Introduction
Pseudoxanthoma elasticum (PXE) is an inherited systemic disease of connective tissue primarily affecting the skin, retina and cardiovascular system. It is characterised pathologically by elastic fibre mineralisation and clinically by angioid streaks, yellow skin papules and cardiovascular involvement. It is important to recognize the disease early to minimize the occurrence of retinal or gastrointestinal haemorrhage and cardiovascular complications. We herein present a case of PXE which manifested with cutaneous features along with renal cortical microcalcifications. Such a finding in isolation has rarely been reported earlier in literature.
Case Report
A 32 year old lady presented to the skin out patient department (OPD) with complaints of skin coloured raised lesions on the neck for the last six years. The lesions were mildly itchy in nature. There was no history of photosensitivity, oral ulcers, laxity of skin/joints, claudication or bleeding from any site. Similar complaints were also present for the last four years in her younger sister aged 26 years. The patient was a product of non-consanguineous marriage. However both her pregnancies were normal and her children did not have similar complaints.
On examination her pulse and blood pressure were within normal limits. There was no pallor. Dermatological examination revealed multiple yellow papules over anterior and lateral aspects of neck (Fig. 1a) along with a solitary irregular plaque on the nape of the neck (Fig. 1b). Similar such papules in a reticular arrangement were found in the axillae (Fig 1c). Reticular hyperpigmentation was also observed in the periumbilical area along with a solitary yellow papule (Fig. 1). Mucosa and appendages were normal. On eye examination, angioid streaks or other associated ophthalmological abnormalities were not present. Systemic examination was also normal.
Fig. 1.
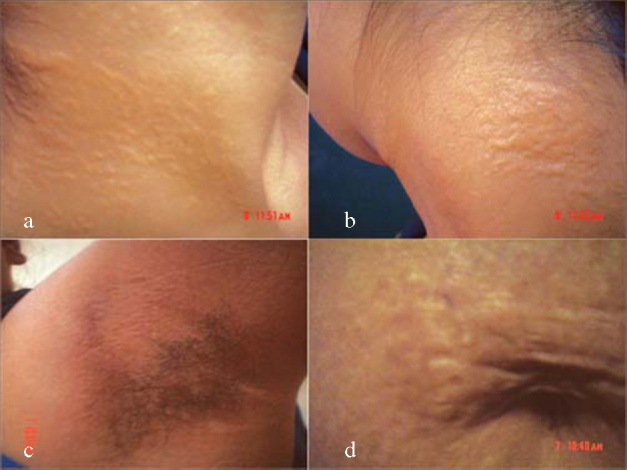
Dermatological manifestations with (a) cobblestone lesions on the neck (b) irregular plaque on the nape of neck (c) axillary reticular papules and (d) periumbilical reticulate pigmentation.
Investigations revealed normal haematological and biochemical parameters. Skin biopsy from the lesion showed numerous elastic fibres with altered morphology in the mid and deep reticular dermis. The elastic fibres were swollen, clumped and stained basophilic with haematoxylin and eosin (H&E) (Fig. 2a). Von Kossa stain confirmed calcification of elastic fibres (Fig. 2b). The collagen fibres were however normal. The diagnosis of PXE was thus confirmed. Stool occult blood and 24 hour urinary protein were normal. Serum calcium, phosphorus and alkaline phosphatase were also normal. Radiograph of the chest, electro and echocardiography did not reveal any cardiovascular abnormality. Ultrasonography of abdomen revealed multiple tiny hyperechoic foci with faint posterior acoustic shadowing involving the renal cortex bilaterally, suggestive of scattered specks of calcifications (Fig. 3a). These could represent early renal microvascular calcifications or cortical nephrocalcinosis. Cortico-medullary differentiation was well maintained bilaterally. There were no other focal lesions. Renal doppler study showed normal flow in all major intrarenal vessels (Fig. 3b). Patient was referred to the urologist who prescribed regular follow up with no active treatment. The patient was also advised to follow up in skin and eye OPD every six months and to report for laboratory investigations regularly.
Fig. 2.
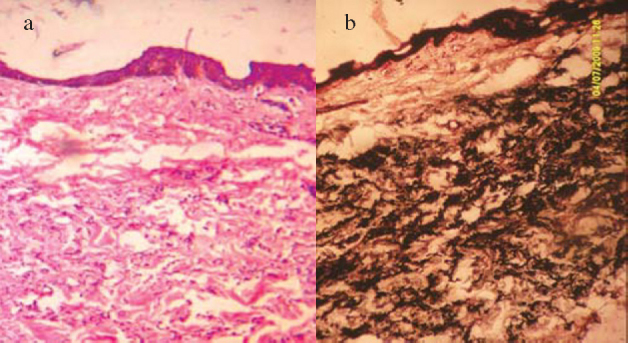
Photomicrographs demonstrating calcified elastic fibres with (a) H & E stain and (b) von Kossa stain (40X).
Fig. 3.
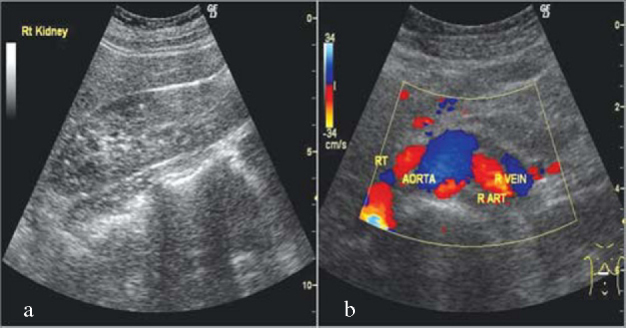
Radiological findings with (a) Ultrasound of the abdomen showing early cortical nephrocalcinosis/renal microvascular calcifications and (b) Renal doppler showing normal blood flow.
Discussion
PXE (Gronblad - Strandberg syndrome) is an inherited systemic connective tissue disorder first described in 1896 by Jean Darier. Prevalence of the disease ranges from 1 in 100,000 to 160,000 with a female to male ratio of 2:1. The inheritance is usually autosomal recessive but may be autosomal dominant or sporadic. PXE is caused by mutations in the ATP-binding ABCC6 linked multidrug resistance associated protein 6 (MRP6) gene which has been mapped to 16p13.1 [1]. Recently, oxidative stress has been reported to play an important role in the pathogenesis of PXE [2].
The cutaneous manifestations of PXE are highly characteristic. The lesions usually develop in early childhood but occasionally they first appear in adulthood as in our case. Clinically yellow papules that coalesce into plaques with a plucked chicken skin or cobblestone appearance occur in flexural regions. Our patient had such features in the neck, axillae and periumbilical region. Later in the course of the disease, redundant folds of skin may develop especially in the neck, axillae and groin. Angioid streaks which are red to brown, curvilinear bands that radiate from the optic disc occur in 85% of patients. Vascular complications include premature atherosclerosis with secondary hypertension, intermittent claudication, angina pectoris, abdominal pain and gastrointestinal haemorrhage. Mitral valve prolapse also has a higher prevalence in PXE [3]. There may be an increased risk of first trimester abortion in women [4]. On H&E stains, elastic fibres are basophilic because of the calcium deposition. Special stains for calcium deposits namely von Kossa and the elastic fibres namely Verhoeff van Gieson and Orcein can confirm the diagnosis. Differential diagnosis includes cutis laxa, actinic damage, mid-dermal elastolysis, anetoderma, post-inflammatory elastosis, perifollicular elastolysis and long term penicillamine therapy [5]. However, these entities are all characterized by loss of elastic fibres in the dermis.
Currently no treatment modalities are available for this devastating disease. A diet low in lipids and calcium (600–1200 mg/d) is recommended to reduce cardiovascular involvement. Prophylactic antibiotics to prevent bacterial endocarditis in cases of mitral valve prolapse and weight reduction with regular exercise for intermittent claudication is also advocated. Avoidance of heavy weight lifting, head trauma and straining is advised to reduce risk of retinal haemorrhage. Aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided to prevent gastrointestinal bleeding. Vitamin C has been found to relieve oxidative stress and Interferon α-2a may be a potential treatment. Genetic counselling is indicated for the patient's relatives. It is very important to recognize the disease early, so that the risk of systemic complications can be minimized [6]. Regular faecal occult blood testing, complete blood count, ophthalmologic and cardiovascular system examination should be performed to detect the abnormalities as early as possible. Visceral calcification in the form of pulmonary, renal, pancreatic and splenic calcification has been rarely reported in PXE [7]. Various cases of nephrocalcinosis along with other features of PXE have been reported earlier though diffuse renal calcification has been mentioned only in a report of two cases [8, 9]. Multiple highly reflective foci in the corticomedullary junction have been reported earlier [10, 11]. However, it is for the first time in literature that a case of PXE is being reported with cutaneous features and renal cortical calcification alone without proteinuria, hypertension, angioid streaks or any other features of the disease. This case could be a phenotypic variation of the normal variants of PXE or a presentation of a very early stage of the disorder.
Conflicts of Interest
None identified
References
- 1.Chassaing N, Martin L, Calvas P, Le-Bert M, Hovnanian A. Pseudoxanthoma elasticum: a clinical, pathophysiological and genetic update including 11 novel ABCC 6 mutations. J Med Genet. 2005;42:881–892. doi: 10.1136/jmg.2004.030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takata T, Ikeda M, Kodama H, Kitagawa N. Treatment of pseudoxanthoma elasticum with tocopherol acetate and ascorbic acid. Paediatr Dermatol. 2007;24:424–425. doi: 10.1111/j.1525-1470.2007.00467.x. [DOI] [PubMed] [Google Scholar]
- 3.Lebwohl MG, Distefano D, Prioleau PG, Uram M, Yannuzzi LA, Fleischmajer R. Pseudoxanthoma elasticum and mitralvalve prolapse. N Engl J Med. 1982;307:228–231. doi: 10.1056/NEJM198207223070406. [DOI] [PubMed] [Google Scholar]
- 4.Bercovitch L, Leroux T, Terry S, Weinstock MA. Pregnancy and obstetrical outcomes in pseudoxanthoma elasticum. British Journal of Dermatology. 2004;151:1011–1018. doi: 10.1111/j.1365-2133.2004.06183.x. [DOI] [PubMed] [Google Scholar]
- 5.Yen A, Wen J, Grau M, Sanchez RL, Smith EB. Elastoderma. J Am Acad Dermatol. 1995;33:389–392. doi: 10.1016/0190-9622(95)91442-0. [DOI] [PubMed] [Google Scholar]
- 6.Laube S, Moss C. Pseudoxanthoma elasticum. Arch Dis Child. 2005;90:754–756. doi: 10.1136/adc.2004.062075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suarez MJ, Garcia JB, Orense M, Raimunde E, Lopez MV, Fernandez O. Sonographic aspects of pseudoxanthoma elasticum. Paediatr Radiol. 1991;21:538–539. doi: 10.1007/BF02011738. [DOI] [PubMed] [Google Scholar]
- 8.Makharia GK, Thapa BR, Poddar U. Pseudoxanthoma elasticum: A rare cause of recurrent gastrointestinal bleeding in a child. Indian J Gastroenterol. 2004;23:231–232. [PubMed] [Google Scholar]
- 9.Chraïbi R, Ismaili N, Belgnaoui F. Pseudoxanthoma elasticum and nephrocalcinosis. Ann Dermatol Venereol. 2007;134:764–766. doi: 10.1016/s0151-9638(07)92534-x. [DOI] [PubMed] [Google Scholar]
- 10.Ortiz Gorraiz MA, Casares Arias A, Tallada Buñuel M, Vicente Prados FJ, Honrubia Vílchez B, Fernández Sánchez A. Urologic findings in pseudoxanthoma elasticum: report one case. Actas Urol Esp. 2005;29:96–99. doi: 10.1016/s0210-4806(05)73205-8. [DOI] [PubMed] [Google Scholar]
- 11.Crespi G, Derchi LE, SafÛoti S. Sonographic detection of renal changes in pseudoxanthoma elasticum. Urol Radiol. 1992;13:223–225. doi: 10.1007/BF02924627. [DOI] [PubMed] [Google Scholar]


