Abstract
Malaria and Cutaneous Leishmaniasis (CL) are co-endemic throughout large regions in tropical countries and co-infection may impact the evolution of host-parasite interactions. In the present study, we evaluate Malaria/Leishmaniasis disease outcome, Th1/Th2 cytokine levels and the CD4 and CD8 T-cell profiles in a co-infection murine model (BALB/c) of Plasmodium yoelii 17XNL (Py) and Leishmania amazonensis (La) or L. braziliensis (Lb). Malaria parasitaemia was assessed through blood strains stained with Giemsa. Leishmania lesions were monitored with a digital caliper and parasite loads determined by limiting-dilution assay. Serum levels of IFN-γ, TNF, IL-2, IL-4, IL-6, IL-10, and IL-17 were determined using multiplexed bead assay and expression of CD3, CD4, and CD8 T-cells markers were determined by Flow Cytometry in the thymus, spleens and lymph nodes. Parasitaemia in Lb+Py co-infected group was lower than in Py single-infected group, suggesting a protective effect of Lb co-infection in Malaria progression. In contrast, La+Py co-infection increased parasitaemia, patent infection and induced mortality in non-lethal Malaria infection. Regarding Leishmaniasis, Lb+Py co-infected group presented smaller lesions and less ulceration than Lb single-infected animals. In contrast, La+Py co-infected group presented only a transitory delay on the development of lesions when compared to La single-infected mice. Decreased levels of IFN-γ, TNF, IL-6, and IL-10 were observed in the serum of co-infected groups, demonstrating a modulation of Malaria immune response by Leishmania co-infections. We observed an intense thymic atrophy in Py single-infected and co-infected groups, which recovered earlier in co-infected animals. The CD4 and CD8 T cell profiles in thymus, spleens and lymph nodes did not differ between Py single and co-infected groups, except for a decrease in CD4+CD8+ T cells which also increased faster in co-infected mice. Our results suggest that Py and Leishmania co-infection may change disease outcome. Interestingly Malaria outcome can be altered according to the Leishmania specie involved. Alternatively Malaria infection reduced the severity or delayed the onset of leishmanial lesions. These alterations in Malaria and CL development seem to be closely related with changes in the immune response as demonstrated by alteration in serum cytokine levels and thymus/spleens T cell phenotypes dynamics during infection.
Keywords: co-infection, Malaria, Leishmaniasis, immunology, cytokine, Plasmodium yoelii, Leishmania braziliensis, Leishmania amazonensis
Introduction
Malaria and Cutaneous Leishmaniasis (CL) are two of the world’s most important vector-borne parasitic diseases (World Health Organization, 2010, 2015; Alvar et al., 2012). Malaria, an infectious disease caused by Plasmodium genus parasites, is an important cause of global mortality and morbidity. Half of the world population is at risk of contracting Malaria, with approximately 214 million cases and 438 000 deaths in 2015, amongst the 3.2 billion people living at risk of infection (World Health Organization, 2015). Humans can be infected by five Plasmodium species: P. falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi. Although P. falciparum accounts for the great majority of morbidity and mortality, P. vivax has a wider geographic distribution and causes considerable symptomatic disease (Battle et al., 2014). Malaria infection has a variable clinical phenotype, ranging from a mild febrile illness to severe disease and death, but infection can also occur in the absence of clinical symptoms. These variations in disease pattern are attributable to many factors, including the genetic background of the host and pathogen, the complex relationship between the parasite and host immune response, the dynamics of parasite transmission and/or the biological interactions of the parasites within the host (Good and Doolan, 2010; van den Bogaart et al., 2012). Leishmaniasis is a complex disease caused by different species of intracellular protozoan parasites from the genus Leishmania, which also induces significant morbidity and mortality throughout the world. According to the World Health Organization (2016) 350 million people in 98 countries are at risk of infection. There are an estimated annual 1.3 million new cases worldwide, of which 300,000 cases are of Visceral Leishmaniasis (VL) and another 1 million of CL. Seventy (70) to 75% of the CL cases occur in Afghanistan, Algeria, Brazil, Colombia, Costa Rica, Ethiopia, Iran, Peru, Sudan, and Syria (World Health Organization, 2016). CL is caused by L. major, L. tropica, and L. aethiopica in the Old World, whereas in the New World it is most frequently caused by L. mexicana, L. amazonensis, L. braziliensis, L. panamensis, and L. guyanensis (World Health Organization, 2010; Hartley et al., 2014). Symptoms range from the more prevalent single self-healing cutaneous lesions to uncontrolled parasite replication, producing non-healing cutaneous, mucosal or even visceral disease as well as chronic metastatic dissemination throughout the skin. This spectrum of manifestations is multifactorial and depends on complex interactions among parasite, host, and environmental factors, including the Leishmania specie, genetic background and immunological status of the host (Hartley et al., 2014).
The overlapping geographic distribution of Malaria and Leishmaniasis, especially in the tropical and subtropical countries demonstrate clearly that the potential for interaction among these parasites may occur and play a role in determining disease outcome (Hotez et al., 2006; van den Bogaart et al., 2012, 2014). Despite this natural coexistence, data from concomitant infections are so far not available in the literature (Ab Rahman and Abdullah, 2011; van den Bogaart et al., 2012, 2014). Therefore, the impact of the dual infections on the human population health remains unassessed particularly in what concerns CL.
In the eighties, two studies in the murine model evaluated the effect of non-lethal P. yoelii and L. amazonensis concomitant infections in the course of each disease (Coleman et al., 1988a,b). These studies demonstrated that severity and susceptibility to both diseases were enhanced during co-infection. Since these parasites do not compete for the same host cells, neither anatomical sites nor resources, the interaction among them is most likely indirect and related mostly to the host’s immune responses induced by each pathogen. However, in both studies (Coleman et al., 1988a,b) the immunological mechanisms involved were not investigated. Alternatively, several studies have shown that outcome of Malaria and CL is determined, in part, by the balance of pro- and anti-inflammatory immune responses (Louis et al., 1998; Li et al., 2001; Silveira et al., 2009; Taylor-Robinson, 2010; Medina et al., 2011; Freitas do Rosario and Langhorne, 2012; Kedzierski and Evans, 2014).
The immune response against the blood stages of Malaria parasites operate in concert and sequentially to control and clear the parasitaemia by an early and strong pro inflammatory, type 1 response (Th1), limiting parasite growth, followed by a shift to anti-inflammatory, type 2 immune (Th2) response. The balance between cytokines produced by pro inflammatory and anti-inflammatory responses during different phases of the blood stage infection determines the outcome of the disease (Li et al., 2001). In contrast to the strong type 1 immune response at the beginning of the Malaria infection, CL caused by L. amazonensis generally results in type 2 cellular immune response polarization (Silveira et al., 2009; Gollob et al., 2014). On the other hand, in CL caused by L. braziliensis, there is evidence that increased production of inflammatory cytokines (IFN-γ and TNF-α) and absence of IL-10 is associated with tissue destruction and the development of mucosal lesions (Dutra et al., 2011; Gollob et al., 2014; Oliveira et al., 2014). In this context, the current study was designed to evaluate the outcome of Malaria-CL infections, the CD4 and CD8 T-cell profiles as well as the Th1/Th2 cytokine levels in a co-infection murine model (BALB/c) of P. yoelii 17XNL (non-lethal) and L. amazonensis or L. braziliensis. Furthermore, as far as we know, this is the first report utilizing L. braziliensis and P. yoelii 17XNL non-lethal strain as a co-infection model.
Materials and Methods
Animals, Parasites, and Infections
Female BALB/c mice (5–6 weeks old) were obtained from the Center for Laboratory Animal Breeding of the Oswaldo Cruz Foundation (FIOCRUZ) (Rio de Janeiro, RJ, Brazil). Mice were maintained under specific pathogen-free conditions in Experimental Animal Center of Leônidas Deane building (FIOCRUZ). This research protocol was approved by the Ethical Committee for Animal Use of FIOCRUZ/MS (license LW-17/11).
P. yoelii 17XNL (non-lethal strain) was provided by Dr Fábio T.M. Costa at Departamento de Genética, Evolução e Bioagentes, Instituto de Biologia, UNICAMP, Campinas, SP, Brazil. Parasite stabilates were stored at -196°C. To obtain the experimental inoculum of P. yoelii 17XNL, parasitized red blood cells (pRBCs) were defrosted and passed through three homologous donor mice.
Promastigotes of L. braziliensis (MCAN/BR/98/R69) and L. amazonensis (IFLA/BR/67/PH8), provided by Laboratório de Pesquisa em Leishmaniose, Instituto Oswaldo Cruz, FIOCRUZ, RJ, Brazil, were cultured in Schneider’s medium supplemented with antibiotics (200 IU penicillin and 200 μg streptomycin/ml) and 10% inactivated fetal calf serum (all from Sigma–Aldrich, St Louis, MO, USA). For L braziliensis 2% of sterile human urine was also added to the cultures (Howard et al., 1991).
Eight to nine-week-old mice (∼22g each) were divided randomly into six groups, Figure 1. Group C: comprised uninfected mice; Group Py: mice were injected intraperitoneally (i.p.) with 106 P. yoelii 17XNL pRBCs (0.2 mL); Groups Lb and La: mice were inoculated intradermally in both ears with either 105 or 104 stationary phase promastigotes of L. braziliensis or L. amazonensis, respectively, as previously described (Belkaid et al., 2000); Groups Lb+Py and La+Py: mice were co-infected with both P. yoelii 17XNL and either L. braziliensis or L. amazonensis. Each group had 25 to 35 mice and experiments were repeated at least three times. First, mice were infected with Leishmania sp. and 3 days later with P. yoelii 17XNL, as shown in Figure 1. At 5, 10, 17, and 25 days post-P. yoelii 17XNL infection, six animals from each group were bled, for serum cytokine assay, and euthanized, for thymus, spleen, ears and lymph nodes removal. Mortality of mice was monitored daily, post-P. yoelii 17XNL infection.
FIGURE 1.
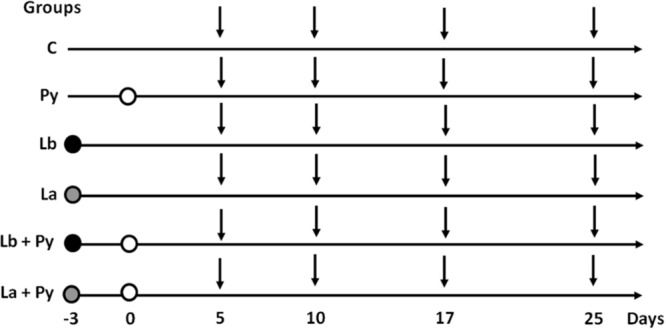
Experimental design. Mice were infected with Leishmania braziliensis. (black circles) or L. amazonensis (gray circles) 3 days before Plasmodium yoelii infection (white circles). On days 5, 10, 17, and 25 post P. yoelii infection (vertical arrows), six mice from Py, Lb, La, Lb+Py, and La+Py groups and four mice from control (C) group were euthanized to evaluate parasite loads in the ears and draining lymph nodes (Lb/La and Lb+Py/La+Py groups), cytokine serum levels (all groups), thymus relative weight (all groups), absolute numbers of thymocytes (all groups) and phenotyping of T cells in the thymus, spleen and lymph nodes (all groups).
Determination of Parasite Load, Leishmania Lesion Size, and Ulceration
Malaria parasitaemia was monitored every 2 days starting at day 3 post-infection. Blood smears were prepared from tail vein, methanol-fixed, stained with Giemsa and microscopically examined to determine parasitaemia in 1,000 erythrocytes. The percentage of infected erythrocytes was calculated as follows: Parasitaemia (%) = (number of infected erythrocytes x 100)/total number of erythrocytes counted (1,000).
The diameter of dermal Leishmania sp. lesions were measured weekly with a digital calipter. We also monitored the time elapsed until ulcer formation in nodules. Parasite numbers in the ears and draining lymph nodes were determined as previously described (Belkaid et al., 2000) and scored as the highest dilution containing viable parasites after incubation for 6 days at 26°C.
Cytokines Quantitation in the Serum
The serum levels of cytokines (interferon – IFN-γ, tumor necrose factor – TNF, interleukin (IL)-2, IL-4, IL-6, IL-10, and IL-17) were determined using the BD Cytometric Bead Array Kit Mouse Th1/Th2/Th17 Cytokines (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. Briefly, 25 μL of mouse serum was incubated for 2 h at room temperature with 25 μL of cytokine capture bead and 25 μL of phycoerythrin (PE) detection reagent. Then, samples were washed with 1 mL of buffer by centrifugation (200 × g, 5 min). The supernatants were carefully aspirated and discarded from each assay tube. Finally, beads were resuspended in 300 μL of buffer for analysis on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA). The serum cytokine concentrations (pg/mL) were determined using a standard curve from recombinant cytokines provided by the kit.
Determination of Thymus Relative Weight
For determination of thymus relative weight, body and thymus of each mouse were weighted. Thymic index (TI) was calculated according to the following formula: TI = thymus weight (mg) / body weight (g).
T Cell Phenotyping by Flow Cytometry
Thymus, spleens, mesenteric nodes, subcutaneous nodes (cervical, axillary, brachial, and inguinal) and submandibular nodes (draining ears lesions) were dissected and mechanically disaggregated. Single-cell suspensions from control, infected and co-infected mice were obtained in RPMI-1640 supplemented with 10% fetal calf serum (Gibco-Invitrogen, USA). Cell numbers and viability were determined by Trypan blue exclusion using Neubauer chamber.
For T lymphocytes phenotypic characterization, 106 cells were stained with FITC labeled anti-mouse CD4, APC labeled anti- mouse CD8 and PE labeled anti-mouse CD3 monoclonal antibodies. After incubation for 30 min, followed by 2 washes with staining buffer (PBS, 0,1% BSA, 0,01% sodium azide, all from Sigma–Aldrich), cells were fixed with 1% paraformaldehyde solution (Sigma–Aldrich), washed and resuspended in staining buffer until acquisition. A minimum of 30,000 events per sample were acquired inside the lymphocytes gate, based on size and granularity properties using a FACSCantoIITM flow cytometer (BD Bioscience, USA) and analyzed using FlowJo 7.5.5 software (Tree Star Inc. Ashland, OR, USA).
Statistical Analysis
Sample size was determined a priori using the software G*Power version 3.1.9.2, based on the Mann–Whitney test, a significance level of 0.05, and a minimum power of 80%. Results were evaluated by a non-parametric test, the Mann–Whitney test, using GraphPad Prism software version 5.0 (GraphPad Software, La Jolla, CA, USA). Data are expressed as means ± SEM and considered to be statistically significant when p < 0.05. Survival curves were analyzed by Mantel Cox and Gehan–Breslow–Wilcoxon tests and Leishmania lesions ulcerations were analyzed by Chi-square test using the same software.
Results
L. braziliensis and L. amazonensis co-Infection exerts opposite effects on P. yoelii 17XNL Infection
Blood stage P. yoelii 17XNL parasites induced Malaria infection in all BALB/c mice from single-infected (Py) and L. braziliensis or L. amazonensis co-infected groups (Lb+Py and La+Py). Parasitaemia peak occurred between 10 and 17 days after infection and no parasite was detected after 23 days post-infection in blood smears of Py single-infected mice. The overall mean parasitaemia observed in Lb+Py co-infected group was lower than that observed in P. yoelii single-infected group (Py). Statistically significant differences observed in some days are suggestive for a protective effect of L. braziliensis co-infection in Malaria progression (Figure 2A). In contrast, co-infection with L. amazonensis appears to have a negative influence in acute Malaria (Figure 2C), and mortality in La+Py co-infected group enhanced in comparison to Py single infected group (Figure 2D). La+Py group exhibited higher parasitaemia on day 5 and a longer course of P. yoelii Malaria patency with mice remaining parasitemic 8 days longer than mice in Py single-infected group (Figure 2C). The higher rate of parasitemia in La+Py coinfeted mice seems to be associated with decreased survival. Four weeks after P. yoelii 17XNL infection, 30% of the co-infected La+Py mice were dead, compared to 100% of survival in the group infected only with P. yoelii 17XNL. Py single-infected groups showed no mortality during the whole experiment (Figures 2B,D).
FIGURE 2.
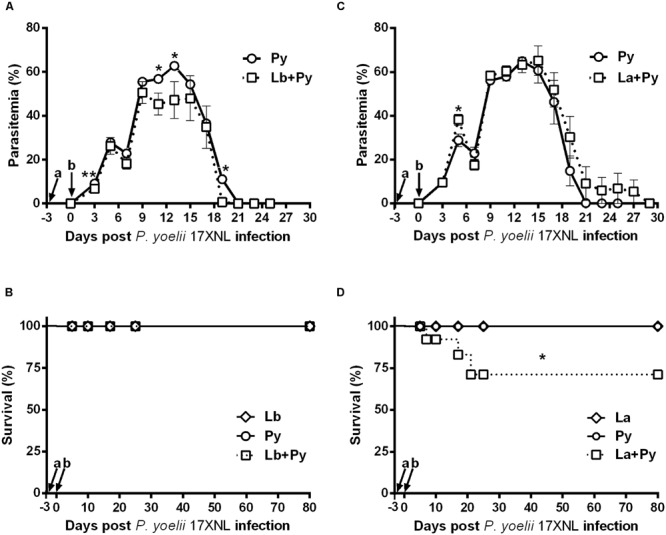
Parasitaemia and survival rate of BALB/c mice infected with blood-stage P. yoelli 17XNL non-lethal strain and co-infected with Leishmania sp. and P. yoelii 17XNL. (A,C) Malaria parasitaemia in P. yoelii single-infected mice (Py) and in mice co-infected with L. braziliensis/P. yoelii (Lb+Py) (A) or with L. amazonensis/P. yoelii (La+Py) (C). (B,D) Comparison of the survival rate in Py single-infected and Lb+Py co-infected (B) or La+Py co-infected mice (D). Parasitaemia results are expressed by the mean ± SEM of at least 10 mice per group. The Mann–Whitney test was used to compare Py single-infected and co-infected groups. Survival rates were compared by Mantel-Cox test. ∗p < 0.05 and ∗∗p < 0.01, respectively. Arrows indicate the time of Leishmania (a) and P. yoelii (b) infection.
Co-Infection is Able to Reduce Severity of L. braziliensis Lesions
BALB/c mice were intradermally infected with 105 stationary phase promastigotes of L. braziliensis. Lb+Py co-infected group showed an overall tendency to present smaller lesion sizes than Lb single infected group (Figure 3A). In both groups, lesions reached maximum size 5 weeks post-infection and started to heal afterward. To evaluate disease severity we followed the onset of open ulcers in Leishmania-infected and co-infected groups (Figure 3B). Ulcers started to appear 21 days post-infection in Lb group and after 28 days in Lb+Py. At week 11 after infection (77 days) Lb single-infected group presented a higher number of ulcerated nodules (83.3%) than Lb+Py (40.9%). At week 17 and 18 post infection, there was a complete resolution of lesions in Lb+Py and Lb groups, respectively (data not shown). Limiting-dilution assays performed in the ears and in draining lymph nodes showed an earlier detection of parasites in the ears of Lb single-infected animals than in co-infected group (14 and 21 days post-infection, respectively) with no difference in parasite loads between groups. On the other hand, in the draining lymph nodes parasites were detected 28 days after infection in both groups, and parasite loads were lower in Lb+Py co-infected group (p = 0.006, Figures 3C,D).
FIGURE 3.
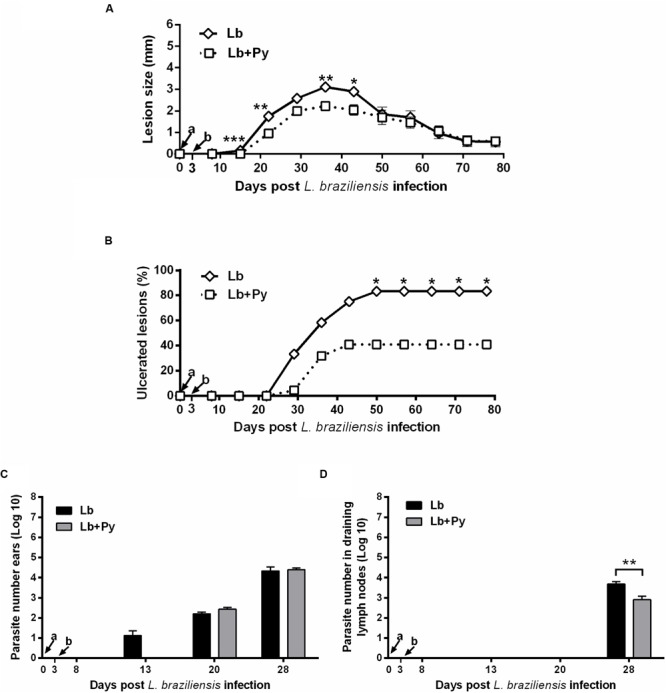
Evaluation of L. braziliensis infection. (A) Lesion development, (B) onset of open ulcers, and (C) parasite quantitation in infected ears and (D) draining lymph nodes of BALB/c mice after intradermal infection in the ears with L. braziliensis promastigotes (Lb) or co-infected with L. braziliensis and P. yoelii (Lb+Py). Parasite loads were individually determined by a limited dilution assay. Results are expressed by the mean ± SEM of twelve ears and lymph nodes. The Mann–Whitney test was used to compare lesion size (A) and parasite loads in ears (C) and draining lymph nodes (D). Chi-square test was used to evaluate ulcerations (B). ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001, respectively. Arrows indicate the time of L. braziliensis (a) and P. yoelii (b) infection.
Co-Infection Reduce Severity of L. amazonensis Lesions Only during Acute Phase Malaria
Infection of BALB/c mice with 104 stationary phase promastigotes of L. amazonensis induced chronic lesions that did not heal over time in La and La+Py groups (Figure 4A). During murine Malaria (from day 0 to day 25) lesions in La+Py co-infected group were significantly smaller than in La single infected group. Although lesions of La group presented greater sizes than La+Py until 28 days of infection, after that time and until the end of the experiment both groups present similar lesion sizes. Ulcerated nodules were detected first in La group (21 days post-infection) while in La+Py group visual detection occurred 1 week later (28 days post-infection) (Figure 4B). However, after 63 days of infection all animals from both groups presented ulcerated nodules. Limiting dilution analysis performed at the site of infection and in draining lymph nodes showed higher parasite loads in La+Py group than La single-infected group (Figures 4C,D).
FIGURE 4.
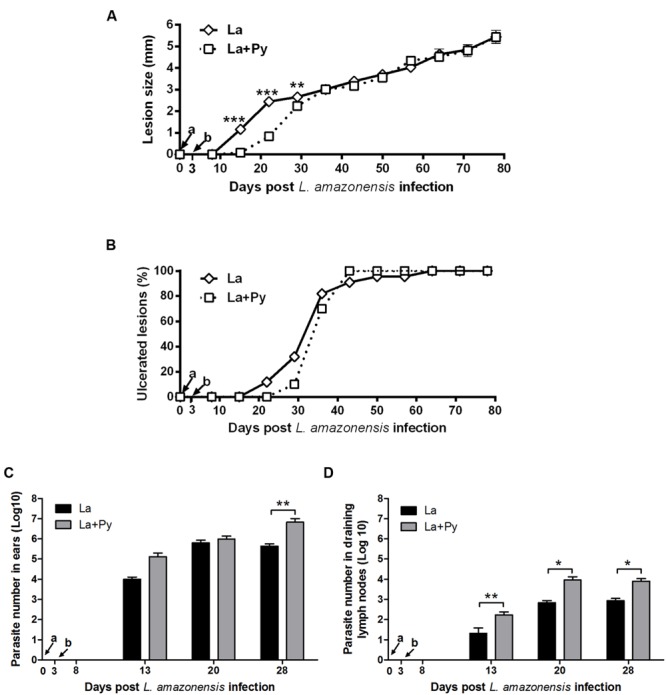
Evaluation of L. amazonensis infection. (A) Lesion development, (B) onset of open ulcers, and (C) parasite quantitation in infected ears and (D) draining lymph nodes of BALB/c mice after intradermal infection in the ears with L. amazonensis promastigotes (La) or co-infected with L. amazonensis and P. yoelii (La+Py). Parasite loads were individually determined by a limited dilution assay. Results are expressed by the mean ± SEM of twelve ears and lymph nodes. The Mann–Whitney test was used to compare parasite loads in ears and draining lymph nodes. ∗p < 0.05 and ∗∗p < 0.01, respectively. Arrows indicate the time of L. braziliensis (a) and P. yoelii (b) infection.
Co-Infection Modulate Serum Cytokine Levels Induced by P. yoelii 17XNL
Cytokine levels were measured in serum samples obtained from 4 to 6 animals of each experimental group. As expected, increased levels of the proinflammatory cytokine IFN-γ, were observed in all groups infected with P. yoelii 17XNL (Py, Lb+Py, and La+Py) in the first week of infection (day 5). The levels of TNF, IL-6 and IL-10 also increased in the serum of the same three groups during blood stage Malaria. Cytokine levels in co-infected groups (Lb+Py and La+Py) were clearly lower than those observed in Malaria single infected group (Py) suggesting a modulatory effect of co-infection (Figure 5). We were not able to detect any of the analyzed cytokines in serum samples from control and Lb or La single infected animals, except for IL-10 in La infected group, only at day 25th post infection. Although we could observe a tendency for an increased production of TNF, IL-6 and IL-10 in the first 10 days of Malaria infection for Lb+Py co-infected group when compared to La+Py co-infected animals, statistic difference was observed only for IL-6 at day 10 post Py infection (P = 0.045). We were not able to detect the cytokines IL-2, IL-4, and IL-17 in serum samples from all experimental groups, at any time of the study.
FIGURE 5.
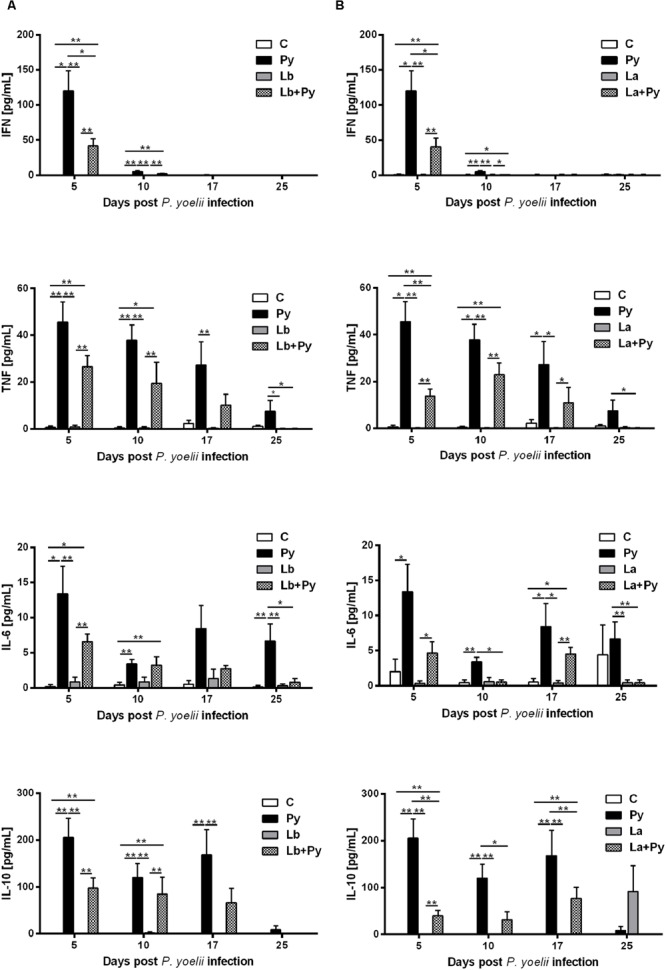
Kinetics of cytokine levels in the serum of mice infected with P. yoelii (Py), L. braziliensis (Lb), L. amazonensis (La) or co-infection with Leishmania sp. and P. yoelii. Serum levels of IFN, TNF, IL-6, and IL-10 were determined by Cytokine Bead Array. Cytokine levels obtained in the serum of non-infected controls (open bars) were compared to those obtained in Py single-infected mice (black bars) and in animals co-infected (checkered bars) or single infected (gray bars) with L. braziliensis (A) or L. amazonensis promastigotes (B). Results are expressed by the mean ± SEM of 4–6 mice per group. The Mann–Whitney test was used to compare serum cytokine levels between groups. ∗p < 0.05 and ∗∗p < 0.01, respectively.
Recovery of Thymic Atrophy Occurs Earlier in Co-Infected Animals
In the course of Malaria infection, we observed a significant reduction of the thymus relative weight in comparison to the uninfected control group. Thymic atrophy was first observed 10 days post P. yoelii infection (dpi) and persisted until day 25th of infection in Py single-infected group. In co-infected groups (Lb+Py and La+Py) thymus relative weight came back to normal values at 25 day post-infection and no significant difference could be observed in comparison to the control group. During the entire experiment, Leishmania single-infected groups showed no differences in thymus relative weight when compared do the uninfected control group (Figure 6).
FIGURE 6.
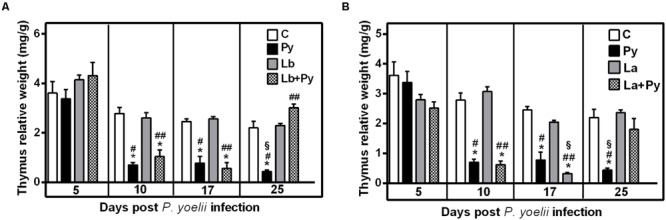
Alterations in the thymus relative weight after infection with P. yoelii (Py), L. braziliensis (Lb), L. amazonensis (La), or co-infection with Leishmania sp. and P. yoelii. Thymus relative weight in uninfected controls (open bars) were compared to those obtained in Py single-infected mice (black bars) and in animals co-infected (checkered bars) or single infected (gray bars) with L. braziliensis (A) or L. amazonensis promastigotes (B). Results are expressed by the mean ± SEM of 4–6 mice per group. The Mann–Whitney test was used to compare thymus relative weight between groups. (∗) statistically different from control, (#) statistically different from Leishmania single infection and (§) statistically different from Leishmania/P. yoelii co-infected group.
Flow cytometry analysis showed that the thymus of Py and co-infected groups underwent a decrease in CD4+CD8+ double-positive (DP) T cell subset and an increase in CD4+ single-positive (SP), CD8+SP and CD4-CD8- double-negative (DN) T cell populations (Figure 7). A significant increase in CD4+SP thymocytes occurred at 17 dpi and persisted up to 25 dpi in Py single-infected animals. In Lb+Py group, CD4+SP thymocytes increased 10 days post infection, and returned to control values by day 25th post infection. In La+Py this transitory increase was observed latter at 17 dpi. The dynamics of CD8+SP thymocytes in single infected and co-infected groups exhibited the same profile of CD4+SP. When we evaluate this results together, it is possible to notice that in Lb+Py co-infection, the increase in CD4+SP and CD8+SP occurred sooner than in La+Py group (10 and 17 dpi, respectively). Double-negative thymocytes increased in Py single-infection at 17 and 25 dpi. The same increase occurred in co-infected groups only at day 17 post infection. In parallel, CD4+CD8+ DP cells significantly decreased in the thymus of Py single infected animals at 17 dpi and remained lower at 25 dpi. This decrease was observed earlier is Lb+Py group (10 dpi) but, at day 25th post infection, DP cells increased in this group and were higher than in control and single infected groups. Interestingly, in La+Py co-infected group, the increase in DP thymocytes occurred earlier (10 dpi) and was followed by a rapid decrease at day 17th post infection, coming back to control values 25 dpi. Overall, thymus recovery seems to occur faster in Malaria/Leishmania co-infection than in Py single infection.
FIGURE 7.
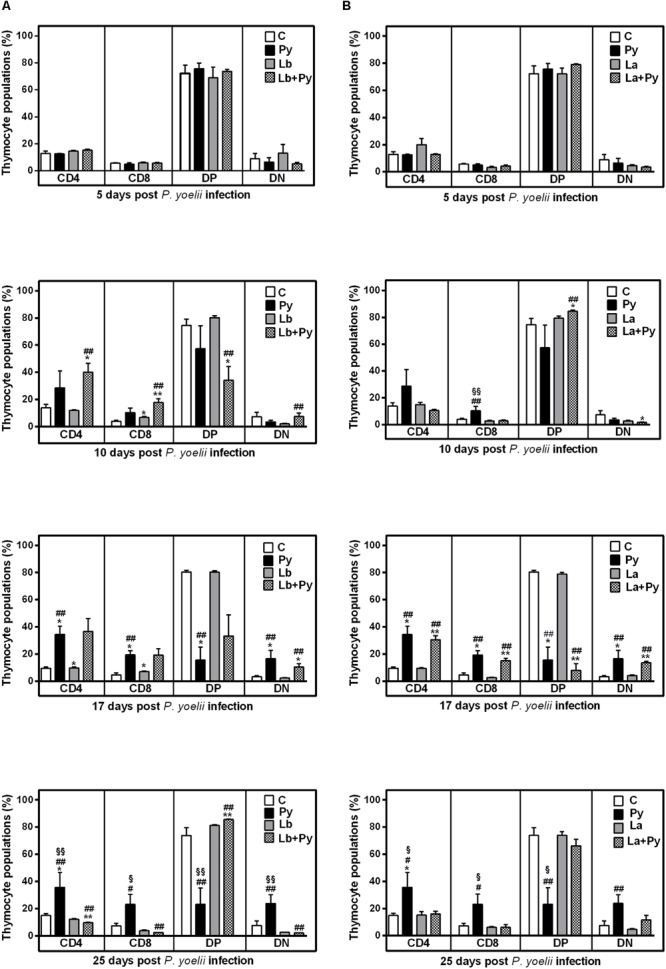
Alterations in thymic T-cell subsets after infection with P. yoelii (Py), L. braziliensis (Lb), L. amazonensis (La), or co-infection with Leishmania sp. and P. yoelii. The phenotype of T subsets in the thymus were determined by flow cytometry at days 5, 10, 17, and 25 post P. yoelii infection. The percentages of CD4+, CD8+, CD4-CD8- double negatives (DN), and CD4+CD8+ double positive cells (DP) obtained in uninfected controls (open bars) were compared to those obtained in Py single-infected mice (black bars) and in animals co-infected (checkered bars) or single infected (gray bars) with L. braziliensis. (A) or L. amazonensis promastigotes (B). Results are expressed by the mean ± SEM of 4–6 mice per group. The Mann–Whitney test was used to compare groups. (∗) statistically different from control, (#) statistically different from Leishmania sp. and (§) statistically different from Leishmania sp./P. yoelii co-infected group.
Leishmania Co-Infection Delay the Enhancement of Double Negative Cells Induced by P. yoelii 17XNL Infection in the Spleen
We also evaluated the T cell phenotypes present in the spleens, mesenteric, subcutaneous and submandibular nodes (the last ones as the draining nodes from ears lesions) in single infected and co-infected animals. No alterations were observed in the T cell phenotypes present in subcutaneous and submandibular nodes among the studied groups, while in mesenteric nodes and spleens we were able to observe similar patterns of alterations that were more evident in the spleens (Figure 8). After a transitory increase at 10 dpi in Py single infected and in co-infected groups, CD4+ T cells dropped in Py single infected group at 17 dpi and remained bellow controls at 25 dpi. In Lb+Py co-infected group a significant decrease was observed only at 25 dpi. Py single infected animals also presented a significant drop in the percentages of CD8+T cells. In co-infected groups, the percentages of CD8+T cells were bellow controls at 10, 17, and 25 dpi. Low percentages of DP splenocytes were observed in Py single infected and co-infected groups, which were different form controls only when animals started to recover from malarial infection (17 and 25 dpi). On the other hand, P. yoelii infection increased the percentage of DN splenocytes during the entire experiment, and Leishmania co-infection was able to delay this enhancement. The percentages of CD4, CD8, DP, and DN splenocytes in Leishmania single infected groups (both Lb and La) remained similar to uninfected controls at all time points.
FIGURE 8.
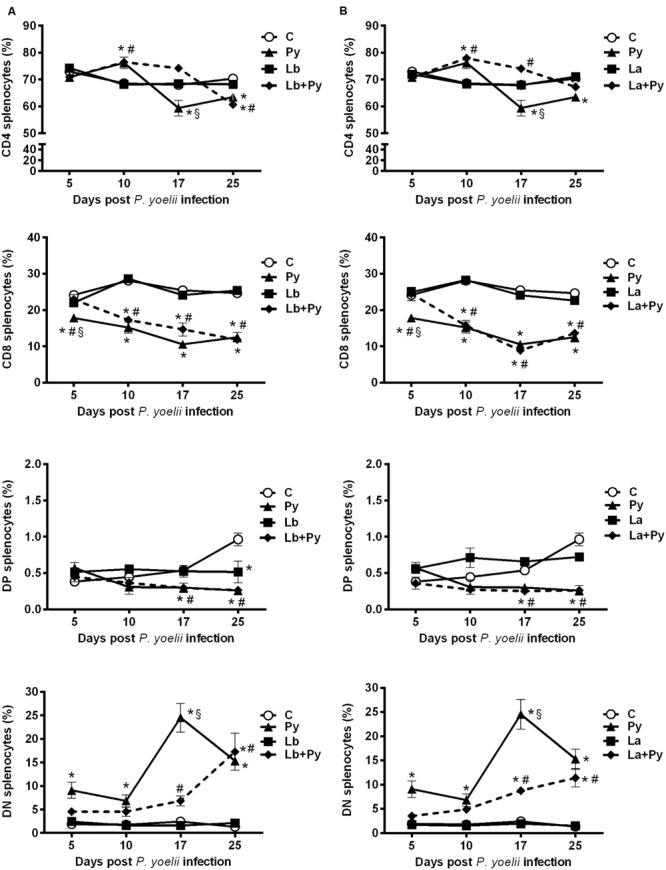
Alterations in spleen T-cell subsets after infection with P. yoelii (Py), L. braziliensis (Lb), L. amazonensis (La), or co-infection with Leishmania sp. and P. yoelii. The phenotype of T subsets in the spleen were determined by flow cytometry at days 5, 10, 17, and 25 post P. yoelii infection. The percentages of CD4+, CD8+, CD4-CD8- double negatives (DN), and CD4+CD8+ double positive cells (DP) obtained in uninfected controls (open circles) were compared to those obtained in Py single-infected mice (black triangles) and in animals co-infected (black diamonds with dashed line) or single infected (black squares) with L. braziliensis. (A) or L. amazonensis promastigotes (B). Results are expressed by the mean ± SEM of 4–6 mice per group. The Mann–Whitney test was used to compare groups. (∗) statistically different from control, (#) statistically different from Leishmania sp. and (§) statistically different from Leishmania sp./P. yoelii co-infected group.
Discussion
Malaria and CL are neglected diseases spread over many tropical and subtropical regions of the World showing extensive overlapping geographic distribution. In addition, the increase in travel, migration and war refugees from endemic regions are factors that also contribute to the risk of simultaneous infections by Plasmodium spp. and Leishmania spp. parasites. Some cases of human Malaria and VL co-infection were described in the past and recently in different parts of the world (Yoeli, 1948; Ab Rahman and Abdullah, 2011; van den Bogaart et al., 2012, 2014; Bin Mohanna, 2015), demonstrating that co-infection may occur more frequently than we would expect. An increasing amount of evidence shows that co-infections may affect the natural outcome and progression of diseases due to the modulation of immune response (La Flamme et al., 2002; Kolbaum et al., 2012; Qi et al., 2013; van den Bogaart et al., 2014). In the present study, we established a co-infection model of American CL and a non-lethal murine Malaria and demonstrated that co-infection is able to affect the pathogenesis and outcome of both diseases.
Pilot studies were carried out in our Laboratory in which mice were co-infected at different times and orders. It was observed that the outcome of malaria or Leishmania infections were the same in any time and order of infections tested. For operational reasons we selected day 3 for Py infection. In addition, our results regarding the outcome of both diseases are similar to those published by Coleman et al. (1988a,b) in P. yoelii and L. amazonensis coinfection experiments.
Rodent Malaria parasites provide great models to investigate pathogenesis and immune mechanisms during infection (Li et al., 2001; Zuzarte-Luis et al., 2014). The non-lethal strain 17XNL of P. yoelii generally causes a transient disease with moderate parasitaemia, weight loss, splenomegaly, hypothermia, and anemia (Coleman et al., 1988b; Kobayashi et al., 1996; Niikura et al., 2008; Kolbaum et al., 2012; Karadjian et al., 2014). In our study, BALB/c mice infected with blood-stage P. yoelii 17XNL develop a self-limiting infection with mild parasitaemia that resolve in 4 weeks. Prior infection with L. braziliensis reduced parasitaemia, while animals previously infected with L. amazonensis showed a tendency to have higher parasitaemia than Py single infected group. Mortality during acute Malaria increased with L. amazonensis co-infection, demonstrating that the existence of intrinsic differences between this two Leishmania species during co-infection, may affect animal survival.
The course of experimental CL is dependent on a combination of factors: Leishmania and host species, parasite strain, site, and size of the inoculum among other aspects (Pereira and Alves, 2008; Pereira et al., 2009; Ribeiro-Gomes et al., 2014). The murine model of subcutaneous infection with L. major generated a great amount of data concerning the immune response in CL. It was in this model that could be demonstrated the importance of a type 1 (proinflammatory) specific immune response for healing the infection in genetically resistant strains of mice (such as C57BL/6), as well as the dominance of a type 2 immune response (with a strong production of IL-4 and IL-10) in susceptible BALC/c mice. On the other hand, all strains of mice that have been tested so far using the subcutaneous route of infection are susceptible to L. amazonensis, and non-permissive hosts for L. braziliensis.
The intradermal rout of infection with low doses of parasites in the ears, first developed for L. major infection (Belkaid et al., 2000), proven also to be an important experimental model for L. braziliensis studies. After intradermal infection in the ears with L. braziliensis promastigotes BALB/c are able to develop ulcerated lesions that heal spontaneously, similar to the most common clinical manifestation of American Tegumentary Leishmaniasis caused by L. braziliensis: the localized cutaneous form (de Moura et al., 2005; Falcão et al., 2012; Carregaro et al., 2013). In the present work, mice infected intradermally with L. braziliensis developed a localized lesion that increased in size, became ulcerated and self-resolved by week 16 post infection, as expected (data not shown). Co-infected mice exhibited similar pattern in lesions development, but those were smaller and presented less ulcerations. L. amazonensis infection, on the other hand induced progressive non-healing lesions, and at the end of the study (11 weeks after L. amazonensis infection) 100% of the animals presented ulcerated lesions. Interestingly, during acute Py infection (in the 1st 4 weeks post L. amazonensis infection), co-infected group presented a transitory delay on the development of lesions as well as smaller lesions when compared to La single-infected mice. As mentioned before, the differences in susceptibility to Leishmania species in murine models, as well as in humans, have multifactorial causes, one of them being the type of immune response generated after infection. For this reason, the differences observed in the disease outcome of L. braziliensis and L. amazonensis infection during Malaria co-infection must be evaluated in the context of the intense systemic immune response elicited by P. yoelii 17XNL in the 1st weeks of Leishmania infection.
It is well described that Malaria erythrocytic stage triggers a strong IFN-γ response in both rodent and human Malaria (Shear et al., 1989; Chen et al., 2010; Perez-Mazliah and Langhorne, 2014). The first wave of proinflammatory cytokines is sustained by the innate immune system, when parasitaemia is still low, and is characterized by the release of IFN-γ, TNF-α, IL-2, and IL-12. The inflammatory environment sustains CD4+ T cell polarization to a Th1 phenotype that provides more IFN-γ for macrophages activation and control of parasitaemia (Artavanis-Tsakonas and Riley, 2002; Artavanis-Tsakonas et al., 2003). In our experiments, we were able to detect an early and pronounced increase of IFN-γ levels in the serum of P. yoelii 17XNL single infected and co-infected groups, mainly in the beginning of the infection, as extensively described in literature (Shear et al., 1989; Kobayashi et al., 1996; Shan et al., 2012; Karadjian et al., 2014). While the enhanced levels of IFN-γ in the serum was only transitory (5 and 10 days after P. yoelii 17XNL) the levels of TNF and IL-6 also increased in animals infected with P. yoelii 17XNL, but remained elevated for a longer period of time (during patent parasitaemia) in the serum. Co-infected groups presented smaller lesions than Leishmania single infected groups and this effect occurred simultaneously with the increased cytokines levels in the serum, during Malaria erythrocytic phase. The strong proinflammatory environment caused by P. yoelii 17XNL infection could explain the delay on lesions development in co-infected groups, given the fact that IFN-γ and TNF play an essential role in controlling Leishmania replication inside macrophages (Ashok and Acha-Orbea, 2014). Obviously a clearly scenario can be achieve if we evaluate the Leishmania-specific immune response developed in Leishmania single infected and Leishmania /P. yoelii 17XNL co-infected groups. This matter is currently under investigation.
In CL as well as in Malaria, excessive inflammatory response can lead to pathology or even death. Therefore, the balance between pro and anti-inflammatory cytokines is important to control pathology and parasitaemia (Artavanis-Tsakonas et al., 2003; Antonelli et al., 2005; Oliveira et al., 2014). Overall we could observe a tendency for reduced levels of IFN-γ, TNF, IL-6, and IL-10 in the serum of co-infected groups, when compared to P. yoelii 17XNL single infected group, suggesting a modulation of P. yoelii 17XNL induced immune response by Leishmania co-infection. On the other hand, L. braziliensis and L. amazonensis co-infection exerts opposite effects on P. yoelii 17XNL infection (Figures 2 and 3). IL-10 is a key regulatory cytokine to protect mice against pathology during acute Malaria (Kobayashi et al., 1996; Freitas do Rosario and Langhorne, 2012). Interestingly the reduction in the serum levels of IL-10 reached statistically significance only in L. amazonensis co-infected group at days 5 and 10 post P. yoelii 17XNL infection. This significant reduction in serum IL-10 levels could be linked to the persistent parasitaemia and deaths observed in L. amazonensis co-infected group (Figure 2).
Similar to several other parasitic diseases, experimental Malaria infections can cause severe thymic alterations (Savino, 2006; De Meis et al., 2012). During infection the organ undergoes a strong atrophy with disruption of its architecture that may influence T cell maturation. It has been already demonstrated that mice infected with P. chabaudi or P. berghei, two other rodent Malaria species, exhibit an important thymic atrophy with cellular depletion and histological disorganization (Seixas and Ostler, 2005; Andrade et al., 2008; Francelin et al., 2011; Lima et al., 2012). Thymic atrophy caused by infectious diseases may occur in combination with one or more of the following events: impairment of thymocyte proliferation, increase in thymocyte death and increase in thymocyte migration to peripheral lymphoid tissue (De Meis et al., 2012). Increase in apoptosis and thymocyte migration has been described in a variety of diseases (Savino, 2006), including Malaria (Francelin et al., 2011).
Accordingly, in P. yoelii 17XNL infection we observed a profound thymic atrophy with a reduction in the percentage of double positive cells (CD4+CD8+) and an increase in the percentage of single positive (CD4+ and CD8+) and double negative cells (CD4-CD8-). We also observed a high degree of apoptosis and necrosis in the thymocytes of P. yoelii single infected and co-infected groups, with no difference between groups (data not shown). Besides the intense atrophy during acute Malaria, thymus was capable to recover its normal size and cellularity after P. yoelii elimination, in accordance with observations with P. chaubaudi non-lethal infection (Seixas and Ostler, 2005). Infections with both Leishmania species were not able to cause thymus involution in mice, probably due to the localized nature of the cutaneous lesions they cause, with no or little impact in the systemic immune environment. Remarkably, thymus recovery occurred faster in co-infected animals, what we believe is a reflection of the minor inflammatory environment observed in co-infected animals.
The reduction in double positive thymocytes has also been described as common feature in infectious diseases, and can be explained by a premature escape of immature cells to blood and peripheral lymphoid organs (De Meis et al., 2012). P. yoelii 17XNL single infected and co-infected groups showed decreased percentages of double positive CD4+CD8+ splenocytes when compared to Leishmania single infected animals and uninfected controls, suggesting that the reduced percentages of double positive cells in the thymus of those groups were not due to increased migration to the peripheral lymphoid organs.
Among the mechanisms described to clear blood-stage Malaria parasites are mature isotypes antibodies and antibody-independent T cell mechanisms (Marsh and Kinyanjui, 2006; Beeson et al., 2008), but all of them require the activation of CD4+ T cells. In our model, CD4+ T cells presented a transitory increase at 10dpi but presented a significant drop on their percentages after 17 dpi when compared to controls. In L. amazonensis co-infected group they equalized controls only at 25 dpi, while in L. braziliensis co-infection they were lower them controls and Leishmania single infected group. The nature of this cells (e.g., if they are regulatory the cells or effector T cells), as well as the cytokines they produce after stimulation with Leishmania antigens or P. yoelii infected erythrocytes are under investigation.
Interestingly the percentages of double negatives CD4 and CD8 splenocytes increased in P. yoelii 17XNL infected groups, and Leishmania co-infection were able to delay this enhancement. We are also performing multiparametric flow cytometry experiments to better understand the phenotype of this CD3+CD4-CD8-cells, since they can be NKT cells or γδT cells. Both cell types are components of the innate immune system and have been proposed to play significant roles in the clearance of blood-stage malarial parasites (Stevenson and Riley, 2004; Urban et al., 2005).
NKT cells are innate-like lymphocytes that account for approximately 5% of T lymphocytes in the spleen. They posses both T cell and NK cells surface markers since they can express CD4 or CD8 co receptors on their surface, or neither one of them (double-negative phenotype) as well as the NK1.1 surface molecule (Balato et al., 2009). Primary P. yoelii infection with non-lethal strain 265BY is able to induce an organ-specific and heterogeneous NKT cells response (Soulard et al., 2007). Hepatic NKT cells consisted mainly of CD1d-dependent CD4+ and double negative NKT cells, whereas splenic NKT cells presented a CD1d-independent TCRhigh CD4high phenotype during infection (Soulard et al., 2007). It was also demonstrated that CD49b+ CD3+ natural killer T (NKT) cells increased in the liver after a primary infection with P. yoelii non-lethal strain (17XNL), and that CD1d-restricted NKT cells, which secrete IFNγ, are critical to reduce liver-stage burden in a secondary infection (Miller et al., 2014). Lack of type 1 IFN-alpha receptor signaling compromise the enhancement of NKT cells in the liver, showing a link between type I IFN signaling, cell recruitment, and subsequent parasite elimination.
Gamma delta T cells are the first to be generated in the ontogeny. They are able to respond quickly through the production of cytokines and can be divided into two types: interferon-producing cells (CD27hi) and IL-17-producing cells (CD27lo) (Zarin et al., 2015). In Malaria they are activated during pre-erythrocytic (liver) and erythrocyte stages and can produce INF-γ in vitro after stimulation with P. falciparum infected erythrocytes (Elloso et al., 1994; Farouk et al., 2004; Scholzen and Sauerwein, 2016). In BALB/c mice, the number of γδT cells increases considerably in the spleen during infection with P. yoelii non-lethal strain (17XNL) (Kopacz and Kumar, 1999), while this increase did not occur to the same extent in mice infected with the lethal strain of P. yoelii (17XL) suggesting that γδT cells play a protective role in Malaria (Kopacz and Kumar, 1999). In another work with P. yoelii non-lethal strain was observed an increase in the percentage of γδT cells in the spleen, liver and peripheral blood, peaking at day 21 of infection (Li et al., 2012). When the mice were depleted from γδT cells with monoclonal antibodies parasite elimination was delayed (Li et al., 2012).
Our results demonstrating an increase in CD3+CD4-CD8- cells during erythrocyte stage in P. yoelii 17XNL non-lethal strain infected animals both in the thymus (Figure 7) and in the spleen (Figure 8) does not discard the possibility that those can be either NK T or γδT cells, and the delay of this enhancement to occur in Leishmania co-infected groups when compared to P. yoelii single infected animals could have a correlation with the differences in cytokines production observed between P. yoelii 17XNL single infected and co-infected groups.
Taken together, our results suggest that coexisting infection with P. yoelii 17XNL and L. braziliensis or L. amazonensis may change diseases outcomes, and that Malaria outcome can be altered according to the Leishmania co-infection specie evaluated. On the other hand Malaria infection produced a transient delay on leishmanial lesions development. These alterations on Malaria and CL progress seem to be closely related to changes in the immune response as verified by alteration in serum cytokine levels and thymus dynamics during infection.
In areas where Malaria and CL are co-endemic, human populations are continuously exposed to Malaria and Leishmaniasis vectors bites and consequently are at risk of suffering repeated infections. These recurrent infections in endemic areas might cause permanent immunomodulatory effects, which can probably interfere in the outcome of both diseases. Moreover, the increase in Malaria parasitaemia, patency and mortality, observed in L. amazonensis/P. yoelii coinfection, might contribute to the severity of disease as well as to the maintenance of the malaria transmission in co-endemic areas. Considering the importance and the geographical distribution of Malaria and CL in tropical regions of the world, further investigation on immunomodulatory mechanisms during co-infection are necessary to understand how co-infection can affect not only the natural history and progression of diseases but also the treatment and prevention of both.
Author Contributions
Conceived and designed the experiments: DB, PL, JF, and RP. Performed the experiments: RP, PL, DS, DV, DP, and DB. Analyzed the data: RP, PL, DV, DS, and DB. Contributed reagents/materials/analysis tools: DB, PL, and DV. Wrote the paper: RP, PL, and DB.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We’d like to express our gratefulness to Professor Dr. Fabio T. M. Costa from Departamento de Genética, Evolução e Bioagentes from Universidade Estadual de Campinas-UNICAMP, SP/Brazil for providing us the P. yoelii 17XNL non-lethal strain utilized in this work. The authors also thank the Center for Laboratory Animal Breeding of the Oswaldo Cruz Foundation (CECAL) and the Center of Animal Experimentation of Leônidas Deane building for providing and keeping animals used in this study. We also thank the Flow Cytometry Core from IOC/FIOCRUZ.
Footnotes
Funding. This work was supported by the IOC/FIOCRUZ and PAEFFIOTEC (IOC-008-FIO-15-47 and IOC-008-FIO-15-66). The publication of this article was funded by IOC/FIOCRUZ. RP, DS, and DP are recipient of CAPES Doctoral Fellowships. DV and JF are recipient of CNPq Productivity Fellowships.
References
- Ab Rahman A. K., Abdullah F. H. (2011). Visceral Leishmaniasis (kala-azar) and Malaria co-infection in an immigrant in the state of Terengganu, Malaysia: a case report. J. Microbiol. Immunol. Infect. 44 72–76. 10.1016/j.jmii.2011.01.014 [DOI] [PubMed] [Google Scholar]
- Alvar J., Velez I. D., Bern C., Herrero M., Desjeux P., Cano J., et al. (2012). Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 7:e35671 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade C. F., Gameiro J., Nagib P. R., Carvalho B. O., Talaisys R. L., Costa F. T., et al. (2008). Thymic alterations in Plasmodium berghei-infected mice. Cell Immunol. 253 1–4. 10.1016/j.cellimm.2008.06.001 [DOI] [PubMed] [Google Scholar]
- Antonelli L. R. V., Dutra W. O., Almeida R. P., Bacellar O., Carvalho E. M., Gollob K. J. (2005). Activated inflammatory T cells correlate with lesion size in human Cutaneous Leishmaniasis. Immunol. Lett. 101 226–230. 10.1016/j.imlet.2005.06.004 [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas K., Riley E. M. (2002). Innate immune response to Malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J. Immunol. 169 2956–2963. 10.4049/jimmunol.169.6.2956 [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas K., Tongren J. E., Riley E. M. (2003). The war between the Malaria parasite and the immune system: immunity, immunoregulation and immunopathology. Clin. Exp. Immunol. 133 145–152. 10.1046/j.1365-2249.2003.02174.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok D., Acha-Orbea H. (2014). Timing is everything: dendritic cell subsets in murine Leishmania infection. Trends Parasitol. 30 499–507. 10.1016/j.pt.2014.08.001 [DOI] [PubMed] [Google Scholar]
- Balato A., Unutmaz D., Gaspari A. A. (2009). Natural killer T cells: an unconventional T-cell subset with diverse effector and regulatory functions. J. Invest. Dermatol. 129 1628–1642. 10.1038/jid.2009.30 [DOI] [PubMed] [Google Scholar]
- Battle K. E., Karhunen M. S., Bhatt S., Gething P. W., Howes R. E., Golding N., et al. (2014). Geographical variation in Plasmodium vivax relapse. Malar. J. 13:144 10.1186/1475-2875-13-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson J. G., Osier F. H., Engwerda C. R. (2008). Recent insights into humoral and cellular immune responses against Malaria. Trends Parasitol. 24 578–584. 10.1016/j.pt.2008.08.008 [DOI] [PubMed] [Google Scholar]
- Belkaid Y., Mendez S., Lira R., Kadambi N., Milon G., Sacks D. (2000). A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165 969–977. 10.4049/jimmunol.165.2.969 [DOI] [PubMed] [Google Scholar]
- Bin Mohanna M. A. (2015). Leishmaniasis, Malaria, and schistosomiasis concurrently in an 8-year-old boy. Saudi Med. J. 36 494–496. 10.15537/smj.2015.4.10757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carregaro V., Costa D. L., Brodskyn C., Barral A. M., Barral-Netto M., Cunha F. Q., et al. (2013). Dual effect of Lutzomyia longipalpis saliva on Leishmania braziliensis infection is mediated by distinct saliva-induced cellular recruitment into BALB/c mice ear. BMC Microbiol. 13:102 10.1186/1471-2180-13-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Feng H., Liu J., Qi Z. M., Wu Y., Guo S. Y., et al. (2010). Characterization of immune responses to single or mixed infections with P. yoelii 17XL and P. chabaudi AS in different strains of mice. Parasitol. Int. 59 400–406. 10.1016/j.parint.2010.05.005 [DOI] [PubMed] [Google Scholar]
- Coleman R. E., Edman J. D., Semprevivo L. H. (1988a). Interactions between Plasmodium yoelii and Leishmania mexicana amazonensis in Leishmania resistant C57B1/6 mice. Am. J. Trop. Med. Hyg. 39 540–544. [DOI] [PubMed] [Google Scholar]
- Coleman R. E., Edman J. D., Semprevivo L. H. (1988b). Leishmania mexicana: effect of concomitant Malaria on Cutaneous Leishmaniasis. Development of lesions in a Leishmania-susceptible (BALB/c) strain of mouse. Exp. Parasitol. 65 269–276. 10.1016/0014-4894(88)90131-2 [DOI] [PubMed] [Google Scholar]
- De Meis J., Aurelio Farias-De-Oliveira D., Nunes Panzenhagen P. H., Maran N., Villa-Verde D. M., Morrot A., et al. (2012). Thymus atrophy and double-positive escape are common features in infectious diseases. J. Parasitol. Res. 2012:574020 10.1155/2012/574020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moura T. R., Novais F. O., Oliveira F., Clarencio J., Noronha A., Barral A., et al. (2005). Toward a novel experimental model of infection to study American Cutaneous Leishmaniasis caused by Leishmania braziliensis. Infect. Immun. 73 5827–5834. 10.1128/IAI.73.9.5827-5834.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra W. O., De Faria D. R., Lima Machado P. R., Guimaraes L. H., Schriefer A., Carvalho E., et al. (2011). Immunoregulatory and effector activities in human cutaneous and mucosal leishmaniasis: understanding mechanisms of pathology. Drug Dev. Res. 72 430–436. 10.1002/ddr.20449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elloso M. M., Van Der Heyde H. C., Vande Waa J. A., Manning D. D., Weidanz W. P. (1994). Inhibition of Plasmodium falciparum in vitro by human gamma delta T cells. J. Immunol. 153 1187–1194. [PubMed] [Google Scholar]
- Falcão S. C., De Moura T. R., Clarencio J., Brodskyn C., Barral A., De Oliveira C. I. (2012). The presence of Tregs does not preclude immunity to reinfection with Leishmania braziliensis. Int. J. Parasitol. 42 771–780. 10.1016/j.ijpara.2012.05.006 [DOI] [PubMed] [Google Scholar]
- Farouk S. E., Mincheva-Nilsson L., Krensky A. M., Dieli F., Troye-Blomberg M. (2004). Gamma delta T cells inhibit in vitro growth of the asexual blood stages of Plasmodium falciparum by a granule exocytosis-dependent cytotoxic pathway that requires granulysin. Eur. J. Immunol. 34 2248–2256. 10.1002/eji.200424861 [DOI] [PubMed] [Google Scholar]
- Francelin C., Paulino L. C., Gameiro J., Verinaud L. (2011). Effects of Plasmodium berghei on thymus: high levels of apoptosis and premature egress of CD4(+)CD8(+) thymocytes in experimentally infected mice. Immunobiology 216 1148–1154. 10.1016/j.imbio.2011.03.009 [DOI] [PubMed] [Google Scholar]
- Freitas do Rosario A. P., Langhorne J. (2012). T cell-derived IL-10 and its impact on the regulation of host responses during Malaria. Int. J. Parasitol. 42 549–555. 10.1016/j.ijpara.2012.03.010 [DOI] [PubMed] [Google Scholar]
- Gollob K. J., Viana A. G., Dutra W. O. (2014). Immunoregulation in human American Leishmaniasis: balancing pathology and protection. Parasite Immunol. 36 367–376. 10.1111/pim.12100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Doolan D. L. (2010). Malaria vaccine design: immunological considerations. Immunity 33 555–566. 10.1016/j.immuni.2010.10.005 [DOI] [PubMed] [Google Scholar]
- Hartley M. A., Drexler S., Ronet C., Beverley S. M., Fasel N. (2014). The immunological, environmental, and phylogenetic perpetrators of metastatic Leishmaniasis. Trends Parasitol. 30 412–422. 10.1016/j.pt.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P. J., Molyneux D. H., Fenwick A., Ottesen E., Ehrlich Sachs S., Sachs J. D. (2006). Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and Malaria. PLoS Med. 3:e102 10.1371/journal.pmed.0030102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M. K., Pharoah M. M., Ashall F., Miles M. A. (1991). Human urine stimulates growth of Leishmania in vitro. Trans. R. Soc. Trop. Med. Hyg. 85 477–479. 10.1016/0035-9203(91)90226-O [DOI] [PubMed] [Google Scholar]
- Karadjian G., Berrebi D., Dogna N., Vallarino-Lhermitte N., Bain O., Landau I., et al. (2014). Co-infection restrains Litomosoides sigmodontis filarial load and plasmodial P. yoelii but not P. chabaudi parasitaemia in mice. Parasite 21:16 10.1051/parasite/2014017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierski L., Evans K. J. (2014). Immune responses during cutaneous and Visceral Leishmaniasis. Parasitology 141 1544–1562. 10.1017/S003118201400095x [DOI] [PubMed] [Google Scholar]
- Kobayashi F., Morii T., Matsui T., Fujino T., Watanabe Y., Weidanz W. P., et al. (1996). Production of interleukin 10 during Malaria caused by lethal and nonlethal variants of Plasmodium yoelii yoelii. Parasitol. Res. 82 385–391. 10.1007/s004360050133 [DOI] [PubMed] [Google Scholar]
- Kolbaum J., Eschbach M. L., Steeg C., Jacobs T., Fleischer B., Breloer M. (2012). Efficient control of Plasmodium yoelii infection in BALB/c and C57BL/6 mice with pre-existing Strongyloides ratti infection. Parasite Immunol. 34 388–393. 10.1111/j.1365-3024.2012.01369.x [DOI] [PubMed] [Google Scholar]
- Kopacz J., Kumar N. (1999). gamma delta T-cells may interfere with a productive immune response in Plasmodium yoelii infections. Int. J. Parasitol. 29 737–742. 10.1016/S0020-7519(99)00026-0 [DOI] [PubMed] [Google Scholar]
- La Flamme A. C., Scott P., Pearce E. J. (2002). Schistosomiasis delays lesion resolution during Leishmania major infection by impairing parasite killing by macrophages. Parasite Immunol. 24 339–345. 10.1046/j.1365-3024.2002.00473.x [DOI] [PubMed] [Google Scholar]
- Li C., Mannoor K., Inafuku M., Taniguchi T., Inamine Y., Miyazaki T., et al. (2012). Protective function of an unconventional gammadelta T cell subset against Malaria infection in apoptosis inhibitor deficient mice. Cell Immunol. 279 151–159. 10.1016/j.cellimm.2012.09.012 [DOI] [PubMed] [Google Scholar]
- Li C., Seixas E., Langhorne J. (2001). Rodent Malarias: the mouse as a model for understanding immune responses and pathology induced by the erythrocytic stages of the parasite. Med. Microbiol. Immunol. 189 115–126. 10.1007/s430-001-8017-8 [DOI] [PubMed] [Google Scholar]
- Lima A. C., Francelin C., Ferrucci D. L., Stach-Machado D. R., Verinaud L. (2012). Thymic alterations induced by Plasmodium berghei: expression of matrix metalloproteinases and their tissue inhibitors. Cell Immunol. 279 53–59. 10.1016/j.cellimm.2012.09.006 [DOI] [PubMed] [Google Scholar]
- Louis J. A., Conceicao-Silva F., Himmelrich H., Tacchini-Cottier F., Launois P. (1998). Anti-leishmania effector functions of CD4+ Th1 cells and early events instructing Th2 cell development and susceptibility to Leishmania major in BALB/c mice. Adv. Exp. Med. Biol. 452 53–60. 10.1007/978-1-4615-5355-7_7 [DOI] [PubMed] [Google Scholar]
- Marsh K., Kinyanjui S. (2006). Immune effector mechanisms in Malaria. Parasite Immunol. 28 51–60. 10.1111/j.1365-3024.2006.00808.x [DOI] [PubMed] [Google Scholar]
- Medina T. S., Costa S. P., Oliveira M. D., Ventura A. M., Souza J. M., Gomes T. F., et al. (2011). Increased interleukin-10 and interferon-gamma levels in Plasmodium vivax Malaria suggest a reciprocal regulation which is not altered by IL-10 gene promoter polymorphism. Malar. J. 10 264 10.1186/1475-2875-10-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. L., Sack B. K., Baldwin M., Vaughan A. M., Kappe S. H. (2014). Interferon-mediated innate immune responses against Malaria parasite liver stages. Cell Rep. 7 436–447. 10.1016/j.celrep.2014.03.018 [DOI] [PubMed] [Google Scholar]
- Niikura M., Kamiya S., Kita K., Kobayashi F. (2008). Co-infection with nonlethal murine Malaria parasites suppresses pathogenesis caused by Plasmodium berghei NK65. J. Immunol. 180 6877–6884. 10.4049/jimmunol.180.10.6877 [DOI] [PubMed] [Google Scholar]
- Oliveira W. N., Ribeiro L. E., Schrieffer A., Machado P., Carvalho E. M., Bacellar O. (2014). The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of human tegumentary Leishmaniasis. Cytokine 66 127–132. 10.1016/j.cyto.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira B. A., Alves C. R. (2008). Immunological characteristics of experimental murine infection with Leishmania (Leishmania) amazonensis. Vet. Parasitol. 158 239–255. 10.1016/j.vetpar.2008.09.015 [DOI] [PubMed] [Google Scholar]
- Pereira C. G., Silva A. L., De Castilhos P., Mastrantonio E. C., Souza R. A., Romao R. P., et al. (2009). Different isolates from Leishmania braziliensis complex induce distinct histopathological features in a murine model of infection. Vet. Parasitol. 165 231–240. 10.1016/j.vetpar.2009.07.019 [DOI] [PubMed] [Google Scholar]
- Perez-Mazliah D., Langhorne J. (2014). CD4 T-cell subsets in Malaria: TH1/TH2 revisited. Front. Immunol. 5:671 10.3389/fimmu.2014.00671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z., Han X., Zhang Y., Wang J., Cao Y. M. (2013). Listeria monocytogenes inoculation protects mice against blood-stage Plasmodium yoelii infection. Tohoku J. Exp. Med. 229 87–96. 10.1620/tjem.229.87 [DOI] [PubMed] [Google Scholar]
- Ribeiro-Gomes F. L., Roma E. H., Carneiro M. B., Doria N. A., Sacks D. L., Peters N. C. (2014). Site-dependent recruitment of inflammatory cells determines the effective dose of Leishmania major. Infect. Immun. 82 2713–2727. 10.1128/IAI.01600-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino W. (2006). The thymus is a common target organ in infectious diseases. PLoS Pathog. 2:e62 10.1371/journal.ppat.0020062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen A., Sauerwein R. W. (2016). Immune activation and induction of memory: lessons learned from controlled human Malaria infection with Plasmodium falciparum. Parasitology 143 224–235. 10.1017/S0031182015000761 [DOI] [PubMed] [Google Scholar]
- Seixas E., Ostler D. (2005). Plasmodium chabaudi chabaudi (AS): differential cellular responses to infection in resistant and susceptible mice. Exp. Parasitol. 110 394–405. 10.1016/j.exppara.2005.03.024 [DOI] [PubMed] [Google Scholar]
- Shan Y., Liu J., Jiang Y. J., Shang H., Jiang D., Cao Y. M. (2012). Age-related susceptibility and resistance to nonlethal Plasmodium yoelii infection in C57BL/6 mice. Folia Parasitol. (Praha) 59 153–161. 10.14411/fp.2012.021 [DOI] [PubMed] [Google Scholar]
- Shear H. L., Srinivasan R., Nolan T., Ng C. (1989). Role of IFN-gamma in lethal and nonlethal Malaria in susceptible and resistant murine hosts. J. Immunol. 143 2038–2044. [PubMed] [Google Scholar]
- Silveira F. T., Lainson R., De Castro Gomes C. M., Laurenti M. D., Corbett C. E. (2009). Immunopathogenic competences of Leishmania (V.) braziliensis and L. (L.) amazonensis in American Cutaneous Leishmaniasis. Parasite Immunol. 31 423–431. 10.1111/j.1365-3024.2009.01116.x [DOI] [PubMed] [Google Scholar]
- Soulard V., Roland J., Sellier C., Gruner A. C., Leite-De-Moraes M., Franetich J. F., et al. (2007). Primary infection of C57BL/6 mice with Plasmodium yoelii induces a heterogeneous response of NKT cells. Infect. Immun. 75 2511–2522. 10.1128/IAI.01818-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. M., Riley E. M. (2004). Innate immunity to Malaria. Nat. Rev. Immunol. 4 169–180. 10.1038/nri1311 [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson A. W. (2010). Regulation of immunity to Plasmodium: implications from mouse models for blood stage Malaria vaccine design. Exp. Parasitol. 126 406–414. 10.1016/j.exppara.2010.01.028 [DOI] [PubMed] [Google Scholar]
- Urban B. C., Ing R., Stevenson M. M. (2005). Early interactions between blood-stage plasmodium parasites and the immune system. Curr. Top. Microbiol. Immunol. 297 25–70. [DOI] [PubMed] [Google Scholar]
- van den Bogaart E., Berkhout M. M., Adams E. R., Mens P. F., Sentongo E., Mbulamberi D. B., et al. (2012). Prevalence, features and risk factors for Malaria co-infections amongst Visceral Leishmaniasis patients from Amudat Hospital, Uganda. PLoS Negl. Trop. Dis. 6:e1617 10.1371/journal.pntd.0001617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaart E., Talha A. B., Straetemans M., Mens P. F., Adams E. R., Grobusch M. P., et al. (2014). Cytokine profiles amongst Sudanese patients with Visceral Leishmaniasis and Malaria co-infections. BMC Immunol. 15:16 10.1186/1471-2172-15-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2010). Control of the leishmaniases. World Health Organ. Tech. Rep. Ser. 949 xii-xiii, 1–186. [PubMed] [Google Scholar]
- World Health Organization (2015). World Malaria Report. Geneva: World Health Organization. [Google Scholar]
- World Health Organization (2016). Global Health Observatory (GHO) Data [Online]. Available: http://www.who.int/gho/neglected_diseases/leishmaniasis/en/ [accessed March 15, 2016]. [Google Scholar]
- Yoeli M. (1948). Non-pigmented Malaria parasites in the bone marrow from a mixed infection of Leishmania and Plasmodium vivax. Trans. R. Soc. Trop. Med. Hyg. 42:99 10.1016/0035-9203(48)90355-1 [DOI] [PubMed] [Google Scholar]
- Zarin P., Chen E. L., In T. S., Anderson M. K., Zuniga-Pflucker J. C. (2015). Gamma delta T-cell differentiation and effector function programming, TCR signal strength, when and how much? Cell Immunol. 296 70–75. 10.1016/j.cellimm.2015.03.007 [DOI] [PubMed] [Google Scholar]
- Zuzarte-Luis V., Mota M. M., Vigario A. M. (2014). Malaria infections: what and how can mice teach us. J. Immunol. Methods 410 113–122. 10.1016/j.jim.2014.05.001 [DOI] [PubMed] [Google Scholar]


