Abstract
Background
Acute kidney injury (AKI) is common after cardiac surgery and is associated with post-operative mortality. Perioperative cardiac biomarkers may predict AKI and mortality.
Objective
We evaluated whether cardiac biomarkers were associated with severe AKI, defined as a doubling in serum creatinine or requiring renal replacement therapy during hospital stay after surgery, and mortality.
Methods
In a prospective multicenter cohort of adults undergoing cardiac surgery, we measured the following biomarkers in pre- and post-operative banked plasma: high-sensitivity troponin T (hs-cTnT), troponin I (cTnI), CK-MB and NT-proBNP.
Results
In the patients who were discharged alive, severe AKI occurred in 37/960 (3.9%) and 43/960 (4.5%) died within 1 year of follow-up. NT-proBNP was the only pre-operative biomarker that was independently associated with severe AKI (with log transformation, adjusted OR=1.4, 95% CI (1.0, 1.9)). Biomarkers measured within 6 hours of surgery (Day 1) were all associated with severe AKI. Pre-operative NT-proBNP was also independently associated with 1-year mortality (with log transformation, adjusted OR=1.7, 95% CI (1.2–2.2)). Patients in the highest tertile for NT-proBNP pre-operatively (>1006.4 ng/L) had marked increases in their risk for 1-year mortality (adjusted OR=27.2, 95%CI (3.5–213.5)). Day 1 NT-proBNP was associated with mortality independently of change in serum creatinine from pre-operative baseline.
Conclusion
Of the studied biomarkers, NT-proBNP was the only pre-operative biomarker independently associated with severe AKI and mortality. Early increases in post-operative cardiac biomarkers were associated with severe AKI after cardiac surgery. Future research should focus on whether interventions that lower NT-proBNP can impact upon post-operative outcomes.
Keywords: Biomarkers, Cardiac Surgery, Acute Kidney Injury, Mortality, Troponin, BNP
Ultramini-abstract
Acute kidney injury (AKI) is common after cardiac surgery and is associated with post-operative mortality. In a prospective cohort of adults undergoing cardiac surgery, early increases in post-operative cardiac biomarkers were associated with severe AKI after cardiac surgery. Pre-operative NT-proBNP levels were independently associated with post-operative severe AKI and mortality.
Introduction
Acute kidney injury (AKI) is a frequent complication after cardiac surgery. In the CORONARY trial, when defined as an increase of 50% or more in baseline serum creatinine, AKI occurs in 21% of patients undergoing cardiac surgery with cardiopulmonary bypass.(1) Dialysis is required in 1–2% of patients undergoing cardiac surgery.(2, 3) The development of post-operative AKI is associated with an increased mortality.(4, 5) Pre-operative and intraoperative risk factors for AKI have been identified and include age, congestive heart failure, proteinuria, longer cardiopulmonary bypass time and post-operative low output state.(3, 5–7) Validated pre-operative risk prediction models for dialysis requiring AKI exist.(8, 9) However, less severe AKI is also associated with morbidity and mortality.(2, 5, 6, 10–12) On an individual patient level, the identification of patients at high risk for AKI could lead to modifications in therapeutic decisions or to a more informed consent before cardiac surgery. For the medical community, it means the possibility of evaluating therapies in a high risk, high response population, thereby increasing the chance to identify potentially promising therapies without large trials. Perioperative cardiac biomarkers, such as CK-MB, cardiac troponin (cTn) and NT-proBNP, are associated with an increased mortality risk after cardiac surgery.(13–15) Higher perioperative NT-proBNP levels have been associated with post-operative AKI, independently of EuroSCORE.(16, 17) With the imminent move towards high-sensitivity cardiac troponin assays, the prognostic value of this biomarker needs to be clarified in patients undergoing cardiac surgery.(18) In a prospective multicenter cohort of adults undergoing cardiac surgery, we aimed to evaluate whether pre- and post- operative cardiac biomarkers, including high-sensitivity cardiac troponin T (hs-cTnT), are associated with the development of severe AKI within the hospital stay, 1-year and long-term mortality after cardiac surgery.
Methods
Study design, setting and patient selection
Detailed methods of the Translational Research Investigating Biomarker Endpoints in AKI (TRIBE-AKI) cardiac surgery cohort (NCT00774137) have been described previously.(19) In summary, following research ethics board approval and informed consent, 1219 patients undergoing cardiac surgery (coronary artery bypass grafting (CABG) or valve) who were at high risk for AKI at six academic medical centers in North America were prospectively enrolled between July 2007 and December 2009. The inclusion and exclusion criteria were designed to capture high-risk patients undergoing cardiac surgery to increase the event rates for AKI. High risk for AKI was defined by the presence of one or more of the following: emergency surgery, preoperative serum creatinine > 2 mg/dl (>177 µmol/L), ejection fraction ≤35% or grade 3 or 4 left ventricular dysfunction, age > 70 years, diabetes mellitus, concomitant CABG and valve surgery, or repeat revascularization surgery. The evaluation of cardiac biomarkers was a pre-specified substudy of the TRIBE-AKI main study. Samples were collected with the a priori defined goal of conducting these analyses.
Clinical and Laboratory Data Collection
Prior to surgery, we recorded demographic and patient history data. Information regarding the surgical procedure was obtained from the medical record using the standardized definitions of the Society of Thoracic Surgeons (STS) data collection tool (www.sts.org).
Following informed consent, samples were collected pre-operatively (less than 60 days before surgery), on day 1 (within 6 hours after surgery), and daily for a maximum of 5 days. The highest measured value was the highest value measured post-operatively. Sample collection was stopped on day 3 in participants transferred out of the intensive care unit (ICU) who did not meet our severe AKI definition. Samples were collected in EDTA tubes, centrifuged to separate plasma and subsequently stored at −80°C.
We obtained vital status after discharge through various mechanisms that allowed complete follow up at one year. For those living in the United States, we phoned to the patients’ homes, searched the National Death Index, and reviewed hospital records. For patients from Canada, we used phone calls, as well as data held at the Institute for Clinical Evaluative Sciences to acquire vital status. These datasets were linked using unique, encoded identifiers and analyzed at the Institute for Clinical Evaluative Sciences (ICES).
Biomarker Measurements
Following one freeze-thaw (storage at −80°C), bioma rker measurements were conducted in batches with an Elecsys 2010 analyzer, Roche Diagnostics for NT-proBNP (ng/L) and hs-cTnT (ng/L), and Beckman Coulter’s Access II instrument for cTnI (ug/L; AccuTnI assay) and CK-MB (ug/L). The imprecision of the assays were within the manufacturers acceptable range and were as follows: NTproBNP inter-assay quality control (QC) level 1 (n=148, mean= 15.7pmol/L) coefficient of variation (CV)=5% and level 2 (n=147, mean= 4423 ng/L) CV=4%, cTnI inter-assay QC level 1 (n= 165, mean= 0.04ug/L) CV=22% and level 3 (n= 167, mean= 3.2ug/L) CV= 6%, CK-MB inter-assay QC level 1 (n= 164, mean= 3.8ug/L) CV=6% and level 3 (n=169, mean= 69.1ug/L) CV= 4% and hs-cTnT inter-assay QC level 1 (n= 135, mean= 25.8ng/L) CV=10% and QC level 2 (n=134, mean= 1993.3ng/L) CV=3%. Samples were analyzed according to the manufacturer’s specifications and laboratory staff were blinded to clinical outcomes. For NTproBNP, the analyzer automatically calculated the concentration in pmol/L; however, to be consistent with the literature preference for this analyte (i.e., mass units) the results were converted to ng/L by the following conversion factor: pmol/L × 8.457 = ng/L. Pre-operative and post-operative serum creatinine was measured as part of routine clinical care in hospital laboratories.
Statistical Analysis
Patient characteristics were compared between groups of patients with and without primary outcomes (severe AKI defined as a doubling in serum creatinine from baseline or requiring renal replacement therapy during hospital stay, and mortality at one year and on long-term follow-up) using two-sample t-test or Wilcoxon-Mann-Whitney rank sum test for continuous variables and chi-squared test for categorical and binary variables. For each biomarker at each time point, we divided the cohort into tertiles to evaluate the association of each specific biomarker at each specific time point with severe AKI and 1-year mortality. After continuous log-transformation of the biomarkers, logistic regression models were used to determine the unadjusted and adjusted odds ratios for severe AKI and 1-year mortality. Cox regression models were used to determine the unadjusted and adjusted hazard ratios for long term mortality (median follow-up of 3 years). Adjustments were made using covariates that predict severe AKI and mortality age (per year), sex, white race, cardiopulmonary bypass (CBP) time > 120 minutes, non-elective surgery, pre-op estimated glomerular filtration rate (eGFR), diabetes, hypertension, center, congestive heart failure (CHF), myocardial infarction (MI), pre-operative urine albumin to creatinine ratio, and type of surgery CABG or valve versus all others)].(5, 6, 8, 9) Each time point was handled as an independent event and analyzed separately.
Receiver operator characteristic (ROC) curves were constructed to find the optimal cut-point of the ROC curve (i.e. the Youden index) for the prediction of severe AKI and 1-year mortality for each biomarker at each time point. We also identified the cut-points corresponding to the lower value of each tertile range.
We stratified the highest measured biomarker values in tertiles and built Kaplan Meier curves in order to visually show the potential association between highest measured biomarker values and long-term mortality.
All two-sided p-values less than 0.05 were considered statistically significant. Statistical analyses were conducted using SAS 9.3 (SAS Institute, Cary, North Carolina). Small cell counts are only presented for data collected by TRIBE-AKI and not from ICES data holdings.
Results
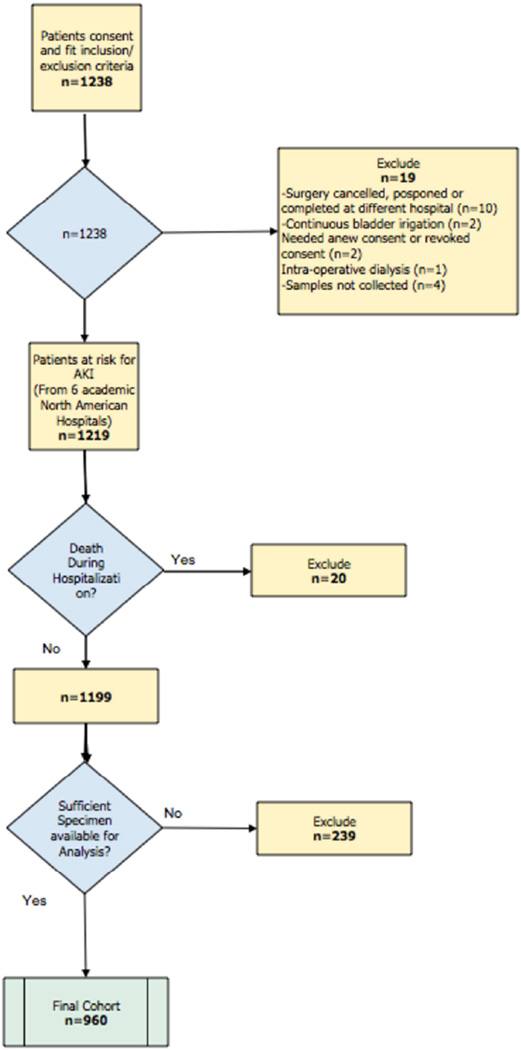
Between July 2007 and December 2009, 1238 eligible patients undergoing cardiac surgery in participating centers consented to participate. Of those, 239 were excluded because samples were insufficient to measure all cardiac biomarkers on three consecutive post-operative days, 19 for various other reasons and 20 additional patients were excluded after they died in hospital (see Figure 1). Of the 960 patients included in our analyses, 37 (3.9%) developed severe AKI, 43 (4.5%) died at 1 year and 104 (10.8%) died on long-term follow-up (Table 1 and Appendix 1). AKI occurred after a median of 3 days (IQR 2–4) after surgery.
Figure 1.
Study Flow Diagram
Table 1.
Baseline Patient Characteristics by Severe AKI
| Characteristics | Severe AKI | No Severe AKI | P-value |
|---|---|---|---|
| N (%) | 37 (3.9) | 923 (96.1) | |
| Age, (years) mean (SD) | 70.7 (10.4) | 71.53 (10) | 0.6 |
| Male, n (%) | 27 (73) | 628 (68) | 0.5 |
| Caucasian, n (%) | 33 (89.2) | 864 (93.6) | 0.3 |
| Previous history, n (%) | |||
| Diabetes | 16 (43.2) | 363 (39.3) | 0.6 |
| Hypertension | 34 (91.9) | 725 (78.5) | 0.05 |
| Congestive Heart Failure | 15(40.5) | 215 (23.3) | 0.02 |
| LVEF<40% | 3 (8.1) | 95 (10.3) | 0.7 |
| eGFR, n (%) | |||
| >60 mL/min per 1.73 m2 | 22 (59.5) | 611 (66.2) | 0.2 |
| 30–60 mL/min per 1.73 m2 | 12 (32.4) | 284 (30.8) | |
| < 30 mL/min per 1.73 m2 | 3 (8.1) | 28 (3) | |
| Urine albumin/creatinine, n (%) | |||
| <10 | 10 (27) | 326 (35.3) | 0.8 |
| 10–30 | 12 (32.4) | 273 (29.6) | |
| 30–300 | 12 (32.4) | 255 (27.6) | |
| >300 | 3 (8.1) | 58 (6.3) | |
| Non-elective surgery, n (%) | 10 (27) | 177 (19.2) | 0.2 |
| Surgery, n (%) | |||
| Isolated CABG or valve | 28 (75.7) | 724 (78.4) | 0.7 |
| CABG + valve | 9 (24.3) | 199 (21.6) | 0.9 |
| Off-pump surgery, n (%) | 4 (10.8) | 83 (9.0) | 0.9 |
| Re-do surgery, n (%) | 1 (2.7) | 17 (1.8) | 0.5 |
| CBP time (minutes), mean (SD) | 177 (101) | 110 (55) | <0.001 |
| Cross-clamp time (minutes), mean (SD) |
121(67) | 75 (42) | <0.001 |
n: Number
SD: Standard Deviation
CPB: Cardiopulmonary Bypass
LVEF: Left Ventricular Ejection Fraction
eGFR: Estimated Glomerular Filtration Rate
CABG: Coronary Artery Bypass Graft
Patients who developed severe AKI were more likely to have a history of hypertension and congestive heart failure. They had longer cardiopulmonary bypass and cross clamp times. Congestive heart failure and post-operative severe AKI were significantly more common in patients who died at one year.
Nine patients (0.9%) required acute dialysis (Table 2 and Appendix 2). Non-renal complications occurred more frequently in the patients who developed severe AKI. Mechanical ventilation duration, ICU and hospital stay were longer for the patients with severe AKI and those who died at 1 year and on long-term follow-up.
Table 2.
Post-Operative Complications During Hospitalization for Cardiac Surgery by Severe AKI
| Complications | Severe AKI | No Severe AKI | P-value |
|---|---|---|---|
| Clinical AKI, n (%) | |||
| Increase > 50% or >0.3 mg/dL | 37 (100.0) | 293 (31.7) | <0.001 |
| Acute dialysis | 9 (24.3) | 0 (0.0) | <0.001 |
| Non-renal complications, n (%) | |||
| none | 19 (51.4) | 572 (62.0) | <0.001 |
| 1 or 2 | 6 (16.2) | 283 (30.7) | |
| >2 | 12 (32.4) | 68 (7.4) | |
| Oliguria on post-operative day 1, n (%) | 0 (0.0) | 11 (1.2) | 0.6 |
| Mechanical ventilation > 48 hours, n (%) | 15 (40.5) | 19 (2.1) | <0.001 |
| ICU length of stay (days), median (IQR) | 5 (3–17) | 2 (1–3) | <0.001 |
| Hospital length of stay (days), median (IQR) |
17 (8–27) | 6 (5–8) | <0.001 |
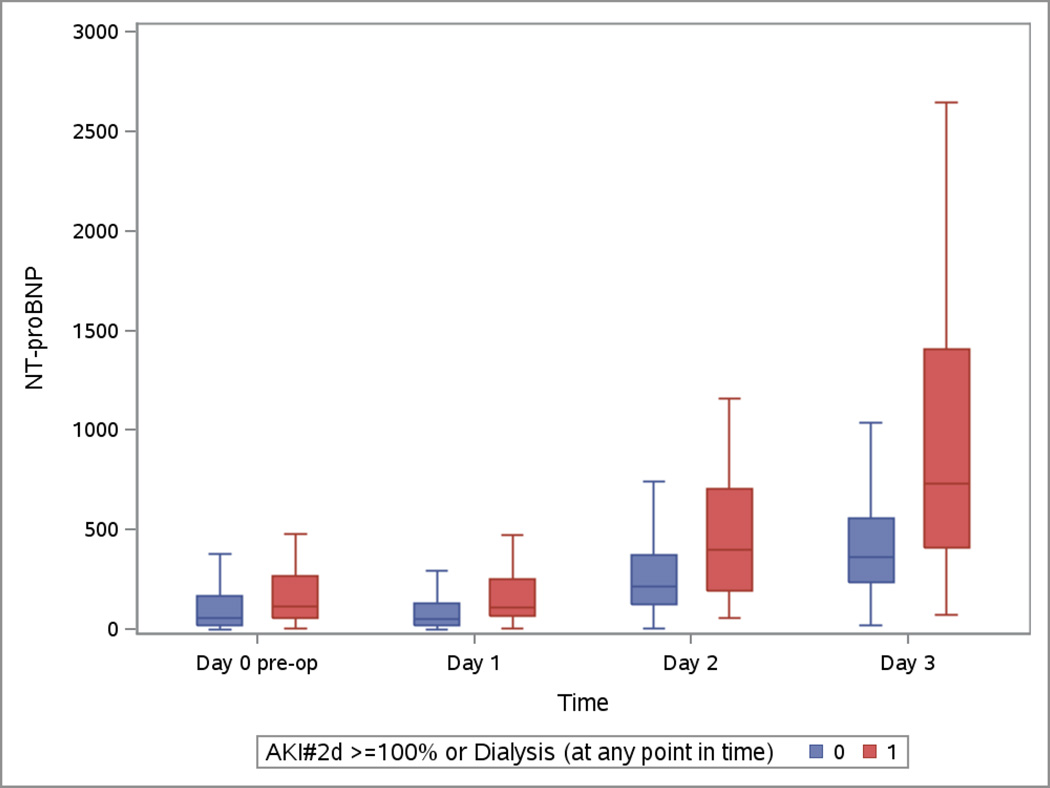
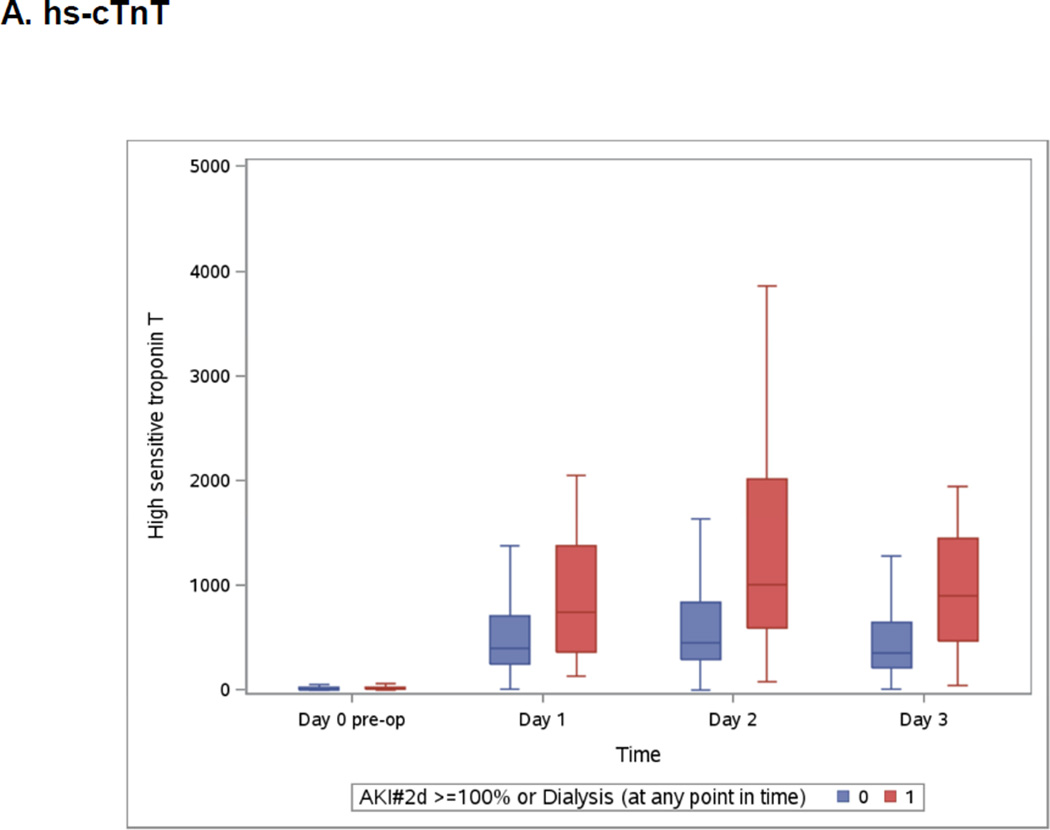
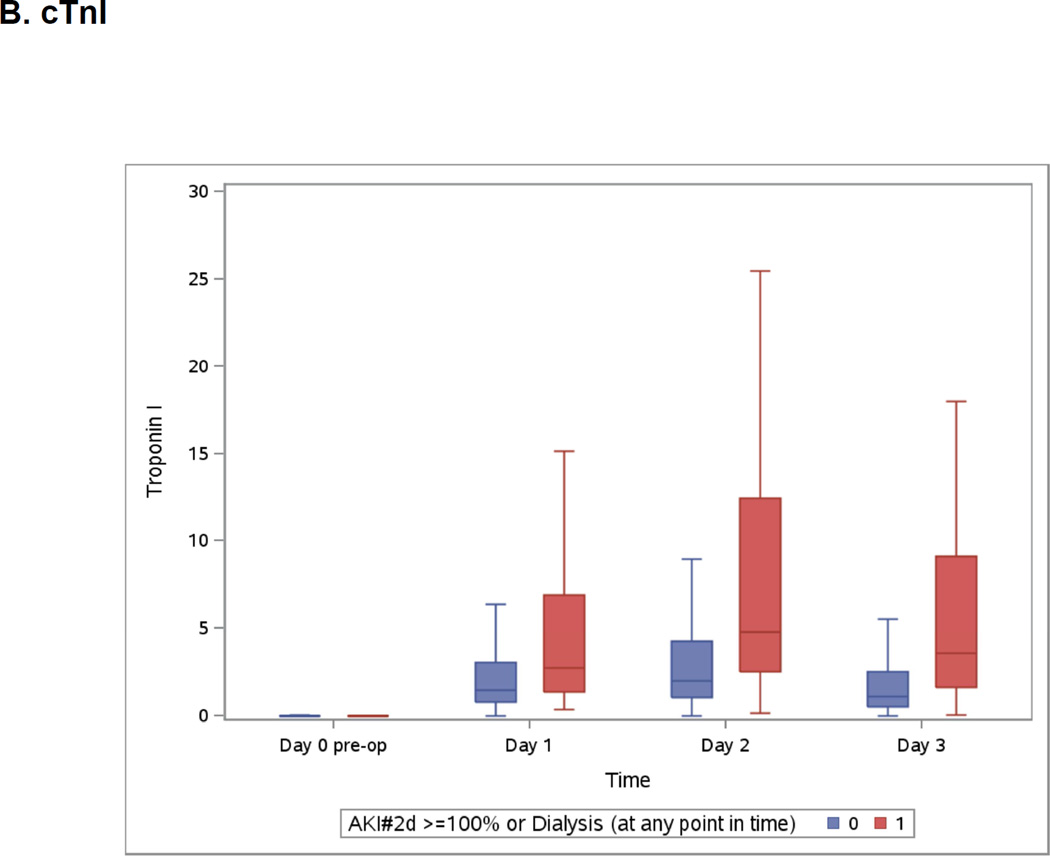
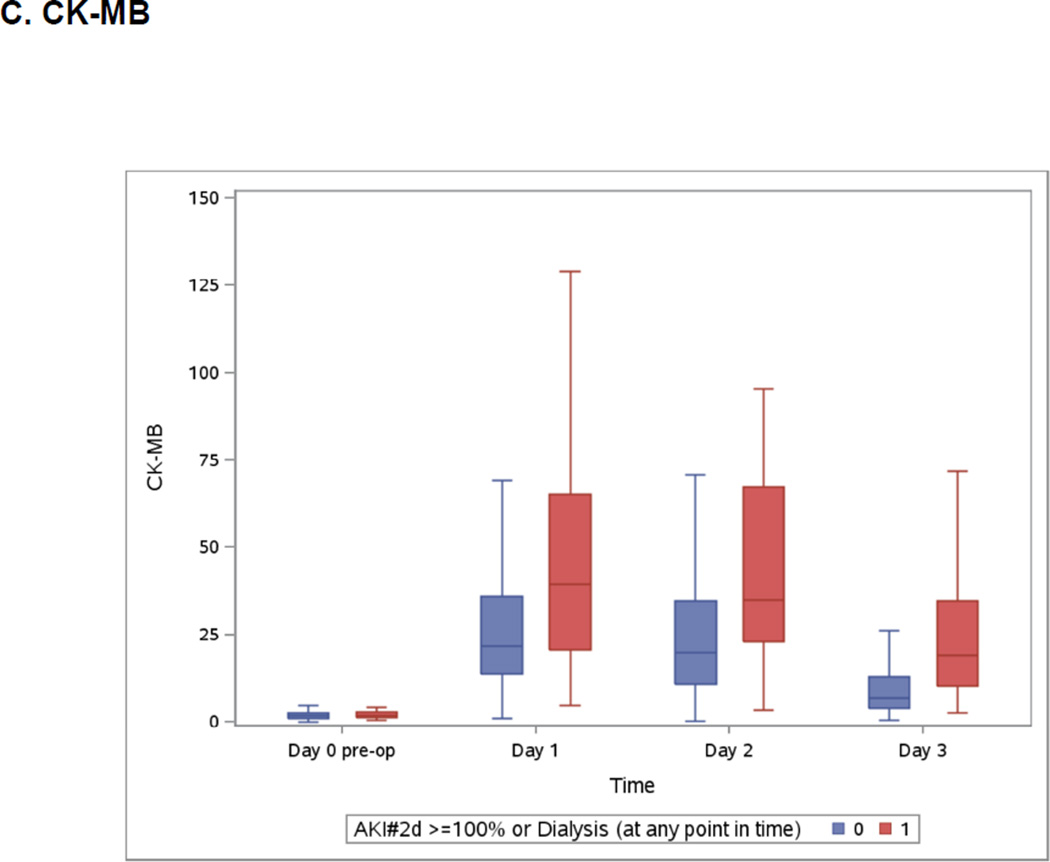
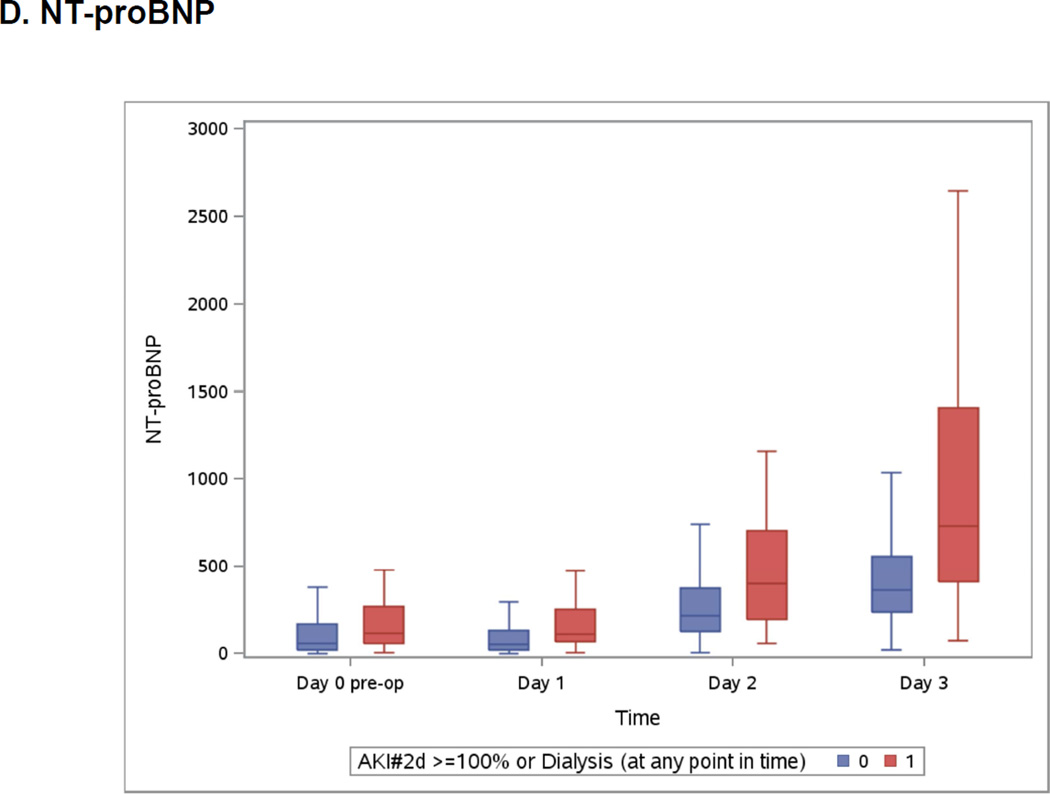
Both highest measured hs-cTnT and cTnI were on day 2 (Appendix 3) whereas highest measured CK-MB was on day 1 and NT-pro-BNP was at its highest on day 3 (Appendix 3). All post-operative biomarkers were higher in the group that developed severe AKI.
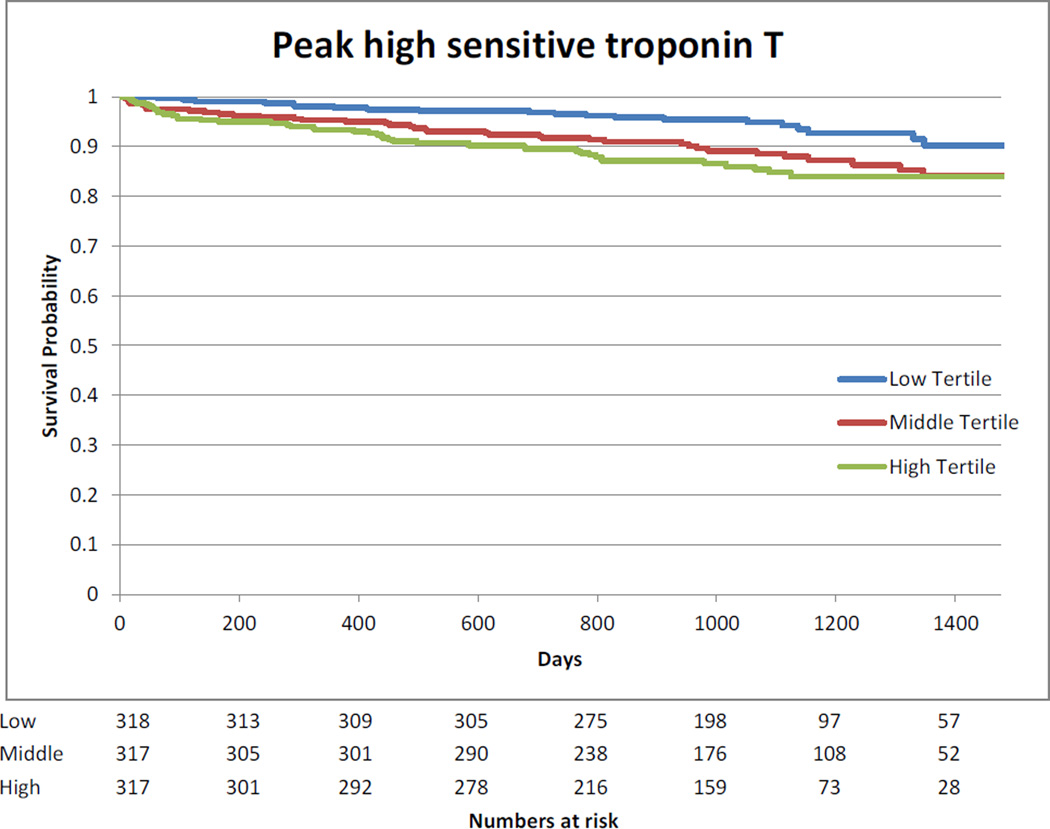
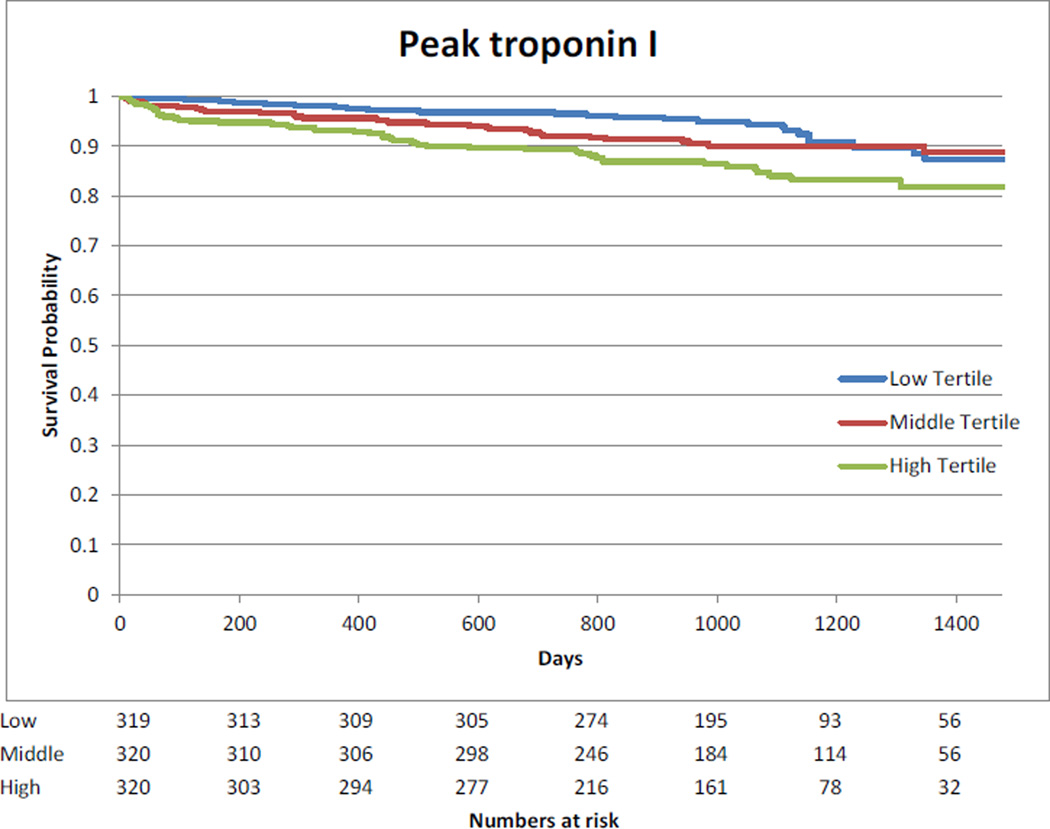
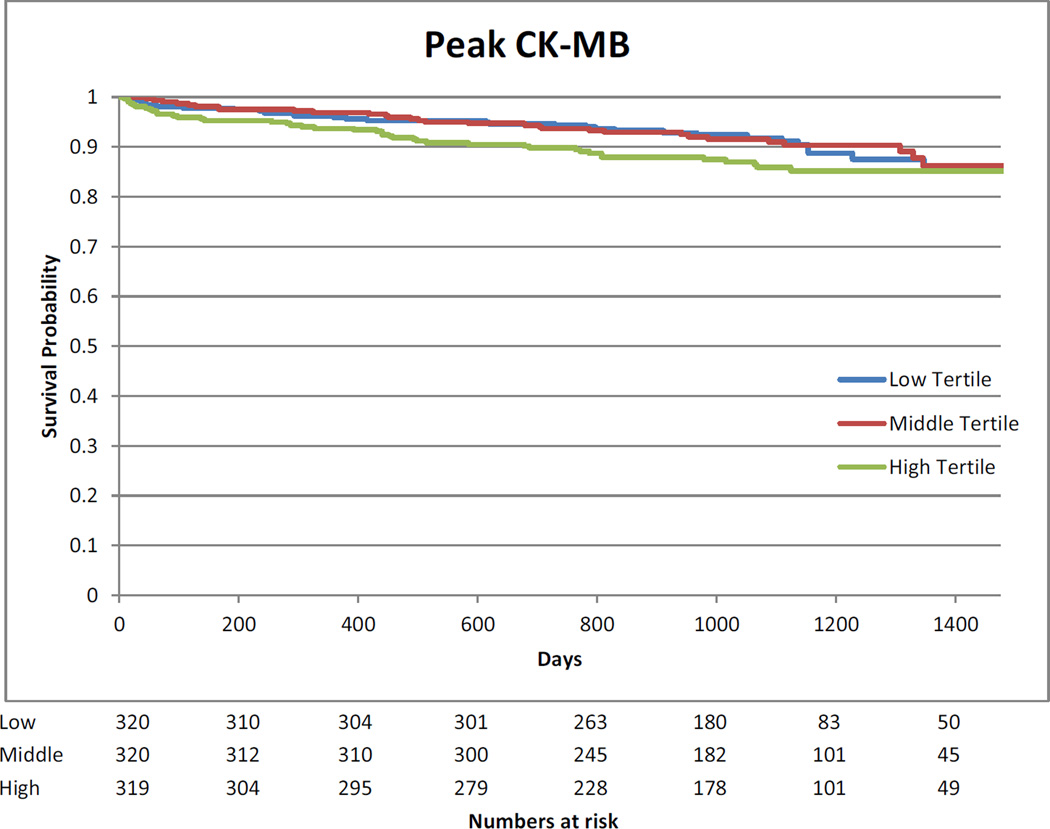
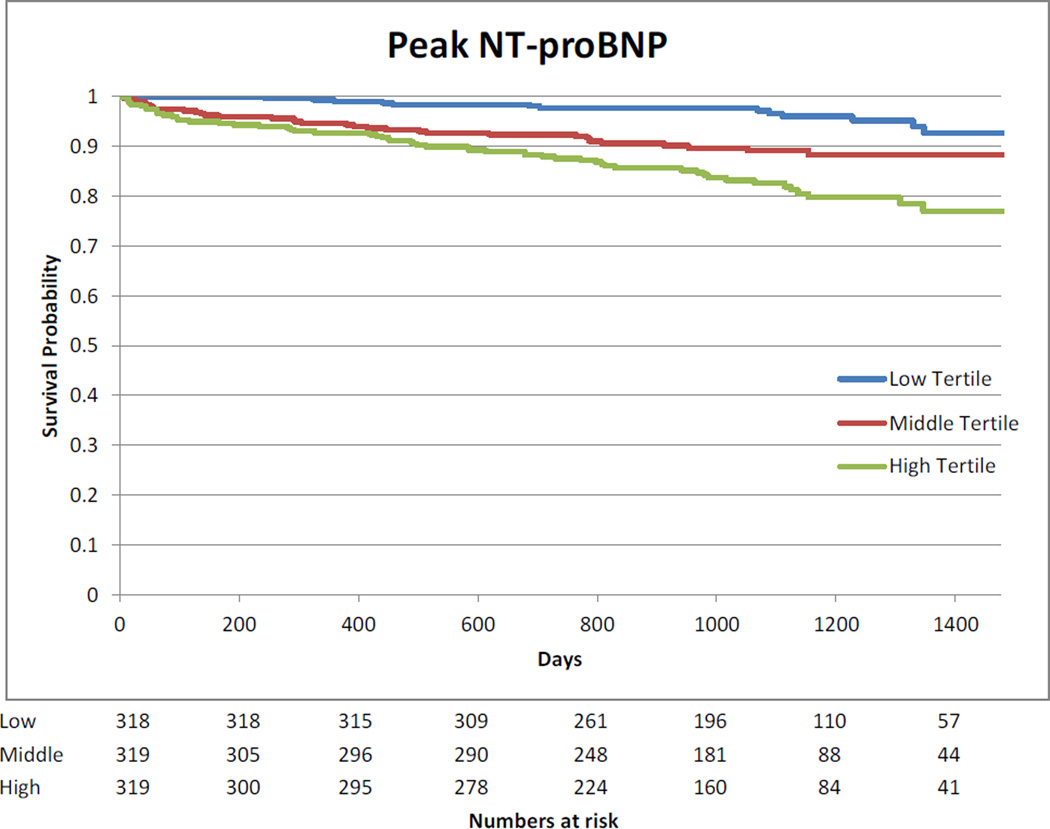
Kaplan-Meier curves showing the association between highest measured biomarkers divided in tertiles and long-term mortality are presented in Appendix 9.
Severe Acute Kidney Injury
Associations of continuous log transformed values of biomarkers at different time peri-operative points with severe AKI are presented in Table 3. NT-proBNP was the only pre-operative biomarker associated with severe AKI and remained statistically significant after adjustment for age, sex, white race, non-elective surgery, diabetes, hypertension, center, congestive heart failure, myocardial infarction, pre-op urine albumin to creatinine ratio, CPB time >120 minutes, pre-op eGFR, and type of surgery. Patients in the highest tertile pre-operatively (NT-proBNP ≥1009.7ng/L) had a more than fourfold increase in severe AKI (adjusted OR=4.5, 95% CI (1.3, 15.1), p = 0.02). First post-operative biomarkers (measured within 6 hours of surgery) including NT-proBNP, hs-cTnT, cTnI and CK-MB were all associated with severe AKI on both univariable and multivariable analyses. On day 1, patients in the third tertile of NT-proBNP (≥ 828.8ng/L) and hs-cTnT (≥591.4 ng/L) had the highest increase in risk of severe AKI (respectively, adjusted OR=4.0, 95%CI (1.2, 13.4), p=0.02 and adjusted OR=4.6, 95%CI (1.5, 14.4), p<0.01) (Appendix 4). The highest measured concentrations of these biomarkers were also associated with severe AKI. However, the highest measured concentration occurred concurrently with the occurrence of AKI. Despite their association with severe AKI, the discriminatory properties of the cardiac biomarkers, as assessed by ROC curve analyses, were modest (Appendix 5).
Table 3.
Log Transformed Biomarkers and Association With Severe Acute Kidney Injury
| Time point |
n | Unadjusted OR (95% CI) |
P-value | Adjusted OR* (95% CI) |
P-value | |
|---|---|---|---|---|---|---|
| hs-cTnT | Pre-op | 928 | 1.11 (0.88, 1.40) | 0.3671 | 1.17 (0.84, 1.61) | 0.4 |
| Day 1 | 949 | 1.96 (1.40, 2.75) | <0.0001 | 2.10 (1.32, 3.34) | 0.002 | |
| cTnI | Pre-op | 956 | 1.10 (0.88, 1.37) | 0.4185 | 1.22 (0.92, 1.63) | 0.2 |
| Day 1 | 959 | 1.56 (1.20, 2.02) | 0.0009 | 1.59 (1.10, 2.28) | <0.0001 | |
| CK-MB | Pre-op | 958 | 1.44 (0.95, 2.19) | 0.0862 | 1.55 (0.94, 2.58) | 0.1 |
| Day 1 | 959 | 2.09 (1.42, 3.06) | 0.0002 | 2.19 (1.31, 3.66) | 0.003 | |
| NT- ProBNP |
Pre-op | 931 | 1.37 (1.08, 1.74) | 0.0113 | 1.39 (1.03, 1.88) | 0.03 |
| Day 1 | 947 | 1.54 (1.19, 2.00) | 0.0009 | 1.46 (1.05, 2.03) | 0.02 |
OR: Odds Ratio
CI: Confidence Interval
Pre-op: Pre-operative
hs-cTnT: High sensitive troponin T
cTnI: Troponin I
Day 1 samples were collected within 6 hours of surgery
Adjusted for age, sex, white race, CPB time > 120 minutes, non-elective surgery, pre-op eGFR, diabetes, hypertension, center, congestive heart failure, myocardial infarction, pre-operative urine albumin to creatinine ratio and type of surgery.
1-Year Mortality
Table 4 presents the association between the biomarkers and 1-year mortality. Pre-operative NT-proBNP and hs-cTnT were associated with 1-year mortality and remained significant after adjustment for age, sex, white race, non-elective surgery, diabetes, hypertension, center, congestive heart failure, myocardial infarction, pre-operative urine albumin to creatinine ratio, and type of surgery.
Table 4.
Log Transformed Biomarkers as Predictors of 1-Year Mortality
| Time point | n | OR (95% CI) | P-value | OR* (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| hs-cTnT | Pre-op | 928 | 1.30 (1.08, 1.58) | 0.0065 | ||
| Day 1 | 949 | 1.45 (1.05, 2.01) | 0.023 | 1.38 (0.90, 2.12) | 0.1 | |
| Highest measured | 952 | 1.64 (1.18, 2.28) | 0.0035 | 1.50 (0.98, 2.29) | 0.1 | |
| cTnI | Pre-op | 956 | 1.16 (0.96, 1.41) | 0.1285 | ||
| Day 1 | 959 | 1.30 (1.02, 1.67) | 0.0353 | 1.25 (0.89, 1.74) | 0.2 | |
| Highest measured | 959 | 1.43 (1.11, 1.85) | 0.006 | 1.29 (0.93, 1.79) | 0.1 | |
| CK-MB | Pre-op | 958 | 0.92 (0.58, 1.47) | 0.7305 | ||
| Day 1 | 959 | 1.24 (0.87, 1.78) | 0.2405 | 1.11 (0.71, 1.75) | 0.7 | |
| Highest measured | 959 | 1.19 (0.82, 1.73) | 0.3499 | 1.01 (0.64, 1.59) | 1 | |
| NT-proBNP | Pre-op | 931 | 1.70 (1.35, 2.14) | <0.0001 | ||
| Day 1 | 947 | 1.68 (1.32, 2.14) | <0.0001 | 1.65 (1.21, 2.23) | 0.001 | |
| Highest measured | 956 | 1.99 (1.37, 2.88) | 0.0003 | 1.99 (1.22, 3.26) | 0.006 |
OR: Odds Ratio
CI: Confidence Interval
Pre-op: Pre-operative
hs-cTnT: High sensitive troponin T
cTnI: Troponin I
Adjusted for age, sex, white race, CPB time > 120 minutes, non-elective surgery, pre-op eGFR, diabetes, hypertension, centre, congestive heart failure, myocardial infarction, pre-operative urine albumin and change in creatinine from baseline.
Patients in the highest tertile for NT-proBNP (range: > 1009.8ng/ L) and hs-cTnT (range: >19.4 ng/L) pre-operatively had marked increases in their risk for 1-year mortality (respectively, adjusted OR=27.2, 95%CI (3.5, 213.5), p<0.01 and adjusted OR=4.2, 95%CI (1.3, 13.7), p=0.02). Day 1 hs-cTnT and cTnI were associated with mortality univariately but not after adjustment for patient characteristics, surgical factors and change in serum creatinine day 1 from baseline. Day 1 NT-proBNP was associated with 1-year mortality independent of clinical characteristics and change in serum creatinine from pre-operative level. The risk increased with higher NT-proBNP concentrations (Appendix 6): the adjusted OR was 15.1 ((95% CI 1.9, 117.9), p<0.01) in the second tertile (265.6–827.9 ng/L) and 25.7 ((95% CI 3.3, 201.2), p<0.01) in the third tertile (≥828.8 ng/L). Highest measured hs-cTnT and cTnI were not associated with 1-year mortality after adjustment whereas highest measured NT-proBNP remained significantly associated with mortality. Highest measured hs-cTnT and NT-proBNP have similar AUC for predicting 1-year mortality (Appendix 7).
Long-Term Mortality
The median follow-up of the cohort was 3 years (2.2 to 3.5 years). The association between log transformed biomarkers and long-term mortality is presented in Appendix 8. Pre-operative hs-cTnT, CK-MB and NT-proBNP were associated with long-term mortality after multivariable analysis. After surgery, no association between mortality and CK-MB was observed. Day 1 and highest measured values of hs-cTnT and NT-pro-BNP were associated with long-term mortality. For NT-proBNP, the risk increased with higher values at all time points.
Discussion
Key Results
NT-proBNP seems to be the most promising of the studied cardiac biomarkers. Higher pre-operative and day-1 post-operative concentrations of NT-proBNP were associated with an increased risk for severe AKI. Mortality at one year and on long-term follow-up was associated with NT-proBNP concentrations at all time points and was independent of increases in serum creatinine after cardiac surgery. All cardiac biomarkers on day 1 were associated with severe AKI. Pre-operative and highest measured hs-cTnT concentrations were associated with 1-year mortality, though after multivariate adjustment only the pre-operative concentration was significant. However, hs-cTnT levels at all time points were independently associated with long-term mortality. cTnI were not associated with 1-year and long-term mortality. CK-MB were not associated with 1-year mortality but the pre-operative level was associated with long-term mortality.
Existing Studies
NT-proBNP is thought to be a predictor of complications after cardiac surgery. A systematic review published in 2012 evaluated the role of pre-operative BNP and NT-proBNP in predicting mortality (4 studies, 1101 patients). The summary AUC was 0.61 (95% CI 0.51, 0.70), suggesting a moderate association.(13) In another observational study of 365 intermediate-risk patients undergoing CABG surgery, pre-operative NT-proBNP was associated with morbidity (including renal failure) and mortality.(16) A prospective cohort including 1,559 patients undergoing on-pump cardiac surgery reported that a post-operative (day 1 and 2) BNP value >790 ng/L was associated with an adjusted HR for mortality of 2.44 (95% CI 1.65–3.62).(17) In that study, post-operative BNP had additional predictive value for 1-year mortality when combined with the logistic EuroSCORE.(17) In another study that included only valvular surgery patients, pre-operative BNP levels were found to be predictive of post-operative inotrope duration (OR=5.9, 95% CI 1.2, 29.68) and ventilation time (OR= 4.7, 95% CI 1.74,17.21).(20) BNP and NT-proBNP levels reflect cardiac filling pressures.(21) These biomarkers therefore provide a surrogate for hemodynamic status, which explains their association with severe AKI and mortality after cardiac surgery. Our results confirm the results of previous smaller studies. However, we have the advantage of a larger sample size that enables adjustment for more variables and greater generalizability. Moreover, the simultaneous measurement of CK-MB, cTnI, hs360 cTnT and NT-proBNP allows head to head comparisons between these biomarkers.
The type of procedure performed and the cross-clamp time affect post-operative CK-MB and cTnI release(22) and have both been shown to predict both AKI and 1-year mortality.(5) Therefore, the association of day 1 biomarkers with severe AKI does not come as a surprise. However, the absence of association between the widely used biomarkers (CK-MB and cTnI) and mortality warrants further discussion. Arbitrarily, a cardiac biomarker value > 10 times the upper limit of normal is used in the Third Universal definition of myocardial infarction for patients who have undergone CABG surgery.(23) A clinically relevant post cardiac surgery myocardial infarction definition should be associated with increased mortality and morbidity. It is of concern that the biomarkers that are currently available in most centers failed to predict 1-year and long-term mortality. The exclusion of patients with very early mortality and the adjustment for other known risk factors might explain this unexpected finding. The inclusion of patients who underwent isolated valvular or aortic procedures could also be in cause. A recent study of patients who underwent isolated valvular surgery found no association between CK-MB and cTnI and short-term morbidity.(20)
Often, clinicians use high-sensitivity cardiac troponin assays interchangeably with sensitive cardiac troponin assays. However, these biomarkers behaved differently in our study. Pre-operative hs-cTnT was associated with 1-year and long-term mortality, whereas cTnI was not. The increased analytical sensitivity of these assays accounts for their capacity to identify high risk patients and their predictive value in cardiac surgery mirrors what was seen in other non acute coronary syndrome populations. (21–25) Interestingly, the cut-point for highest measured hs-cTnT identified on our ROC curve for 1-year mortality was approximately 40 times the upper limit of normal (i.e., 40 × 99th percentile) suggesting the currently used MI definition (10 × 99th percentile) may fail to identify patients at higher mortality risk. The transition towards hs-cTn assays means that understanding their perioperative predictive value is crucial. The VISION (Vascular events In Surgery patIents cOhort evaluatioN) Cardiac Surgery Study (NCT01842568), a 15,000-patient cohort that is currently recruiting will inform on the predictors of complications after cardiac surgery, including hs-cTn.
Strengths and Limitations
The included patients are representative of a high-risk North American cardiac surgery population. We minimized the variation in biomarker measurement by centralizing the analyses. This study is the largest prospective cohort evaluating the association of multiple pre and post-operative cardiac biomarkers with severe AKI and mortality in cardiac surgery. However, despite including high-risk patients, relatively few events occurred and our model might be over-fitted. The exclusion of patients needing emergent surgery may have contributed to the low event rate. It would be interesting to evaluate the association of cardiac biomarkers with other cardiovascular events in order to increase the power and number of possible analyses. Moreover, patients may see stroke and myocardial infarction, given the associated morbidity, as being more important than a doubling in serum creatinine. Combining a doubling in serum creatinine with the need for acute dialysis may be questioned but aims to use the RIFLE criteria for injury while accounting for patients needing temporary renal replacement therapy.(24) The association of cardiac biomarkers with in-hospital mortality, probably the highest risk group, also warrants further research.
Future Directions
The integration of biomarkers to pre-operative risk prediction tools could refine their predictive value and help patients and clinicians weight the surgical procedure associated risks and benefits. Given the proven benefit to BNP-guided therapy in congestive heart failure,(25, 26) NT-proBNP modulation to prevent post-operative AKI and mortality warrants further investigation.
Conclusions
NT-proBNP is the most promising of the biomarkers we studied for the prediction of post-operative severe AKI and mortality. The commonly used CK-MB and cTnI did not appear to provide prognostic information in addition to clinical characteristics, while pre-operative hs-cTn did have prognostic utility for mortality beyond such parameters. Widespread update of hs-cTn should help physicians better inform patients and their families of risk.
Figure 2.
Acknowledgments
This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
Members of TRIBE-AKI Consortium are as follows: Dr. Prasad Devarajan, (University of Cincinnati Children’s Hospital); Dr. Charles Edelstein (University of Colorado); Dr. Cary Passik (Danbury Hospital); Dr. Madhav Swaminathan and Dr. Uptal Patel (Duke University); Dr. Michael Zappitelli (Montreal Children’s Hospital); and Dr. Isabel Butrymowicz (Yale-New Haven Hospital).
The Evidence InvestigatorTM Cytokine Custom Array 4 kits were donated by Randox Laboratories Ltd. The granting agencies, Randox Laboratories Ltd. did not participate in the design and conduct of the study, collection, management, analysis, and interpretation of the data, and preparation, review, or approval of the manuscript.
This study was supported by the National Institutes of Health (NIH) R01HL085757 to C.R.P.) to fund the TRIBE-AKI Consortium to study novel biomarkers of AKI in cardiac surgery. S.G.C. has been supported by an NIH Career Development Award (K23DK080132). C.R.P. is supported by the NIH K24DK090203) and P30 DK079310-07 O'Brien Center Grant. S.G.C., A.X.G., and C.R.P. are also members of the NIH-sponsored Assess, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury Consortium (U01DK082185).
Dr. Parikh is a consultant for Cardiorentis and Abbvie. He is also a scientific advisor for Thrasos. Dr. Kavsak has obtained grants/honorarium/advisory or consultant fees from Abbott Laboratories, Abbott Point of Care, Beckman Coulter, Ortho Clinical Diagnostics, Roche Diagnostics, and the Canadian Agency for Drugs and Technologies in Health. Dr. Garg has received research funding from Astellas, Roche, Fresenius, Ortho-Biotech, Pfizer Canada. John W Eikelboom reports grants and personal fees from Bayer, Boehringer-Ingelheim, Astra Zeneca, Bristol-Myer-Squibb, Glaxo-Smith-Kline, Pfizer, Janssen, Sanofi-Aventis, and personal fees from Daiichi-Sankyo and Eli Lilly.
List of abbreviations
- AKI
Acute kidney injury
- CABG
Coronary artery bypass grafting
- CHF
Congestive heart failure
- CK-MB
Creatinine kinase-MB
- CBP
Cardiopulmonary bypass
- CV
Coefficient of variation
- cTnI
Troponin I
- eGFR
Estimated glomerular filtration rate
- hs-cTnT
High-sensitivity troponin T
- ICES
Institute for Clinical Evaluative Sciences
- ICU
Intensive care unit
- MI
Myocardial infarction
- NT-proBNP
N-terminal prohormone of brain natriuretic peptide
- OR
Odds ratio
- QC
Quality control
- ROC
Receiver operator characteristic
- STS
Society of Thoracic Surgeons
- TRIBE-AKI
Translational Research Investigating Biomarker Endpoints in AKI
- VISION
Vascular events In Surgery patIents cOhort evaluatioN
Appendix 1
Baseline Patient Characteristics by 1-Year Mortality
| Characteristics | Dead at 1 year |
Alive at 1 year | P-value |
|---|---|---|---|
| N (%) | 43 (4.5) | 917 (95.5) | |
| Age, (years) mean (SD) | 74 (10) | 71 (10) | 0.1 |
| Male, n (%) | 29 (67.4) | 626 (68.3) | 0.9 |
| Caucasian, n (%) | 39 (90.7) | 858 (93.6) | 0.5 |
| Previous history, n (%) | |||
| Diabetes | 20 (46.5) | 359 (39.1) | 0.3 |
| Hypertension | 36 (83.7) | 723 (78.8) | 0.4 |
| Congestive Heart Failure | 18 (41.9) | 212 (23.1) | 0.005 |
| LVEF<40% | 5 (11.6) | 93 (10.1) | 0.8 |
| eGFR, n (%) | |||
| >60 mL/min per 1.73 m2 | 25 (58.1) | 608 (66.3) | 0.3 |
| 30–60 mL/min per 1.73 m2 | 15 (34.9) | 281 (30.6) | |
| < 30 mL/min per 1.73 m2 | 3 (7.0) | 28 (3.1) | |
| Urine albumin/creatinine, n (%) | |||
| <10 | 5 (11.6) | 331 (36.1) | 0.01 |
| 10–30 | 19 (44.2) | 266 (29.0) | |
| 30–300 | 13 (30.2) | 254 (27.7) | |
| >300 | 5 (11.6) | 56 (6.1) | |
| Non-elective surgery, n (%) | 8 (18.6) | 179 (19.5) | 0.9 |
| Surgery, n (%) | |||
| Isolated CABG or valve | 15 (34.9) | 193 (21.0) | 0.1 |
| CABG + valve | 28 (65.1) | 724 (78.0) | |
| Off-pump surgery, n (%) | 3 (7.0) | 84 (9.2) | 0.5 |
| Re-do surgery, n (%) | 0 (0.0) | 18 (2) | 0.4 |
| CBP time (minutes), mean (SD) | 138 (56) | 111 (58) | 0.004 |
| Cross-clamp time (minutes), mean (SD) |
99 (46) | 76 (44) | <0.001 |
Appendix 2
Post-Operative Complications During Hospitalization for Cardiac Surgery by 1-Year Mortality
| Complications | Dead at 1 year | Alive at 1 year | P-value |
|---|---|---|---|
| Clinical AKI, n (%) | |||
| Increase > 50% or >0.3 mg/dL | 20 (46.5) | 310 (33.8) | 0.09 |
| Acute dialysis | 2 (4.7) | 7 (0.8) | 0.01 |
| Non-renal complications, n (%) | |||
| none | 20 (46.5) | 571 (62.3) | 0.02 |
| 1 or 2 | 15 (34.9) | 274 (29.9) | |
| >2 | 8 (18.6) | 72 (7.9) | |
| Oliguria on post-operative day 1, n (%) | 0 (0.0) | 11 (1.2) | 0.5 |
| Mechanical ventilation > 48 hours, n (%) | 4 (9.3) | 30 (3.3) | 0.04 |
| ICU length of stay (days), median (IQR) | 4 (2–5) | 2 (1–3) | <0.001 |
| Hospital length of stay (days), median (IQR) |
8 (6–13) | 6 (5–8) | <0.001 |
Appendix 3
Boxplots of the Peri-operative Cardiac Biomarkers According to Severe AKI
The patients who developed the events are represented in blue. The patients free events are in green. Day 1 samples were collected within 6 hours of surgery. Horizontal line is the median, box encourages the 25th to 75th percentile.
Appendix 4
Biomarkers as Predictors of Severe AKI Divided by Tertiles
| Time point | Tertile | Range | Adjusted OR* (95% CI) | p-value | |
|---|---|---|---|---|---|
| hs-cTnT (ng/L) |
Pre-op | T1 | 3.0 – 7.9 | 1.0 | |
| T2 | 8–19.3 | 0.93 (0.36, 2.41) | 0.9 | ||
| T3 | >19.4 | 1.54 (0.60, 3.99) | 0.4 | ||
| Day 1 | T1 | 8.0–291.0 | 1.0 | ||
| T2 | 291.1–588.0 | 1.67 (0.51, 5.45) | 0.4 | ||
| T3 | >591.0 | 4.57 (1.46, 14.36) | 0.009 | ||
| NT-proBNP (ng/L) |
Pre-op | T1 | 6.77–287.5 | 1.0 | |
| T2 | 288.4 – 1006.4 |
4.13 (1.30, 13.06) | 0.02 | ||
| T3 | >1007.2 | 4.48 (1.33, 15.07) | 0.02 | ||
| Day 1 | T1 | 15.2–264.7 | 1.0 | ||
| T2 | 265.6–827.9 | 2.52 (0.77, 8.28) | 0.1 | ||
| T3 | ≥828.8 | 4.02 (1.21, 13.38) | 0.02 |
Adjusted for Age (per year), sex, white race, non-elective surgery, diabetes, hypertension, center, congestive heart failure, myocardial infarction, pre-op urine albumin to creatinine ratio, and type of surgery
Appendix 5
Association Between Biomarkers and Severe Acute Kidney Injury
| Biomarkers | Time point | n | AUC (SE) |
cut point | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| hs-cTnT (ng/L) |
Day 1 | 949 | 0.68 (0.05) | 714.4 | 0.59 | 0.8 |
| cTnI (ug/L) |
Day 1 | 959 | 0.65 (0.05) | 1.8 | 0.70 | 0.6 |
| CK-MB (ug/L) |
Pre-op | 958 | 0.57 (0.05) | 1.9 | 0.59 | 0.5 |
| Day 1 | 959 | 0.67 (0.05) | 39.5 | 0.51 | 0.8 | |
| NT-proBNP (ng/L) |
Pre-op | 931 | 0.64 (0.04) | 504.0 | 0.78 | 0.5 |
| Day 1 | 947 | 0.68 (0.04) | 556.5 | 0.81 | 0.6 |
AUC: Area Under the Receiver Operative Curve
Pre-op: Pre-operative
SE: Standard Error
hs-cTnT: High sensitive troponin T
cTnI: Troponin I
Appendix 6
Biomarkers as Predictors of 1-Year Mortality Divided by Tertiles
| Time point | Tertile | Range | OR* (95% CI) | p-value | |
|---|---|---|---|---|---|
| h-cTnT (ng/L) |
Pre-op | T1 | 3.0–7.9 | 1.0 | |
| T2 | 8.0–19.3 | 3.78 (1.21, 11.77) | 0.02 | ||
| T3 | >19.4 | 4.18 (1.27, 13.74) | 0.02 | ||
| Day 1 | T1 | 8.0–290.8 | 1.0 | ||
| T2 | 291.1–587.8 | 1.04 (0.40, 2.68) | 0.9 | ||
| T3 | ≥591.4 | 1.66 (0.62, 4.42) | 0.3 | ||
| Highest measured |
T1 | 9.1–393.7 | 1.0 | ||
| T2 | 393.9–802.6 | 2.23 (0.76, 6.52) | 0.1 | ||
| T3 | >804 | 2.95 (0.96, 9.06) | 0.1 | ||
| NT- proBNP (ng/L) |
Pre-op | T1 | 6.8–287.5 | 1.0 | |
| T2 | 288.4–1006.4 | 15.24 (1.95, 118.91) | 0.009 | ||
| T3 | ≥1009.8 | 27.20 (3.46, 213.50) | 0.002 | ||
| Day 1 | T1 | 15.2–264.7 | 1.0 | ||
| T2 | 265.6–827.9 | 15.10 (1.93, 117.94) | 0.01 | ||
| T3 | ≥828.8 | 25.74 (3.29, 201.18) | 0.002 | ||
| Highest measured |
T1 | 70.2–2462.7 | 1.0 | ||
| T2 | 2468.6–4260.6 | 9.83 (2.18, 44.24) | 0.003 | ||
| T3 | ≥8263.2 | 11.68 (2.50, 54.55) | 0.002 |
For pre-op biomarkers: Adjusted for Age (per year), sex, white race, non-elective surgery, diabetes, hypertension, center, congestive heart failure, myocardial infarction, pre-op urine albumin to creatinine ratio, and type of surgery
For day 1 and highest measured biomarkers: Adjusted for Age (per year), sex, white race, non-elective surgery, diabetes, hypertension, center, congestive heart failure, myocardial infarction, pre-op urine albumin to creatinine ratio, and type of surgery and change in serum creatinine day 1 0–6 hours from pre-op.
Appendix 7
Association Between Biomarkers and 1-Year Mortality
| Time point | n | AUC (SE) |
cut point | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| hs-cTnT (ng/L) |
Pre-op | 928 | 0.64 (0.04) | 9.0 | 0.88 | 0.39 |
| Day 1 | 949 | 0.59 (0.05) | 912.6 | 0.38 | 0.82 | |
| Highest measured |
952 | 0.64 (0.04) | 542.9 | 0.74 | 0.51 | |
| cTnI (ug/L) |
Pre-op | 956 | 0.59 (0.04) | <0.01 | 0.64 | 0.53 |
| Day 1 | 959 | 0.59 (0.05) | 5.0 | 0.35 | 0.85 | |
| Highest measured |
959 | 0.63 (0.04) | 4.9 | 0.49 | 0.74 | |
| CK-MB (ug/L) |
Pre-op | 958 | 0.51 (0.04) | 3.2 | 0.98 | 0.17 |
| Day 1 | 959 | 0.55 (0.05) | 38.3 | 0.40 | 0.77 | |
| NT-proBNP (ng/L) |
Pre-op | 931 | 0.72 (0.03) | 843.6 | 0.81 | 0.56 |
| Day 1 | 947 | 0.71 (0.03) | 751.8 | 0.71 | 0.64 | |
| Highest measured |
956 | 0.68 (0.04) | 2940.5 | 0.84 | 0.47 |
AUC: Area Under the Receiver Operative Curve
SE: Standard Error
Pre-op: Pre-operative
hs-cTnT: High sensitive troponin T
cTnI: Troponin I
Appendix 8
Log Transformed Biomarkers as Predictors of Long-Term Mortality, Median Follow-up of 3 Years
| Time point | HR* (95% CI) | |
|---|---|---|
| hs-cTnT | Pre-op | 1.31 (1.14, 1.51) |
| Day 1 | 1.28 (1.03, 1.58) | |
| Highest measured | 1.25 (1.05, 1.47) | |
| cTnI | Pre-op | 1.04 (0.9, 1.21) |
| Day 1 | 1.14 (0.98, 1.31) | |
| Highest measured | 1.12 (0.96, 1.32) | |
| CK-MB | Pre-op | 1.17 (1.02, 1.33) |
| Day 1 | 1.1 (0.91, 1.32) | |
| Highest measured | 1.02 (0.84, 1.23) | |
| NT-proBNP | Pre-op | 1.44 (1.30, 1.59) |
| Day 1 | 1.37 (1.26, 1.49) | |
| Highest measured | 1.93 (1.42, 2.62) |
HR: Hazard Ratio
CI: Confidence Interval
Pre-op: Pre-operative
hs-cTnT: High sensitive troponin T
cTnI: Troponin I
For pre-op biomarkers: Adjusted for Age (per year), sex, white race, non-elective surgery, diabetes, hypertension, center, congestive heart failure, myocardial infarction, pre-op urine albumin to creatinine ratio, and type of surgery
For day 1 and highest measured biomarkers : Adjusted for Age (per year), sex, white race, non-elective surgery, diabetes, hypertension, center, congestive heart failure, myocardial infarction, pre-op urine albumin to creatinine ratio, and type of surgery and change in serum creatinine day 1 0–6 hours from pre-op.
Appendix 9
Kaplan-Meier Curve – Association between highest measured hs-cTnT tertiles and long-term mortality
Kaplan-Meier Curve – Association between highest measured cTnI tertiles and long-term mortality
Kaplan-Meier Curve – Association between highest measured CK-MB tertiles and long-term mortality
Kaplan-Meier Curve – Association between highest measured NT-proBNP tertiles and long-term mortality
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None of the other authors had relationships with the industry and financial associations that might pose a conflict of interest
References
- 1.Garg AX, Devereaux PJ, Yusuf S, et al. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2014 Jun 4;311(21):2191–2198. doi: 10.1001/jama.2014.4952. [DOI] [PubMed] [Google Scholar]
- 2.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clinical journal of the American Society of Nephrology : CJASN. 2006 Jan;1(1):19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 3.Ostermann ME, Taube D, Morgan CJ, Evans TW. Acute renal failure following cardiopulmonary bypass: a changing picture. Intensive care medicine. 2000 May;26(5):565–571. doi: 10.1007/s001340051205. [DOI] [PubMed] [Google Scholar]
- 4.Conlon PJ, Stafford-Smith M, White WD, et al. Acute renal failure following cardiac surgery. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1999 May;14(5):1158–1162. doi: 10.1093/ndt/14.5.1158. [DOI] [PubMed] [Google Scholar]
- 5.Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Annals of internal medicine. 1998 Feb 1;128(3):194–203. doi: 10.7326/0003-4819-128-3-199802010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. The American journal of medicine. 1998 Apr;104(4):343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 7.Li SY, Chuang CL, Yang WC, Lin SJ. Proteinuria predicts postcardiotomy acute kidney injury in patients with preserved glomerular filtration rate. The Journal of thoracic and cardiovascular surgery. 2015 Mar;149(3):894–899. doi: 10.1016/j.jtcvs.2014.10.054. [DOI] [PubMed] [Google Scholar]
- 8.Fortescue EB, Bates DW, Chertow GM. Predicting acute renal failure after coronary bypass surgery: cross-validation of two risk-stratification algorithms. Kidney international. 2000 Jun;57(6):2594–2602. doi: 10.1046/j.1523-1755.2000.00119.x. [DOI] [PubMed] [Google Scholar]
- 9.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. Journal of the American Society of Nephrology : JASN. 2005 Jan;16(1):162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Delgado JC, Esteve F, Torrado H, et al. Influence of acute kidney injury on short- and long-term outcomes in patients undergoing cardiac surgery: risk factors and prognostic value of a modified RIFLE classification. Critical care. 2013 Dec 13;17(6):R293. doi: 10.1186/cc13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng SY, Sanagou M, Wolfe R, Cochrane A, Smith JA, Reid CM. Prediction of acute kidney injury within 30 days of cardiac surgery. The Journal of thoracic and cardiovascular surgery. 2014 Jun;147(6):1875–1883. 83 e1. doi: 10.1016/j.jtcvs.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 12.Pacini D, Pantaleo A, Di Marco L, et al. Risk factors for acute kidney injury after surgery of the thoracic aorta using antegrade selective cerebral perfusion and moderate hypothermia. The Journal of thoracic and cardiovascular surgery. 2015 Apr 9; doi: 10.1016/j.jtcvs.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Litton E, Ho KM. The use of pre-operative brain natriuretic peptides as a predictor of adverse outcomes after cardiac surgery: a systematic review and meta-analysis. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2012 Mar;41(3):525–534. doi: 10.1093/ejcts/ezr007. [DOI] [PubMed] [Google Scholar]
- 14.Lurati Buse GA, Koller MT, Grapow M, Bolliger D, Seeberger M, Filipovic M. The prognostic value of troponin release after adult cardiac surgery - a meta-analysis. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2010 Feb;37(2):399–406. doi: 10.1016/j.ejcts.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 15.Domanski MJ, Mahaffey K, Hasselblad V, et al. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA : the journal of the American Medical Association. 2011 Feb 9;305(6):585–591. doi: 10.1001/jama.2011.99. [DOI] [PubMed] [Google Scholar]
- 16.Holm J, Vidlund M, Vanky F, et al. EuroSCORE II and N-terminal pro-B-type natriuretic peptide for risk evaluation: an observational longitudinal study in patients undergoing coronary artery bypass graft surgery. British journal of anaesthesia. 2014 Jul;113(1):75–82. doi: 10.1093/bja/aeu088. [DOI] [PubMed] [Google Scholar]
- 17.Lurati Buse GA, Bolliger D, Seeberger E, et al. Troponin T and B-type natriuretic Peptide after on-pump cardiac surgery: prognostic impact on 12-month mortality and major cardiac events after adjustment for postoperative complications. Circulation. 2014 Sep 16;130(12):948–957. doi: 10.1161/CIRCULATIONAHA.113.007253. [DOI] [PubMed] [Google Scholar]
- 18.Kavsak PA, Jaffe AS, Hickman PE, et al. Canadian Institutes of Health Research dissemination grant on high-sensitivity cardiac troponin. Clinical biochemistry. 2014 Oct 7; doi: 10.1016/j.clinbiochem.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Parikh CR, Coca SG, Thiessen-Philbrook H, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. Journal of the American Society of Nephrology : JASN. 2011 Sep;22(9):1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh S, Kapoor A, Agarwal SK, Pande S, Sinha A, Rai H. Differential Release Kinetics of Cardiac Biomarkers in Patients Undergoing Valve Replacement Surgery. Journal of Cardiac Surgery. 2014;29(2):134–140. doi: 10.1111/jocs.12280. [DOI] [PubMed] [Google Scholar]
- 21.Iwanaga Y, Nishi I, Furuichi S, et al. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. Journal of the American College of Cardiology. 2006 Feb 21;47(4):742–748. doi: 10.1016/j.jacc.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Mastro F, Guida P, Scrascia G, et al. Cardiac troponin I and creatine kinase-MB release after different cardiac surgeries. Journal of cardiovascular medicine. 2014 Jul 11; doi: 10.2459/JCM.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 23.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. European heart journal. 2012 Oct;33(20):2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 24.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P Acute Dialysis Quality Initiative w. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical care. 2004 Aug;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jourdain P, Jondeau G, Funck F, et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. Journal of the American College of Cardiology. 2007 Apr 24;49(16):1733–1739. doi: 10.1016/j.jacc.2006.10.081. [DOI] [PubMed] [Google Scholar]
- 26.Troughton RW, Frampton CM, Yandle TG, Espine EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. The Lancet. 2000;355(9210):1126–1130. doi: 10.1016/s0140-6736(00)02060-2. [DOI] [PubMed] [Google Scholar]