Abstract
Positron emission tomography (PET) and PET/ computed tomography (CT) are emerging as important imaging techniques and their popularity is growing within the medical fraternity. Though PET has been a useful research tool for many decades its real growth into clinical applications has occurred in the last one decade or so. Currently its major use is in oncologic imaging. However it has a multitude of clinical applications in cardiology, neurology and psychiatry as well. In oncologic imaging, a major advantage of PET is that a single whole-body examination can provide accurate assessment of disease activity and spread. PET/CT amalgamates the functional information of PET with the structural details of the CT scan, thus greatly aiding in accurate staging, therapy response assessment and early detection of recurrent disease.
Key Words: Positron emission tomography
Introduction
Medical imaging continues to evolve at a blistering pace and recent developments in molecular imaging have created a lot of excitement in the medical fraternity. The availability of metabolic functional imaging has greatly enhanced our understanding of various pathologic processes like cancers and opened newer options for incorporation of this information into patient management protocols. Of all the tools available for molecular imaging, Positron Emission Tomography (PET) has emerged at the forefront for various clinical applications.
Historical Perspective
The clinical impact and use of PET remained restricted until availability of Medical/Baby cyclotrons, in the eighties, made it feasible to produce the inherently short-lived PET tracers, at a reasonable cost, at the hospital PET facilities itself. The early clinical applications of PET emerged in the field of neurology and cardiology (in the 1980s), and in oncology (in the 1990s) [1]. In the late 1990s 18F- fluorodeoxyglucose (FDG) as the radiopharmaceutical began to be used widely in evaluation of oncology patients. The clinical use of PET received a major boost in 1998, when reimbursement for PET scanning was approved by health care agencies in USA [1]. More recently, integration of Computed Tomography (CT) with PET into a composite inline PET/CT scanner has led to creation of a technological wonder that amalgamates functional information of PET with the anatomic information provided by the CT.
Pathophysiological Basis of PET Imaging
The basis of PET imaging is the detection of altered metabolism in biological tissues. By using tracers that target physiological parameters such as glucose metabolism, PET enables imaging and quantification of cellular function. In cancerous cells metabolic changes occur much before the cells undergo changes like dysplasia, metaplasia or anaplasia. This is finally followed by structural changes at a later stage. PET scan detects the disease at the metabolic level while anatomical imaging techniques like CT or MRI detect the disease at the structural level.
PET Tracers
Several positron emitting radio-isotopes have been used for PET imaging (Table 1). The first four isotopes in Table 1 are of particular note as each of these can be easily incorporated into biological compounds to create useful bio-tracers [2].
Table 1.
PET Radionuclides
| Isotope | Half life | Positron energy (MeV) |
|---|---|---|
| C-11 | 20 minutes | 0.385 |
| N-13 | 10 minutes | 0.492 |
| O-15 | 2 minutes | 0.735 |
| F-18 | 110 minutes | 0.250 |
| K-38 | 8 minutes | 1.216 |
| Cu-62 | 10 minutes | 1.315 |
| Cu-64 | 12.7 hours | 0.278 |
| Ga-68 | 68.1 hours | 0.836, 0.352 |
| Rb-82 | 1.3 minutes | 1.523, 1.157 |
| I-124 | 4.2 days | 1.691, 7.228,1.509,1.376 |
Glucose Metabolism
18F-Fluorodeoxyglucose (FDG), an analog of glucose that allows assessment of glucose metabolism in body tissues is the most commonly used PET tracer. FDG enters cells by the same transport mechanism as glucose and is intracellularly phosphorylated by hexokinase to FDG-6-phosphate (FDG-6-P). Intracellularly FDG-6-P does not get metabolized further and accumulates in proportion to the glycolytic rate of the cells. Most malignant cells have higher rate of glycolysis and higher levels of glucose transporter proteins (GLUT) than do normal cells and therefore accumulate FDG-6-P to higher levels than do the normal tissues.
Tumor Proliferation
Carbon-11 thymidine and F-18 Fluorothymidine (FLT) an analog of thymidine are markers of cellular proliferation. Uptake of FLT has considerable analogy with FDG uptake in that FLT is taken up by actively proliferating cells but is not incorporated further into DNA synthesis and hence accumulates intracellularly in tumor cells. It has shown promising ability to predict tumor grade in lung cancers, evaluate brain tumors and may be a good predictor of tumor response [3]. 11C-methionine and amino acid, has shown great promise in evaluating brain tumors and other cancers. 11C-choline and 11C-acetate have been used in prostate cancer to evaluate the primary and metastatic disease [2].
Myocardial Perfusion Imaging
Rubidium-82 is a potassium analog and is used as a first pass extraction agent to assess myocardial perfusion in the same way as Thallium 201 or Technetium -99 labelled compounds. Nitrogen-13 labelled ammonia is another PET tracer used for myocardial perfusion studies.
Skeletal Imaging
F-18 Sodium fluoride has shown great promise as a bone scan agent, comparable to or even superior to Technetium -99 labelled MDP.
Brain Imaging
A large number of PET tracers have been investigated to study the brain (Table 2) [3].
Table 2.
PET radiopharmaceuticals for brain imaging
| Compound | Application |
|---|---|
| O-15 H2O | Blood flow |
| O-15 O2 | Oxygen metabolism / flow |
| O-15 or C-11 Carboxy haemoglobin | Blood volume |
| C-11 Methionine | Amino acid metabolism |
| C-11 Ephedrine | Adrenergic terminals |
| C-11 Carfentanil | Opiate receptor activity |
| C-11 Flunitrazepam | Benzodiazepine receptor activity |
| C-11 Methylspiperone | Dopamine receptor activity |
| C-11 Scopolamine | Muscarinic cholinergic receptor activity |
| F-18 Fluorodeoxyglucose (FDG) | Glucose metabolism |
| F-18 Fluoro-dopa | Presynaptic dopamine system |
| F-18 Fluorothymidine (FLT) | DNA synthesis |
Clinical Applications
Oncology
PET and PET/CT have of late taken the centre stage in oncologic imaging. FDG as a tracer has been the workhorse for most oncologic applications. The goals of oncologic imaging remain lesion detection, lesion characterization, staging of malignant lesions and assessment of the therapeutic response. Staging includes lesion localization, evaluation of proximity to vessel and detection of nodal and distant metastases. Some of these goals are better achieved with high-resolution anatomic imaging techniques like CT and others with molecular imaging by PET. PET helps in differentiation of benign from malignant lesions, aids accurate staging, reliably detects recurrence, and can be used to assess response to therapy in malignant lesions [4]. PET/CT fusion images have the potential to guide biopsies of the most metabolically active regions of tumors and provide better maps of viable cancer than CT alone, for modulating the field and dose of radiation therapy [5, 6] (Figs. 1, 2).
Fig. 1.
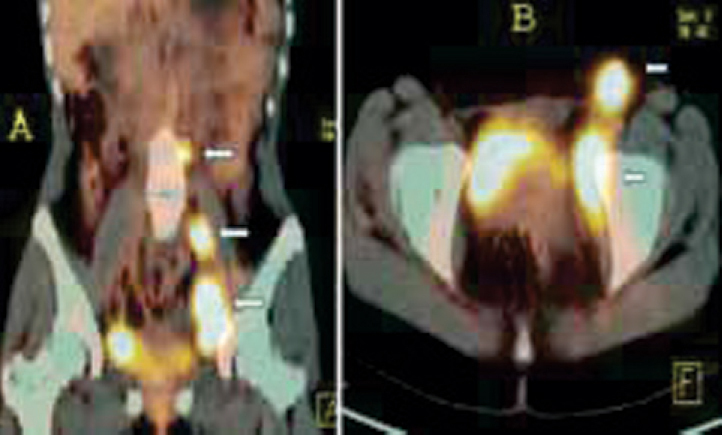
(A and B): FDG PET/CT for staging a case of NHL. Coronal (A) and axial (B) fused PET/CT images show metabolically active disease in multiple groups of enlarged lymph nodes below the diaphragm (arrows). Spleen and bone marrow were normal (Stage II disease).
Fig. 2.
Coronal plain CT (2A) and fused PET/CT (2B) images shows FDG uptake at the periphery of an abdominal mass lesion with central cold area of necrosis. Biopsy from the periphery confirmed adenocarcinoma. Hepatic metastatic lesion was picked up (black arrow).
Brain
Gliomas comprise more than 90% of primary brain tumors in adults. There is a correlation between intensity of FDG uptake on PET, the histological grades and survival in glioma patients [7, 8]. In clinical practice FDG PET is being used for initial grading, evaluating response to therapy, assessing for malignant transformation of low grade tumors and for differentiating residual/ recurrent tumor from post therapy changes (Fig. 3). Though the reliability of FDG-PET has been good in high grade tumors, the poor sensitivity in detecting low grade tumors has been a limitation. Other PET radiotracers for evaluating brain tumors include 11C-Methionine and FLT (amino acid transport), 11C-Thymidine (RNA incorporation) and 11C-Choline (lipid metabolism). 11C-Methionine has been found promising as it has the advantage of higher target to background ratio enabling detection of low grade tumors [2].
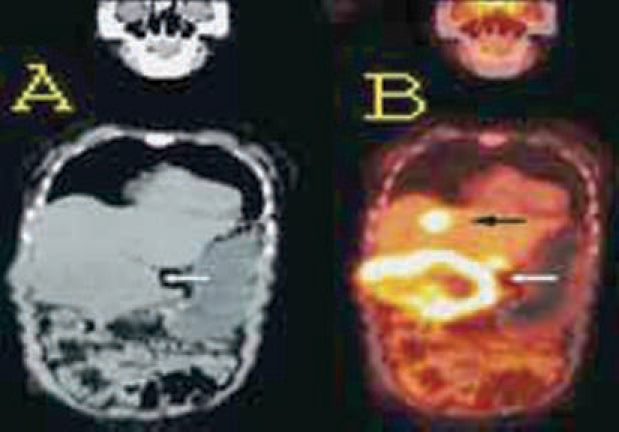
Fig. 3.
(A and B): Brain FDG PET/CT scan in an operated, post radiotherapy case of high grade glioma. Axial plain CT (A) and PET (B) images show focal areas of increased metabolism in an ill defined, hypodense lesion with calcification, in Right frontal lobe (arrows) indicating residual/recurrent tumor.
Head and Neck
Apart from staging, PET/CT is increasingly being used for planning radiation treatment in head and neck cancers [5]. Squamous cell carcinoma is the most common cancer in the head and neck region. It is highly FDG avid and easily detected by FDG PET. PET has been found useful in localizing the site of unknown primary tumor in 25%-35% of patients with metastatic cervical lymphadenopathy [9, 10]. The biggest advantage of PET over conventional imaging modalities is in follow up imaging, for detection of residual or recurrent disease. The timing of PET scanning after therapy is an important factor to improve the specificity of PET in differentiating acute post therapy inflammatory changes from tumor. Whole body PET scanning approximately 12 weeks after completion of radiotherapy and six weeks after combined regimen of chemo and radiotherapy can reliably identify residual tumor.
Lungs
Solitary pulmonary nodule (SPN) is a common diagnostic dilemma in clinical practice. FDG-PET has been found useful in differentiating benign from malignant nodules (Fig. 4). Semiquantitative analysis by using standardized uptake value (SUV) to assess the FDG avidity of the lesion and cut off value of 2.5 (to differentiate benign from malignant nodule) has been found to be accurate [11]. Most lung cancers are highly FDG avid except broncheo-alveolar carcinoma and pulmonary carcinoid. PET is useful in determining the nodal stage of non small cell lung carcinoma (NSCLC), though the use is limited in small cell lung carcinoma (SCLC). Conventionally the staging has been based on anatomical imaging with CT which uses a short axis size greater than 10mm as criterion for a positive mediastinal lymph node. FDG PET can identify both positive nodes smaller than 10mm size and negative nodes that are larger in size. The distinction between N1 nodes (hilar and intrapulmonary nodes) and N2nodes in (ipsilateral mediastinum) is critical from the therapy point of view as this separates the resectable from non resectable lung cancer. Buck et al [12] concluded that FDG uptake correlates well with tumor proliferation in lung cancers. FDG avidity of a lung tumor is a valuable prognostic factor and can be used to identify patients requiring aggressive therapy. An important role of FDG PET is in detection of distant metastases, the presence of which upstages the disease to stage IV and precludes any surgical therapy.
Fig. 4.
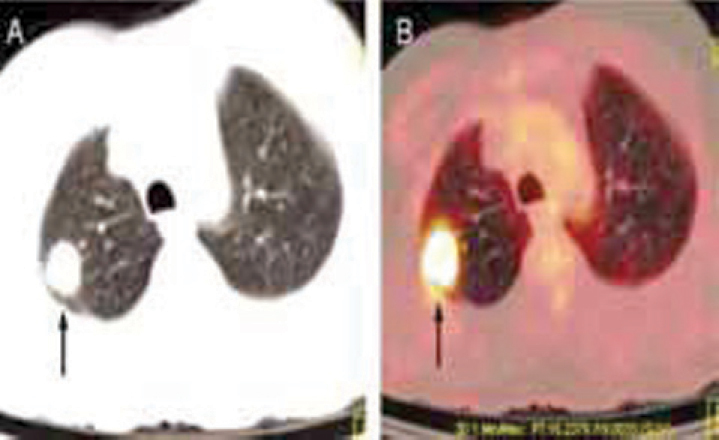
(A and B): FDG PET/CT Scan for solitary pulmonary nodule (SPN): Axial, lung window CT image (A) shows a nodular parenchymal lesion in right lung (arrow). Axial PET/CT fused image (B) reveals intense FDG uptake in the nodule (arrow) indicating malignant nature of the nodule.
Gastrointestinal Tract
Esophageal Cancer
Squamous cell carcinoma involves upper 2/3rd where as adenocarcinoma typically occurs in the lower third of esophagus. Overall sensitivity of PET CT in carcinoma esophagus is 95%. In adenocarcinoma, 10 -15 % patients may be falsely negative on PET due to low uptake in mucinous and signet cell variants. Sensitivity of PET for lymph node involvement is approximately 70% and specificity greater than 90% [2]. Identification of paraesophageal nodes by PET, though, is limited as these may be inseparable from the primary mass. However PET is superior to CT in detection of distant metastases and may result in change in patient management in approx 20 % of cases [13]. PET has proven to be of value in assessing patients during therapy and following therapy for recurrence. Following neoadjuvant chemotherapy (NACT), PET is a better predictor of patient survival than standard imaging methods like CT and endoscopic ultrasonography (EUS) [14].
Colorectal Carcinoma
PET can detect primary colon carcinoma in more than 90% cases as compared to 60% sensitivity of CT. PET is superior to CT in initial staging, identifying nodal disease and detection of local recurrence. PET may directly alter patient management in 29% to 36% of cases with colorectal carcinoma [2].
Lymphoma
Lymphomas are classified as Hodgkin's (15%) and the more common Non Hodgkin's Lymphoma (NHL) (85%). PET has emerged as a useful modality for staging, monitoring therapy and restaging in lymphomas (Fig. 1). For staging, accuracy of PET is reported to be 96% vs 80% for CT. PET imaging leads to change in staging in approx 20- 40 % of cases [3, 15]. PET is useful in detecting extranodal disease including bone marrow and spleen. It can accurately evaluate the effectiveness of therapy. Decreased tumor FDG uptake, seen in patients responding well to therapy, can be evaluated as early as after the first cycle of chemotherapy and is an accurate predictor of prognosis. FDG PET can detect non responders and guide timely changes in therapy [16].
Carcinoma Breast
Though PET is generally not recommended in the initial diagnosis and screening of most patients it has proven efficacy in staging of advanced disease, monitoring therapy (Fig. 5), restaging and detecting recurrent disease. It can provide additional information over CT, MRI and bone scan in upto 29% cases [3]. PET may not be able to detect primary lesions if they are small in size or well differentiated and slow growing e.g. lobular carcinoma and ductal carcinoma in situ. PET may fail to detect microscopic metastases in normal sized axillary nodes leading to false negatives. Therefore a PET scan negative for axillary nodes in a case of breast cancer does not exclude the need for further work up. False positives in axillary nodes may result due to inflammatory conditions.
Fig. 5.
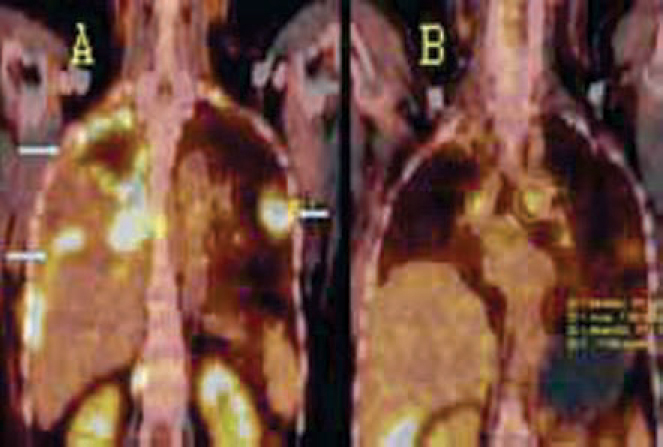
(A and B): FDG PET/CT for therapeutic assessment in a case of metastatic breast carcinoma. Coronal PET / CT images: Pretherapy image (A) shows a large pleural effusion with FDG avid pleural metastases in right hemithorax and a metastatic nodule in the left lung (arrows). Post therapy image (B) six months later, reveals marked resolution of the disease.
Cervical and Ovarian Carcinoma
FDG-PET has shown high sensitivity in detection of cervical cancers and can reliably demonstrate the extent of nodal involvement, thus aiding correct planning of therapy [17]. In ovarian cancers direct spread and seeding of the omentum and organ surfaces is common. PET-CT can detect abnormal activity in small peritoneal lesions that are not discernible on CT. Though FDG PET has been used in staging and restaging, it is widely used to detect recurrent disease. The reported sensitivity of PET varies from 50-90% and specificity from 60-80% [3].
Renal, Prostate and Bladder Cancers
PET is not useful in diagnosis of primary tumors of kidney, prostate and bladder due to low FDG avidity of these tumors and interference by high FDG concentration in urine. Role of PET in these cancers is mainly to detect distant metastases. Recently, modification of the PET technique by injection of a diuretic (frusemide) followed by delayed imaging has shown promising results in detection of kidney and bladder tumors. Newer tracers like 11C-acetate and 11C-choline have shown promising results in demonstrating the primary lesion in prostatic cancers.
Testicular Cancers
Both seminoma and non seminomatous germ cell tumors (NSGCT) spread most commonly to the retroperitoneal nodes. Accurate staging and surveillance to detect recurrence is aided quite well by FDG-PET. High sensitivity and negative predictive value for detecting nodal disease has been reported for PET [3].
Melanoma
Survival in melanoma cases depends on the stage at the time of diagnosis and thickness of the primary lesion. PET is useful in detecting metastatic disease and alters therapy in approximately one fourth of the patients [15].
Musculoskeletal Tumors
Malignant primary bone tumors are usually FDG avid as are many benign conditions like giant cell tumor (GCT), fibrous dysplasia and eosinophilic granuloma (EG). PET may be useful in patients who cannot undergo MR imaging and in monitoring the effects of therapy. If a non-responder is identified early, the course of therapy can be altered. In soft tissue sarcomas the accuracy of PET is related to grade of tumor. FDG avid tumors like malignant fibrous histiocytoma (MFH) allow detection with a high degree of sensitivity.
Neurology
Dementias
PET can help diagnose the etiology of dementia much earlier than the clinical criteria or MRI. In early Alzheimer's disease (AD) FDG PET reveals hypometabolism in superior parieto-temporal cortices, usually bilateral and often symmetrical. With progression of disease there is involvement of frontal cortices. Sparing of occipital visual cortex, somatosensory and motor cortex, basal ganglia and thalami is the norm [18]. On PET, Dementia with Lewy bodies (DBL) is shown to have greater involvement of occipital lobes, with preserved hippocampal activity. PET can differentiate frontotemporal dementias from AD by demonstrating reduced glucose metabolism in frontal and temporal lobes with preserved metabolism in parietal and occipital regions, in the former.
Epilepsy
Intractable seizures may require surgical therapy. Precise localization of the seizure focus is a prerequisite for such an intervention and FDG-PET has been found useful in localizing the seizure focus. Ictal PET imaging usually reveals focally increased glucose uptake at the site of seizure focus and interictal images reveal relative hypometabolism in the same area. Ictal study is more sensitive but technically demanding as it requires patient admission, continuous monitoring off medication and injection of radiotracer within seconds of seizure onset.
Movement Disorders
PET can image the dopaminergic system and 18F-Fluoro DOPA scan is used to evaluate cases of Parkinsons disease (PD). In PD there is reduced striatal metabolic activity which affects the posterior striatum first and gradually progresses anteriorly. New tropane agents derived from cocaine have been investigated to image the presynaptic membrane dopamine transporter (DAT) activity. PET is useful to assess the severity and monitor response to therapy in PD [3]. Huntingtons disease has characteristic findings on FDG-PET, marked by isolated hypometabolism in the caudate nuclei.
Stroke and Cerebrovascular Disease (CVD)
Although MRI and CT are the pre-eminent modalities for diagnosing stroke there is renewed interest in assessing the CVD patients with PET, to determine the role of therapeutic interventions. PET may show positivity earlier than CT in cases of stroke. Functional imaging offers the ability to visualize the relative cerebral blood flow (rCBF) and relative glucose metabolism (rCGM). It can help determine which patients are at risk for stroke, most likely to benefit from intervention and even predict stroke recovery. In the early hours following stroke PET can be used to detect potentially salvageable brain tissue by demonstrating active metabolism (glucose/ oxygen) in tissues with markedly reduced perfusion. Patients with such demonstrable areas of mismatch/misery perfusion are strong candidates for thrombolytic therapy [19].
Cardiology
Myocardial Viability
FDG/perfusion-PET imaging can be used to identify regions of myocardium that will improve with revascularization by comparing the regional FDG uptake with regional blood flow. Following an acute episode of ischemia, the vascular supply may return to normal, but persistent dysfunction of myocardial contractility, called myocardial stunning, may remain. Chronically ischemic area of myocardium may also show contractile dysfunction and is called myocardial hibernation. FDG PET can detect viability in the stunned and hibernating myocardium by demonstrating glucose uptake. FDG-perfusion mismatch pattern, i.e normal/ increased FDG in area of decreased perfusion indicates hibernating viable myocardium whereas areas with reduced perfusion and reduced FDG uptake indicate a scar. This assessment is important prior to revascularization procedures (Fig. 6).
Fig. 6.
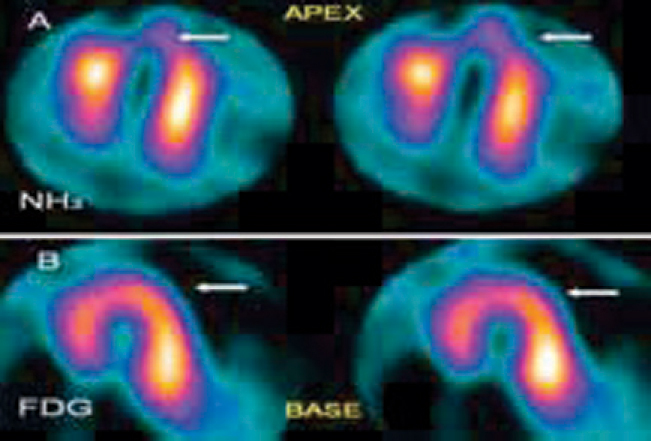
(A and B): Cardiac PET/CT Scan, horizontal long axis sections of the left ventricle: NH3 rest perfusion images (A, Top Row) show a perfusion defect (arrow) in the apex of left ventricle. FDG metabolism images (B, Bottom Row) reveal normal FDG uptake in the apex. Inference: hypo-perfused but viable (hibernating) myocardium in the apex.
Coronary Artery Evaluation
Myocardial perfusion imaging (MPI) was initially used for the detection of coronary artery disease (CAD). Now it is more often used in evaluation of known CAD to evaluate the hemodynamic significance of individual stenosis by studying the regional blood flow [20]. Classically MPI-PET is performed as a combination of post pharmacological stress and rest studies. Commonly used tracers are rubidium-82 or 13N -ammonia.
Coronary Perfusion Reserve
Absolute myocardial blood flow, measured in units of flow per gram of tissue (ml/min/g) provides additional information compared to relative perfusion. The ratio of maximum flow to the resting flow gives the coronary flow reserve. This is useful for evaluating individual coronary artery lesion and for evaluating the overall coronary vasculature [21]. Absolute myocardial blood flow is obtained directly from the washout of diffusible tracers like 15O-water and 11C- butanol [2].
The Future
For the future, integrating the high resolution PET scanner with high-performance multiple row detectors CT (MDCT) is an attractive concept that offers unparalleled performance for both modalities, with the potential for under 5-min imaging times. Apart from enhancing patient comfort and minimizing the effects of patient movement, a further advantage will be increased throughput of patients. With availability of newer, more specific tracers, PET and PET/CT will facilitate the transformation of imaging techniques from the current non-specific methods to patient specific imaging evaluation based on morphologic, molecular, physiologic and genetic markers of the disease.
Conclusion
Over the years PET has emerged as an important molecular imaging technique with useful clinical applications in oncology, cardiology and neurology. A major evolution in medical imaging has been the combination of anatomical and functional imaging in the form of PET/CT. It is fast becoming a powerful imaging tool and has already taken the centre stage in oncologic imaging. More promising clinical applications will be possible in the future with newer PET tracers becoming available for routine clinical use. However for the optimal use of these techniques it is important to understand their clinical applications, advantages and limitations. Ultimately the optimal use of multimodality imaging systems and specific imaging agents will achieve the task of accurate diagnosis, treatment evaluation, surveillance and prognostication.
Conflicts of Interest
None identified
References
- 1.Coleman RE, Delbeke D, Guiberteau MJ. Concurrent PET/CT with an integrated imaging System: Intersociety Dialogue from the Joint Working Group of the American College of Radiology, the society of nuclear medicine and the society of computed body tomography and magnetic resonance. J Nucl Med. 2005;46:1225–1239. [PubMed] [Google Scholar]
- 2.Jadvar H, Parker JA. In Clinical PET and PET/CT. first ed. Springer; Philadelphia: 2005. PET radiotracers; pp. 45–67. [Google Scholar]
- 3.Zeissmann HA, O'Malley JP, Thrall JH. Third ed. Elsevier; London: 2006. In Nuclear Medicine the requisites. [Google Scholar]
- 4.Gambhir SS, Czernin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med. 2001;42:1S–93S. [PubMed] [Google Scholar]
- 5.Ciernik IF, Dizendorf E, Baumert BG. Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): a feasibility study. Int J Radiat Oncol Biol Phys. 2003;57:853–863. doi: 10.1016/s0360-3016(03)00346-8. [DOI] [PubMed] [Google Scholar]
- 6.Esthappan J, Mutic S, Malyapa RS. Treatment planning guidelines regarding the use of CT/PET-guided IMRT for cervical carcinoma with positive paraaortic lymph nodes. Int J Radiat Oncol Biol Phys. 2004;58:1289–1297. doi: 10.1016/j.ijrobp.2003.09.074. [DOI] [PubMed] [Google Scholar]
- 7.Padma MV, Said S, Jacobs M. Prediction of pathology and survival by FDG PET in gliomas. J Neurooncol. 2003;64:227–237. doi: 10.1023/a:1025665820001. [DOI] [PubMed] [Google Scholar]
- 8.Schifter T, Hoffman JM, Hanson MW. Serial FDG PET studies in prediction of survival in patients with primary brain tumors. J Comput assisted Tomogr. 1993;17:509–516. doi: 10.1097/00004728-199307000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Jadvar H, Segall GM, Norbash AM. Unknown head and neck primary tumors: identification with F-18 FDG PET. Radiology. 1996;201:239. [Google Scholar]
- 10.Regelink G, Brouwer J, De Bree R. Detection of unknown primary tumors and distant metastases in patients with cervical metastases: value of FDG PET versus conventional modalities. Eur J Nucl Mol Imaging. 2002;29:1024–1030. doi: 10.1007/s00259-002-0819-0. [DOI] [PubMed] [Google Scholar]
- 11.Gould MK, Maclean CC, Kushner WG. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. Jama. 2001;285:914–924. doi: 10.1001/jama.285.7.914. [DOI] [PubMed] [Google Scholar]
- 12.Buck AK, Halter G, Schirrmeister H. Imaging proliferation in lung tumors with PET: (18) F-FLT versus (15) F-FDG. J Nucl Med. 2003;44:1426–1431. [PubMed] [Google Scholar]
- 13.Block MI, Patterson GA, Sundaresan RS. Improvement in staging of esophageal cancer with addition of positron emission tomography. Ann Thorac Surg. 1997;64:770–776. doi: 10.1016/s0003-4975(97)00619-x. [DOI] [PubMed] [Google Scholar]
- 14.Arslan N, Miller TR, Dehdashti F. Evaluation of response to neoadjuvant therapy by quantitative 2-deoxy-2 [18F] fluoro-D-glucose with positron emission tomography in patients with esophageal c. Mol Imaging Biol. 2002;4:301–310. doi: 10.1016/s1536-1632(02)00011-2. [DOI] [PubMed] [Google Scholar]
- 15.Macapinlac HA. FDG PET and PET/CT imaging in lymphoma and melanoma. Cancer J. 2004;10:262–270. doi: 10.1097/00130404-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Kostakoglu L, Coleman M, Leonard JP. PET predicts prognosis after 1 cycle of chemotherapy in aggressive lymphoma and Hodgkin's disease. J Nucl Med. 2002:1018–1827. [PubMed] [Google Scholar]
- 17.Kumar R, Alavi A. PET imaging in gynaecologic malignancies. Radiol Clin North Am. 2004;42:1155–1167. doi: 10.1016/j.rcl.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Silverman DH, Small GW, Chang CY. Positron emission tomography in evaluation of dementia: regional brain metabolism and long term outcome. JAMA. 2002;286:2120–2127. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 19.Mountz JM, Liu HG, Deutch G. Neuroimaging in cerebrovasular disorders: measurement of cerebral physiology after stroke and assessment of recovery. Sem Nucl Med. 2003;33:56–60. doi: 10.1053/snuc.2003.127293. [DOI] [PubMed] [Google Scholar]
- 20.Strauss HW, Grewal RK, Pandit-Taskar N. Molecular imaging in nuclear cardiology. Semin Nucl Med. 2004:47–55. doi: 10.1053/j.semnuclmed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Wyss CA, Koepfli P, Mikolajczyk Bicycle exercise stress PET for assessment of coronary flow reserve: repeatability and comparison with adenosine stress. J Nucl med. 2003;44:146–154. [PubMed] [Google Scholar]


