Abstract
Purpose
To evaluate the patient, disease and tumor characteristics of the three morphologically distinct groups of vitreous seeds in retinoblastoma presenting for treatment with ophthalmic artery chemosurgery: dust (class 1), spheres (class 2) and clouds (class 3) in primary and recurrent vitreous seeds.
Design
Retrospective cohort study of patients treated for vitreous seeds at Memorial Sloan Kettering Cancer Center between May 2006 and March 2015.
Participants
135 eyes with active vitreous seeds, presenting for either primary treatment with ophthalmic artery chemosurgery, or with recurrent vitreous disease.
Methods
Vitreous seeds were classified into three groups: dust, spheres and clouds. Indirect ophthalmoscopy, fundus photography, ultrasonography and ultrasonic biomicroscopy were used to locate and evaluate the extent of retinal and vitreous disease. Patient and disease characteristics (age, laterality of disease, treatment status) were compared between classification groups. A two-tailed Fisher's exact test and paired Student's t-test were used for statistical analysis.
Main Outcome Measures
Age of patient, laterality of disease, location of retinal disease, extent of vitreous disease and treatment status.
Results
Primary treated disease: Patients with eyes containing class 3 (cloud) vitreous seeds were significantly older compared to patients with class 1 or 2 (p<0.05) seeds: median age for class 1, 2 and 3 was 11, 15.5 and 32 mos, respectively. Eyes containing class 3 seeds were significantly more likely to occur in the equator-ora region of the fundus (p<0.0001), in a diffuse pattern (p<0.0001), and in patients with unilateral disease (p<0.05), compared to class 1 and 2 seeds. Recurrent disease: Recurrent vitreous seeds were significantly more common to class 2 (p<0.05), occurring in a diffuse pattern (p=0.01) and in patients with bilateral disease (p<0.001).
Conclusion
The three classes of vitreous seeds have distinct clinical characteristics associated with age of patient, laterality of disease, extent and location of tumor-producing seeds. Furthermore, recurrent vitreous seeds appear to have a unique clinical profile compared to seeds receiving primary treatment.
Keywords: Retinoblastoma, intravitreal, intra-arterial, chemotherapy, retina, melphalan, classification, vitreous seeds, vitreous seed classification
Precis
Vitreous seed classification in retinoblastoma is associated with unique patient, disease and tumor characteristics.
Introduction
A number of temporal and spatial relationships have been established in retinoblastoma, particularly with regard to patient age at diagnosis and intraocular tumor location1,2. For example, it is known that patients with germline (usually bilateral) disease typically present at a younger age, with multifocal tumors, compared to patients with (usually unilateral and single foci) disease restricted to a somatic mutation. Stallard recognized that new tumor foci may form following diagnosis of the presenting tumor, and these subsequent tumors are typically peripheral to the presenting disease3. These temporal and spatial relationships focus on retinal disease, and little is known about how these principles may apply to vitreous disease.
As our success rate for treating vitreous disease has improved with the use of intravitreal chemotherapy, so too has our knowledge of vitreous seeds. Based on their distinct morphological characteristics, vitreous seeds can be classified as follows: dust (class 1), spheres (class 2), and clouds (class 3)4,5. With intravitreal melphalan, each seed class varies not only in the amount and dose of intravitreal melphalan used to control the seeds, but also in regression pattern and time to final response to treatment5. A similar analysis has not yet been reported for seed response to ophthalmic artery chemosurgery (OAC). The present study evaluated whether there are additional clinical features that distinguish these classes of vitreous seeds, particularly with regard to age of patient, laterality of disease, intraocular tumor location, extent of disease, and treatment status.
Methods
This Memorial Sloan Kettering Institutional Review Board (IRB)-approved retrospective study included all eyes with active vitreous seeds presenting for either primary treatment with ophthalmic artery chemosurgery, or with recurrent vitreous disease, at Memorial Sloan Kettering Cancer Center (MSKCC) from May 30th, 2006 to March 25th, 2015. Informed consent was obtained for each patient from a guardian, caregiver or parent. The study was Health Insurance Portability and Accountability Act (HIPAA)-compliant. Research adhered to the tenets of the Declaration of Helsinki.
Three hundred sixty eyes of 289 patients were retrospectively reviewed for vitreous seeds. Eyes were excluded if there was an absence of active vitreous seeds, or if vitreous hemorrhage precluded an assessment of the fundus, if clinical history was insufficient or if fundus photography was unavailable to judge vitreous seed status. The clinical status was reviewed using clinical notes documented during the examination under anesthesia with indirect ophthalmoscopy, RetCam fundus photography (Clarity, Pleasanton, CA, USA), B-scan ultrasonography (OTI Scan 2000 Ophthalmic Technologies) and ultrasonic biomicroscopy (OTI Scan 2000 Ophthalmic Technologies, North York, ON, Canada). While all eyes presented for and received treatment with ophthalmic artery chemosurgery, some eyes may have subsequently received intravitreal chemotherapy. Unlike our prior publication on this topic, this present study does not report on the response to treatment{Francis:2015he}.
Patient data included age, sex, laterality and treatment status. Treatment status was divided into eyes receiving primary treatment (these eyes could be naïve to treatment, or could have received prior treatment with intraarterial chemosurgery at an outside hospital or systemic chemotherapy, but contain seeds that were not from a new source nor deemed recurrent: for instance, an eye that received a cycle of systemic chemotherapy while arranging travel to our center); and eyes with recurrent vitreous disease (defined as recurrent tumor after two months of progression-free follow-up at monthly examinations at an outside hospital, or following ophthalmic artery chemosurgery (OAC) at our institution). Tumor data included Reese-Ellsworth (RE) classification, International Classification (IC) using the Children’s Oncology grouping, seed classification (class 1 = dust, class 2 = spheres +/− dust, or class 3 = clouds +/− spheres or dust), extent of disease (localized [< or = 1 quadrant] or diffuse [>1 quadrant]), and location of retinal tumor/source of vitreous seeds (defined by tumor center located in macula or macula-equator or equator-ora; tumors nasal to the disc could only be defined as macula-equator or equator-ora).
The inter-observer variability of vitreous seed classification is unknown. However, the system is purposefully based on morphological features (much in the same way that the existing classification systems for retinoblastoma are constructed) and as such, places emphasis on objective clinical characteristics. Nonetheless, it is open to interpretation and thereby may have inherent variability between observers, like the Reese-Ellsworth and International Classifications.
Outcome measures were compared for all three seed classifications, and included the patient and tumor data described above. Statistical analysis was performed with Fisher’s exact test or two-tailed Student’s t-test using GraphPad software (www.graphpad.com, Feb 10th 2014) and NCSS software (www.ncss.com, Feb 10th 2014).
Results
One hundred thirty-five eyes of 129 patients met the inclusion criteria for this study. All eyes were Reese-Ellsworth group VB; eyes were classified according to the Children’s Oncology Group International Classification as C in 11 eyes, D in 97 eyes, and E in 27 eyes.
Treatment status
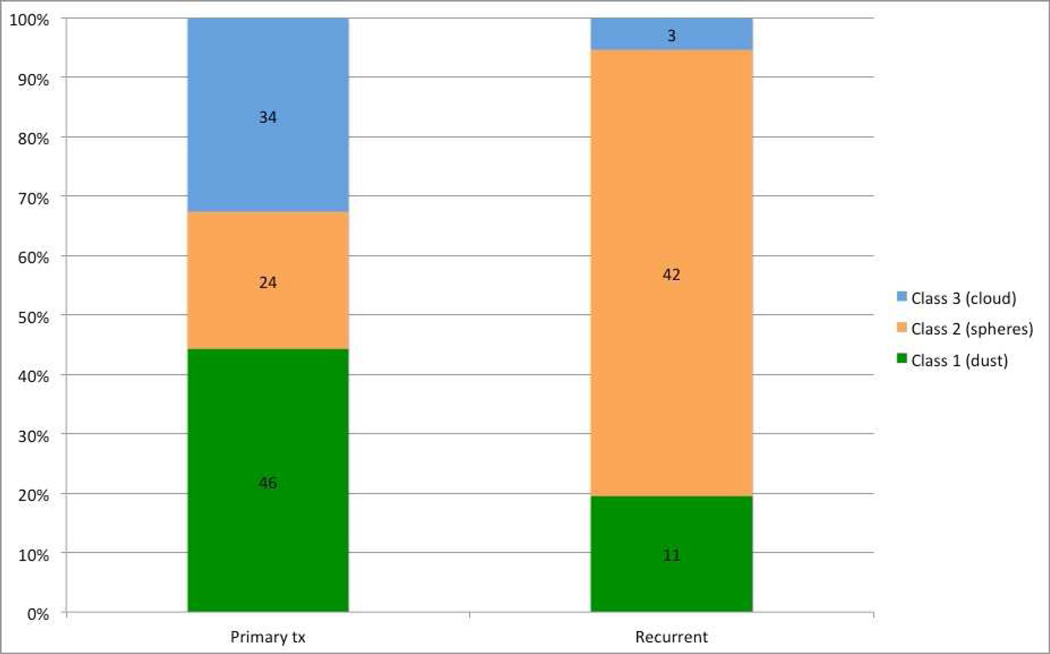
One hundred ten eyes presented for primary treatment with OAC: 67 had naïve disease and 37 were previously treated for a total of 104 eyes. Fifty-six eyes had recurrent vitreous disease: 31 of these eyes presented for treatment of recurrent disease following outside treatment, and 25 eyes (of the original 104 eyes) had recurrent disease following primary treatment with OAC at MSKCC. Seed classification according to treatment status is depicted in Figure 1.
Figure 1.
Seed classification according to treatment status. This chart demonstrates the distribution of seed classes for both primary treated and recurrent vitreous seeds.
Age of patient
Table 1 shows age of patient at presentation of vitreous disease, according to vitreous seed classification. For the primary treated group, age differs significantly between all three classes. Patients with class 1 seeds (dust) are significantly younger; by contrast, patients with class 3 seeds (clouds) are significantly older; patients’ ages in the class 2 group fall in between (p<0.05).
Table 1.
Median, mean and range of age at presentation of primary or recurrent vitreous disease
| Treatment Status |
No. of eyes |
Age* (mos) | 1 (dust) | 2 (spheres) | 3 (cloud) |
|---|---|---|---|---|---|
| median | 11 | 15.5 | 32 | ||
| Primary | 104 | mean | 12.8 | 19.2 | 39.3 |
| range | 3–41 | 5–59 | 6–120 | ||
| median | 31 | 34.4 | 85 | ||
| Recurrent | 56 | mean | 27.2 | 48.1 | 102.7 |
| range | 11–41 | 15–215 | 31–192 | ||
Age at presentation of primary or recurrent vitreous disease. Bolded numbers represent those that are statistically significant p<0.05
Laterality of disease
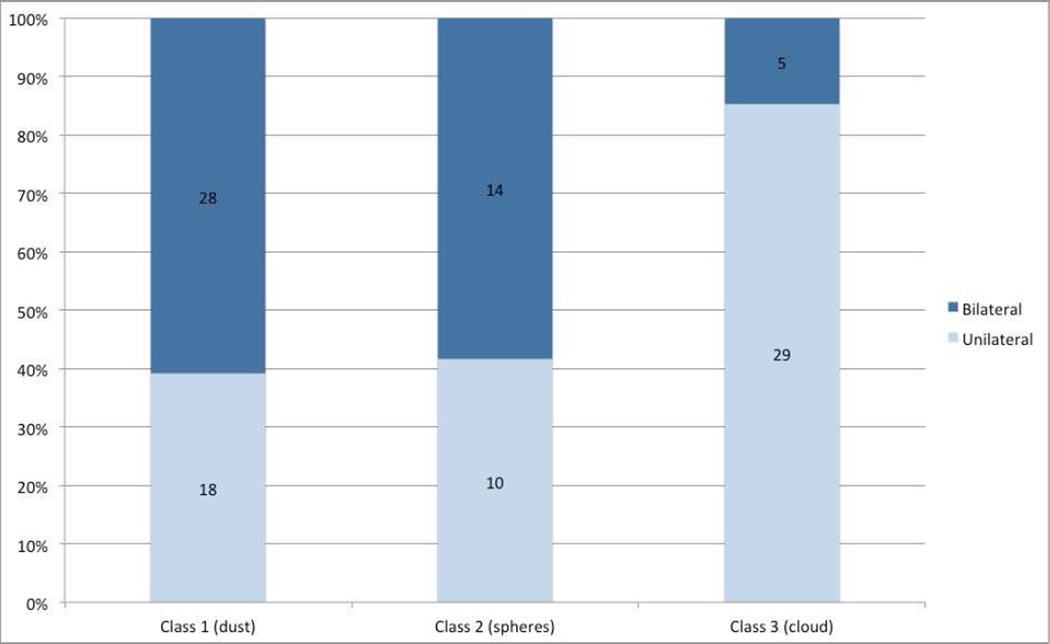
Figure 2 shows laterality of disease, according to seed classification for primary treated eyes. Eyes with class 3 seeds (clouds) were significantly more likely to have unilateral disease (85% unilateral vs 15% bilateral) compared to class 1 (dust) and 2 (spheres) (40% unilateral vs 60% bilateral) (p<0.0001).
Figure 2.
Seed classification and laterality of disease. This chart demonstrates the laterality of retinoblastoma for each seed class.
Location of tumor-producing seeds
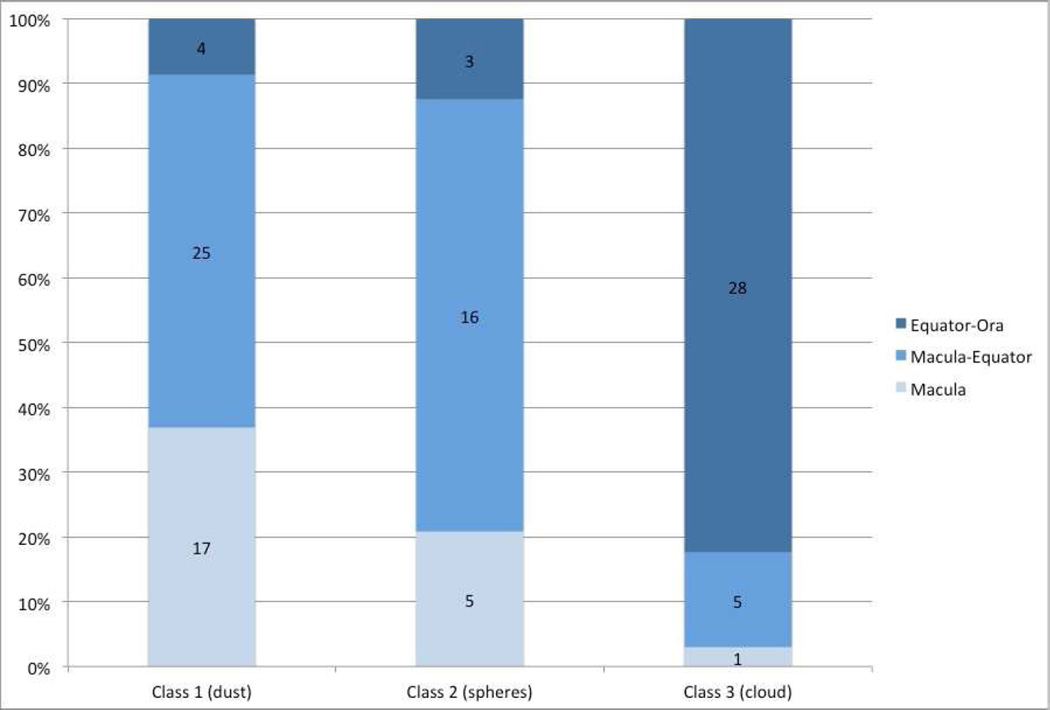
Figure 3 shows location of tumor-producing seeds, according to seed classification for primary treated eyes. Class 3 seeds (clouds) were significantly more likely to be located in the equator-ora region of the fundus; in comparison, class 1 and 2 seeds were significantly more likely to be located in the macula or macula-equator region (p<0.0001).
Figure 3.
Seed classification and location of tumor. This chart demonstrates the location of the tumor producing seeds for each seed class.
Extent of disease
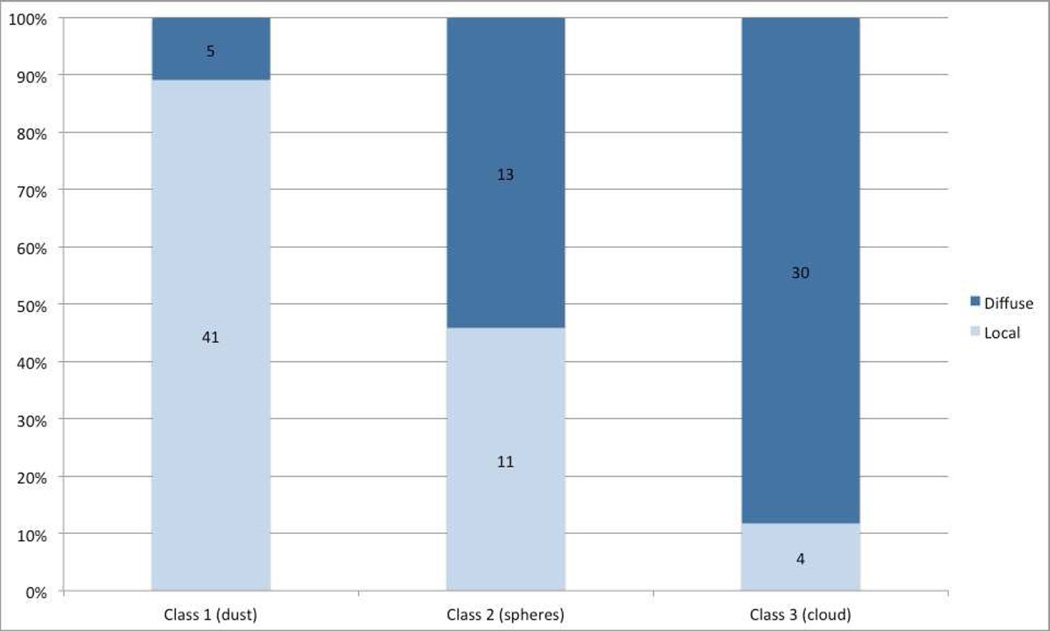
Figure 4 shows extent of vitreous disease, according to seed classification for primary treated eyes. Class 3 seeds (clouds) are significantly more likely to be diffuse, compared with class 1 seeds (dust), which are more likely to be localized to a single quadrant of the eye (p<0.0001).
Figure 4.
Seed classification and extent of disease. This chart demonstrates the extent of disease (localized or diffuse) for each seed class.
Recurrent disease
In the recurrent disease group, patient age did not differ significantly between the three vitreous seed classes. As depicted in Figure 1, recurrent seeds are more likely to be class 2 (spheres), compared to primary treated seeds (p<0.0001). Table 2 compares disease laterality, location of tumor-producing seeds, and extent of disease between primary and recurrent vitreous disease. Recurrent vitreous seeds are significantly more likely to occur in patients with bilateral disease and in a diffuse pattern, compared to primary treated disease. Ninety-six percent of eyes that contained recurrent vitreous seeds following OAC at MSKCC were categorized as class 2.
Table 2.
A comparison of disease and tumor characteristics between primary treated and recurrent vitreous disease
| Variable | Primary tx | Recurrent | p-value | |
|---|---|---|---|---|
| Laterality of disease |
Unilateral | 55% (57) | 20% (11) | 0.0001 |
| Bilateral | 45% (47) | 80% (45) | ||
| Location of tumor producing seeds |
Macula/Macula-Equator | 66% (69) | 79% (44) | 0.15 |
| Equator-Ora | 34% (35) | 21% (12) | ||
| Extent of disease | Localized | 54% (56) | 32% (18) | 0.01 |
| Diffuse | 46% (48) | 68% (38) |
Discussion
For the past several decades, over half of the retinoblastoma eyes with vitreous seeds would have been enucleated due to inadequate response rates to historical treatments6,7. However, the increased use of OAC alone, or in conjunction with intravitreal chemotherapy, now enables us to salvage a vast majority of eyes with vitreous seeds 8–11. As a result of this increased ocular survival, our knowledge of vitreous seeds has deepened; we can now distinguish three classes of vitreous seeds based on morphological characteristics. Furthermore, we can use this classification to predict response to treatment4,5.
The present study broadens our knowledge of vitreous seeds by establishing an association with seed class and unique patient, tumor and treatment characteristics (Table 3). With each successive seed class (for primary treated eyes), there is a significant increase in median age of the patient: the median age for patients with eyes containing class 1, 2 and 3 seeds is 11, 15.5, and 32 months, respectively. In addition to the fact that they occur in older patients, class 3 seeds (clouds) are significantly more likely to derive from tumors located in the equator-ora region of the fundus, are associated with diffuse (as opposed to localized) seeding, and occur in patients with unilateral disease. By contrast, class 1 seeds (dust) are more likely to be localized to a single quadrant of the eye; along with class 2 seeds (spheres), they are more likely to occur in the macula or macula-equator region of the fundus, and are equally distributed among patients with bilateral and unilateral disease. The finding that class 1 seeds are preponderantly localized, and class 3 seeds diffuse, is consistent with our previously published data5.
Table 3.
Summary of vitreous seed classification and clinical characteristics
| Class | Type | Description5 | Response to Intravitreal Melphalan5 |
Median age (mos) |
Typical location |
Typical laterality |
Typical distribution |
|---|---|---|---|---|---|---|---|
| Type I |
Dust | Small granules of vitreous opacities Can be seen as a vitreous haze overlying tumor |
2–3 weeks to regress, receives least drug/injections |
11 | All | U/B | Localized |
| Type 2 |
Spheres | Spherically shaped opacities within vitreous Dust may be present around spheres Can be homogenously opaque or have a translucent/opaque outer shell with contrasting center |
6–7 weeks to regress, receives medium amount of drug/injections |
15.5 | Macula- Equator |
U/B | Localized/ Diffuse |
| Type 3 |
Cloud | Dense collection of punctate vitreous opacities Can appear as a sheet or globule of seed granules and often with wispy edge Dust and spheres are sometimes also visible |
30–32 weeks to regress, receives most drug/injections |
32 | Equator-Ora | U | Diffuse |
U = unilateral, B = bilateral
We might speculate on the pathogenesis associated with the unique features of class 3 seeds. Perhaps the genetic underpinnings related to somatic mutations are more conducive to forming clouds; environmental factors present in the anterior fundus might aid in cultivating cloud morphology. Perhaps the architecture of the vitreous in older patients, and/or an anterior location, allows for more dispersive properties; maybe a prolonged duration of active vitreous disease (as could be true in a patient with unilateral disease, and no family history) results in the formation of a cloud.
Recurrent seeds have features that are significantly different from those of primary treated seeds. Recurrent seeds are more likely to fall into the class 2 (spheres) category, to occur in a diffuse configuration, and to occur in patients with bilateral disease. It is intriguing that all but one of the eyes that developed recurrent seeds following ophthalmic artery chemosurgery were categorized as having class 2 seeds. This begs the question as to how prior treatment predisposes to the generation of spheres, and how prior treatment breeds a genetically resistant clone that forms spheres. However, it should also be noted that distinguishing between regrowth of previously treated seeds and growth of seeds from a new source is difficult to clinically discern with accuracy, and even though these seeds were considered together under the category “recurrent”, there may be differences between them.
In summary, grouping vitreous seeds into three distinct classes based on morphological features can provide valuable clinical information. In addition to predicting response to intravitreal melphalan, this classification scheme is associated with unique patient, tumor and treatment characteristics. Now the relationships between disease patterns and age of patient, or location of tumor, can be expanded to include vitreous seeds. We encourage other groups to utilize the proposed classification scheme, and prospectively validate our findings.
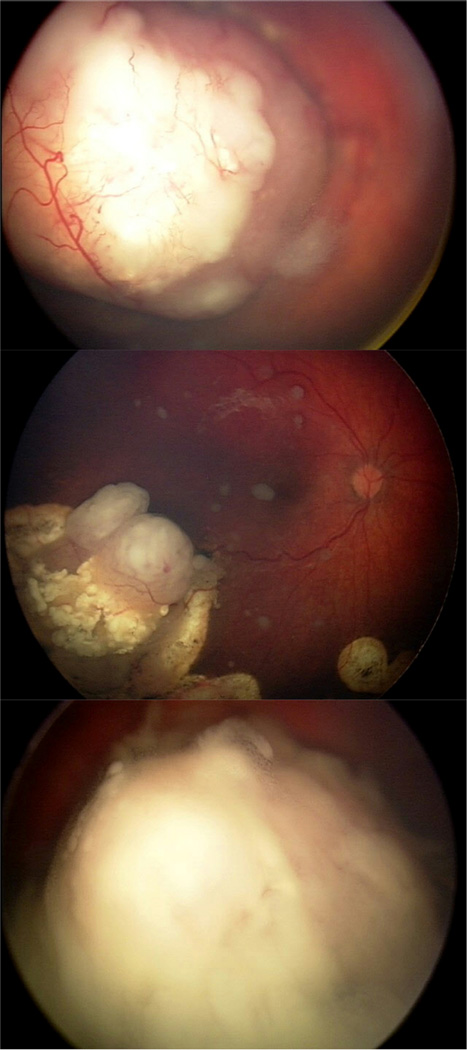
Figure 5.
Representative images of the three seed class. A. Class I (dust) seeds in a 10-month-old male with unilateral disease, in a localized pattern and emanating from a tumor located in the macula-equator. B. Class II (spheres) seeds in a 30-month-old female with bilateral disease, in a diffuse pattern and recurring from a tumor located in the macula-equator that was previously treated with ophthalmic artery chemosurgery. C. Class III (cloud) seeds in a 36-month-old male with unilateral disease, in a diffuse pattern and deriving from a tumor located in the equator-ora.
Acknowledgments
This study was supported by The Fund for Ophthalmic Knowledge, Perry’s Promise Fund, Research to Prevent Blindness and Cancer Center Support Grant (P30 CA008748). The sponsors/funding organizations had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
JHF and DHA have no conflicting relationships. BPM is a consultant for AURA Biosciences.
References
- 1.Abramson DH, Greenfield DS, Ellsworth RM. Bilateral retinoblastoma. Correlations between age at diagnosis and time course for new intraocular tumors. Ophthalmic Paediatr Genet. 1992;13:1–7. doi: 10.3109/13816819209070046. [DOI] [PubMed] [Google Scholar]
- 2.Schueler AO, Anastassiou G, Jurklies C, et al. De novo intraocular retinoblastoma development after chemotherapy in patients with hereditary retinoblastoma. Retina (Philadelphia, Pa) 2006;26:425–431. doi: 10.1097/01.iae.0000238548.97497.4c. [DOI] [PubMed] [Google Scholar]
- 3.Stallard HB. Multiple islands of retinoblastoma; incidence rate and time span of appearance. Br J Ophthalmol. 1955;39:241–243. doi: 10.1136/bjo.39.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munier FL. Classification and management of seeds in retinoblastoma. Ellsworth Lecture Ghent August 24th 2013. Ophthalmic Genet. 2014;35:193–207. doi: 10.3109/13816810.2014.973045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francis JH, Abramson DH, Gaillard M-C, et al. The Classification of Vitreous Seeds in Retinoblastoma and Response to Intravitreal Melphalan. Ophthalmology. 2015;122:1173–1179. doi: 10.1016/j.ophtha.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Bartuma K, Pal N, Kosek S, et al. A 10-year experience of outcome in chemotherapy-treated hereditary retinoblastoma. Acta Ophthalmol. 2013 doi: 10.1111/aos.12282. [DOI] [PubMed] [Google Scholar]
- 7.Berry JL, Jubran R, Kim JW, et al. Long-term outcomes of Group D eyes in bilateral retinoblastoma patients treated with chemoreduction and low-dose IMRT salvage. Pediatr Blood Cancer. 2013;60:688–693. doi: 10.1002/pbc.24303. [DOI] [PubMed] [Google Scholar]
- 8.Munier FL, Gaillard M-C, Balmer A, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012 doi: 10.1136/bjophthalmol-2011-301450. [DOI] [PubMed] [Google Scholar]
- 9.Ghassemi F, Shields CL, Ghadimi H, et al. Combined intravitreal melphalan and topotecan for refractory or recurrent vitreous seeding from retinoblastoma. JAMA Ophthalmol. 2014;132:936–941. doi: 10.1001/jamaophthalmol.2014.414. [DOI] [PubMed] [Google Scholar]
- 10.Shields CL, Douglass AM, Beggache M, et al. INTRAVITREOUS CHEMOTHERAPY FOR ACTIVE VITREOUS SEEDING FROM RETINOBLASTOMA: Outcomes After 192 Consecutive Injections. The 2015 Howard Naquin Lecture. Retina (Philadelphia, Pa) 2015 doi: 10.1097/IAE.0000000000000903. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Han JW, Hahn SM, et al. Combined intravitreal melphalan and intravenous/intra-arterial chemotherapy for retinoblastoma with vitreous seeds. Graefes Arch Clin Exp Ophthalmol. 2015 doi: 10.1007/s00417-015-3202-0. [DOI] [PubMed] [Google Scholar]