence of traditional risk factors is seen among HIV patients. Studies also suggest that non-traditional, HIV-specific risk factors may play a critical role. Although older cART regimens may result in more adverse metabolic effects and may contribute to the risk of CVD, early and continuous use of modern cART regimens may help minimize the risk of MI by maintaining viral suppression and decreasing immune activation. Nonetheless, data suggests that immune activation persists to a degree among HIV-infected patients on cART, potentially contributing to accelerated and vulnerable coronary atherosclerotic plaque morphology more prone to rupture and resulting MI. A comprehensive management strategy to prevent CVD in HIV will likely require addressing traditional risk factors as well as non-traditional, HIV-specific mechanisms such as immune activation and inflammation.
Search Strategy and Selection Criteria.
We searched the authors’ personal files in addition to Pubmed and Google Scholar using the search term “HIV” along with one or several of the following terms: atherosclerosis, subclinical atherosclerosis, cardiovascular disease, coronary heart disease, myocardial infarction, acute coronary syndrome, metabolic, traditional risk factors, smoking, hypertension, blood pressure, diabetes, insulin resistance, dyslipidemia, lipodystrophy, reverse cholesterol transport, cholesterol efflux capacity, lipids, antiretroviral therapy, ART initiation, ART interruption, delayed ART, early ART, ART naive, ART treated, protease inhibitor, nucleoside reverse transcriptase inhibitor, non-nucleoside reverse transcriptase inhibitor, entry inhibitor, fusion inhibitor, integrase inhibitor, immune activation, inflammation, biomarkers, monocytes, macrophages, T-cells, co-infection, microbial translocation, viral replication, coronary computed tomography angiography, coronary artery calcium, carotid intima-media thickness, carotid ultrasound, non-calcified plaque, calcified plaque, mixed plaque, vulnerable plaque, high-risk plaque, low attenuation plaque, positively remodeled plaque, spotty calcified plaque, napkin ring sign, carotid intima-media thickness, flow-mediated dilation, FDG-PET, FDG, arterial inflammation, cardiovascular risk prediction, cardiovascular risk calculators, smoking cessation, quit smoking, metformin, glitazones, lifestyle modification, exercise, diet, renin, angiotensin, aldosterone, statins, tesamorelin, CCR5 antagonists, methotrexate, probiotics, sevelamer. There were no date restrictions. Full-text, original articles and reviews resulting from these searches and relevant references cited in those articles were reviewed. UNAIDS and WHO were also reviewed for relevant HIV global statistics. Relevant abstracts from the 2015 and 2014 Conference on Retroviruses and Opportunistic Infections were also included. We also searched ClinicalTrials.gov for ongoing clinical trials of HIV and immune activation or cardiovascular disease.
Footnotes
Contributors
EN did the initial literature review and wrote the first draft of the manuscript. JL, CH, and SKG assisted with additional literature review and revised the manuscript.
Declaration of Interests
There was no role of any funding source in the creation of this review article. SKG has consulted for BMS, Merck, Navidea, Theratechnologies, Gilead, NovoNordisk, AstraZeneca, Aileron, received speaker fees from Takeda, and research funding from BMS, Gilead, Amgen, Navidea, Kowa Pharmaceuticals and Theratechnologies over the past 3 years, all unrelated to this manuscript. EN, JL, and CH have no competing interests. This work was supported in part by the National Institute of Allergies and Infectious Diseases Intramural Program. SKG is a co-investigator for the currently enrolling REPRIEVE trial.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. HIV/AIDS: Data and Statistics [Online] 2014 [cited 2015 August 3rd]; Available from: http://www.who.int/hiv/data/en/
- 2.Miller CJ, Baker JV, Bormann AM, Erlandson KM, Huppler Hullsiek K, Justice AC, et al. Adjudicated Morbidity and Mortality Outcomes by Age among Individuals with HIV Infection on Suppressive Antiretroviral Therapy. PLoS One. 2014;9(4):e95061. doi: 10.1371/journal.pone.0095061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem A, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15(7):810–8. doi: 10.1016/S1473-3099(15)00056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morlat P, Roussillon C, Henard S, Salmon D, Bonnet F, Cacoub P, et al. Causes of death among HIV-infected patients in France in 2010 (national survey): trends since 2000. AIDS. 2014;28(8):1181–91. doi: 10.1097/QAD.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 5.Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53(11):1130–9. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 6.Klein D, Hurley LB, Quesenberry CP, Jr, Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr. 2002;30(5):471–7. doi: 10.1097/00126334-200208150-00002. [DOI] [PubMed] [Google Scholar]
- 7.Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33(4):506–12. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 8.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sorensen HT, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis. 2007;44(12):1625–31. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 10.Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24(8):1228–30. doi: 10.1097/QAD.0b013e328339192f. [DOI] [PubMed] [Google Scholar]
- 11.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec’s public health insurance database. J Acquir Immune Defic Syndr. 2011;57(3):245–53. doi: 10.1097/QAI.0b013e31821d33a5. [DOI] [PubMed] [Google Scholar]
- 12.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paisible AL, Chang CC, So-Armah KA, Butt AA, Leaf DA, Budoff M, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68(2):209–16. doi: 10.1097/QAI.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanni MV, Schouten J, Grinspoon SK, Reiss P. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol. 2014;11(12):728–41. doi: 10.1038/nrcardio.2014.167. [DOI] [PubMed] [Google Scholar]
- 15.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Sr, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hulten E, Mitchell J, Scally J, Gibbs B, Villines TC. HIV positivity, protease inhibitor exposure and subclinical atherosclerosis: a systematic review and meta-analysis of observational studies. Heart. 2009;95(22):1826–35. doi: 10.1136/hrt.2009.177774. [DOI] [PubMed] [Google Scholar]
- 17.Grunfeld C, Delaney JA, Wanke C, Currier JS, Scherzer R, Biggs ML, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23(14):1841–9. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24(2):243–53. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Post WSBM, Kingsley L, Palella FJ, Witt MD, Xiuhong Li, George RT, Brown TT, Jacobson LP. Associations Between HIV Infection and Subclinical Coronary Atherosclerosis Associations Between HIV Infection and Subclinical Coronary Atherosclerosis. Ann Intern Med. 2014;160(7):458–67. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanni MV, Abbara S, Lo J, Wai B, Hark D, Marmarelis E, et al. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS. 2013;27(8):1263–72. doi: 10.1097/QAD.0b013e32835eca9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motoyama S, Sarai M, Narula J, Ozaki Y. Coronary CT angiography and high-risk plaque morphology. Cardiovasc Interv Ther. 2013;28(1):1–8. doi: 10.1007/s12928-012-0140-1. [DOI] [PubMed] [Google Scholar]
- 22.Soliman EZ, Sharma S, Arasteh K, Wohl D, Achhra A, Tambussi G, et al. Baseline cardiovascular risk in the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med. 2015;16(Suppl 1):46–54. doi: 10.1111/hiv.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tesoriero JM, Gieryic SM, Carrascal A, Lavigne HE. Smoking among HIV positive New Yorkers: prevalence, frequency, and opportunities for cessation. AIDS Behav. 2010;14(4):824–35. doi: 10.1007/s10461-008-9449-2. [DOI] [PubMed] [Google Scholar]
- 24.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165(10):1179–84. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 25.Butt AA, McGinnis K, Rodriguez-Barradas MC, Crystal S, Simberkoff M, Goetz MB, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23(10):1227–34. doi: 10.1097/QAD.0b013e32832bd7af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galli L, Salpietro S, Pellicciotta G, Galliani A, Piatti P, Hasson H, et al. Risk of type 2 diabetes among HIV-infected and healthy subjects in Italy. Eur J Epidemiol. 2012;27(8):657–65. doi: 10.1007/s10654-012-9707-5. [DOI] [PubMed] [Google Scholar]
- 27.De Wit S, Sabin CA, Weber R, Worm SW, Reiss P, Cazanave C, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care. 2008;31(6):1224–9. doi: 10.2337/dc07-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calza L, Masetti G, Piergentili B, Trapani F, Cascavilla A, Manfredi R, et al. Prevalence of diabetes mellitus, hyperinsulinaemia and metabolic syndrome among 755 adult patients with HIV-1 infection. Int J STD AIDS. 2011;22(1):43–5. doi: 10.1258/ijsa.2010.010256. [DOI] [PubMed] [Google Scholar]
- 29.Worm SW, Kamara DA, Reiss P, Kirk O, El-Sadr W, Fux C, et al. Elevated triglycerides and risk of myocardial infarction in HIV-positive persons. AIDS. 2011;25(12):1497–504. doi: 10.1097/QAD.0b013e32834917c6. [DOI] [PubMed] [Google Scholar]
- 30.Worm SW, De Wit S, Weber R, Sabin CA, Reiss P, El-Sadr W, et al. Diabetes mellitus, preexisting coronary heart disease, and the risk of subsequent coronary heart disease events in patients infected with human immunodeficiency virus: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D Study) Circulation. 2009;119(6):805–11. doi: 10.1161/CIRCULATIONAHA.108.790857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasmussen LD, Helleberg M, May MT, Afzal S, Kronborg G, Larsen CS, et al. Myocardial infarction among Danish HIV-infected individuals: population-attributable fractions associated with smoking. Clin Infect Dis. 2015;60(9):1415–23. doi: 10.1093/cid/civ013. [DOI] [PubMed] [Google Scholar]
- 32.Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356(17):1723–35. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 33.Worm SW, Sabin C, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201(3):318–30. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 34.Monforte A, Reiss P, Ryom L, El-Sadr W, Dabis F, De Wit S, et al. Atazanavir is not associated with an increased risk of cardio- or cerebrovascular disease events. AIDS. 2013;27(3):407–15. doi: 10.1097/QAD.0b013e32835b2ef1. [DOI] [PubMed] [Google Scholar]
- 35.Stein JH, Ribaudo HJ, Hodis HN, Brown TT, Tran TT, Yan M, et al. A prospective, randomized clinical trial of antiretroviral therapies on carotid wall thickness. AIDS. 2015;29(14):1775–83. doi: 10.1097/QAD.0000000000000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. AIDS. 2008;22(14):F17–24. doi: 10.1097/QAD.0b013e32830fe35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi AI, Vittinghoff E, Deeks SG, Weekley CC, Li Y, Shlipak MG. Cardiovascular risks associated with abacavir and tenofovir exposure in HIV-infected persons. AIDS. 2011;25(10):1289–98. doi: 10.1097/QAD.0b013e328347fa16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young J, Xiao Y, Moodie EE, Abrahamowicz M, Klein MB, Bernasconi E, et al. Effect of Cumulating Exposure to Abacavir on the Risk of Cardiovascular Disease Events in Patients From the Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2015;69(4):413–21. doi: 10.1097/QAI.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 39.Desai M, Joyce V, Bendavid E, Olshen RA, Hlatky M, Chow A, et al. Risk of Cardiovascular Events Associated With Current Exposure to HIV Antiretroviral Therapies in a US Veteran Population. Clin Infect Dis. 2015;61(3):445–52. doi: 10.1093/cid/civ316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cruciani M, Zanichelli V, Serpelloni G, Bosco O, Malena M, Mazzi R, et al. Abacavir use and cardiovascular disease events: a meta-analysis of published and unpublished data. AIDS. 2011;25(16):1993–2004. doi: 10.1097/QAD.0b013e328349c6ee. [DOI] [PubMed] [Google Scholar]
- 41.Ding X, Andraca-Carrera E, Cooper C, Miele P, Kornegay C, Soukup M, et al. No association of abacavir use with myocardial infarction: findings of an FDA meta-analysis. J Acquir Immune Defic Syndr. 2012;61(4):441–7. doi: 10.1097/QAI.0b013e31826f993c. [DOI] [PubMed] [Google Scholar]
- 42.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 43.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210(8):1248–59. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7(9):e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lang S, Mary-Krause M, Simon A, Partisani M, Gilquin J, Cotte L, et al. HIV replication and immune status are independent predictors of the risk of myocardial infarction in HIV-infected individuals. Clin Infect Dis. 2012;55(4):600–7. doi: 10.1093/cid/cis489. [DOI] [PubMed] [Google Scholar]
- 47.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr. 2010;55(5):615–9. doi: 10.1097/QAI.0b013e3181f4b752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sabin CA, Ryom L, De Wit S, Mocroft A, Phillips AN, Worm SW, et al. Associations between immune depression and cardiovascular events in HIV infection. AIDS. 2013;27(17):2735–48. doi: 10.1097/01.aids.0000432457.91228.f3. [DOI] [PubMed] [Google Scholar]
- 49.INSIGHT-START. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015 doi: 10.1056/NEJMoa1506816. Published Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hileman CO, Longenecker CT, Carman TL, McComsey GA. C-reactive protein predicts 96-week carotid intima media thickness progression in HIV-infected adults naive to antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;65(3):340–4. doi: 10.1097/QAI.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 51.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51(3):268–73. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Longenecker CT, Funderburg NT, Jiang Y, Debanne S, Storer N, Labbato DE, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013;14(6):385–90. doi: 10.1111/hiv.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012;206(10):1558–67. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ, Jr, Kingsley LA, et al. Elevated Levels of Monocyte Activation Markers Are Associated With Subclinical Atherosclerosis in Men With and Those Without HIV Infection. J Infect Dis. 2015;211(8):1219–28. doi: 10.1093/infdis/jiu594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parra S, Coll B, Aragones G, Marsillach J, Beltran R, Rull A, et al. Nonconcordance between subclinical atherosclerosis and the calculated Framingham risk score in HIV-infected patients: relationships with serum markers of oxidation and inflammation. HIV Med. 2010;11(4):225–31. doi: 10.1111/j.1468-1293.2009.00766.x. [DOI] [PubMed] [Google Scholar]
- 56.Ross Eckard A, Longenecker CT, Jiang Y, Debanne SM, Labbato D, Storer N, et al. Lipoprotein-associated phospholipase A2 and cardiovascular disease risk in HIV infection. HIV Med. 2014;15(9):537–46. doi: 10.1111/hiv.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zanni MV, Kelesidis T, Fitzgerald ML, Lo J, Abbara S, Wai B, et al. HDL redox activity is increased in HIV-infected men in association with macrophage activation and non-calcified coronary atherosclerotic plaque. Antivir Ther. 2014;19(8):805–11. doi: 10.3851/IMP2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204(8):1227–36. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Merlini E, Luzi K, Suardi E, Barassi A, Cerrone M, Martinez JS, et al. T-cell phenotypes, apoptosis and inflammation in HIV+ patients on virologically effective cART with early atherosclerosis. PLoS One. 2012;7(9):e46073. doi: 10.1371/journal.pone.0046073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203(6):780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baker JV, Hullsiek KH, Singh A, Wilson E, Henry K, Lichtenstein K, et al. Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS. 2014;28(6):831–40. doi: 10.1097/QAD.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Westhorpe CL, Maisa A, Spelman T, Hoy JF, Dewar EM, Karapanagiotidis S, et al. Associations between surface markers on blood monocytes and carotid atherosclerosis in HIV-positive individuals. Immunol Cell Biol. 2014;92(2):133–8. doi: 10.1038/icb.2013.84. [DOI] [PubMed] [Google Scholar]
- 63.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203(4):452–63. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaplan RC, Kingsley LA, Gange SJ, Benning L, Jacobson LP, Lazar J, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22(13):1615–24. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baker JV, Henry WK, Patel P, Bush TJ, Conley LJ, Mack WJ, et al. Progression of carotid intima-media thickness in a contemporary human immunodeficiency virus cohort. Clin Infect Dis. 2011;53(8):826–35. doi: 10.1093/cid/cir497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187(10):1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 67.Tebas P, Henry WK, Matining R, Weng-Cherng D, Schmitz J, Valdez H, et al. Metabolic and immune activation effects of treatment interruption in chronic HIV-1 infection: implications for cardiovascular risk. PLoS One. 2008;3(4):e2021. doi: 10.1371/journal.pone.0002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, et al. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol. 2012;60(16):1512–20. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 69.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120(23):4599–608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308(4):379–86. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mujawar Z, Rose H, Morrow MP, Pushkarsky T, Dubrovsky L, Mukhamedova N, et al. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol. 2006;4(11):e365. doi: 10.1371/journal.pbio.0040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El Khoury P, Ghislain M, Villard EF, Le Goff W, Lascoux-Combe C, Yeni P, et al. Plasma cholesterol efflux capacity from human THP-1 macrophages is reduced in HIV-infected patients: impact of HAART. J Lipid Res. 2015;56(3):692–702. doi: 10.1194/jlr.M054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lo J, Rosenberg ES, Fitzgerald ML, Bazner SB, Ihenachor EJ, Hawxhurst V, et al. High-density lipoprotein-mediated cholesterol efflux capacity is improved by treatment with antiretroviral therapy in acute human immunodeficiency virus infection. Open Forum Infect Dis. 2014;1(3):ofu108. doi: 10.1093/ofid/ofu108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beltran LM, Munoz Hernandez R, de Pablo Bernal RS, Garcia Morillo JS, Egido J, Noval ML, et al. Reduced sTWEAK and increased sCD163 levels in HIV-infected patients: modulation by antiretroviral treatment, HIV replication and HCV co-infection. PLoS One. 2014;9(3):e90541. doi: 10.1371/journal.pone.0090541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Freiberg MS, Cheng DM, Kraemer KL, Saitz R, Kuller LH, Samet JH. The association between hepatitis C infection and prevalent cardiovascular disease among HIV-infected individuals. AIDS. 2007;21(2):193–7. doi: 10.1097/QAD.0b013e3280118a0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 77.Srinivasa S, Fitch KV, Lo J, Kadar H, Knight R, Wong K, et al. Plaque burden in HIV-infected patients is associated with serum intestinal microbiota-generated trimethylamine. AIDS. 2015;29(4):443–52. doi: 10.1097/QAD.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pereyra F, Lo J, Triant VA, Wei J, Buzon MJ, Fitch KV, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS. 2012;26(18):2409–12. doi: 10.1097/QAD.0b013e32835a9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23(9):1059–67. doi: 10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging. 2013;6(12):1250–9. doi: 10.1016/j.jcmg.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 81.Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105(23):2708–11. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 82.Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol. 2014;11(7):390–402. doi: 10.1038/nrcardio.2014.60. [DOI] [PubMed] [Google Scholar]
- 83.Tawakol A, Lo J, Zanni MV, Marmarelis E, Ihenachor EJ, MacNabb M, et al. Increased Arterial Inflammation Relates to High-Risk Coronary Plaque Morphology in HIV-Infected Patients. J Acquir Immune Defic Syndr. 2014;66(2):164–71. doi: 10.1097/QAI.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.D’Agostino RB., Sr Cardiovascular risk estimation in 2012: lessons learned and applicability to the HIV population. J Infect Dis. 2012;205(Suppl 3):S362–7. doi: 10.1093/infdis/jis196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zanni MV, Fitch KV, Feldpausch M, Han A, Lee H, Lu MT, et al. 2013 American College of Cardiology/American Heart Association and 2004 Adult Treatment Panel III cholesterol guidelines applied to HIV-infected patients with/without subclinical high-risk coronary plaque. AIDS. 2014;28(14):2061–70. doi: 10.1097/QAD.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Metkus TS, Brown T, Budoff M, Kingsley L, Palella FJ, Jr, Witt MD, et al. HIV infection is associated with an increased prevalence of coronary noncalcified plaque among participants with a coronary artery calcium score of zero: Multicenter AIDS Cohort Study (MACS) (Published Online) HIV Med. 2015 doi: 10.1111/hiv.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Friis-Moller N, Ryom L, Smith C, Weber R, Reiss P, Dabis F, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: The Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study (Published Online) Eur J Prev Cardiol. 2015 doi: 10.1177/2047487315579291. [DOI] [PubMed] [Google Scholar]
- 88.Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58(1):1–10. doi: 10.1093/cid/cit757. [DOI] [PubMed] [Google Scholar]
- 89.Jea Lundgren. European AIDS Clinical Society Guidelines [Online] 2014 [cited 2015 August 3rd]; Available from: http://www.eacsociety.org/Portals/0/140601_EACS%20EN7.02.pdf.
- 90.Klein DB, Leyden WA, Xu L, Chao CR, Horberg MA, Towner WJ, et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis. 2015;60(8):1278–80. doi: 10.1093/cid/civ014. [DOI] [PubMed] [Google Scholar]
- 91.Petoumenos K, Worm S, Reiss P, de Wit S, d’Arminio Monforte A, Sabin C, et al. Rates of cardiovascular disease following smoking cessation in patients with HIV infection: results from the D:A:D study(*) HIV Med. 2011;12(7):412–21. doi: 10.1111/j.1468-1293.2010.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Helleberg M, May MT, Ingle SM, Dabis F, Reiss P, Fatkenheuer G, et al. Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS. 2015;29(2):221–9. doi: 10.1097/QAD.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cropsey KL, Jardin BF, Burkholder GA, Clark CB, Raper JL, Saag MS. An Algorithm Approach to Determining Smoking Cessation Treatment for Persons Living With HIV/AIDS: Results of a Pilot Trial. J Acquir Immune Defic Syndr. 2015;69(3):291–8. doi: 10.1097/QAI.0000000000000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carr A, Miller J, Law M, Cooper DA. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS. 2000;14(3):F25–32. doi: 10.1097/00002030-200002180-00001. [DOI] [PubMed] [Google Scholar]
- 95.Thiebaut R, Daucourt V, Mercie P, Ekouevi DK, Malvy D, Morlat P, et al. Lipodystrophy, metabolic disorders, and human immunodeficiency virus infection: Aquitaine Cohort, France, 1999. Groupe d’Epidemiologie Clinique du Syndrome d’Immunodeficience Acquise en Aquitaine. Clin Infect Dis. 2000;31(6):1482–7. doi: 10.1086/317477. [DOI] [PubMed] [Google Scholar]
- 96.Henry K, Melroe H, Huebsch J, Hermundson J, Levine C, Swensen L, et al. Severe premature coronary artery disease with protease inhibitors. Lancet. 1998;351(9112):1328. doi: 10.1016/S0140-6736(05)79053-X. [DOI] [PubMed] [Google Scholar]
- 97.Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12(7):F51–8. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 98.Guaraldi G, Scaglioni R, Zona S, Orlando G, Carli F, Ligabue G, et al. Epicardial adipose tissue is an independent marker of cardiovascular risk in HIV-infected patients. AIDS. 2011;25(9):1199–205. doi: 10.1097/QAD.0b013e3283474b9f. [DOI] [PubMed] [Google Scholar]
- 99.Lo J, Abbara S, Rocha-Filho JA, Shturman L, Wei J, Grinspoon SK. Increased epicardial adipose tissue volume in HIV-infected men and relationships to body composition and metabolic parameters. AIDS. 2010;24(13):2127–30. doi: 10.1097/QAD.0b013e32833c055a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stanley TL, Feldpausch MN, Oh J, Branch KL, Lee H, Torriani M, et al. Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat accumulation: a randomized clinical trial. JAMA. 2014;312(4):380–9. doi: 10.1001/jama.2014.8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Falutz J, Allas S, Blot K, Potvin D, Kotler D, Somero M, et al. Metabolic effects of a growth hormone-releasing factor in patients with HIV. N Engl J Med. 2007;357(23):2359–70. doi: 10.1056/NEJMoa072375. [DOI] [PubMed] [Google Scholar]
- 102.Hadigan C, Corcoran C, Basgoz N, Davis B, Sax P, Grinspoon S. Metformin in the treatment of HIV lipodystrophy syndrome: A randomized controlled trial. JAMA. 2000;284(4):472–7. doi: 10.1001/jama.284.4.472. [DOI] [PubMed] [Google Scholar]
- 103.Hadigan C, Meigs JB, Rabe J, D’Agostino RB, Wilson PW, Lipinska I, et al. Increased PAI-1 and tPA antigen levels are reduced with metformin therapy in HIV-infected patients with fat redistribution and insulin resistance. J Clin Endocrinol Metab. 2001;86(2):939–43. doi: 10.1210/jcem.86.2.7410. [DOI] [PubMed] [Google Scholar]
- 104.van Wijk JP, de Koning EJ, Cabezas MC, op’t Roodt J, Joven J, Rabelink TJ, et al. Comparison of rosiglitazone and metformin for treating HIV lipodystrophy: a randomized trial. Ann Intern Med. 2005;143(5):337–46. doi: 10.7326/0003-4819-143-5-200509060-00009. [DOI] [PubMed] [Google Scholar]
- 105.Slama L, Palella FJ, Jr, Abraham AG, Li X, Vigouroux C, Pialoux G, et al. Inaccuracy of haemoglobin A1c among HIV-infected men: effects of CD4 cell count, antiretroviral therapies and haematological parameters. J Antimicrob Chemother. 2014;69(12):3360–7. doi: 10.1093/jac/dku295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Srinivasa S, Fitch KV, Wong K, Torriani M, Mayhew C, Stanley T, et al. RAAS Activation Is Associated With Visceral Adiposity and Insulin Resistance Among HIV-infected Patients. J Clin Endocrinol Metab. 2015;100(8):2873–82. doi: 10.1210/jc.2015-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fitch K, Abbara S, Lee H, Stavrou E, Sacks R, Michel T, et al. Effects of Lifestyle Modification and Metformin on Atherosclerotic Indices among HIV-Infected Patients with the Metabolic Syndrome. AIDS. 2012;26(5):587–97. doi: 10.1097/QAD.0b013e32834f33cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dube MP, Wu JW, Aberg JA, Deeg MA, Alston-Smith BL, McGovern ME, et al. Safety and efficacy of extended-release niacin for the treatment of dyslipidaemia in patients with HIV infection: AIDS Clinical Trials Group Study A5148. Antivir Ther. 2006;11(8):1081–9. [PMC free article] [PubMed] [Google Scholar]
- 109.Munoz MA, Liu W, Delaney JA, Brown E, Mugavero MJ, Mathews WC, et al. Comparative effectiveness of fish oil versus fenofibrate, gemfibrozil, and atorvastatin on lowering triglyceride levels among HIV-infected patients in routine clinical care. J Acquir Immune Defic Syndr. 2013;64(3):254–60. doi: 10.1097/QAI.0b013e3182a60e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 111.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 112.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Funderburg NT, Jiang Y, Debanne SM, Labbato D, Juchnowski S, Ferrari B, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;68(4):396–404. doi: 10.1097/QAI.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stein JH, Merwood MA, Bellehumeur JL, Aeschlimann SE, Korcarz CE, Underbakke GL, et al. Effects of pravastatin on lipoproteins and endothelial function in patients receiving human immunodeficiency virus protease inhibitors. Am Heart J. 2004;147(4):E18. doi: 10.1016/j.ahj.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 115.Boccara F, Simon T, Lacombe K, Cohen A, Laloux B, Bozec E, et al. Influence of pravastatin on carotid artery structure and function in dyslipidemic HIV-infected patients receiving antiretroviral therapy. AIDS. 2006;20(18):2395–8. doi: 10.1097/QAD.0b013e32801120e3. [DOI] [PubMed] [Google Scholar]
- 116.Moore RD, Bartlett JG, Gallant JE. Association between use of HMG CoA reductase inhibitors and mortality in HIV-infected patients. PLoS One. 2011;6(7):e21843. doi: 10.1371/journal.pone.0021843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Calza L, Manfredi R, Colangeli V, Trapani FF, Salvadori C, Magistrelli E, et al. Two-year treatment with rosuvastatin reduces carotid intima-media thickness in HIV type 1-infected patients receiving highly active antiretroviral therapy with asymptomatic atherosclerosis and moderate cardiovascular risk. AIDS Res Hum Retroviruses. 2013;29(3):547–56. doi: 10.1089/aid.2012.0015. [DOI] [PubMed] [Google Scholar]
- 118.Rasmussen LD, Kronborg G, Larsen CS, Pedersen C, Gerstoft J, Obel N. Statin therapy and mortality in HIV-infected individuals; a Danish nationwide population-based cohort study. PLoS One. 2013;8(3):e52828. doi: 10.1371/journal.pone.0052828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Overton ET, Kitch D, Benson CA, Hunt PW, Stein JH, Smurzynski M, et al. Effect of statin therapy in reducing the risk of serious non-AIDS-defining events and nonaccidental death. Clin Infect Dis. 2013;56(10):1471–9. doi: 10.1093/cid/cit053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. The Lancet HIV. 2015;2(2):e52–e63. doi: 10.1016/S2352-3018(14)00032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McComsey G. Rosuvastatin Arrests Progression of Carotid Intima-Media Thickness in Treated HIV. Presented at Conference on Retroviruses and Opportunistic Infections (CROI); 2015 February 26th; Seattle, WA. 2015. [Google Scholar]
- 122.Lang S, Lacombe JM, Mary-Krause M, Partisani M, Bidegain F, Cotte L, et al. Is Impact of Statin Therapy on All-Cause Mortality Different in HIV-Infected Individuals Compared to General Population? Results from the FHDH-ANRS CO4 Cohort. PLoS One. 2015;10(7):e0133358. doi: 10.1371/journal.pone.0133358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Krsak M, Kent DM, Terrin N, Holcroft C, Skinner SC, Wanke C. Myocardial Infarction, Stroke, and Mortality in cART-Treated HIV Patients on Statins. AIDS Patient Care STDS. 2015;29(6):307–13. doi: 10.1089/apc.2014.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.U.S. National Library of Medicine. Clinicaltrails.gov [online] 2015 [cited 2015 August 13th]; Available from: https://clinicaltrials.gov/ct2/show/NCT02344290.
- 125.Moncunill G, Negredo E, Bosch L, Vilarrasa J, Witvrouw M, Llano A, et al. Evaluation of the anti-HIV activity of statins. AIDS. 2005;19(15):1697–700. doi: 10.1097/01.aids.0000183517.60384.db. [DOI] [PubMed] [Google Scholar]
- 126.Aberg JA, Zackin RA, Brobst SW, Evans SR, Alston BL, Henry WK, et al. A randomized trial of the efficacy and safety of fenofibrate versus pravastatin in HIV-infected subjects with lipid abnormalities: AIDS Clinical Trials Group Study 5087. AIDS Res Hum Retroviruses. 2005;21(9):757–67. doi: 10.1089/aid.2005.21.757. [DOI] [PubMed] [Google Scholar]
- 127.Silverberg MJ, Leyden W, Hurley L, Go AS, Quesenberry CP, Jr, Klein D, et al. Response to newly prescribed lipid-lowering therapy in patients with and without HIV infection. Ann Intern Med. 2009;150(5):301–13. doi: 10.7326/0003-4819-150-5-200903030-00006. [DOI] [PubMed] [Google Scholar]
- 128.Calza L, Manfredi R, Colangeli V, Pocaterra D, Pavoni M, Chiodo F. Rosuvastatin, pravastatin, and atorvastatin for the treatment of hypercholesterolaemia in HIV-infected patients receiving protease inhibitors. Curr HIV Res. 2008;6(6):572–8. doi: 10.2174/157016208786501481. [DOI] [PubMed] [Google Scholar]
- 129.Malvestutto CD, Ma Q, Morse GD, Underberg JA, Aberg JA. Lack of pharmacokinetic interactions between pitavastatin and efavirenz or darunavir/ritonavir. J Acquir Immune Defic Syndr. 2014;67(4):390–6. doi: 10.1097/QAI.0000000000000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, Donate-Correa J, Cazana-Perez V, Garcia-Perez J. Effect of phosphate binders on serum inflammatory profile, soluble CD14, and endotoxin levels in hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(9):2272–9. doi: 10.2215/CJN.01650211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sandler NG, Zhang X, Bosch RJ, Funderburg NT, Choi AI, Robinson JK, et al. Sevelamer does not decrease lipopolysaccharide or soluble CD14 levels but decreases soluble tissue factor, low-density lipoprotein (LDL) cholesterol, and oxidized LDL cholesterol levels in individuals with untreated HIV infection. J Infect Dis. 2014;210(10):1549–54. doi: 10.1093/infdis/jiu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Villar-Garcia J, Hernandez JJ, Guerri-Fernandez R, Gonzalez A, Lerma E, Guelar A, et al. Effect of probiotics (Saccharomyces boulardii) on microbial translocation and inflammation in HIV-treated patients: a double-blind, randomized, placebo-controlled trial. J Acquir Immune Defic Syndr. 2015;68(3):256–63. doi: 10.1097/QAI.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 133.U.S. National Library of Medicine. Clinicaltrials.gov [Online] 2015 [cited 2015 August 4th]; Available from: https://clinicaltrials.gov/ct2/show/NCT01949116.
- 134.Fernandez-Sender L, Alonso-Villaverde C, Rull A, Rodriguez-Gallego E, Riera-Borrull M, Hernandez-Aguilera A, et al. A possible role for CCR5 in the progression of atherosclerosis in HIV-infected patients: a cross-sectional study. AIDS Res Ther. 2013;10(1):11. doi: 10.1186/1742-6405-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cipriani S, Francisci D, Mencarelli A, Renga B, Schiaroli E, D’Amore C, et al. Efficacy of the CCR5 antagonist maraviroc in reducing early, ritonavir-induced atherogenesis and advanced plaque progression in mice. Circulation. 2013;127(21):2114–24. doi: 10.1161/CIRCULATIONAHA.113.001278. [DOI] [PubMed] [Google Scholar]
- 136.Rusconi S, Vitiello P, Adorni F, Colella E, Foca E, Capetti A, et al. Maraviroc as intensification strategy in HIV-1 positive patients with deficient immunological response: an Italian randomized clinical trial. PLoS One. 2013;8(11):e80157. doi: 10.1371/journal.pone.0080157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hunt PW, Shulman NS, Hayes TL, Dahl V, Somsouk M, Funderburg NT, et al. The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial. Blood. 2013;121(23):4635–46. doi: 10.1182/blood-2012-06-436345. [DOI] [PMC free article] [PubMed] [Google Scholar]