Abstract
Background
Whether patients with reduced left ventricular function present worse outcome after transcatheter aortic valve implantation (TAVI) is controversial. The aim of this study was to assess the impact of baseline severe impairment of left ventricular ejection fraction (LVEF) on mortality after TAVI.
Methods
Six-hundred-forty-nine patients with aortic stenosis underwent TAVI with the CoreValve system (92.8%) or the Edwards SAPIEN valve system (7.2%). Baseline LVEF was measured by the echocardiographic Simpson method. The impact of LVEF ≤ 30% on mortality was assessed by Cox regression.
Results
Patients with LVEF ≤ 30% (n = 63), as compared to those with LVEF > 30% (n = 586), had a higher prevalence of NHYA class > 2 (P < 0.001) and presented with a higher Euroscore (P < 0.001). Procedural success was similar in both groups (98.4% vs. 97.2%, P = 1). After a median follow-up of 436 days (25th–75th percentile, 357–737 days), all-cause mortality [23.8% vs. 23.7%, P = 0.87, hazard ratios (HR): 0.96, 95% confidence intervals (CI): 0.56–1.63] and cardiac mortality (19.1% vs. 17.6%, P = 0.89, HR: 1.04, 95% CI: 0.57–1.90) were similar in patients with LVEF ≤ 30% as compared to those with LVEF > 30%. Thirty-day all-cause mortality was not significantly different between the two groups (11.1% vs. 6.3%, P = 0.14, HR: 1.81, 95% CI: 0.81–4.06). Patients with LVEF ≤ 30% had a trend toward higher risk of 30-day cardiac mortality (11.1% vs. 5.3%; P = 0.06, HR: 2.16, 95% CI: 0.95–4.90), which disappeared after multivariable adjustment (P = 0.22).
Conclusions
Baseline severe impairment of LVEF is not a predictor of increased short-term and mid-term mortality after TAVI. Selected patients with severe impairment of left ventricular function should not be denied TAVI.
Keywords: Left ventricular ejection fraction, Mortality, Transcatheter aortic valve implantation
1. Introduction
Transcatheter aortic valve implantation (TAVI) is becoming a widespread therapeutic option alternative to surgery in selected high-risk patients with aortic stenosis.[1]–[5] Patients with severe aortic stenosis and low baseline left ventricular ejection fraction (LVEF) have been found to be at high risk of mortality after surgical aortic valve replacement.[6],[7] Evidence addressing the risk profile and efficacy of TAVI in this setting is limited, in particular among those patients with severe impairment of left ventricular function. Recently, in a large registry comprising 1432 patients, which were treated with TAVI from 2009 to 2010 in Germany, mortality at 30 days and at 1 year was higher in patients with LVEF ≤ 30% as compared to those with LVEF > 30%.[8] However, results from other studies addressing whether patients with impaired LVEF present worse outcome after TAVI are controversial.[4],[5],[9]–[15]
The aim of this study was assess the impact of baseline severe impairment of left ventricular function on mortality at mid-term follow-up after TAVI in a large registry of consecutive patients.
2. Methods
2.1. Study design and patient population
Consecutive patients with severe aortic stenosis undergoing TAVI at five Italian tertiary cardiac centers between 2004 and 2011 were enrolled. A heart team of experienced interventional cardiologists, cardiovascular surgeons, and anesthesiologists was involved in the process of patient eligibility assessment for TAVI procedure. A TAVI procedure was considered to be appropriate when the patient presented a symptomatic severe aortic stenosis (valve area < 1 cm2) and the patient was considered not to be suitable to undergo conventional surgical aortic-valve replacement, due to high risk of irreversible complications and/or mortality. Logistic Euroscore was used to assess surgical risk of mortality. Significant liver disease defined as Child-Pugh Class B and C, the presence of porcelain aorta, severe patient frailty assessed by heart team, were also considered for surgical risk stratification. Life expectancy below one year due to cancer diseases was excluded. This study was approved by the local Ethics Committee. Written informed consent was obtained from all the patients in the study. The authors had full access to and take full responsibility for the integrity of the data.
2.2. Device and procedure
Both the CoreValve Revalving System (Medtronic, Santa Rosa, California) and the SAPIEN heart-valve system (Edwards Lifesciences, Irvine, California) were used in this study. Technical features of the third generation CoreValve and of Edwards SAPIEN valve system and technical details of the TAVI procedure have been reported previously.[16]–[18] Procedures were performed in an operating room with a fixed fluoroscopic imaging system with the use of general anesthesia or local anesthesia according to the center practice. Transesophageal echocardiography was employed according to local practice. Main arterial access was the common femoral artery. The trans-subclavian approach was used as alternative to unsuitable iliofemoral approach routes. No valve was implanted using the transapical or transaortic route. Hemostasis was achieved with the use of a Prostar XL 10 (Abbott Vascular, Abbott Park, IL) in the majority of the patients, for percutaneous femoral access. The subclavian approach was obtained by surgical exposure of the artery. All patients received 75–100 mg acetylsalicylic acid daily, for at least five days before the procedure (or a loading dose of 300 mg the day before the procedure), and lifelong thereafter, as well as clopidogrel (loading dose of 300 mg administered the day before the procedure followed by 75 mg daily for three to six months, depending on patient compliance, bleeding risk, center practice). Intravenous heparin was administered during the procedure to achieve an activated clotting time of 200–250 s.
2.3. Echocardiography
The Doppler echocardiographic measurements included left ventricular end-diastolic volume, LVEF, transvalvular pressure gradient, aortic valve area. LVEF was measured by the Simpson method from four- and two-chamber views. Patients were classified as those with LVEF ≤ 30% and those with LVEF > 30%, as this cutoff is considered an indicator of severe impairment of LV function. In the apical five-chamber view, peak and mean pressure gradients across the aortic valve were calculated by using the Bernoulli equation. In a subgroup analysis restricted to patients with LVEF ≤ 30% only, patients were further classified in two groups as those with low gradient (i.e., if baseline mean gradient was < 40 mmHg) and those with high gradient (i.e., if baseline mean gradient ≥ 40 mmHg). Effective aortic valve area was calculated by means of the continuity equation. Paravalvular aortic regurgitation was assessed by angiography immediately after the procedure and by transthoracic or transesophageal echocardiography until day 3 after TAVI. The incidence of postprocedural aortic regurgitation ≥ 2 was reported. Aortic annulus measurements were performed by computed tomography scan in the large majority of patients, and by transesophageal echocardiography in the remainder.
2.4. Study endpoints
The primary endpoint of this study was all-cause mortality at follow-up. Secondary endpoints included cardiovascular mortality, myocardial infarction, any cerebrovascular accident which was classified as stroke or transient ischemic attack. Procedural success was defined as successful device delivery with deployment of prosthesis in correct position. Peri-procedural complications included major vascular complications, cardiac tamponade, conversion to open heart surgery, major bleeding. The incidences of post-procedural pacemaker implantation and of post-procedural paravalvular aortic regurgitation ≥ 2 were reported. Endpoints were classified according to the Valve Academic Research Consortium and Valve Academic Research Consortium-2 consensus documents.[19] However, life-threatening or disabling bleeding and major bleeding were pooled together in this study. In order to minimize the risk of misclassification error of minor bleeding toward major bleeding, transfusing of whole blood/packed red blood cells ≥ 4 was used as indicator of major bleeding in this study. The process of event adjudication was based on information collected by each participating center without an independent clinical committee. Clinical follow-up was obtained by clinical visits and/or through telephone contacts. Referring cardiologists, general practitioners and patients were contacted whenever necessary for further information.
2.5. Statistical analysis
The distribution of continuous variables was assessed by visual inspection of their frequency histograms and with the use of the Shapiro-Wilk test. Continuous variables are expressed as mean ± SD or median and 25th–75th percentile, according to a Gaussian or non-normal distribution, respectively. Categorical variables are expressed as percentage and were compared by chi square or Fisher's exact tests as appropriate. The incidence of events over time was studied with the use of Kaplan-Meier method, and log-rank test was performed to assess differences.
For each endpoint, we performed time to first event analysis. When assessing each individual endpoint, we censored patients who died from any cause beyond the time of death—that is, they were not at risk anymore for the endpoint assessed after they died.
To assess the impact of baseline LVEF ≤ 30% on mortality, Cox regression analyses with calculation of hazard ratios (HR) of events with 95% confidence intervals (CI) were performed. If baseline LVEF was associated with mortality at simple Cox regression analysis (P-value ≤ 0.1), the impact of LVEF was tested at multivariable Cox regression model entering variables which emerged as predictors at simple Cox regression (P-value ≤ 0.1), and variables that presented a significant imbalance between patients with LVEF ≤ 30% and those with LVEF > 30% (P < 0.05) (Table 1), respecting the rule of thumb of entering 1 predictor each 10 events in the model.
Table 1. Baseline clinical characteristics.
| Overall population (n = 649) | LVEF ≤ 30% (n = 63) | LVEF > 30% (n = 586) | P | |
| Age, yrs | 83.6 (79.6–86.4) | 83.0 (77.0–86.4) | 83.6 (79.8–86.4) | 0.36 |
| Male | 305 (47.0%) | 30 (47.6%) | 275 (46.9%) | 0.92 |
| BSA, mean ± SD | 1.76 ± 0.19 | 1.72 ± 0.18 | 1.76 ± 0.20 | 0.12 |
| BMI | 24.9 (22.6–28.0) | 24.2 (21.5–26.3) | 25.2 (22.8–28.3) | 0.01 |
| Hypertension | 510 (87.6%) | 43 (86%) | 467 (87.8%) | 0.71 |
| Coronary artery disease | 329 (50.7%) | 38 (60.3%) | 291 (49.7%) | 0.11 |
| Peripheral vascular disease | 191 (29.4%) | 23 (36.5%) | 168 (28.7%) | 0.19 |
| Prior surgical aortic valve implantation | 32 (4.9%) | 2 (3.2%) | 30 (5.1%) | 0.76 |
| Prior myocardial infarction | 103 (15.9%) | 21 (33.3%) | 82 (13.9%) | < 0.001 |
| Prior stroke | 53 (8.2%) | 5 (7.9%) | 48 (8.2%) | 1.0 |
| Prior bypass graft surgery | 89 (13.7%) | 15 (23.8%) | 74 (12.6%) | 0.014 |
| Prior PCI | 214 (32.9%) | 24 (38.1%) | 190 (32.4%) | 0.36 |
| Prior pacemaker | 60 (9.2%) | 7 (11.1%) | 53 (9.0%) | 0.59 |
| Euroscore | 20.3 (13–30.2) | 37 (27.8–51.0) | 19.0 (12.8–27.0) | < 0.0001 |
| NYHA class ≥ 3 | 491 (75.8%) | 59 (93.6%) | 432 (73.8%) | < 0.0001 |
Data are presented as n (%) or median (25th–75th percentile) unless other indicated. BMI: body mass index; BSA: body surface area; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; PCI: percutaneous coronary intervention.
Multivariable predictors of mortality were also calculated and reported entering variables associated with mortality (P ≤ 0.1) at simple Cox regression analysis (i.e., postprocedural aortic regurgitation ≥ 2, body mass index, Euroscore, NYHA class, major vascular complications, previous myocardial infarction, previous stroke, valve size, major bleeding, peripheral artery disease, and previous coronary artery bypass surgery). The validity of the proportional-hazards assumptions was verified for all covariates by a test based on the scaled Schoenfeld residuals that checks for each variable, the slope in the regression of residuals on time is zero. A prespecified landmark analysis assessing all-cause death and cardiac death at 30 days was performed. A prespecified subgroup analysis was performed comparing patients with LVEF ≤ 30% to those with LVEF ≥ 50% with respect to mortality. In another prespecified subgroup analysis restricted to patients with LVEF ≤ 30% only, mortality rates were assessed in patients with low gradient vs. those with high gradient. A two-tailed P-value < 0.05 was the pre-specified level of statistical significance. All analyses were performed with STATA 11.2 (StataCorp LP, College Station, Texas).
3. Results
3.1. Patients
A total of 649 patients, 63 (9.7%) with LVEF ≤ 30%, and 586 (90.3%) with LVEF > 30% were enrolled. Baseline clinical characteristics of the entire study population and of the two groups of patients are reported in Table 1. Briefly, patients with LVEF ≤ 30%, as compared to those with LVEF > 30%, had a higher prevalence of previous myocardial infarction (P < 0.001), of NHYA class > 2 (P < 0.001), presented with higher Euroscore (P < 0.001). In terms of morphometric parameters, patients with LVEF ≤ 30%, as compared to those with LVEF > 30%, had a significantly smaller body mass index (P = 0.01) (Table 1). Baseline transvalvular peak and mean gradient were significantly lower in patients with LVEF ≤ 30% as compared to patients with LVEF > 30% (P < 0.001) (Table 2). Aortic valve areas were similar in the two groups (Table 2). No significant differences in aortic annulus measurements were found between the two groups of patients (Table 2). The large majority of patients received the CoreValve system (92.8%). The Edwards system was implanted in the remainder (7.2%) (Table 1). No significant difference was found with respect to valve size between the two groups (Table 2). Vascular access and the type of anesthesia were similar in the two groups of patients (Table 2).
Table 2. Baseline procedural and echocardiographic characteristics.
| Overall population (n = 649) | LVEF ≤ 30% (n = 63) | LVEF > 30% (n = 586) | P | |
| Baseline peak pressure gradient, mmHg | 81 (69–97) | 59.5 (44.5–69.5) | 84.0 (71–100) | < 0.0001 |
| Baseline mean pressure gradient, mmHg | 50 (41–60) | 34 (25–41) | 50 (43–60) | < 0.0001 |
| Valve area, cm2 | 0.68 (0.50–0.80) | 0.67 (0.51–0.80) | 0.67 (0.51–0.95) | 0.50 |
| Aortic annulus, mm | 22 (21–24) | 23 (20.6–24) | 22 (21–24) | 0.37 |
| Vascular access | 0.7 | |||
| Femoral | 556 (85.7%) | 53 (84.1%) | 503 (85.8%) | |
| Subclavian | 93 (14.3%) | 10 (15.9%) | 83 (14.2%) | |
| *Anesthesia | 0.69 | |||
| General | 355 (54.8%) | 33 (52.4%) | 322 (55.0%) | |
| Local | 293 (45.2%) | 30 (47.6%) | 263 (44.9%) | |
| Valve size, mm | 0.27 | |||
| 23 | 28 (4.3%) | 3 (1%) | 25 (7.2%) | |
| 26 | 366 (55.8%) | 134 (43.5%) | 232 (66.7%) | |
| 29 | 262 (39.9%) | 171 (55.5%) | 91 (26.1%) | |
| Valve type | 1.0 | |||
| CoreValve | 602 (92.8%) | 59 (93.6%) | 543 (92.7%) | |
| Edwards | 47 (7.2%) | 4 (6.4%) | 43 (7.3%) |
Data are presented as n (%) or median (25th–75thpercentile). *Information missing in one case. LVEF: left ventricle ejection fraction.
3.2. Procedural success and clinical outcome
The list of clinical endpoints is reported in Table 3. Procedural success was similar in patients with LVEF ≤ 30% as compared to those with LVEF > 30%. The incidence of major vascular complications and of major bleeding was similar in the two groups. Numerically non-significant higher incidence of post-procedural aortic regurgitation ≥ 2 was found in patients with LVEF ≤ 30% as compared to those with LVEF > 30%. The incidence of pacemaker implantation was numerically lower in patients with LVEF ≤ 30%, but differences did not reach the statistical significance. No myocardial infarction or cerebrovascular event was recorded among patients with LVEF ≤30%; the differences between the two groups being not statistically significant(Table 3).
Table 3. Clinical and procedural outcomes in the overall population and in both subgroups of LVEF ≤ 30% and LVEF > 30%.
| Overall population (n = 649) | LVEF ≤ 30% (n = 63) | LVEF > 30% (n = 586) | P | Test | |
| Procedural success | 632 (97.4%) | 62 (98.4%) | 570 (97.3%) | 0.59 | χ2 = 0.29 |
| All-cause death | 154 (23.7%) | 15 (23.8%) | 139 (23.7%) | 0.87 | Log-rank 0.03 |
| Cardiovascular death | 115 (17.7%) | 12 (19.1%) | 103 (17.6%) | 0.89 | Log-rank 0.02 |
| Myocardial infarction | 5 (0.77%) | 0 (0%) | 5 (0.85%) | 0.42 | Log-rank 0.64 |
| Any CVA | 18 (2.8%) | 0 (0%) | 18 (3.1%) | 0.16 | Log-rank 1.95 |
| Stroke | 16 (2.5%) | 0 (0%) | 16 (2.7%) | 0.19 | Log-rank 1.74 |
| Major vascular complication | 77 (11.9%) | 9 (14.3%) | 68 (11.6%) | 0.53 | χ2 = 0.39 |
| Conversion to open heart surgery | 4 (0.6%) | 0 | 4 (0.7%) | 1 | Fisher |
| Cardiac tamponade | 14 (2.2%) | 0 | 14 (2.4%) | 0.21 | χ2 = 1.54 |
| Transfusions ≥ 4 units | 54 (8.3%) | 3 (4.8%) | 51 (8.7%) | 0.35 | Fisher |
| Post-procedural AR ≥ 2 | 164 (25.3%) | 20 (31.7%) | 144 (24.6%) | 0.21 | χ2 = 1.55 |
| New pacemaker implantation | 102 (16.1%) | 6 (9.7%) | 96 (16.8%) | 0.15 | χ2 = 2 |
Data are presented as n (%) unless other indicated. P-value calculated with log-rank method, χ2 or fisher's exact test, as appropriate. The following endpoints were assessed at the end of follow-up: all-cause death, cardiac death, myocardial infarction, stroke, and CVA. The remaining endpoints were assessed during in-hospital stay. AR: aortic regurgitation; CVA: cerebrovascular accident; LVEF: left ventricular ejection fraction.
3.3. Mortality
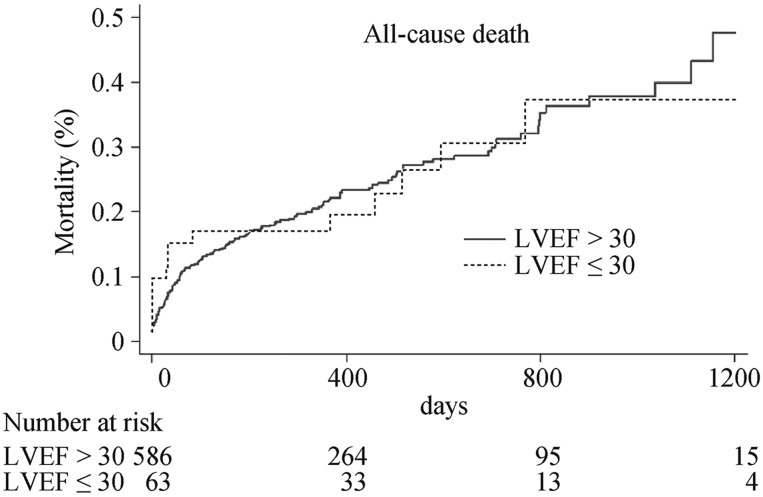
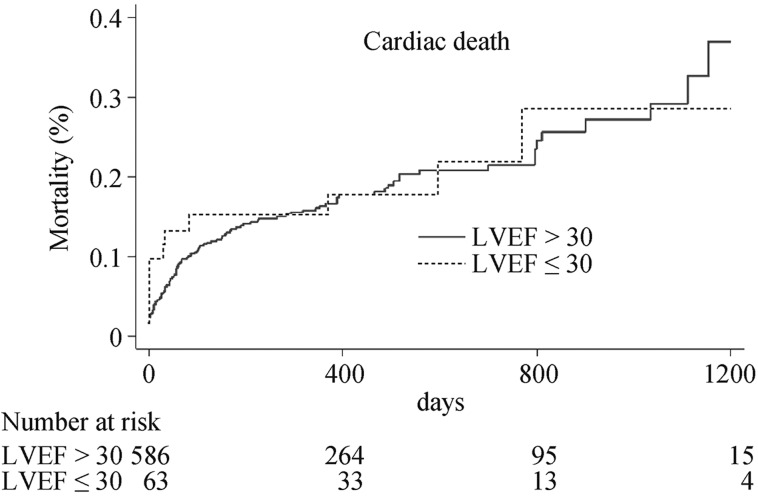
After a median follow-up of 436 days (25th–75th percentile, 357–737 days), all-cause mortality (23.8% vs. 23.7%, P = 0.87, HR: 0.96, 95% CI: 0.56–1.63) and cardiac mortality (19.1% vs.17.6%, P = 0.89, HR: 1.04, 95% CI: 0.57–1.90) were similar in patients with LVEF ≤ 30% as compared to those with LVEF > 30% (Figure 1 and 2).
Figure 1. Cumulative hazard curve of all-cause mortality in patients with baseline LVEF ≤ 30% and LVEF > 30%.
LVEF: left ventricular ejection fraction.
Figure 2. Cumulative hazard curve of cardiac mortality in patients with baseline LVEF ≤ 30% and LVEF > 30%.
LVEF: left ventricular ejection fraction.
At 30 days, no significant difference in all-cause mortality was found between the two groups (11.1% in patients with LVEF ≤ 30% vs. 6.3% in patients with LVEF > 30%, P = 0.14, HR: 1.81, 95% CI: 0.81–4.06). The risk of 30-day cardiac mortality tended to be higher in patients with LVEF ≤ 30% as compared to those with LVEF > 30% (11.1% vs. 5.3%, P = 0.06, HR: 2.16, 95% CI: 0.95–4.90). However, at multivariable cox regression, after adjustment for covariates, LVEF ≤ 30% was not associated with 30-day cardiac mortality (HR: 1.72, 95% CI: 0.73–4.04, P = 0.22).
Prespecified subgroup analysis comparing patients with LVEF ≥ 50% to patients with LVEF ≤ 30% found similar risk of all-cause death (23.7% vs. 23.8%, P = 0.88, HR: 1.04, 95% CI: 0.60–1.79) and of cardiac mortality (17.3% vs. 19.0%, P = 0.85, HR: 0.94, 95% CI: 0.51–1.74).
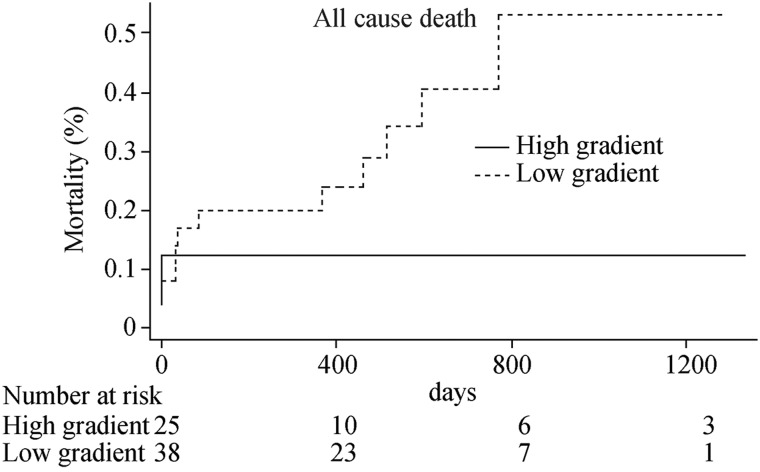
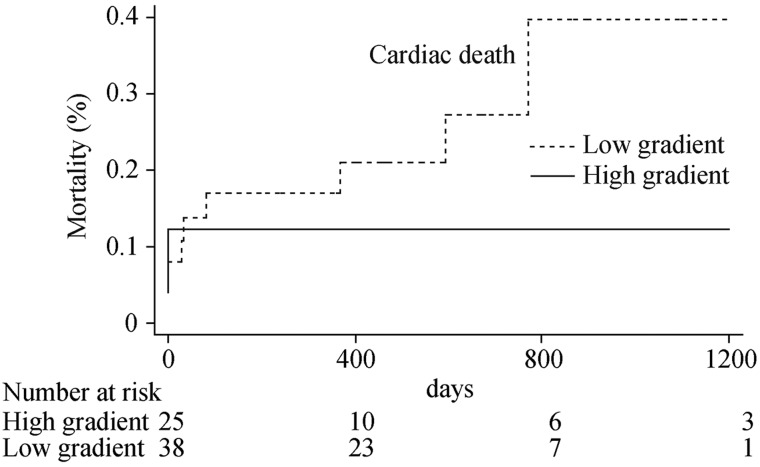
In the prespecified subgroup analysis restricted to patients with LVEF ≤ 30%, 38 patients were classified as with low gradient, and 25 patients presented high gradient. Non-significantly numerically higher rates of all-cause death (31.6% vs. 12.0%, P = 0.14, HR: 2.46, 95% CI: 0.69– 8.74) and of cardiac death (23.7% vs. 12.0%, P = 0.32, HR: 1.90, 95% CI: 0.51–7.03) were observed in patients with low gradient as compared to those with high gradient (Figure 3 and 4).
Figure 3. Cumulative hazard curve of all-cause mortality among patients with LVEF ≤ 30% with high gradient or low gradient.
LVEF: left ventricular ejection fraction.
Figure 4. Cumulative hazard curve of cardiac death in patients with LVEF ≤ 30% and low gradient and in patients with LVEF ≤ 30% and high gradient.
LVEF: left ventricular ejection fraction.
3.4. Predictors of mortality
At multivariable Cox regression analysis aortic regurgitation ≥= 2, major vascular complications and major bleeding emerged as independent predictor of all-cause death, while aortic regurgitation ≥ 2 and major bleedings were found to be independent predictors of cardiac death (Table 4 and 5).
Table 4. Univariable and multivariable predictors for all-cause death.
| Simple Cox regression |
P | Multiple Cox regression |
P | Multiple Cox regression |
P | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| LVEF ≤ 30% | 0.96 (0.56–1.63) | 0.87 | Including LVEF ≤ 30% | |||
| Age | 1.0 (0.97–1.03) | 1 | ||||
| Post-procedural AR ≥ 2 | 1.97 (1.42–2.73) | < 0.001 | 1.76 (1.26–2.45) | 0.001 | 1.73 (1.24–2.43) | 0.001 |
| BMI | 0.97 (0.93–1.004) | 0.09 | 0.96 (0.93–1.001) | 0.06 | 0.96 (0.93–1.0) | 0.05 |
| Coronary artery disease | 0.93 (0.68–1.28) | 0.68 | ||||
| Euroscore | 1.01 (1.005–1.02) | 0.003 | 1.007 (0.99–1.02) | 0.19 | 1.01 (1.0–1.02) | 0.048 |
| Female sex | 0.77 (0.56–1.06) | 0.11 | ||||
| Major vascular complications | 1.98 (1.32–2.96) | 0.001 | 1.62 (1.04–2.53) | 0.03 | 1.72 (1.09–2.71) | 0.018 |
| NYHA > 2 | 1.73 (1.15–2.59) | 0.008 | 1.45 (0.95–2.21) | 0.09 | 1.51 (0.98–2.31) | 0.06 |
| Previous CABG | 1.03 (0.65–1.62) | 0.90 | ||||
| Previous MI | 1.53 (1.05–2.24) | 0.03 | 1.31 (0.88–1.96) | 0.19 | 1.37 (0.91–2.05) | 0.13 |
| Previous PCI | 1.01 (0.72–1.41) | 0.95 | ||||
| Previous Stroke | 1.61 (0.98–2.63) | 0.06 | 1.35 (0.81–2.24) | 0.24 | 1.29 (0.77–2.14) | 0.33 |
| Peripheral vascular disease | 1.29 (0.92–1.81) | 0.13 | ||||
| Valve size | 1.45 (1.08–1.94) | 0.01 | 1.35 (0.99–1.83) | 0.05 | 1.37 (1.01–1.86) | 0.04 |
| Transfusions ≥ 4 | 1.89 (1.19–2.99) | 0.007 | 1.74 (1.05–2.89) | 0.03 | 1.65 (0.99–2.74) | 0.05 |
AR: aortic regurgitation; BMI: body mass index; CABG: coronary artery bypass graft; HR: hazard ratio; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NYHA: New York Heart Association; PCI: percutaneous coronary intervention.
Table 5. Univariable and multivariable predictors for cardiac death.
| Simple Cox regression |
P | Multiple Cox regression |
P | Multiple Cox regression |
P | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| LVEF ≤ 30% | 1.04 (0.57–1.90) | 0.89 | Including LVEF ≤ 30% | |||
| Age | 0.99 (0.96–1.02) | 0.76 | ||||
| Post-procedural AR ≥ 2 | 1.85 (1.26–2.71) | 0.002 | 1.68 (1.14–2.48) | 0.009 | 1.65 (1.12–2.45) | 0.012 |
| BMI | 0.98 (0.94–1.02) | 0.39 | ||||
| Coronary artery disease | 1.06 (0.73–1.53) | 0.76 | ||||
| Euroscore | 1.02 (1.005–1.03) | 0.004 | 1.01 (0.99–1.02) | 0.14 | 1.01 (0.99–1.02) | 0.05 |
| Female sex | 0.77 (0.53–1.11) | 0.16 | ||||
| Major vascular complications | 2.0 (1.26–3.19) | 0.003 | 1.56 (0.93–2.60) | 0.09 | 1.66 (0.98–2.79) | 0.06 |
| NYHA > 2 | 1.63 (1.02–2.59) | 0.04 | 1.29 (0.79–2.11) | 0.29 | 1.34 (0.82–2.18) | 0.24 |
| Previous CABG | 1.16 (0.70–1.92) | 0.56 | ||||
| Previous MI | 1.72 (1.12–2.64) | 0.01 | 1.43 (0.91–2.24) | 0.12 | 1.49 (0.95–2.34) | 0.08 |
| Previous PCI | 1.09 (0.74–1.59) | 0.66 | ||||
| Previous stroke | 1.57 (0.88–2.79) | 0.13 | ||||
| Peripheral vascular disease | 1.38 (0.94–2.02) | 0.09 | 1.17 (0.78–1.75) | 0.45 | 1.13 (0.75–1.71) | 0.54 |
| Valve size | 1.44 (1.02–2.01) | 0.03 | 1.30 (0.91–1.85) | 0.15 | 1.32 (0.93–1.88) | 0.12 |
| Transfusions ≥ 4 | 2.22 (1.34–3.67) | 0.002 | 1.95 (1.12–3.39) | 0.02 | 1.86 (1.07–3.24) | 0.03 |
AR: aortic regurgitation; BMI: body mass index; CABG: coronary artery bypass graft; HR: hazard ratio; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NYHA: New York Heart Association; PCI: percutaneous coronary intervention.
4. Discussion
In this large registry of patients undergoing TAVI with a prevalent use of CoreValve system, patients with baseline LVEF ≤ 30% as compared to those with LVEF > 30% presented similar all-cause mortality and cardiac mortality at mid-term follow-up. These findings were confirmed after exclusion of patients with LVEF > 30% and < 50%. A trend toward higher crude cardiac mortality at 30 days was observed in patients with LVEF ≤ 30%, as compared to patients with LVEF > 30%, which however disappeared at multivariable Cox regression.
4.1. LVEF and mortality
The impact of left ventricular dysfunction on outcome has been controversial in previous studies.[4],[5],[9]–[15] Several studies did not report significant differences in all-cause mortality between patients with normal LVEF and those with impaired left ventricular function.[10]–[12],[14],[15] In the study of Ewe, et al.[10] however, reduced LVEF was a predictor of combined major adverse cardiovascular events. These studies differed with respect to cutoff values of LVEF for the definition of impaired left ventricular function, as cutoff values varied from 50%,[9] to 30%,[10] or 35%.[12] In the cohort A of the PARTNER trial,[15] left ventricular dysfunction was defined as LVEF > 20% and < 50%. In this study, an association with borderline significance, between LV dysfunction and increased risk of repeat hospitalization within the first year after TAVI was reported.[15] In contrast with these findings, Tamburino, et al.[5] found that LVEF < 40% was an independent predictor of 30-day mortality (odds ratio: 3.51). Gotzmann, et al.[13] found a significant impact of reduced LVEF on all-cause death and mortality at 1-year follow-up. Fraccaro, et al.[14] also reported significantly higher in-hospital mortality (14% vs. 4%, P = 0.004) and from discharge to 30-days mortality (10% vs. 3%, P = 0.013) in patients with severe impairment in LVEF as compared to the remainder.
It could be speculated that the use of higher cutoff values of LVEF for the definition of left ventricular dysfunction, might contribute to minimize a negative effect of impaired left ventricular function on mortality, thus leading to heterogenous findings across studies. However, our data does not support this hypothesis. In our study, we used a cutoff LVEF of 30% and patients with very low LVEF were included in this registry at odds with the Cohort A of the Partner trial.[15] Patients with LEVF ≤ 30%, as compared to those with LVEF > 30%, had worse admission NYHA class, had a higher risk as predicted by Euroscore and more frequently a previous myocardial infarction. Despite such higher risk profile, mortality was not increased at mid-term follow-up in patients with LVEF ≤ 30%. Potential explanations for the lack of mortality differences are the excellent procedural success achieved in this high risk population, the comparable incidence of major bleeding or major vascular complications and of significant post-procedural paravalvular aortic regurgitation which were found to be independent predictors of mortality in this registry. Furthermore, it has been consistently reported in previous studies that improvement in LVEF occurs after TAVI, which might contribute to abolish a potential negative prognostic impact of baseline impairment of left ventricular function.[9],[14],[15],[20] However, we could not assess this phenomenon in our study due to the lack of complete echocardiographic data at follow-up.
We have also ruled out that the inclusion of patients with impaired LVEF among those with LVEF ≥ 30% could have had a dilution effect on mortality differences between the two groups of patients, as in a prespecified subgroup analysis comparing patients with LVEF ≤ 30% to those with LVEF ≥ 50% no difference in mortality was found.
4.2. Low transvalvular gradient
In order to identify other factors which could stratify patients with LVEF ≤ 30%, we have explored the impact of baseline low mean transvalvular gradient on mortality. We found numerically higher all-cause and cardiac mortality rates at mid-term follow-up in patients with low gradient, as compared to patients with high gradient, the difference however not achieving the statistically significance. It is plausible that the small sample size of these subgroups and the small event rate may have blunted the existence of a true clinically difference between groups. Indeed, from a physiopathological standpoint, patients with reduced LVEF and low gradient have been found to differ from those with high gradient as they present a very high arterial afterload despite a low systolic arterial pressure, they develop a less pronounced improvement in LVEF following TAVI, which has been hypothesized to be related to a lack of contractile reserve in a proportion of patients.[21] Furthermore, several studies have reported a negative prognostic impact of low gradient.[20]–[25] Ben-Dor, et al.[20] found that among patients with LVEF < 40%, those with low (≤ 40 mmHg) gradient presented higher mortality than those with high gradient (53.8% vs. 41%, P = 0.01) at mid-term follow-up. O'Sullivan, et al.[21] found that LVEF < 30% and low gradient was an independent predictor of 12-month mortality. Interestingly, Le Ven, et al.[26] pointed out the importance of the presence of low flow state, defined as stroke volume index < 35 mL/m2, showing that the prognostic impact of low LVEF and of low gradient documented at univariable analysis disappeared after adjustment for other risk factors including stroke volume index, thus suggesting that the association between low gradient and mortality could indeed be related to the presence of a low flow state. Recently, Elhmidi, et al.[22] found that patients with low flow/low gradient aortic stenosis exhibited a 3.8-fold higher 6-month and 2.8-fold higher 1-year mortality rate than patients with high gradient aortic stenosis. Similarly, low flow/low gradient was associated with higher mortality in previous studies.[23]–[25] We did not have availability of echocardiographic stroke volume index measurements to correctly identify the proportion of patients with low flow among the two subgroups to verify this data.
4.3. Limitations
In addition to the aforementioned limitations we acknowledge the following limitations. Dobutamine stress echocardiography was performed only in a minority of patients with reduced LVEF and low gradient, in order to assess the presence of contractile reserve before TAVI. The lack of an independent core laboratory for the assessment of echocardiograms represents another limitation. Differences in mortality at 30 days between patients with LVEF ≤ 30% and those with LVEF > 30% were not statistically significant, although such comparison could be underpowered owing to a reduction in the overall event rate at this time point. Echocardiographic data on baseline mitral regurgitation was available only in approximately 60% of patients enrolled in this registry. Therefore, the relation between baseline mitral regurgitation, LVEF and outcomes following TAVI could not be investigated. Follow-up duration was heterogeneous. The heterogeneity in TAVI experience among the participating centers may represent a source of bias. Despite the use of VARC-2 classification to standardize event adjudication, such process was performed using clinical source document reported by each participating center without an independent clinical event committee. However, our incidence of major vascular complications is in line with previous studies.[4],[5] The incidence of cerebrovascular accidents in our study may have been underestimated due to the lack of systematic assessment of all cerebrovascular events by a neurology specialist. In this registry, there was heterogeneity of follow-up duration, although loss at follow-up was minimal (i.e., < 1%).
4.4. Conclusions
In this multicenter registry of TAVI patients, baseline severe impairment of left ventricular function was not a predictor of increased short-term and mid-term mortality after TAVI. Among patients with severe impairment of left ventricular function, those with low transvalvular gradient deserve a careful evaluation owing to numerically higher mortality rates.
Larger studies with patient stratification according to the magnitude of transvalvular gradient, the presence of low-flow state, the assessment of baseline mitral regurgitation and of contractility reserve are required to better identify those patients with severe impairment of left ventricular function who can mostly benefit from TAVI procedures. Selected patients with severe impairment of left ventricular function should not be denied TAVI.
References
- 1.Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–1704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 2.Gilard M, Eltchaninoff H, Iung B, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012;366:1705–1715. doi: 10.1056/NEJMoa1114705. [DOI] [PubMed] [Google Scholar]
- 3.Moat NE, Ludman P, de Belder MA, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol. 2011;58:2130–2138. doi: 10.1016/j.jacc.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 4.Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 5.Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308. doi: 10.1161/CIRCULATIONAHA.110.946533. [DOI] [PubMed] [Google Scholar]
- 6.Powell DE, Tunick PA, Rosenzweig BP, et al. Aortic valve replacement in patients with aortic stenosis and severe left ventricular dysfunction. Arch Intern Med. 2000;160:1337–1341. doi: 10.1001/archinte.160.9.1337. [DOI] [PubMed] [Google Scholar]
- 7.Halkos ME, Chen EP, Sarin EL, et al. Aortic valve replacement for aortic stenosis in patients with left ventricular dysfunction. Ann Thorac Surg. 2009;88:746–751. doi: 10.1016/j.athoracsur.2009.05.078. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer U, Zahn R, Abdel-Wahab M, et al. Comparison of outcomes of patients with left ventricular ejection fractions ≤ 30% versus ≥ 30% having transcatheter aortic valve implantation (from the German Transcatheter Aortic Valve Interventions Registry) Am J Cardiol. 2015;115:656–663. doi: 10.1016/j.amjcard.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Clavel MA, Webb JG, Rodés-Cabau J, et al. Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation. 2010;122:1928–1936. doi: 10.1161/CIRCULATIONAHA.109.929893. [DOI] [PubMed] [Google Scholar]
- 10.Ewe SH, Ajmone Marsan N, Pepi M, et al. Impact of left ventricular systolic function on clinical and echocardiographic outcomes following transcatheter aortic valve implantation for severe aortic stenosis. Am Heart J. 2010;160:1113–1120. doi: 10.1016/j.ahj.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Pilgrim T, Wenaweser P, Meuli F, et al. Clinical outcome of high-risk patients with severe aortic stenosis and reduced left ventricular ejection fraction undergoing medical treatment or TAVI. PLoS One. 2011;6:e27556. doi: 10.1371/journal.pone.0027556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Boon RM, Nuis RJ, Van Mieghem NM, et al. Clinical outcome following transcatheter aortic valve implantation in patients with impaired left ventricular systolic function. Catheter Cardiovasc Interv. 2012;79:726–732. doi: 10.1002/ccd.23423. [DOI] [PubMed] [Google Scholar]
- 13.Gotzmann M, Rahlmann P, Hehnen T, et al. Heart failure in severe aortic valve stenosis: Prognostic impact of left ventricular ejection fraction and mean gradient on outcome after transcatheter aortic valve implantation. Eur J Heart Fail. 2012;14:1087–1089. doi: 10.1093/eurjhf/hfs108. [DOI] [PubMed] [Google Scholar]
- 14.Fraccaro C, Al-Lamee R, Tarantini G, et al. Transcatheter aortic valve implantation in patients with severe left ventricular dysfunction: immediate and mid-term results, a multicenter study. Circ Cardiovasc Interv. 2012;5:253–260. doi: 10.1161/CIRCINTERVENTIONS.111.964213. [DOI] [PubMed] [Google Scholar]
- 15.Elmariah S, Palacios IF, McAndrew T, et al. Outcomes of transcatheter and surgical aortic valve replacement in high-risk patients with aortic stenosis and left ventricular dysfunction: results from the Placement of Aortic Transcatheter Valves (PARTNER) Trial (Cohort A) Circ Cardiovasc Interv. 2013;6:604–614. doi: 10.1161/CIRCINTERVENTIONS.113.000650. [DOI] [PubMed] [Google Scholar]
- 16.Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol. 2007;50:69–76. doi: 10.1016/j.jacc.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 17.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 18.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 19.Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Dor I, Maluenda G, Iyasu GD, et al. Comparison of outcome of higher versus lower transvalvular gradients in patients with severe aortic stenosis and low (< 40%) left ventricular ejection fraction. Am J Cardiol. 2012;109:1031–1037. doi: 10.1016/j.amjcard.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 21.O'Sullivan CJ, Stortecky S, Heg D, et al. Clinical outcomes of patients with low-flow, low-gradient, severe aortic stenosis and either preserved or reduced ejection fraction undergoing transcatheter aortic valve implantation. Eur Heart J. 2013;34:3437–3450. doi: 10.1093/eurheartj/eht408. [DOI] [PubMed] [Google Scholar]
- 22.Elhmidi Y, Piazza N, Krane M, et al. Clinical presentation and outcomes after transcatheter aortic valve implantation in patients with low flow/low gradient severe aortic stenosis. Catheter Cardiovasc Interv. 2014;84:283–290. doi: 10.1002/ccd.25366. [DOI] [PubMed] [Google Scholar]
- 23.Amabile N, Ramadan R, Ghostine S, et al. Early and mid-term cardiovascular outcomes following TAVI: impact of pre-procedural transvalvular gradient. Int J Cardiol. 2012;167:687–692. doi: 10.1016/j.ijcard.2012.03.066. [DOI] [PubMed] [Google Scholar]
- 24.Lauten A, Zahn R, Horack M, et al. Transcatheter aortic valve implantation in patients with low-flow, low-gradient aortic stenosis. JACC Cardiovasc Interv. 2012;5:552–559. doi: 10.1016/j.jcin.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann HC, Pibarot P, Hueter I, et al. Predictors of mortality and outcomes of therapy in low flow severe aortic stenosis: A PARTNER trial analysis. Circulation. 2013;127:2316–2326. doi: 10.1161/CIRCULATIONAHA.112.001290. [DOI] [PubMed] [Google Scholar]
- 26.Le Ven F, Freeman M, Webb J, et al. Impact of low flow on the outcome of high-risk patients undergoing transcatheter aortic valve replacement. J Am Coll Cardiol. 2013;62:782–788. doi: 10.1016/j.jacc.2013.05.044. [DOI] [PubMed] [Google Scholar]