Abstract
Background
Ticagrelor provides enhanced antiplatelet efficacy but increased risk of bleeding and dyspnea. This study aimed to display the relationship between ADP-induced platelet-fibrin clot strength (MAADP) and clinical outcomes in acute coronary syndrome (ACS) patients treated by ticagrelor.
Methods
Consecutive Chinese-Han patients with ACS who received maintenance dose of ticagrelor on top of aspirin were recruited. After 5-day ticagrelor maintenance treatment, MAADP measured by thrombelastography (TEG) were recorded for the evaluation of ticagrelor anti-platelet reactivity. Pre-specified cutoffs of MAADP > 47 mm for high on-treatment platelet reactivity (HTPR) and MAADP < 31 mm for low on-treatment platelet reactivity (LTPR) were applied for evaluation. The occurrences of primary ischemic cardiovascular events (including a composite of cardiac death, non-fatal myocardial infarction and stroke), the Thrombolysis in Myocardial Infarction (TIMI) defined bleeding events, and ticagrelor related dyspnea were recorded after a follow-up of three months.
Results
Overall, 176 ACS patients (Male: 79.55%, Age: 59.91 ± 10.54 years) under ticagrelor maintenance treatment were recruited. The value of MAADP ranged from 4.80% to 72.90% (21.27% ± 12.07% on average), with the distribution higher skewed towards the lower values. Using the pre-specific cutoffs for HTPR and LTPR, seven patients (3.98%) were identified as HTPR and 144 patients (81.82%) as LTPR. After a follow-up of three months in 172 patients, major cardiovascular events occurred in no patient, but TIMI bleeding events in 81 (47.09%) with major bleedings in three patients. All patients with major bleedings were classified as LTPR. Ticagrelor related dyspnea occurred in 31 (18.02%) patients, with 30 (21.28%) classified as LTPR and no one as HTPR (P = 0.02).
Conclusions
In ticagrelor treated ACS patients, MAADP measured by TEG might be valuable for the prediction of major bleeding and ticagrelor related dyspnea. Due to the small number of patients with HTPR after ticagrelor maintenance treatment, larger scale study should be warranted to verify the relationship between MAADP defined HTPR and ticagrelor related ischemic events.
Keywords: Clinical outcomes, Platelet reactivity, Thrombelastography, Ticagrelor
1. Introduction
Dual antiplatelet therapy, with a P2Y12 receptor inhibitor on top of asipirin, is the recommended therapy for secondary prevention of cardiovascular ischemic events in patients with acute coronary syndrome (ACS) and those undergoing percutaneous coronary intervention (PCI).[1],[2] Ticagrelor, a member of cyclopentyl-triazolo-pyrimidines, is the first reversibly binding oral antiplatelet P2Y12 receptor inhibitor. It could achieve more rapid and great platelet inhibition than high-loading-dose clopidogrel, with the strong effect sustaining during the maintenance phase.[3],[4] In the PLATelet inhibition and patient Outcomes (PLATO) trial, treatment with ticagrelor as compared with clopidogrel significantly reduced the rate of major cardiovascular events (MACE), without an increase in the rate of overall major bleeding.[5] Therefore, ticagrelor is recommended for the treatment of patients with ACS and PCI management in the updated guidelines.[1],[2] However, with more potent antiplatelet effects, ticagrelor has been challenged and administrated with caution for its association with a higher rate of bleeding events and the dyspnea side effect due to the off-target effects of ticagrelor induced by adenosine.[5] Therefore, the methods for the evaluation and prediction of the risks for bleeding and dyspnea side effects related to ticagrelor could be favorable for the personalized application of P2Y12 receptor inhibitors.
As we know, the variability in response to clopidogrel has drawn forth the notion of high on-treatment platelet reactivity (HTPR) and its tendency to ischemic outcomes in ACS patients undergoing PCI.[6]–[9] Conversely, the occurrence of bleeding events in patients treated with P2Y12 receptor inhibitors is related to the excessive platelet inhibition with the consequence of low on-treatment platelet reactivity (LTPR).[10]–[12] In the recent updated consensus on the definition of on-treatment platelet reactivity induced by adenosine diphosphate (ADP), the cutoffs values for HTPR and LTPR with respect to various platelet function tests were proposed as the therapeutic window in personalized antiplatelet therapy of P2Y12 receptor inhibitors.[13]–[15] Thrombelastography (TEG) measured ADP-induced platelet-fibrin clot strength (MAADP) values with > 47 mm defined for HTPR and < 31 mm for LTPR were reported as the important predictors for post-PCI ischemic or bleeding events, respectively.[16],[17] TEG is more reflective of the physiologic character of a blood clot in vivo, however, the utility of TEG as well as the therapeutic window for the measurement of antiplatelet effect in ticagrelor treated patients has not been evaluated. Therefore, the present study aimed to display the relationship between MAADP measured by TEG and clinical outcomes in ticagrelor treated ACS patients.
2. Methods
2.1. Study population
All Patients with ACS who received maintenance dose (MD) of ticagrelor (90 mg, twice daily) and aspirin (100 mg, once daily) in-hospital and out-hospital were consecutively recruited at the Department of Cardiology, General Hospital of Chinese People's Liberation Army (GH-PLA), from January 2014 to April 2015. Those patients that planed to drug eluting stent (DES) placement were pretreated with a loading dose of 180 mg ticagrelor and 300 mg aspirin before PCI according to current standard guidelines.[1],[2] Intravenous anticoagulant and glycoprotein IIb/IIIa inhibitor during and after PCI were administered according to the interventional cardiologists' discretion. Exclusion criteria included patients' age younger than 18 years old, known contraindication to aspirin or ticagrelor treatment, cardiac arrest, severe dyspnea, platelet count < 100 × 109/L, history of bleeding diathesis, concurrent severe illness with expected survival of < 1 month, surgery within one month or scheduled in the year, stroke within one month, and liver or renal dysfunction. The present study complied with the Declaration of Helsinki was approved by the institutional ethics committee. All of the patients gave written informed consent for the study before the inclusion.
2.2. Platelet reactivity measurements
After 5-day maintenance ticagrelor treatment, peripheral venous whole blood was drawn by venipuncture into vacutainer tubes containing 3.2% sodium citrate (Becton-Dickinson, San Jose, CA). The first 2–4 mL of blood was discarded to avoid spontaneous platelet activation. Blood sample measurements were carried out using the TEG Haemostasis System (Haemoscope Corporation, Niles, IL, USA). A detailed operating instruction of this method has been outlined previously.[18] The TEG Haemostasis Analyzer with automated analytical software provides viscoelastic quantitative and qualitative measurements of the physical properties of a clot. Time to fibrin formation (R), angle constant (α), clot formation time (K), maximum amplitude (MA) including MAADP, MAk and MAf. MAk and MAf, were recorded, representing the maximum platelet fibrin clot strength and fibrin clot strength. MAADP was transformed into the actual measure of clot strength (G scale, dyne/cm2), which is calculated from [(5000 × MAADP)/(100 – MAADP)]. ADP induced platelet inhibition (PIADP) was calculated by the formula as PIADP (%) = 100% – [(MAADP – MAf)/(MAk – MAf)] × 100%. PIADP and MAADP values were confirmed at the central clinical laboratory for platelet function studies in the Department of Cardiology, GH-PLA. All measurement procedures were carried out within two hours. HTPR was defined as the value of MAADP > 47 mm, and LTPR defined as the value of MAADP < 31 mm, according to the previous reported consensus and update on the definition of on-treatment platelet reactivity to ADP associated with ischemic and bleeding.[15]
2.3. Study end points and follow-up
The primary efficacy outcomes included a composite of cardiac death, non-fatal myocardial infarction (MI) and stroke. Secondary efficacy outcomes included a composite of defined or probable stent thrombosis, coronary revascularization, and re-hospitalization for unstable angina. The safety outcomes included major and minor bleeding events defined according to the updated Thrombolysis in Myocardial Infarction (TIMI) criteria.[19],[20] The side effect of dyspnea related to ticagrelor was justified according to the standard procedures reported in previous literature.[21] Clinical follow-up was performed through telephone interview at the outpatient clinics at one and three months. All collected data were input to the database by well-trained staffs, with source documentation double-checked to ensure accurate data input.
2.4. Statistical method
All statistical tests were performed with the use of SPSS Statistics 17.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables were presented as the mean ± SD and compared using the Student's t test, Mann–Whitney U test, or one-way analysis of variance (ANOVA) test, as appropriate. Categorical variables were expressed as frequencies and percentages, which were compared with a chi-square test or Fisher exact test. Multivariate linear regression analysis with calculation of the adjusted β coefficient was used to test the independent contribution of each covariate to the value of TEG-MAADP. Adjustments were made for the possible confounding effects, including baseline demographic [gender, age (in decades), body mass index (BMI, per 5 kg/m2), smoking status, and comorbidities (diabetes mellitus, renal dysfunction), co-medications [pump inhibitor (PPI), statins, or calcium channel blockers (CCBs)], and laboratory examination [left ventricular ejection fraction (LVEF), platelet count and creatinine-based estimates of the glomerular filtration rate (eGFR) (per 30 mL/min per 1.73 m2)]. Comparisons of clinical outcomes among patients were analyzed using the chi-square test. A two-sided P value < 0.05 was used to test for the significance.
3. Results
3.1. Patients' baseline characteristics
Baseline characteristics were detailed in Table 1. A total of 176 eligible ticagrleor treated ACS patients were included in the study, with 79.55% male and a mean age of 59.91 ± 10.54 years old. ST-elevated myocardial infarction (STEMI) was diagnosed in 31 (17.61%), Non-STEMI in 10 (5.68%), and unstable angina in 135 (76.70%) patients. After admission, a total of 156 (88.64%) patients underwent the treatment of PCI.
Table 1. Demographic and clinical characteristics of the enrolled ACS patients.
| Characteristics | Patients (n = 176) |
| Male | 140 (79.55%) |
| Age, yrs | 59.91 ± 10.54 |
| BMI, kg/m2 | 25.75 ± 2.78 |
| Cardiovascular risk factor | |
| Current smoker | 80 (45.45%) |
| Hypertension | 101 (57.39%) |
| Diabetes | 57 (32.39%) |
| Chronic renal failure | 2 (1.14%) |
| Hypercholesterolemia | 56 (31.82%) |
| Other medical history | |
| Prior MI | 28 (15.91%) |
| Prior PCI | 50 (28.41%) |
| Prior CABG | 4 (2.27%) |
| Final diagnosis of ACS | |
| ST-elevation MI | 31 (17.61%) |
| Non–ST-elevation MI | 10 (5.68%) |
| Unstable angina | 135 (76.70%) |
| PCI with coronary stent placement | 156 (88.64%) |
| Laboratory evaluation | |
| LVEF, % | 57.71 ± 7.57 |
| Platelet count, × 105/µL | 224.26 ± 63.21 |
| Total cholesterol, mmol/L | 3.96 ± 1.15 |
| Triglycerides, mmol/L | 1.79 ± 1.68 |
| HDL-C, mmol/L | 1.04 ± 0.29 |
| LDL-C, mmol/L | 2.36 ± 0.93 |
| Creatinie, µmol/L | 77.75 ± 19.69 |
| Antithrombotic treatment in hospital | |
| Aspirin | 172 (97.73%) |
| Glycoprotein IIb/IIIa inhibitor | 94 (53.41%) |
| Heparin | 138 (78.41%) |
| Other medication administered in hospital | |
| ARB | 30 (17.05%) |
| ACE inhibitors | 43 (24.43%) |
| Beta-blockers | 138 (78.41%) |
| CCBs | 62 (35.23%) |
| Statins | 171 (97.16%) |
| Diuretics | 8 (4.55%) |
| Nitrates | 103 (58.52%) |
| Proton pump inhibitor | 22 (12.50%) |
Data are presented as n (%) or median ± SD. ACE: angiotensin-converting enzyme; ACS: acute coronary syndrome; ARB: angiotensin receptor bloker; BMI: body mass index; CABG: coronary artery bypass grafting; CCBs: calcium channel blockers; HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; LVEF: left ventricular ejection fraction; MI: myocardiac infarction; PCI: percutaneous coronary intervention.
3.2. Anti-platelet reactivity measured by TEG
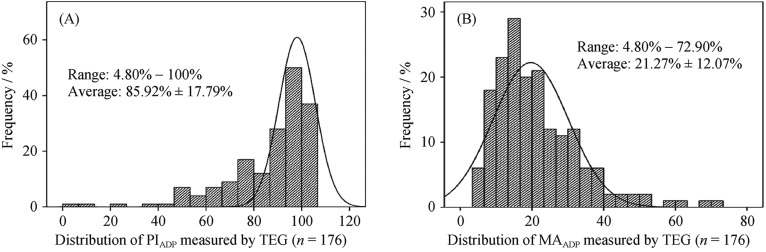
PIADP measured by TEG was 85.92% ± 17.79% on average (ranged from 4.8% to 100%) during the maintenance treatment of ticagrelor. The value of MAADP was 21.27% ± 12.07% on average, ranged from 4.80% to 72.90%. The distribution of PIADP was skewed toward higher values, while MAADP measured by TEG was skewed toward lower values (Figure 1). With the pre-specific cutoffs for HTPR (TEG-MAADP > 47 mm) and LTPR (TEG-MAADP < 31 mm), seven patients (3.98%) were identified as HTPR and 144 patients (81.82%) as LTPR.
Figure 1. Distribution of ticagrelor anti-platelet reactivity measured by TEG in ACS patients.
Figure 1A and figure 1B represent the distribution of PIADP and MAADP measured by TEG, respectively. ACS: acute coronary syndrome; MAADP: ADP-induced platelet-fibrin clot strength; PIADP: ADP induced platelet inhibition; TEG: thrombelastography.
3.3. Factors associated with anti-platelet reactivity measured by TEG-MAADP
Variables affecting anti-platelet reactivity of ticagrelor were displayed in Table 2. By multiple linear regression analysis, we found the concomitant therapy with CCBs [β coefficient: –4.08, 95% CI: (–7.96 to –0.20), P = 0.04] and LVEF [β coefficient: –0.31, 95% CI: (–0.57 to –0.05), P = 0.02] were independently associated with lower probability effect on platelet reactivity, in other words, lower probability for HTPR. No significant influence on the value of MAADP was found for the characteristics of age (P = 0.29), gender (P = 0.34), BMI (P = 0.65), comorbidity of diabetes mellitus (P = 0.46), renal dysfunction (P = 0.43), co-medications with PPI (P = 0.19), platelet count (P = 0.29) and eGFR (P = 0.38).
Table 2. Clinical factors related to TEG-MAADP measured after 5-day ticagrelor maintenance treatment in ticagrelor treated ACS patients (n = 176).
| Variables | β coefficient | 95% CI | *Adjusted P value |
| Age (in decades) | 1.13 | 0.99 to 3.26 | 0.29 |
| Female gender | 2.64 | –2.79 to 8.07 | 0.34 |
| BMI (per 5 kg/m2) | 1.11 | –3.81 to 15.90 | 0.65 |
| Current smoking status | –1.72 | –5.87 to 2.43 | 0.41 |
| Diabetes mellitus | –1.52 | –5.37 to 2.32 | 0.46 |
| Renal dysfunction | 9.75 | –14.37 to 33.87 | 0.43 |
| PPI | 3.80 | –1.85 to 9.45 | 0.19 |
| Statins | –3.19 | –14.04 to 7.66 | 0.56 |
| CCB | –4.08 | –7.96 to –0.20 | 0.04 |
| LVEF | –0.31 | –0.57 to –0.05 | 0.02 |
| Platelet count | 0.02 | –0.01 to 0.05 | 0.29 |
| eGFR (per 30 mL/min per 1.73 m2) | 0.05 | –0.06 to 0.15 | 0.38 |
*Adjusted by the baseline demographic [gender, age (in decades), BMI (per 5 kg/m2), smoking status and comorbidities (diabetes mellitus, renal dysfunction), co-medications (PPI, statins, CCB), laboratory examination [LVEF, platelet count and creatinine-based eGFR (per 30 mL/min per 1.73 m2)]. ACS: acute coronary syndrome; BMI: body mass index; CCB: calcium channel blockers; eGFR: estimates of the glomerular filtrationrate; LVEF: left ventricular ejection; MAADP: ADP-induced platelet-fibrin clot strength; PPI: proton pump inhibitor; TEG: thrombelastography.
3.4. Clinical outcomes in relation to HTPR and LTPR defined by MAADP
A total of 172 (97.73%) patients completed the 3-month follow-up, with 7 (4.07%) classified as HTPR, 24 (13.95%) in therapeutic range, and 141 (81.98%) as LTPR. We did not found the occurrence of primary ischemic event in any patients. Secondary ischemic events occurred in seven patents, with two (28.57%) classified as HTPR, two (8.33%) in therapeutic range and three (2.13%) classified as LTPR (P = 0.03). Bleeding events occurred in 81 patients, with major bleeding in three patients, and minor bleeding in 78 patients. All patients with major bleeding events were classified as LTPR. No relationship was found between the occurrence of minor bleeding events and TEG-MAADP defined antiplatelet responsiveness (P = 0.65). Ticagrelor related dyspnea occurred in 31 patients, with 30 (21.28%) classified as LTPR, one (4.17%) in therapeutic range, but no one as HTPR (P = 0.02) (Table 3).
Table 3. Relationship between clinical outcomes and TEG-MAADP defined antiplatelet responsiveness in ticagrelor treated ACS patients (n = 172).
| Clinical outcomes during 3-month follow-up | LTPR MAADP < 31mm (n = 141) |
In therapeutic range 31 ≤ MAADP ≤47 mm (n = 24 ) |
HTPR MAADP > 47 mm (n =7) |
P value |
| Ischemic events | ||||
| Primary events | 0 | 0 | 0 | - |
| Secondary events | 3 (2.13%) | 2 (8.33%) | 2 (28.57%) | 0.03 |
| Bleeding events | ||||
| Major bleeding | 3 (2.13%) | 0 | 0 | - |
| Minor bleeding | 62 (43.97%) | 13 (54.17%) | 3 (42.86%) | 0.65 |
| Adverse events | ||||
| Ticagrelor related dyspnea | 30 (21.28%) | 1 (4.17%) | 0 | 0.02 |
Data are presented as n (%). Primary ischemic endpoints included a composite of cardiac death, non-fatal myocardial infarction and stroke; Secondary efficacy endpoints included a composite of defined or probable stent -thrombosis, coronary revascularization, and re-hospitalization for unstable angina; the major and minor bleeding events are defined according to the updated TIMI criteria. ACS: acute coronary syndrome; HTPR: high on-treatment platelet reactivity; LTPR: low on-treatment platelet reactivity; MAADP: ADP-induced platelet-fibrin clot strength; TEG: thrombelastography; TIMI: thrombolysis in myocardial infarction.
4. Discussion
The present study showed the relationship between MAADP measured by TEG and anti-platelet responsiveness in ticagrelor treated ACS patients. According to the consensus on the therapeutic window for P2Y12 receptor inhibitors, LTPR defined by MAADP could predict major bleeding and dyspnea side effect in the present ticagrelor treated ACS patients. To the best of our knowledge, this study is the first to evaluate the relationship between the measurement of MAADP by TEG and the antiplatelet effects of ticagrelor in ACS patients.
The relationship between on-treatment platelet reactivity evaluated by ex vivo platelet function tests and clinical outcomes in coronary artery disease (CAD) has been set up.[6],[22]–[28] In the present study, the distribution of MAADP measured by TEG during ticagrelor treatment was highly skewed toward lower values, indicating the strong antiplatelet efficacy of ticagrelor in ACS patients. Similar distribution of platelet reactivity measured with different platelet function assays in ticagrelor treated patients could be found in previous studies.[29]–[33] As we know, antiplatelet responsiveness of P2Y12 inhibitors may be greatly influenced by the different assays used for evaluation.[6],[34] Similar prevalence of HTPR (2.7%–3.5%) in ticagrelor treated ACS patients was reported in previous studies with the cutoff of 50% platelet reactivity index using vasodilator-stimulated phosphoprotein (VASP) assay,[9],[35] but higher prevalence of HTPR (9.2% and 13.3%) when using multiple electrode aggregometry test with cutoff of ADP induced area under the curve (AUC) of 468 or 417,[36] and rare occurrence of HTPR (0–0.6%) by VerifyNow P2Y12 assay with the cutoff of ADP induced P2Y12 reaction units (PRU) of 208 to 230.[30],[37] The prevalence of LTPR is high in present study, which indicates the higher bleeding risk in ticagrelor treated patients.[29] The incidence of LTPR in ticagrelor treated ACS patients has been reported previously in two studies using different platelet function assays, with 25% using a VerifyNow P2Y12 cutoff < 10 PRU,[30] and 65.6% using a VASP cutoff < 16%.[35] In addition, variable time for the platelet function measurement in ACS patients may also influence the different prevalence of HTPR and LTPR in these reported studies.[9],[30],[35]–[37]
In the present study, we found concomitant administration with CCBs was independently associated with lower MAADP values. As we know, CCBs are substrates for CYP3A4, with non-dihydropyridine CCBs being the moderate CYP3A4 inhibitors as well.[38] In addition, in-vitro studies have shown that ticagrelor is both a substrate and weak inhibitor of the cytochrome CYP3A4 isoenzyme,[39] suggesting a potential drug interaction of ticagrelor with other CYP3A4 substrates. Therefore, CCBs potentially interact with ticagrelor and change the plasma levels of ticagrelor metabolite.[2] Future studies should be warranted to elucidate whether CCBs could show a pharmacokinetic interaction on ticagrelor. As for the influence of LVEF, the serious status of heart failure may frequently reduce gastrointestinal motility and thereby delay oral absorption and decrease peak plasma concentration of ticagrelor.[40] The preliminary study reported that LVEF < 35% was independently associated with high on-clopidogrel platelet reactivity,[41] indirectly supported the influence of LVEF on platelet reactivity in ticagrelor treated ACS patients. Actually, we did not find the association between platelet reactivity and age, gender, BMI, diabetes mellitus or renal dysfunction, co-medications with PPI, platelet count and eGFR in ticagrelor treated patients. However, these variables have been shown to influence platelet reactivity in ticagrelor-treated patients in the previous studies.[30],[36],[42],[43] The paradox might partly attribute to the different included patients and platelet function assay applied in the studies.
We did not observe the occurrence of MACE in the present study, which might attribute to the relatively lower-risk patients included and the shorter follow-up intervals. However, the low rate of ischemic events in the study indicated the strong antithrombotic effect of ticagrelor in the ACS patients. We observed that all patients with major bleeding events in the study were classified as LTPR, indicating that TEG-MAADP defined antiplatelet responsiveness might be predictive for major bleeding. However, more than 80% of ticagrelor treated ACS patients in the present study were classified as bleeding-related LTPR when the consensus cut-off of MAADP < 31 mm was used.[16],[17] Due to the binding characteristics of ticagrelor associated with a wider separation between antithrombotic and bleeding effects than that seen with irreversibly binding of thienopyridines,[44] we suspected that the therapeutic window for ticagrelor might be wider and the optimal predicted values of MAADP for ticagrelor-related bleeding might be lower than 31mm. Therefore, the novel therapeutic window for ticagrelor should be established and validated in a large cohort of ACS patients.
In the present study, we found ticagrelor related dyspnea occurred in 31(18.02%) patients, with 30 (21.28%) classified as LTPR, one (4.17%) in therapeutic range, but no one as HTPR. Preliminary studies suggested that ticagrelor inhibits adenosine taken up into erythrocytes, leading to changes in regional blood flow, along with the symptom of dyspnea.[45] Moreover, ticagrelor may inhibit platelets not only by P2Y12 receptor inhibition but also by interacting with adenosine, which is a potent aggregation inhibitor.[46] Therefore, it is reasonable to understand that patients presented with LTPR could have a higher rate of dyspnea due to the potential increased plasma adenosine by ticagrelor. The potential higher risk for ticagrelor related major bleedings and dyspnea side effects in patients with MAADP defined LTPR suggests the need to verify the predictive effects of the cutoff in larger cohorts.
Several limitations in the present study should be mentioned. Firstly, this was a single-center study with small sample size, which may introduce bias into the primary findings. The duration of the follow-up was relative shorter with limited data on efficacy and safety outcomes. Thirdly, TEG-MAADP defined HTPR and LTPR during ticagrelor maintenance treatment were determined based on the updated consensus definitions originated mainly from studies of clopidogrel therapy, and thus may not appropriately extend to ticagrelor. Future large-scale prospective designed trials with appropriate clinical follow-up intervals should be warranted to establish the new therapeutic windows defining HTPR and LTPR in patients receiving ticagrelor.
In conclusion, MAADP as measured by TEG could potentially predict major bleeding and dyspnea side effects in ticagrelor treated ACS patients. Due to the small number of patients with HTPR after ticagrelor maintenance treatment, larger scale study should be warranted to verify the relationship between MAADP defined HTPR and ticagrelor related ischemic events.
Acknowledgments
This work was supported by grants from the Beijing Natural Science Foundation of China (No. 7152129) and National Natural Science Foundation of China (No. 30971259), the Clinical Research Supportive Fund General Hospital of Chinese People's Liberation Army (No. 2012FC-TSYS-3042). There are no potential conflicts of interest to declare.
References
- 1.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394. doi: 10.1161/CIR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 2.Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 3.Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577–2585. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 4.Storey RF, Angiolillo DJ, Patil SB, et al. Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: the PLATO (PLATelet inhibition and patient Outcomes) PLATELET substudy. J Am Coll Cardiol. 2010;56:1456–1462. doi: 10.1016/j.jacc.2010.03.100. [DOI] [PubMed] [Google Scholar]
- 5.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 6.Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–933. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 7.Brar SS, ten Berg J, Marcucci R, et al. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. A collaborative meta-analysis of individual participant data. J Am Coll Cardiol. 2011;58:1945–1954. doi: 10.1016/j.jacc.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 8.Parodi G, Marcucci R, Valenti R, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011;306:1215–1223. doi: 10.1001/jama.2011.1332. [DOI] [PubMed] [Google Scholar]
- 9.Stone GW, Witzenbichler B, Weisz G, et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet. 2013;382:614–623. doi: 10.1016/S0140-6736(13)61170-8. [DOI] [PubMed] [Google Scholar]
- 10.Cuisset T, Cayla G, Frere C, et al. Predictive value of post-treatment platelet reactivity for occurrence of post-discharge bleeding after non-ST elevation acute coronary syndrome. Shifting from antiplatelet resistance to bleeding risk assessment? EuroIntervention. 2009;5:325–329. doi: 10.4244/51. [DOI] [PubMed] [Google Scholar]
- 11.Sibbing D, Schulz S, Braun S, et al. Antiplatelet effects of clopidogrel and bleeding in patients undergoing coronary stent placement. J Thromb Haemost. 2010;8:250–256. doi: 10.1111/j.1538-7836.2009.03709.x. [DOI] [PubMed] [Google Scholar]
- 12.Patti G, Pasceri V, Vizzi V, et al. Usefulness of platelet response to clopidogrel by point-of-care testing to predict bleeding outcomes in patients undergoing percutaneous coronary intervention (from the Antiplatelet Therapy for Reduction of Myocardial Damage During Angioplasty-Bleeding Study) Am J Cardiol. 2011;107:995–1000. doi: 10.1016/j.amjcard.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 13.Tantry US, Gurbel PA. Assessment of oral antithrombotic therapy by platelet function testing. Nat Rev Cardiol. 2011;8:572–579. doi: 10.1038/nrcardio.2011.107. [DOI] [PubMed] [Google Scholar]
- 14.Jeong YH, Bliden KP, Antonino MJ, et al. Usefulness of thrombelastography platelet mapping assay to measure the antiplatelet effect of P2Y(12) receptor inhibitors and high on-treatment platelet reactivity. Platelets. 2013;24:166–169. doi: 10.3109/09537104.2012.675108. [DOI] [PubMed] [Google Scholar]
- 15.Tantry US, Bonello L, Aradi D, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62:2261–2273. doi: 10.1016/j.jacc.2013.07.101. [DOI] [PubMed] [Google Scholar]
- 16.Gurbel PA, Bliden KP, Guyer K, et al. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study. J Am Coll Cardiol. 2005;46:1820–1826. doi: 10.1016/j.jacc.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 17.Gurbel PA, Bliden KP, Navickas IA, et al. Adenosine diphosphate-induced platelet-fibrin clot strength: a new thrombelastographic indicator of long-term poststenting ischemic events. Am Heart J. 2010;160:346–354. doi: 10.1016/j.ahj.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hobson AR, Petley GW, Dawkins KD, et al. A novel fifteen minute test for assessment of individual time-dependent clotting responses to aspirin and clopidogrel using modified thrombelastography. Platelets. 2007;18:497–505. doi: 10.1080/09537100701329162. [DOI] [PubMed] [Google Scholar]
- 19.Mega JL, Braunwald E, Mohanavelu S, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374:29–38. doi: 10.1016/S0140-6736(09)60738-8. [DOI] [PubMed] [Google Scholar]
- 20.Sabatine MS, Antman EM, Widimsky P, et al. Otamixaban for the treatment of patients with non-ST-elevation acute coronary syndromes (SEPIA-ACS1 TIMI 42): a randomised, double-blind, active-controlled, phase 2 trial. Lancet. 2009;374:787–795. doi: 10.1016/S0140-6736(09)61454-9. [DOI] [PubMed] [Google Scholar]
- 21.Parodi G, Storey RF. Dyspnoea management in acute coronary syndrome patients treated with ticagrelor. Eur Heart J Acute Cardiovasc Care. 2015;4:555–560. doi: 10.1177/2048872614554108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price MJ, Endemann S, Gollapudi RR, et al. Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J. 2008;29:992–1000. doi: 10.1093/eurheartj/ehn046. [DOI] [PubMed] [Google Scholar]
- 23.Patti G, Nusca A, Mangiacapra F, et al. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention results of the ARMYDA-PRO (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity Predicts Outcome) study. J Am Coll Cardiol. 2008;52:1128–1133. doi: 10.1016/j.jacc.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 24.Valgimigli M, Campo G, de Cesare N, et al. Intensifying platelet inhibition with tirofiban in poor responders to aspirin, clopidogrel, or both agents undergoing elective coronary intervention: results from the double-blind, prospective, randomized tailoring treatment with tirofiban in patients showing resistance to aspirin and/or resistance to clopidogrel study. Circulation. 2009;119:3215–3222. doi: 10.1161/CIRCULATIONAHA.108.833236. [DOI] [PubMed] [Google Scholar]
- 25.Marcucci R, Gori AM, Paniccia R, et al. Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up. Circulation. 2009;119:237–242. doi: 10.1161/CIRCULATIONAHA.108.812636. [DOI] [PubMed] [Google Scholar]
- 26.Breet NJ, van Werkum JW, Bouman HJ, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA. 2010;303:754–762. doi: 10.1001/jama.2010.181. [DOI] [PubMed] [Google Scholar]
- 27.Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097–1105. doi: 10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- 28.Suh JW, Lee SP, Park KW, et al. Multicenter randomized trial evaluating the efficacy of cilostazol on ischemic vascular complications after drug-eluting stent implantation for coronary heart disease: results of the CILON-T (influence of CILostazol-based triple antiplatelet therapy ON ischemic complication after drug-eluting stenT implantation) trial. J Am Coll Cardiol. 2011;57:280–289. doi: 10.1016/j.jacc.2010.08.631. [DOI] [PubMed] [Google Scholar]
- 29.Alexopoulos D, Galati A, Xanthopoulou I, et al. Ticagrelor versus prasugrel in acute coronary syndrome patients with high on-clopidogrel platelet reactivity following percutaneous coronary intervention: a pharmacodynamic study. J Am Coll Cardiol. 2012;60:193–199. doi: 10.1016/j.jacc.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 30.Alexopoulos D, Xanthopoulou I, Storey RF, et al. Platelet reactivity during ticagrelor maintenance therapy: a patient-level data meta-analysis. Am Heart J. 2014;168:530–536. doi: 10.1016/j.ahj.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 31.Perl L, Zemer-Wassercug N, Rechavia E, et al. Comparison of platelet inhibition by prasugrel versus ticagrelor over time in patients with acute myocardial infarction. J Thromb Thrombolysis. 2015;39:1–7. doi: 10.1007/s11239-014-1119-9. [DOI] [PubMed] [Google Scholar]
- 32.Vaduganathan M, Zemer-Wassercug N, Rechavia E, et al. Relation between ticagrelor response and levels of circulating reticulated platelets in patients with non-ST elevation acute coronary syndromes. J Thromb Thrombolysis. 2015;40:211–217. doi: 10.1007/s11239-015-1178-6. [DOI] [PubMed] [Google Scholar]
- 33.Alexopoulos D, Perperis A, Koniari I, et al. Ticagrelor versus high dose clopidogrel in ST-segment elevation myocardial infarction patients with high platelet reactivity post fibrinolysis. J Thromb Thrombolysis. 2015;40:261–267. doi: 10.1007/s11239-015-1183-9. [DOI] [PubMed] [Google Scholar]
- 34.Lemesle G, Landel JB, Bauters A, et al. Poor agreement between light transmission aggregometry, Verify Now P2Y(1)(2) and vasodilatator-stimulated phosphoprotein for clopidogrel low-response assessment: a potential explanation of negative results of recent randomized trials. Platelets. 2014;25:499–505. doi: 10.3109/09537104.2013.840363. [DOI] [PubMed] [Google Scholar]
- 35.Laine M, Toesca R, Berbis J, et al. Platelet reactivity evaluated with the VASP assay following ticagrelor loading dose in acute coronary syndrome patients undergoing percutaneous coronary intervention. Thromb Res. 2013;132:e15–e18. doi: 10.1016/j.thromres.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 36.Verdoia M, Sartori C, Pergolini P, et al. Prevalence and predictors of high-on treatment platelet reactivity with ticagrelor in ACS patients undergoing stent implantation. Vascul Pharmacol. 2016;77:48–53. doi: 10.1016/j.vph.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Lemesle G, Schurtz G, Bauters C, et al. High on-treatment platelet reactivity with ticagrelor versus prasugrel: a systematic review and meta-analysis. J Thromb Haemost. 2015;13:931–942. doi: 10.1111/jth.12907. [DOI] [PubMed] [Google Scholar]
- 38.Katoh M, Nakajima M, Shimada N, et al. Inhibition of human cytochrome P450 enzymes by 1,4-dihydropyridine calcium antagonists: prediction of in vivo drug-drug interactions. Eur J Clin Pharmacol. 2000;55:843–852. doi: 10.1007/s002280050706. [DOI] [PubMed] [Google Scholar]
- 39.Zhou D, Andersson TB, Grimm SW. In vitro evaluation of potential drug-drug interactions with ticagrelor: cytochrome P450 reaction phenotyping, inhibition, induction, and differential kinetics. Drug Metab Dispos. 2011;39:703–710. doi: 10.1124/dmd.110.037143. [DOI] [PubMed] [Google Scholar]
- 40.Sica DA. Pharmacotherapy in congestive heart failure: drug absorption in the management of congestive heart failure: loop diuretics. Congest Heart Fail. 2003;9:287–292. doi: 10.1111/j.1527-5299.2003.02399.x. [DOI] [PubMed] [Google Scholar]
- 41.Motovska Z, Ondrakova M, Doktorova M, et al. Severe left ventricular systolic dysfunction is independently associated with high on-clopidogrel platelet reactivity. Am J Cardiovasc Drugs. 2014;14:313–318. doi: 10.1007/s40256-014-0074-3. [DOI] [PubMed] [Google Scholar]
- 42.Parodi G, Valenti R, Bellandi B, et al. Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients: RAPID (Rapid Activity of Platelet Inhibitor Drugs) primary PCI study. J Am Coll Cardiol. 2013;61:1601–1606. doi: 10.1016/j.jacc.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 43.Motovska Z, Ondrakova M, Bednar F, et al. Selection of P2Y12 antagonist, treatment initiation, and predictors of high on-treatment platelet reactivity in a “Real World” registry. Thromb Res. 2015;135:1093–1099. doi: 10.1016/j.thromres.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Becker RC, Gurbel PA. Platelet P2Y12 receptor antagonist pharmacokinetics and pharmacodynamics: A foundation for distinguishing mechanisms of bleeding and anticipated risk for platelet-directed therapies. Thromb Haemost. 2010;103:535–544. doi: 10.1160/TH09-07-0491. [DOI] [PubMed] [Google Scholar]
- 45.Wittfeldt A, Emanuelsson H, Brandrup-Wognsen G, et al. Ticagrelor enhances adenosine-induced coronary vasodilatory responses in humans. J Am Coll Cardiol. 2013;61:723–727. doi: 10.1016/j.jacc.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 46.Dillinger JG, Manzo Silberman S, Bal dit Sollier C, et al. Ticagrelor effectiveness overestimated by VASP index: platelet inhibition by ticagrelor versus prasugrel in acute coronary syndrome patients according to platelet function tests. Int J Cardiol. 2014;176:557–559. doi: 10.1016/j.ijcard.2014.07.019. [DOI] [PubMed] [Google Scholar]