Abstract
Objective
To assess in older persons with concomitant health conditions the impact of retinopathy on mortality.
Design
Population-based prospective cohort study.
Participants
4966 individuals aged 67–96 years old (43.2% male) from the Age, Gene/Environment Susceptibility-Reykjavik Study (AGES-RS).
Methods
Retinopathy was evaluated from digital fundus images (2002–2006) using the modified Airlie House adaptation of the Early Treatment Diabetic Retinopathy Study protocol. Mortality was assessed through September 2013 (cause-of-death assigned through 2009). Cox proportional hazards regression models, with age as the time scale, estimated the association between retinopathy and death while controlling for risk factors and the presence of concomitant health conditions.
Main Outcome Measures
Mortality from all-causes and cardiovascular disease (CVD).
Results
Among the 4966 participants, 503 (10.1%) had diabetes and 614 (12.4%) had retinopathy at baseline. A subset of these, 136 (2.7%), had both diabetes and retinopathy. After a median follow-up of 8.6 years, 1763 persons died, 276 (45.0%) with retinopathy and 1487 (34.2%) without retinopathy, of whom 76 and 162 persons, respectively, also had diabetes. There were 366 deaths from CVD through 2009; 72 (11.7%) in persons with retinopathy and 294 (6.8%) without retinopathy. In multivariable analyses, retinopathy was significantly associated with all-cause mortality [Hazard Ratio (HR) 1.26 (95% Confidence Interval (CI): 1.10, 1.43); p<0.01] and CVD-related mortality [HR 1.57 (95% CI: 1.20, 2.06); p<0.01]. Findings were more striking in men: all-cause HR 1.33 (95% CI: 1.11, 1.60) and CVD HR 1.81 (95% CI: 1.25, 2.63). Risk of mortality was further increased among those with retinopathy concomitant with microalbuminuria [all-cause HR 1.70 (95% CI: 1.03, 2.23) and CVD HR 2.04 (95% CI: 1.27, 3.28)] and those with retinopathy, microalbuminuria and diabetes [all-cause HR 2.01 (95% CI: 1.22, 3.31) and CVD HR 5.24 (95% CI: 1.91, 14.42)]. History of clinical stroke increased the risk of CVD-related mortality among persons with retinopathy [HR 3.30 (95% CI: 2.05, 5.32)], particularly those with retinopathy and diabetes [HR 5.38 (95% CI: 1.80, 16.06)].
Conclusions
Even minimal retinopathy was a significant predictor of increased mortality in older persons, particularly men, irrespective of diabetes status. Persons with retinopathy may warrant closer clinical management of general health.
Keywords: AGES-Reykjavik Study, retinopathy, mortality, kidney dysfunction, cerebrovascular disease
INTRODUCTION
Retinopathy, a common condition, has been independently associated with several adverse health conditions, including diabetes, hypertension, coronary heart disease, and chronic kidney disease.1–8 In the Age, Gene/Environment Susceptibility – Reykjavik Study (AGES-RS), retinopathy was present in one or both eyes in 27% of participants with diabetes mellitus and 10.7% of those without diabetes; among the latter group, microalbuminuria was the only statistically significant factor associated cross-sectionally with increased risk of retinopathy.9 Another analysis from the same cohort showed that retinopathy was associated with multiple cerebral microbleeds, particularly in participants with diabetes.10 Results from the Lipid Research Clinic’s Coronary Primary Prevention Trial suggested that retinopathy predicts coronary heart disease in high risk men, independent of other risk factors,5 and more recently, the Chronic Renal Insufficiency Cohort Study demonstrated, after adjustment for a wide array of risk factors, a strong cross-sectional association between severity of retinopathy and chronic kidney disease.3
Retinopathy has also been associated with an increased risk of mortality, specifically, cardiovascular disease (CVD)-related mortality, in older adults.11–17 The Beijing Eye Study11 followed 3224 Chinese participants with a mean age of 56 years (range 40–101 years) over five years and found the mortality rate among the 8.8% of persons who, at baseline, had retinopathy was twice that of those without retinopathy. Similarly, the Blue Mountains Eye Study12 followed 2967 participants aged 49+ years over 12 years and reported retinopathy to be a significant predictor of coronary heart disease deaths in the 28.6% of persons with retinopathy and diabetes and the 9.7% of persons with retinopathy without diabetes. In the Ibaraki Prefectural Health Study in Japan,13 87,890 participants aged 40–79 years were followed for 15 years, and retinopathy was a significant risk factor for death due to cardiovascular causes independent of other cardiovascular risk factors, including hypertension.
Further, among individuals with diabetes, a Finnish cohort study of persons aged 45–64 years suggested that retinopathy predicted all-cause, cardiovascular, and coronary disease related mortality in men and women14 while a meta-analysis of 20 studies involving retinopathy determined that the presence of retinopathy was associated with an increased risk of all-cause mortality and cardiovascular events in diabetic patients.15 Data from the Third National Health and Nutrition Survey reported a synergistically increased all-cause mortality risk among adults having both retinopathy and chronic kidney disease.16
It is unclear why retinopathy, particularly mild signs that are the most common, would be associated with mortality. We speculate that the influence of common concomitant age-related health conditions, particularly diabetes and cardiovascular disease, may affect associations between retinopathy and mortality, but there are few published reports on the topic. The current study extends previous work on retinopathy in the well-phenotyped AGES-RS cohort of Icelandic elders by investigating the relationship between retinopathy and mortality in the context of concomitant health conditions.
METHODS
Study Population
The AGES-RS is a population-based prospective cohort study designed to investigate the contribution of interacting genetic and environmental factors on common age-related conditions and has been described in detail elsewhere.18 Participants were recruited between 2002 and 2006 from randomly selected, surviving men and women born between 1907 and 1935 (n=11,549) from the Reykjavik Study that was initiated by the Icelandic Heart Association (IHA) in 1967. Of the 5764 individuals (aged 67 years or older) examined as part of the AGES-RS, retinal images were collected from 4994 (86.6%) persons. Ungradable or incomplete images from both eyes excluded 28 participants from retinopathy assessment, resulting in an analytic sample of 4966 persons for the current study.
In adherence to the Tenets of the Declaration of Helsinki, the AGES Study was approved by the Icelandic National Bioethics Committee (VSN: 00–063), the Icelandic Data Protection Authority, Iceland, and by the Institutional Review Board for the National Institute of Aging, National Institutes of Health, USA. Written informed consent was obtained from all participants.
Retinal Examination
Following pharmacologic dilation of the pupils, a Canon CR6 nonmydriatic camera (US Canon Inc, Lake Success, NY) with a Canon D60 camera back was used to capture two 45-degree images of each retina, one centered on the optic disc (field 1) and the other centered on the fovea (field 2), from both eyes using a standardized protocol. Procedures have been described in detail.19 Graders, masked to health status, at the Ocular Epidemiology Reading Center at the University of Wisconsin evaluated retinal images using EyeQ Lite image processing software (Digital Healthcare Inc., Cambridge, UK) and the modified Airlie House Classification adaption of the Early Treatment Diabetic Retinopathy Study protocol20, the same retinopathy grading method used for the Multi-Ethnic Study of Atherosclerosis.21
Retinopathy lesions were classified as definite if the grader was at least 90% certain that a retinal lesion was present, and once classified as definite, a retinopathy level was assigned to the lesion based on the presence of hard exudates (HE), dot or blot hemorrhages (HEM), microaneurysms (MA), cotton wool spots (CWS), intraretinal microvascular abnormalities (IRMA), venous beading (VB), and new vessels.21 Grading levels were categorized as no retinopathy (levels 10–13, findings of no or questionable retinopathy), mild non-proliferative retinopathy (levels 14–31, findings indicative of HE, IRMA, CWS or VB without MA or HEM; MA only; HEM only), moderate-to-severe non-proliferative retinopathy (levels 41–51, findings of MA and at least one other of these lesions CWS, IRMA, VB or HEM; multiple MA) or proliferative retinopathy (levels 60–80, findings indicative of fibrous proliferation; panretinal photocoagulation laser scars; vitreous hemorrhage). Unless otherwise stated, retinopathy was dichotomized as no retinopathy or any retinopathy (levels 14–80) for the participant’s worse eye (i.e. the eye with the most severe retinopathy lesion). If fundus images were unavailable or ungradable for one eye, the retinopathy level from the other eye was used. Since retinopathy signs in the presence of diabetes and hypertension share many similarities,22 care was taken not to attribute an underlying cause of the retinopathy but rather to consider concomitant conditions which co-occurred with it.
Mortality Outcome
Records for AGES-RS participants were linked, with the permission of the Icelandic Data Protection Authority, to the complete adjudicated registry of deaths of all Icelanders, dying in country and abroad, whose names appear in the Icelandic National Roster maintained by Statistics Iceland (http://www.statice.is/Statistics/Population/Births-and-deaths), from which IHA ascertained mortality events. All-cause mortality was assessed through September 27, 2013, but cause-of-death was available only for deaths recorded through December 31, 2009 when Icelandic funding for such classification ceased. IHA determined cause-of-death due to CVD using guidelines from the SCORE project23 and the International Classification of Disease (ICD) (versions 9), where deaths with the following ICD codes: 401 through 414, 426 through 443, and 798.1 and 798.2, with the exception of the following codes for definitely non-atherosclerotic causes of death: 426.7, 429.0, 430.0, 432.1, 437.3, 437.4, and 437.5, were attributed to CVD.
Assessment of Participant Characteristics
A wide array of information was assessed through detailed in-person interviews and clinical examinations. Education was dichotomized as completed secondary school or less than secondary school. Smoking status was classified as never smoked, former smoker, or current smoker. Hypertension was defined as a self-reported history of hypertension, use of antihypertensive drugs, or blood pressure ≥ 140/90 mm Hg. Body mass index (BMI) was calculated as weight (kg) divided by height (meters) squared. Self-reported health status was determined from the interviewed participant’s response when asked to rate it using a Likert scale (e.g. excellent, very good, good, fair, or poor), and subsequently, dichotomized as poor or good (not poor). A three-step procedure was used to identify cases of dementia, which were adjudicated in a consensus conference adhering to international guidelines.24 Cognitive status was dichotomized into impaired (dementia/mild cognitive impairment) and not impaired for the purposes of this analysis. Self-reported history of difficulty walking or use of walking aids was used to define a walking disability. Fasting blood serum was collected, and total cholesterol (mmol/L), high-density lipoprotein (HDL) cholesterol (mmol/L), triglycerides (mmol/L), glucose (mmol/L), and HbA1c (%) were analyzed on a Hitachi 912 (Mito, Japan), using reagents from Roche Diagnostics (Mannheim, Germany) and following the manufacturer’s instructions.9 A standard from Roche Diagnostics and also an in-house serum standard were used to determine a coefficient of variation (%) for each measurement. They are, respectively, for total cholesterol 2.5% and 2.9%, HDL 3.5% and 2.1%, triglycerides 2.7% and 2.2%, and glucose 1.9% and 2.0%. For HbA1c, the % coefficient of variation using the manufacturer’s standard was 3.1%. Diabetes mellitus was determined by a self-reported history of diabetes, use of glucose-modifying medications, or HbA1c level of ≥ 6.5%. Self-reported history of angina and CVD were determined from participant’s interview response (yes or no). Documented hospital reports of a myocardial infarction, coronary artery bypass surgery, or angioplasty were used to ascertain a participant’s record of a clinical cardiovascular event (yes or no). All adjudicated cases of clinical stroke, from prior to entry into the AGES study and following entry into the study through December 31, 2012, were determined from hospital records. Microalbuminuria was defined as a urine albumin: creatinine ratio (UACR) ≥ 30 mg/g, and chronic kidney disease (CKD) was considered to be present if the participant had an estimated glomerular filtration rate (eGFR)25 < 60 ml/min/1.73m2. Cerebral microbleeds and brain infarcts, presence of and number, were detected by a neuroradiologist from magnetic resonance imaging (MRI) and characterized by trained radiographers at IHA as none, single or multiple (≥ 2) using a detailed grading protocol.26 Total brain tissue volume was estimated by summing grey matter, white matter, and white matter lesion volumes from brain MRIs and was corrected for total intracranial volume in order to calculate a standardized percentage tissue volume.26
Statistical Analysis
Statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, North Carolina, USA). Differences between groups with and without retinopathy data and by retinopathy status, adjusting for age and sex, were tested using analysis of covariance (ANCOVA) and logistic regression. Kaplan-Meier plots were generated to illustrate age-adjusted mortality risk by retinopathy status over the ten year follow-up period for men and women separately. Hazard ratios (HR) for all-cause mortality, and specifically, due to CVD, and retinopathy were estimated using Cox proportional hazards regression. Age was used as the time scale in all regression analyses instead of follow-up time, allowing the Cox proportional hazards models to compare risk for persons of comparable age in order to mitigate the substantial impact of increasing age on mortality risk.
Risk factors significantly associated with retinopathy or mortality due to any cause in preliminary analyses (p<0.05) were included in multivariable models and these included sex, smoking status, BMI, hypertension, diabetes, self-reported health status, cognitive status, walking disability, number of medications, and serum total cholesterol. Self-reported history of angina and cardiovascular disease were also included in multivariable analyses for cardiovascular-disease related mortality. Additionally, in order to examine the specific influence of kidney dysfunction (e.g. chronic kidney disease and microalbuminuria) and cerebrovascular conditions (e.g. total brain tissue volume, cerebral microbleeds, brain infarcts, and clinical stroke) on the associations between retinopathy and mortality, the interactions between each condition and retinopathy were formally tested and separate regression models were utilized to adjust for these factors. Stratified analyses were also performed for men and women to account for sex differences and to assess the dual impact of concomitant health conditions and retinopathy on mortality. Cox proportional hazards models were tested for violations of proportionality assumptions by graphical inspection and by generating time-dependent covariates using the interactions of each variable with the logarithm of the follow-up time. No violations were found. Other variables examined in preliminary analyses, but ultimately excluded as important to the association between retinopathy and mortality due to lack of significance included marital status, physical activity, depressive symptomology, self-reported history of falls, hearing impairment, cod liver oil use, antidepressant use, aspirin use, glucose level, self-reported history of cancer or arthritis, hand osteoarthritis, coronary calcium score, and Vitamin D levels.
All analyses were two-sided at the 95% confidence level with hazard ratio (HR) and confidence intervals (CI) reported.
RESULTS
Characteristics of participants with and without data on retinopathy are reported in Supplemental Table A. Study participants with retinopathy data included in this analysis (N=4966) were significantly younger and more likely to be male compared to the 798 excluded participants. After adjusting for age and sex, included participants were also significantly more likely to be better educated, a former smoker, currently consume alcohol, and have a greater total brain tissue volume but were less likely to currently smoke, self-report poor health, be cognitively impaired, have a walking disability, take more medications, have diabetes, have had a stroke, or have low serum HDL cholesterol. Among participants with data on retinopathy, 96.7% had graded images from both eyes.
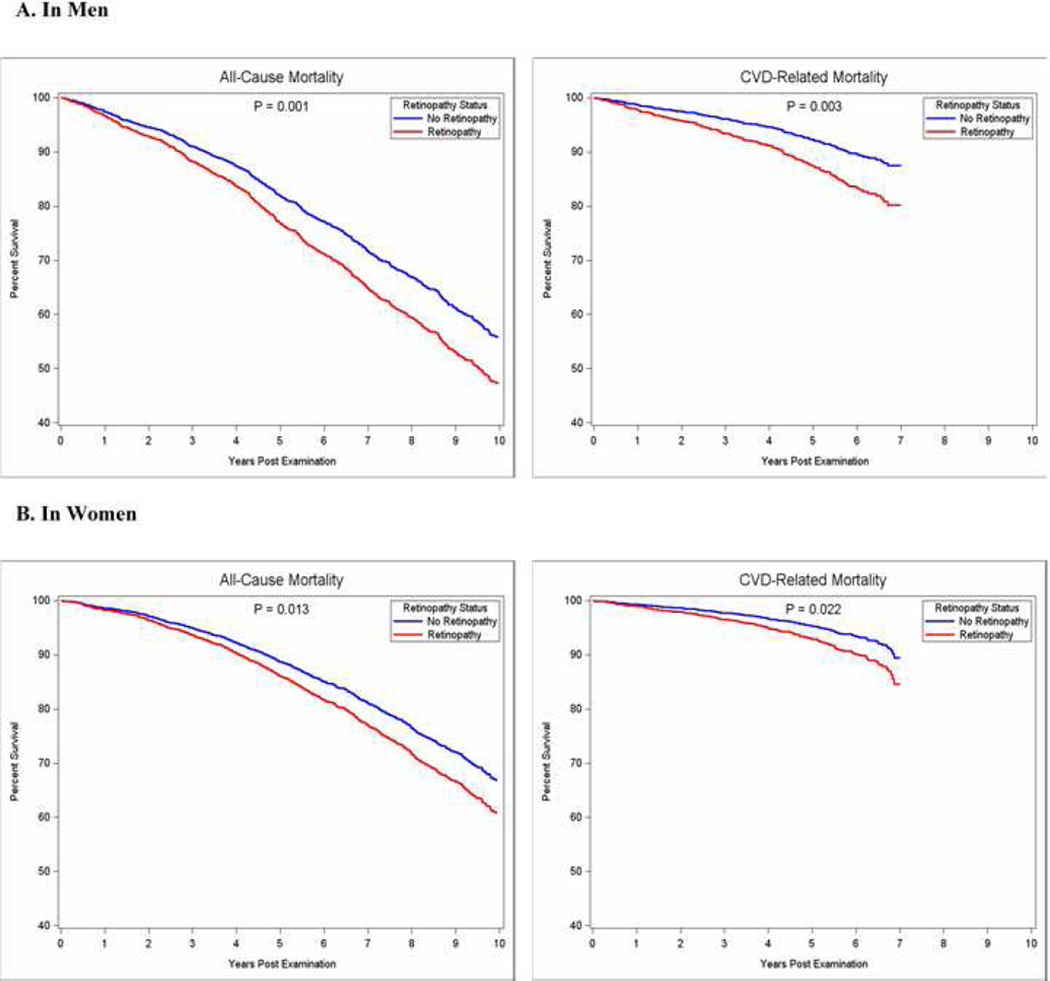
Retinopathy was present at the baseline examination in 614 (12.4%) of the 4966 study participants; of whom, 593 (11.9%) had mild non-proliferative retinopathy, 16 (0.3%) had moderate-to-severe non-proliferative retinopathy, and 5 (0.1%) had proliferative retinopathy. Of those with retinopathy, 96.6% exhibited mild signs of disease, predominantly blot/dot hemorrhages or microaneurysms. Due to the small number of advanced cases of retinopathy, all analyses considered retinopathy as yes or no. Only 136 persons (22.1%) with retinopathy at baseline also had diabetes (all but 17 individuals had mild signs of retinopathy). During the ten-year follow-up period (median follow-up 8.6 years, range 3–10 years), 1763 (35.5%) individuals died (900 men and 863 women). The mean time-to-death from baseline exam was 4.8 years and the mean age-at-death was 84.0 years. Through 2009, 860 deaths occurred, of which 366 (42.6%) were attributed to CVD. Mean time-to-death and mean age-at-death did not differ significantly by retinopathy status, but persons with retinopathy were significantly more likely to die compared to peers of comparable age and sex. Figure 1 depicts mortality by retinopathy status, over the ten year follow-up period, stratified by sex and adjusted for age. Mortality risk was more pronounced for men compared to women, suggesting that separate analyses for men and women were warranted.
Figure 1. Kaplan-Meier Plot for All-Cause and Cardiovascular Disease Related Mortality Rates by Retinopathy Status in Men and Women, Adjusted for Age.
All-cause mortality was assessed through September 27, 2013; CVD-related mortality was assessed through December 31, 2009.
Among individuals who died, 15.7% had retinopathy at the baseline exam (16.1% of men and 15.2% of women). Participants with retinopathy who died during follow-up were not significantly older at baseline than participants without retinopathy (79.5±5.7, range 67–92 years, versus 79.1±5.6, range 67–96 years, p=0.34); however, they were more likely to be in poorer health (e.g. self-report of poor health and greater medication use) and have concomitant health conditions, such as hypertension, diabetes, chronic kidney disease, microalbuminuria, and clinical stroke. Characteristics for those who died are provided by retinopathy status, stratified by sex, in Table 1.
Table 1.
Participant Characteristics at Baseline by Retinopathy Status among those who Died, Overall and by Sex (N=1763)
| No Retinopathy |
Any Retinopathy |
||||||
|---|---|---|---|---|---|---|---|
| Characteristic at Baseline Exam | All Participants (N=1487) |
Men (N=755) |
Women (N=732) |
All Participants (N=276) |
Men (N=145) |
Women (N=131) |
p-value* |
| Mean Age in years | 79.1 (5.6) | 79.2 (5.6) | 79.0 (5.7) | 79.5 (5.7) | 78.6 (5.6) | 80.4 (5.6) | 0.34 |
| Male | 50.8% (755) | -- | -- | 52.5% (145) | -- | -- | 0.58 |
| Education Level, Completed Secondary or More | 73.9% (1080) | 81.4% (604) | 66.1% (476) | 76.8% (208) | 84.6% (121) | 68.0% (87) | 0.31 |
| Former Smoker | 47.5% (704) | 59.4% (448) | 35.1% (256) | 42.4% (117) | 53.8% (78) | 29.8% (39) | 0.09 |
| Current Smoker | 14.4% (214) | 13.5% (102) | 15.4% (112) | 14.9% (41) | 17.2% (25) | 12.2% (16) | 0.70 |
| Current Alcohol Consumption | 60.4% (891) | 67.1% (504) | 53.5% (387) | 58.7% (162) | 67.6% (98) | 48.9% (64) | 0.62 |
| Hypertension | 83.0% (1233) | 81.9% (618) | 84.1% (615) | 92.4% (255) | 92.4% (134) | 92.4% (121) | < 0.01 |
| Mean Diastolic Blood Pressure, mmHG | 73.1 (9.9) | 74.7 (9.6) | 71.4 (9.9) | 74.1 (11.5) | 75.5 (10.5) | 72.6 (12.3) | 0.10 |
| Mean Systolic Blood Pressure, mmHG | 142.8 (21.4) | 141.6 (20.7) | 144.1 (22.1) | 147.5 (22.5) | 148.3 (21.1) | 146.6 (24.1) | < 0.01 |
| Mean Body Mass Index | 26.5 (4.5) | 26.5 (3.9) | 26.4 (5.1) | 26.7 (4.7) | 26.4 (3.9) | 27.1 (5.4) | 0.27 |
| Self-Reported Health Status, Poor | 8.6% (128) | 7.2% (54) | 10.1% (74) | 12.4% (34) | 9.0% (13) | 16.0% (21) | 0.04 |
| Cognitive Status, Impaired | 24.5% (357) | 27.4% (203) | 21.5% (154) | 29.5% (79) | 25.5% (36) | 33.9% (43) | 0.12 |
| Walking Disability | 28.2% (418) | 25.4% (192) | 31.0% (226) | 31.5% (87) | 24.8% (36) | 38.9% (51) | 0.36 |
| Median Number of Medications (Range) | 4.0 (0–19) | 4.0 (0–15) | 4.0 (0–19) | 5.0 (0–15) | 5.0 (0–15) | 6.0 (0–14) | < 0.01 |
| Mean High-Density Lipoprotein Cholesterol, mmol/L | 1.6 (0.5) | 1.4 (0.4) | 1.7 (0.5) | 1.5 (0.4) | 1.5 (0.5) | 1.6 (0.4) | 0.35 |
| Mean Total Cholesterol, mmol/L | 5.5 (1.2) | 5.1 (1.0) | 5.9 (1.2) | 5.3 (1.1) | 5.0 (1.1) | 5.7 (1.1) | 0.03 |
| History of Angina, by Self-Report | 17.9% (258) | 20.9% (152) | 14.9% (106) | 21.1% (56) | 19.9% (28) | 22.6% (28) | 0.22 |
| History of Cardiovascular Disease, by Self-Report | 30.1% (446) | 38.4% (289) | 21.5% (157) | 32.4% (89) | 34.0% (49) | 30.5% (40) | 0.48 |
| Record of Clinical Cardiovascular Event | 19.8% (293) | 28.6% (215) | 10.7% (78) | 22.3% (61) | 28.0% (40) | 16.2% (21) | 0.37 |
| Clinical Strokea | 15.9% (236) | 16.2% (122) | 15.6% (114) | 22.3% (61) | 21.7% (31) | 23.1% (30) | 0.01 |
| HbA1c ≥ 6.5% | 6.0% (82) | 7.0% (49) | 4.9% (33) | 15.0% (38) | 18.3% (25) | 11.1% (13) | < 0.01 |
| Diabetes Mellitusb | 10.9% (162) | 13.3% (100) | 8.5% (62) | 27.5% (76) | 31.7% (46) | 22.9% (30) | < 0.01 |
| Microalbuminuriac | 12.1% (176) | 16.5% (122) | 7.5% (54) | 27.1% (72) | 31.2% (44) | 22.4% (28) | < 0.01 |
| Chronic Kidney Diseased | 37.7% (561) | 31.8% (240) | 43.9% (321) | 49.6% (137) | 41.4% (60) | 58.8% (77) | < 0.01 |
| Cerebral Microbleeds | 14.8% (187) | 17.8% (112) | 11.8% (75) | 18.7% (44) | 20.5% (26) | 16.7% (18) | 0.16 |
| Mean Total Brain Tissue Volume (corrected for intracranial volume) |
70.1 (4.1) | 68.8 (4.1) | 71.4 (3.7) | 69.7 (4.0) | 68.6 (4.1) | 70.9 (3.5) | 0.43 |
| Brain Infarcts, Single | 17.2% (222) | 19.4% (125) | 15.0% (97) | 20.3% (48) | 22.1% (28) | 18.4% (20) | 0.15 |
| Brain Infarcts, Multiple (≥ 2) | 23.4% (302) | 29.9% (193) | 16.9% (109) | 27.1% (64) | 33.9% (43) | 19.3% (21) | 0.14 |
Data are presented as % (N) or mean (SD) except where noted as median (range). All-cause mortality was assessed through 27 September 2013.
Comparisons are between retinopathy and no retinopathy group for all participants, adjusted for age and sex.
Clinical stroke was defined as having a stroke, as determined from hospital records, before entry to or during the AGES study up to 31 December 2012.
Diabetes mellitus was defined as the self-reported history of diabetes, use of glucose-modifying medications, or HbA1c ≥ 6.5%.
Microalbuminuria was defined as a urine albumin: creatinine ratio (UACR) ≥ 30 mg/g.
Chronic kidney disease was defined as an estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73m2.
Other variables, including marital status, physical activity level, depressive symptomology, self-reported history of falls, hearing impairment, cod liver oil use, antidepressant use, aspirin use, glucose level, vitamin D level, self-reported history of cancer or arthritis, hand osteoarthritis, and coronary calcium were also considered, but due to the lack of significance in preliminary analyses, were not included.
In multivariable Cox proportional hazards regression models, the relationship between retinopathy and certain concomitant health conditions on mortality was also apparent (Table 2). Results from initial analytic models that included the mortality-susceptibility risk factors (age, sex, smoking status, BMI, hypertension, diabetes, self-reported health status, cognitive status, walking disability, number of medications, serum total cholesterol, and for CVD-related mortality analyses, self-reported history of CVD and angina) suggested that having retinopathy significantly increased the risk of mortality [HR 1.26 (95% CI: 1.10, 1.43)], particularly CVD-mortality [HR 1.57 (95% CI: 1.20, 2.06)]. Among men, those with retinopathy had a significantly higher risk of mortality compared to those without retinopathy [all-cause HR 1.33 (95% CI: 1.11, 1.60) and CVD HR 1.81 (95% CI: 1.25, 2.63)]. Mortality risk was also elevated for women with retinopathy but was not statistically significantly different compared to women without retinopathy.
Table 2.
Cox Proportional Hazards Regression Models of All-Cause and Cardiovascular Disease Related Mortality by Retinopathy Status, Overall and by Sex
| Retinopathy, Present versus Absent |
Model 1 (HR and 95% CI) |
Model 2 (HR and 95% CI) |
Model 3 (HR and 95% CI) |
Model 4 (HR and 95% CI) |
|---|---|---|---|---|
| All-Cause Mortality | ||||
| All Participants | 1.26 (1.10, 1.43)** | 1.20 (1.05, 1.37)** | 1.16 (1.01, 1.35)* | 1.12 (0.97, 1.30) |
| Men | 1.33 (1.11, 1.60)** | 1.30 (1.08, 1.57)** | 1.30 (1.06, 1.60)* | 1.28 (1.04, 1.57)* |
| Women | 1.19 (0.98, 1.44) | 1.13 (0.93, 1.38) | 1.04 (0.84, 1.30) | 0.98 (0.78, 1.22) |
|
Cardiovascular-Disease Related Mortality |
||||
| All Participants | 1.57 (1.20, 2.06)** | 1.46 (1.11, 1.93)** | 1.42 (1.05, 1.93)* | 1.24 (0.91, 1.69) |
| Men | 1.81 (1.25, 2.63)** | 1.72 (1.18, 2.50)** | 1.81 (1.19, 2.73)** | 1.74 (1.14, 2.66)** |
| Women | 1.37 (0.92, 2.07) | 1.31 (0.87, 1.99) | 1.20 (0.76, 1.91) | 0.95 (0.59, 1.53) |
HR=hazard ratio; CI = confidence interval;
p-value < 0.05;
p-value < 0.01.
All-cause mortality was assessed through 27 September 2013 and cardiovascular disease related mortality was assessed through 31 December 2009.
Model 1 adjusted for age, sex, smoking status, BMI, hypertension, diabetes, self-reported health status, cognitive status, walking disability, number of medications, and serum total cholesterol. For cardiovascular disease related mortality analyses, it further adjusted for self-reported history of angina or cardiovascular disease.
Model 2 adjusted for all variables in model 1 and microalbuminuria and chronic kidney disease.
Model 3 adjusted for all variables in model 2 and total brain tissue volume (corrected for intracranial volume), cerebral microbleeds, and brain infarcts.
Model 4 adjusted for all variables in model 3 and clinical stroke.
In subsequent models (Table 2), indicators of kidney dysfunction (chronic kidney disease and microalbuminuria) and cerebrovascular conditions (total brain tissue volume, cerebral microbleeds and brain infarcts) were included. These concomitant conditions mitigated some of the mortality risk attributed to retinopathy; nonetheless, the resulting associations between retinopathy and mortality due to any cause and CVD remained statistically significant when data for men and women were combined, as well as when men were examined alone (p<0.05). Increased risk of death attributable to retinopathy was further attenuated when clinical stroke was considered, although it remained statistically significant in men [all-cause HR 1.28 (95% CI: 1.04, 1.57) and CVD HR 1.74 (95% CI: 1.14, 2.66)].
Interactions between retinopathy and the health conditions microalbuminuria and clinical stroke were tested in the final all-cause and CVD models, and no statistically significant interactions for either condition in any of the models were found, suggesting that the relationship between retinopathy and mortality was consistent in persons with and without the health condition. Nonetheless, the impact on mortality of having both retinopathy and the condition may be greater than that which is seen in participants not having the health condition. In stratified analyses assessing the impact of concomitant kidney dysfunction, specifically microalbuminuria, with retinopathy on mortality (Table 3), individuals with both retinopathy and microalbuminuria were at significantly increased risk of all-cause and CVD-related mortality compared to those without retinopathy and microalbuminuria [all-cause HR 1.70 (95% CI: 1.30, 2.23) and CVD HR 2.04 (95% CI: 1.27, 3.28)] while only having retinopathy but not microalbuminuria did not significantly increase risk of mortality [HR 1.08 (95% CI: 0.91, 1.27)]. This relationship between retinopathy and microalbuminuria on mortality was particularly evident among persons with diabetes [HR 2.01 (95% CI: 1.22, 3.31)], but mortality risk was also elevated in those without diabetes [HR 1.52 (95% CI: 1.08, 2.14)]. Risk of mortality associated with retinopathy concomitant with microalbuminuria was also significantly elevated among persons with hypertension [HR 1.71 (95% CI: 1.30, 2.25) and was elevated but not statistically so among the few normotensive people [HR 1.88 (95% CI: 0.25, 13.97)]. Risk of mortality among men with retinopathy and concomitant microalbuminuria was also significantly greater [all-cause HR 2.06 (95% CI: 1.44, 2.94) and CVD HR 4.12 (95% CI: 2.21, 7.68)] compared to men without either condition; this was most predominant when co-present with diabetes or hypertension. Women who died were considerably less likely than men to have microalbuminuria or when present to have it concomitant with retinopathy. Furthermore, women did not exhibit similarly significant increased risk of death associated with retinopathy when concomitant with microalbuminuria compared to men.
Table 3.
Cox Proportional Hazards Regression Models of All-Cause and Cardiovascular Disease Related Mortality by Retinopathy Status and Microalbuminuria, Overall and by Sex and Diabetes Status
| All-Cause Mortality |
Cardiovascular Disease - Related Mortality |
|||||
|---|---|---|---|---|---|---|
| All Participants (N=4953) | All Participants (HR and 95% CI)a |
Men (HR and 95% CI)a |
Women (HR and 95% CI)a |
All Participants (HR and 95% CI)b |
Men (HR and 95% CI)b |
Women (HR and 95% CI)b |
| No Retinopathy & No Microalbuminuria (N=4018, 41.1% male) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| No Retinopathy & Microalbuminuria (N=334, 63.5% male) | 1.32 (1.10, 1.58)** | 1.34 (1.07, 1.68)* | 1.37 (0.99, 1.88) | 1.42 (0.97, 2.08) | 1.35 (0.82, 2.21) | 1.54 (0.81, 2.92) |
| Retinopathy & No Microalbuminuria (N=505, 42.4% male) | 1.08 (0.91, 1.27) | 1.20 (0.94, 1.52) | 0.98 (0.77, 1.25) | 1.15 (0.80, 1.68) | 1.29 (0.75, 2.22) | 1.09 (0.65, 1.84) |
| Retinopathy & Microalbuminuria (N=96, 61.5% male) | 1.70 (1.30, 2.23)** | 2.06 (1.44, 2.94)** | 1.32 (0.86, 2.03) | 2.04 (1.27, 3.28)** | 4.12 (2.21, 7.68)** | 0.87 (0.37, 2.02) |
| Participants without Diabetes (N=4453) | ||||||
| No Retinopathy & No Microalbuminuria (N=3712, 40.6% male) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| No Retinopathy & Microalbuminuria (N=273, 61.2% male) | 1.34 (1.10, 1.64)** | 1.44 (1.12, 1.84)** | 1.24 (0.87, 1.78) | 1.83 (1.23, 2.71)** | 1.82 (1.09, 3.04)* | 1.91 (0.99, 3.69) |
| Retinopathy & No Microalbuminuria (N=410, 40.7% male) | 1.07 (0.89, 1.29) | 1.29 (0.99, 1.67) | 0.91 (0.69, 1.18) | 1.14 (0.76, 1.71) | 1.29 (0.72, 2.33) | 1.10 (0.63, 1.91) |
| Retinopathy & Microalbuminuria (N=58, 53.5% male) | 1.52 (1.08, 2.14)* | 1.81 (1.11, 2.96)* | 1.32 (0.81, 2.17) | 1.74 (0.95, 3.19) | 2.48 (1.00, 6.13)* | 1.38 (0.59, 3.21) |
| Participants with Diabetes (N=500) | ||||||
| No Retinopathy & No Microalbuminuria (N=306, 47.4% male) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| No Retinopathy & Microalbuminuria (N=61, 73.8% male) | 1.23 (0.78, 1.95) | 0.94 (0.53, 1.68) | 2.23 (0.95, 5.21) | 0.58 (0.15, 2.26) | 0.36 (0.06, 2.22) | 3.92 (0.02, 7.55) |
| Retinopathy & No Microalbuminuria (N=95, 49.5% male) | 1.14 (0.74, 1.76) | 0.83 (0.43, 1.59) | 1.50 (0.81, 2.79) | 1.27 (0.45, 3.55) | 0.71 (0.14, 3.76) | 3.44 (0.60, 9.18) |
| Retinopathy & Microalbuminuria (N=38, 73.7% male) | 2.01 (1.22, 3.31)** | 2.06 (1.11, 3.80)* | 1.86 (0.64, 5.43) | 5.24 (1.91, 14.42)** | 11.03 (3.10, 39.29)** | 2.75 (0.08, 9.83) |
HR=hazard ratio; CI=confidence interval;
p-value < 0.05;
p-value < 0.01.
Microalbuminuria was defined as a urine albumin: creatinine ratio (UACR) ≥ 30 mg/g. Diabetes mellitus was defined as the self-reported history of diabetes, use of glucose-modifying medications, or HbA1c ≥ 6.5%.
All-cause mortality was assessed through 27 September 2013, and analyses adjusted for age, sex, smoking status, BMI, hypertension, diabetes (for all participants), self-reported health status, cognitive status, walking disability, number of medications, serum total cholesterol, clinical stroke, chronic kidney disease, cerebral microbleeds, total brain tissue volume, and brain infarcts.
Cardiovascular disease related mortality was assessed through 31 December 2009, and analyses adjusted for age, sex, smoking status, BMI, hypertension, diabetes (for all participants), self-reported health status, cognitive status, walking disability, number of medications, serum total cholesterol, clinical stroke, chronic kidney disease, cerebral microbleeds, total brain tissue volume, brain infarcts, self-reported history of angina or cardiovascular disease.
Clinical stroke, equally common among men and women who died, mitigated the association between retinopathy and increased risk of death in multivariable analyses (Table 2). Investigating this further in stratified analyses (Table 4), there was, in men and women combined and in men only, a significant association between retinopathy and all-cause mortality irrespective of the co-presence of clinical stroke [compared to persons without retinopathy and clinical stroke: for all participants, HR 1.22 (95% CI: 1.03, 1.44) retinopathy without clinical stroke and HR 1.41 (95% CI: 1.05, 1.90) retinopathy and clinical stroke, and for men, HR 1.32 (95% CI: 1.06, 1.65) retinopathy without clinical stroke and HR 1.65 (95% CI: 1.07, 2.56) retinopathy and clinical stroke; all p<0.05]. In contrast, although CVD-mortality risk was increased in persons with retinopathy and no clinical stroke [HR1.64 (95% CI: 1.12, 2.40), p<0.05], persons with concomitant retinopathy and clinical stroke were much more likely to die of CVD than their counterparts without retinopathy and clinical stroke [HR 3.30 (95% CI: 2.05, 5.32), p<0.01], particularly in men [HR 4.49 (95% CI: 2.26, 8.90), p<0.01], although the association was also significant in women [HR 2.91 (95% CI: 1.47, 5.78), p<0.01]. Among persons without diabetes, retinopathy was associated with CVD-mortality only in persons with concomitant retinopathy and clinical stroke [HR 3.34 (95% CI: 1.92, 5.81), p<0.01, for all participants; HR 3.18 (95% CI: 1.26, 8.05), p<0.05 for men only; and HR 3.85 (95% CI: 1.88, 7.88), p<0.01, for women]. Among persons with diabetes, retinopathy with and without clinical stroke was associated with increased risk of CVD-mortality [HR 5.38 (95% CI: 1.80, 16.06) and HR 3.23 (95% CI: 1.26, 8.33), respectively]. When stratified by sex, the risk attributable to retinopathy was statistically significant, irrespective of the presence of clinical stroke, only for men. Women with retinopathy but no clinical stroke history experienced significantly increased risk of CVD-mortality, but while elevated, when concomitant retinopathy and clinical stroke were present, the association between retinopathy and increased mortality was not statistically significant. Among persons with hypertension, retinopathy with and without clinical stroke was significantly associated with all-cause mortality in all persons [HR 1.52 (95% CI: 1.12, 2.07) and HR 1.30 (95% CI: 1.09, 1.54), respectively] but was more strongly associated with CVD-mortality [HR 3.88 (95% CI: 2.36, 6.38) and HR 1.75 (95% CI: 1.18, 2.59), respectively]. In men with concomitant retinopathy, hypertension and clinical stroke, the risk of CVD-mortality was significantly elevated [HR 4.71 (95% CI: 2.35, 9.47), p<0.01] and the same was true for women [HR 3.61 (95% CI: 1.70, 7.68), p<0.01]. There were only eight normotensive participants with clinical stroke who also had retinopathy, too few to assess risk.
Table 4.
Cox Proportional Hazards Regression Models of All-Cause and Cardiovascular Disease Related Mortality by Retinopathy Status and Clinical Stroke, Overall and by Sex and Diabetes Status
| All-Cause Mortality |
Cardiovascular Disease - Related Mortality |
|||||
|---|---|---|---|---|---|---|
| All Participants (N=4916) | All Participants (HR and 95% CI)a |
Men (HR and 95% CI)a |
Women (HR and 95% CI)a |
All Participants (HR and 95% CI)b |
Men (HR and 95% CI)b |
Women (HR and 95% CI)b |
| No Retinopathy & No Clinical Stroke (N=3923, 42.2% male) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| No Retinopathy & Clinical Stroke (N=384, 51.0% male) | 1.64 (1.39, 1.93)** | 1.43 (1.13, 1.80)** | 1.91 (1.51, 2.42)** | 3.93 (2.91, 5.30)** | 3.19 (2.07, 4.92)** | 4.68 (3.06, 7.18)** |
| Retinopathy & No Clinical Stroke (N=525, 45.3% male) | 1.22 (1.03, 1.44)* | 1.32 (1.06, 1.65)* | 1.12 (0.87, 1.43) | 1.64 (1.12, 2.40)* | 1.96 (1.19, 3.25)** | 1.37 (0.75, 2.50) |
| Retinopathy & Clinical Stroke (N=84, 46.4% male) | 1.41 (1.05, 1.90)* | 1.65 (1.07, 2.56)* | 1.28 (0.85, 1.94) | 3.30 (2.05, 5.32)** | 4.49 (2.26, 8.90)** | 2.91 (1.47, 5.78)** |
| Participants without Diabetes (N=4416) | ||||||
| No Retinopathy & No Clinical Stroke (N=3594, 41.4% male) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| No Retinopathy & Clinical Stroke (N=347, 49.6% male) | 1.71 (1.44, 2.04)** | 1.49 (1.16, 1.91)** | 1.98 (1.55, 2.53)** | 4.55 (3.35, 6.19)** | 3.64 (2.34, 5.68)** | 5.38 (3.49, 8.29)** |
| Retinopathy & No Clinical Stroke (N=418, 42.6% male) | 1.19 (0.99, 1.43) | 1.37 (1.06, 1.75)* | 1.05 (0.80, 1.39) | 1.42 (0.92, 2.19) | 1.61 (0.89, 2.91) | 1.28 (0.67, 2.46) |
| Retinopathy & Clinical Stroke (N=57, 38.6% male) | 1.30 (0.91, 1.88) | 1.44 (0.79, 2.61) | 1.27 (0.79, 2.04) | 3.34 (1.92, 5.81)** | 3.18 (1.26, 8.05)* | 3.85 (1.88, 7.88)** |
| Participants with Diabetes (N=500) | ||||||
| No Retinopathy & No Clinical Stroke (N=329, 50.5% male) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| No Retinopathy & Clinical Stroke (N=37, 64.9% male) | 1.17 (0.69, 2.01) | 1.24 (0.63, 2.45) | 1.19 (0.44, 3.26) | 2.97 (0.85, 10.44) | 4.02 (0.80, 20.25) | 17.86 (0.88, 36.36) |
| Retinopathy & No Clinical Stroke (N=107, 56.1% male) | 1.30 (0.87, 1.94) | 1.19 (0.68, 2.10) | 1.48 (0.78, 2.83) | 3.23 (1.26, 8.33)* | 4.31 (1.23, 15.11)* | 8.33 (1.07, 64.57)* |
| Retinopathy & Clinical Stroke (N=27, 63.0% male) | 1.55 (0.89, 2.69) | 1.80 (0.85, 3.82) | 1.17 (0.46, 2.97) | 5.38 (1.80, 16.06)** | 9.44 (2.24, 39.70)** | 2.96 (0.13, 66.45) |
HR=hazard ratio; CI=confidence interval;
p-value < 0.05;
p-value < 0.01.
Clinical stroke was defined as having a stroke, as determined from hospital records, before entry to or during the AGES study up to 31 December 2012. Diabetes mellitus was defined as the self-reported history of diabetes, use of glucose-modifying medications, or HbA1c ≥ 6.5%.
All-cause mortality was assessed through 27 September 2013, and analyses adjusted for age, sex, smoking status, BMI, hypertension, diabetes (for all participants), self-reported health status, cognitive status, walking disability, number of medications, serum total cholesterol, chronic kidney disease, microalbuminuria, cerebral microbleeds, total brain tissue volume, and brain infarcts.
Cardiovascular disease related mortality was assessed through 31 December 2009, and analyses adjusted for age, sex, smoking status, BMI, hypertension, diabetes (for all participants), self-reported health status, cognitive status, walking disability, number of medications, serum total cholesterol, chronic kidney disease, microalbuminuria, cerebral microbleeds, total brain tissue volume, brain infarcts, self-reported history of angina or cardiovascular disease.
DISCUSSION
Our findings show that mortality risk due to any cause, and specifically CVD, was significantly increased in older persons with retinopathy compared to similarly-aged individuals without retinopathy, even after adjusting for diabetes, hypertension, and other factors, like smoking, which are commonly associated with decreased survival. This was particularly true in men. Women with retinopathy had a higher but not statistically significantly increased risk of dying relative to women of comparable age without retinopathy. Furthermore, our results show that retinopathy concomitant with microalbuminuria or history of clinical stroke posed higher mortality risk than was observed with retinopathy alone, and it was irrespective of diabetes status.
The current study corroborates findings from published studies examining the association between retinopathy and mortality in studies of persons with and without diabetes.11–14, 31–33 Results from a population-based cohort study of Chinese adults, aged 40–101 years (mean age 56.1 years), suggested that in addition to older age, male sex, and living in a rural region, retinopathy was a risk factor for increased mortality over a five year period.11 Similarly, the Beaver Dam Eye Study found that retinopathy was associated with decreased survival over 14 years in men and women without diabetes, aged 43–84 years, with the strongest association reported for ischemic heart disease mortality.31 The relationship between CVD-mortality and retinopathy was also significant in another analysis from the Beaver Dam Eye Study that found retinopathy predicted 10-year cardiovascular mortality independent of other retinal lesions, blood pressure, diabetes, glycosylated hemoglobin and other risk factors, and even for persons without a history of myocardial infarction or stroke.32 Increased cardiovascular mortality risk (over a 14 year follow-up period) was also independently associated with mild hypertensive retinopathy among men and women with and without hypertension in the Ibaraki Prefectural Health Study.13
It is well known, however, that CVD and retinopathy are complications of diabetes,27–29 and multiple studies have considered the impact of retinopathy on mortality risk, particularly resulting from CVD, in persons with diabetes.15–17,30 Proliferative retinopathy predicted all-cause, CVD, and CHD death in Finnish men and women, aged 45–64 years, with diabetes mellitus who were free of CVD at baseline, even after adjustment for traditional CVD risk factors, glycemic control, duration of diabetes, and proteinuria.15 In the Wisconsin Epidemiologic Study of Diabetic Retinopathy, retinopathy was strongly associated with all-cause and ischemic heart disease mortality in persons with early and late onset diabetes, when controlling for age and sex.30 Mortality risk was mitigated when other factors were included, but the presence of severe retinopathy in individuals with diabetes remained a significant indicator for increased risk of ischemic heart disease death. Among those with diabetes in the current study, retinopathy was a significant risk factor for mortality due to any cause and, specifically CVD, consistent with these earlier findings. However, while the relationship was stronger among persons with diabetes, there were considerably more persons with retinopathy in our sample who did not have diabetes. In this latter group, signs of retinopathy were exclusively mild, yet this group also exhibited increased risk of death due to all-causes and CVD.
The current study attempts to unravel patterns of multi-morbidity that are common in old age and if better understood may provide insight into the conditions which co-occur and ultimately result in poor health. Previous reports indicated that kidney disease and reduced total brain volume predicted mortality in the Icelandic population by increasing risk of cardiovascular and cerebrovascular disease.34–35 In a separate analysis, microalbuminuria was found to be a statistically significant factor associated with increased risk of retinopathy in participants without diabetes.9 This analysis therein considered the overall influence of retinopathy on mortality, as well as possible joint interactive effects of microalbuminuria, a marker of kidney dysfunction, and clinical stroke, an indicator of reduced functional brain volume, and considered the possibility that there might be sex-specific differences influenced by whether or not diabetes was also present. As a result, the current study’s sex-specific results extend findings from a recent analysis of National Health and Nutrition Examination Survey (NHANES) 1988–1994 data reporting that in the presence of chronic kidney disease, retinopathy was a strong predictor of all-cause and CVD-related mortality in the adult population,14 as well as earlier results suggesting that the joint impact of retinopathy and macroalbuminuria or proteinuria significantly increased the risk of death on individuals with diabetes.36–37
The association between retinopathy and decreased survival was more prominent in men than women in our analysis involving older people, reflecting, in part, differential patterns of multi-morbidity, with men exhibiting more concomitance of conditions. Hence, rather than representing true biological differences between the sexes, point estimates elevated above one but not statistically significant for women due to wide confidence intervals may reflect the smaller number of women with these constellation of conditions, and given a larger sample of women, confidence intervals around the estimates may narrow becoming more consistent with results seen in men. Studies reporting on sex differences in retinopathy and mortality analyses are limited,15, 38 but in contrast to our results, a Finnish study examining CVD-mortality in men and women with diabetes found that proliferative retinopathy predicted all-cause, CVD, and CHD mortality in all participants and in women alone, independent of current smoking, hypertension, total cholesterol, HDL cholesterol, glycemic control of diabetes, duration of diabetes, and proteinuria.15 Participants in that study were, however, younger (ages ranged from 45–64 years) and all had type 2 diabetes.
The mechanisms underlying a direct association between retinopathy and mortality is not known. Retinopathy may be a biomarker indicative of structural or functional abnormalities in the systemic microvasculature, particularly in organs such as the kidney, heart and brain, related to endothelial dysfunction.12 It may also represent the severity of an individual’s allostatic load of cardiovascular risk factors, including smoking, hypertension, dyslipidemia, obesity, chronic kidney disease, and microalbuminuria, all of which have been associated with retinopathy in persons with and without diabetes.33 Consistent with this notion, men in our study were significantly more likely to have retinopathy, a history of cardiovascular disease, clinical evidence of a previous cardiovascular event, diabetes, microalbuminuria, and multiple brain infarcts compared to the women. Furthermore, men were also more likely to have multiple conditions concurrently compared to women, indicating poorer health and a greater propensity toward decreased survival.
The current study’s strengths include a large, randomly selected, population-based cohort of older adults, retinal examinations that were completed following standardized objective protocols with grading of retinopathy masked to the health status of participants, a vast range of data on participant characteristics and concomitant health conditions, and linkage to a complete, adjudicated registry of deaths with no loss to follow-up, as well as access to hospital data. Limitations include the availability of cause-of-death only through December 31, 2009, the cross-sectional study collection of health characteristics at a single time point in a cohort with European ancestry, and a possible selection bias between persons who completed the retinal examination and those who did not, all of which could have influenced the results. Additionally, data on clinical stroke events were collected and adjudicated only through December 31, 2012, which may have resulted in an underestimate of the number of strokes occurring throughout the entire follow-up period. The relatively small number of cases of diabetes with retinopathy could also be considered by some a study limitation, but fortunately for the populace, the low limitation is that the rate of hypertension in this sample of Icelandic elders was very high and although 78.7% of participants reported use of hypertensive medications and we adjusted for hypertension in our analytic models, we were unable to assess fully the impact of retinopathy and concomitant conditions specifically in normotensive persons.
In conclusion, findings from this study suggest that persons with retinopathy are at increased risk of mortality, particularly due to CVD. Concomitant health conditions in old age such as kidney dysfunction, cerebrovascular disease, and diabetes further increased risk of death among those with retinopathy, particularly for men. While the underlying reasons for the association between retinopathy and decreased survival remain unclear, it is possible that even minimal retinopathy indicates more extensive disease in the microcirculation that warrants referral for closer clinical management of general health in order to reduce the risk of clinical cardiovascular events and therein prolong healthy survival. Detailed study of mid-life factors will prove useful to ascertain the biology underlying how retinopathy, kidney dysfunction, diabetes, and hypertension come to manifest concomitantly and impact on survival in late life.
Supplementary Material
Acknowledgments
Financial Support
This work was supported by the National Institutes of Health (Intramural Research Programs of the National Institute of Aging and the National Eye Institute, ZIAEY00401), National Institutes of Health contract number N01-AG-1-2100, the Icelandic Heart Association, the Icelandic Parliament, the University of Iceland Research Fund and the Helga Jonsdottir and Sigurlidi Kristjansson Research Fund. The funders had no role in data collection, management, analysis and interpretation of the data, preparation, writing and approval of the manuscript, and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no proprietary or commercial interest in any materials discussed in this article.
REFERENCES
- 1.Wong TY, McIntosh R. Hypertensive retinopathy signs as risk indicators of cardiovascular morbidity and mortality. British Medical Bulletin. 2005;73 and 74:57–70. doi: 10.1093/bmb/ldh050. [DOI] [PubMed] [Google Scholar]
- 2.Henderson AD, Biousse V, Newman NJ, et al. Grade III or grade IV hypertensive retinopathy with severely elevated blood pressure. West J Emerg Med. 2012;13:529–534. doi: 10.5811/westjem.2011.10.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grunwald JE, Alexander J, Ying GS, et al. Retinopathy and chronic kidney disease in the Chronic Renal Insufficiency Cohort Study (CRIC) Arch Ophthalmol. 2012;130:1136–1144. doi: 10.1001/archophthalmol.2012.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grunwald JE, Ying GS, Maguire M, et al. Association between retinopathy and cardiovascular disease in patients with chronic kidney disease (from the Chronic Renal Insufficiency Cohort [CRIC] Study) Am J Cardiol. 2012;110:246–253. doi: 10.1016/j.amjcard.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan BB, Wong TY, Tyroler HA, Davis CE, Fuchs FD. Hypertensive retinopathy and incident coronary heart disease in high risk men. Br J Ophthalmol. 2002;86:1002–1006. doi: 10.1136/bjo.86.9.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung N, Rogers S, Couper DJ, Klein R, Sharrett AR, Wong TY. Is diabetic retinopathy an independent risk factor for ischemic stroke? Stroke. 2007;38:398–401. doi: 10.1161/01.STR.0000254547.91276.50. [DOI] [PubMed] [Google Scholar]
- 7.Bello NA, Pfeffer MA, Skali H, et al. Retinopathy and clinical outcomes in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia. BMJ Open Diabetes Research and Care. 2014;2:e000011. doi: 10.1136/bmjdrc-2013-000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawasaki R, Tanaka S, Tanaka S, et al. Risk of cardiovascular disease is increased even with mild diabetic retinopathy. The Japan Diabetes Complications Study. Ophthalmology. 2013;120:574–582. doi: 10.1016/j.ophtha.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 9.Gunnlaugsdottir E, Halldorsdottir S, Klein R, et al. Retinopathy in older persons with and without diabetes mellitus: the Age, Gene/Environment Susceptibility-Reykjavik Study (AGES-R) Diabetologia. 2012;55:671–680. doi: 10.1007/s00125-011-2395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu C, Cotch MF, Sigurdsson S, et al. Retinal and cerebral microvascular signs and diabetes: the age, gene/environment susceptibility-Reykjavik study. Diabetes. 2008;57:1645–1650. doi: 10.2337/db07-1455. [DOI] [PubMed] [Google Scholar]
- 11.Xu L, Wang YX, Xie XW, Jonas JB. Retinopathy, mortality. The Beijing Eye Study. Graefes Arch Clin Exp Ophthalmol. 2008;246:923–925. doi: 10.1007/s00417-008-0773-z. [DOI] [PubMed] [Google Scholar]
- 12.Liew G, Wong TY, Mitchell P, Cheung N, Wang JJ. Retinopathy predicts coronary heart disease mortality. Heart. 2009;95:391–394. doi: 10.1136/hrt.2008.146670. [DOI] [PubMed] [Google Scholar]
- 13.Sairenchi T, Iso H, Yamagishi K, et al. Mild retinopathy is a risk factor for cardiovascular mortality in Japanese with and without hypertension. The Ibaraki Prefectural Health Study. Circulation. 2011;124:2502–2511. doi: 10.1161/CIRCULATIONAHA.111.049965. [DOI] [PubMed] [Google Scholar]
- 14.Juutilainen A, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Retinopathy predicts cardiovascular mortality in type 2 diabetic men and women. Diabetes Care. 2007;30:292–299. doi: 10.2337/dc06-1747. [DOI] [PubMed] [Google Scholar]
- 15.Kramer CK, Rodrigues TC, Canani LH, Gross JL, Azevedo MJ. Diabetic retinopathy predicts all-cause mortality and cardiovascular events in both type 1 and type 2 diabetes: meta-analysis of observational studies. Diabetes Care. 2011;34:1238–1244. doi: 10.2337/dc11-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricardo AC, Grunwald JE, Parvathaneni S, Goodin S, Ching A, Lash JP. Retinopathy and CKD as predictors of all-cause and cardiovascular mortality: National Health and Nutrition Examination Survey (NHANES) 1988–1994. Am J Kidney Dis. 2014;64:198–203. doi: 10.1053/j.ajkd.2014.01.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Hecke MV, Dekker JM, Stehouwer CD, et al. Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB prospective complications study. Diabetes Care. 2005;28:1383–1389. doi: 10.2337/diacare.28.6.1383. [DOI] [PubMed] [Google Scholar]
- 18.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein R, Davis MD, Magli YL, et al. The Wisconsin Age-Related Maculopathy Grading System. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 20.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs-an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 21.Wong TY, Klein R, Islam FM, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141:446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grosso A, Cheung N, Veglio F, Wong TY. Similarities and differences in early retinal phenotypes in hypertension and diabetes. J Hypertens. 2011;29:1667–1675. doi: 10.1097/HJH.0b013e3283496655. [DOI] [PubMed] [Google Scholar]
- 23.Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987e–1003e. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 24.Saczynski JS, Jonsdottir MK, Garcia ME, et al. Cognitive impairment: an increasingly important complication of type 2 diabetes The Age, Gene/Environment Susceptibility-Reykjavik Study. Am J Epidemiol. 2008;168:1132–1139. doi: 10.1093/aje/kwn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 26.Sigurdsson S, Aspelund T, Forsberg L, et al. Brain tissue volumes in the general population of the elderly: the AGES-Reykjavik study. Neuroimage. 2012;59:3862–3870. doi: 10.1016/j.neuroimage.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson PW, Cupples LA, Kannel WB. Is hyperglycemia associated with cardiovascular disease? The Framingham Study. Am Heart J. 1991;121:586–590. doi: 10.1016/0002-8703(91)90729-2. [DOI] [PubMed] [Google Scholar]
- 28.Seeman T, Mendes de Leon C, Berkman L, Ostfeld A. Risk factors for coronary heart disease among older men and women: a prospective study of community-dwelling elderly. Am J Epidemiol. 1993;138:1037–1049. doi: 10.1093/oxfordjournals.aje.a116822. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Prevention of blindness from diabetes mellitus. Report of a WHO consultation. Geneva: WHO; 2006. [Accessed 10 July 2015]. http://whqlibdoc.who.int/publications/2006/924154712x_eng.pdf. [Google Scholar]
- 30.Klein R, Klein BEK, Moss SE, Cruickshanks KJ. Association of ocular disease and mortality in a diabetic population. Arch Opthalmol. 1999;117:1487–1495. doi: 10.1001/archopht.117.11.1487. [DOI] [PubMed] [Google Scholar]
- 31.Hirai FE, Moss Se, Knudtson MD, Klein BEK, Klein R. Retinopathy and survival in a population without diabetes. Am J Epidemiol. 2007;166:724–730. doi: 10.1093/aje/kwm126. [DOI] [PubMed] [Google Scholar]
- 32.Wong TY, Klein R, Nieto FJ, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality. Ophthalmology. 2003;110:933–940. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 33.Van Hecke MV, Dekker JM, Nijpels G, Moll AC, Van Leiden HA, Heine RJ. Retinopathy is associated with cardiovascular and all-cause mortality in both diabetic and nondiabetic subjects: the Hoorn Study. Diabetes Care. 2003;26:2958. doi: 10.2337/diacare.26.10.2958. [DOI] [PubMed] [Google Scholar]
- 34.Di Angelantonio E, Chowdhury R, Sarwar N, Aspelund T, Danesh J, Gudnason V. Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: prospective population based cohort study. BMJ. 2010;341:c4986. doi: 10.1136/bmj.c4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Elderen SS, Zhang Q, Sigurdsson S, et al. Brain volume as an integrated marker for the risk of death in a community-based sample: Age, Gene/Environment Susceptibility-Reykjavik study. J Gerontol A Biol Sci Med Sci. 2014:1–7. doi: 10.1093/gerona/glu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grauslund A, Green A, Sjolie AK. Proliferative retinopathy and proteinuria predict mortality rate in type 1 diabetic patients from Fyn County, Denmark. Diabetologia. 2008;51:583–588. doi: 10.1007/s00125-008-0953-8. [DOI] [PubMed] [Google Scholar]
- 37.Tong PCY, Kong AP, So WY, et al. Interactive effect of retinopathy and macroalbuminuria on all-cause mortality, cardiovascular and renal endpoints in Chinese patients with Type 2 diabetes mellitus. Diabet Med. 2007;24:741–746. doi: 10.1111/j.1464-5491.2007.02145.x. [DOI] [PubMed] [Google Scholar]
- 38.Wong TY, Klein R, Sharrett AR, et al. The prevalence and risk factors of retinal microvascular abnormalities in older persons: the Cardiovascular Health Study. Ophthalmology. 2003;110:658–666. doi: 10.1016/S0161-6420(02)01931-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.