Abstract
Background
The influence of homocysteine (Hcy) on the migration and proliferation of vascular smooth muscle cells has been well established. However, the impact of Hcy levels on the progression of non-culprit coronary lesions (NCCLs) is controversial. This study aims to evaluate whether the plasma level of Hcy is related to the progression of NCCLs after percutaneous coronary stent implantation in elderly patients with acute coronary syndrome (ACS).
Methods
A total of 223 elderly patients (≥ 65 years old) with ACS undergoing stent implantation and follow-up coronary angiography were enrolled. Laboratory determination comprised of blood sample evaluation for Hcy was carried out before baseline coronary intervention. The patients were classified into two groups according to the blood Hcy tertiles (≥ 15 mmol/L or < 15 mmol/L). Patients were followed up for 12.2 months. NCCL progression was assessed by three-dimensional quantitative coronary angiography.
Results
A significantly higher ratio of NCCL progression was observed in the group with baseline Hcy concentrations above 15 mmol/L compared to the group with concentrations below 15 mmol/L (41/127, 32.3% vs. 14/96, 14.6%, P = 0.002). Multivariate Cox regression analysis showed that Hcy and diabetes mellitus were independent risk factors for NCCL progression. The crude hazard ratio (HR) of NCCL progression for Hcy level was 1.056 (95% CI: 1.01–1.104, P = 0.015). The adjusted HR of NCCL progression for Hcy level was 1.024 (95% CI: 1.007–1.042, P = 0.007). The adjusted HR of NCCL progression for diabetes mellitus was 1.992 (95% CI: 1.15–3.44, P = 0.013).
Conclusions
Hcy is an independent risk factor for NCCL progression after 12 months of follow-up in elderly patients with ACS who has undergone percutaneous coronary stenting.
Keywords: Coronary angiography, Elderly patients, Homocysteine, Non-culprit coronary lesion, Percutaneous coronary intervention
1. Introduction
Culprit lesions, the treatment of which is the primary goal of percutaneous coronary intervention (PCI), have been studied for decades and have been found to be responsible for most of the adverse events of ACS, while other plaques, which are referred to as non-culprit lesions (NCCLs), are considered to be innocent.[1] However, even after successful PCI for the thrombotic arteries, adverse cardiac events still occur. Recent studies have demonstrated that NCCLs were associated with cardiovascular events in patients who received PCI.[2] To date, there are few studies evaluating the effects of NCCLs on patients and how to recognize high risk NCCLs in patients with ACS, especially in elderly patients who are more susceptible to multi-vessel coronary disease.
Serum levels of homocysteine (Hcy) were associated with pathogenesis and progression of coronary atherosclerosis.[3]–[7] Several studies validated that hyper-Hcy was an independent risk factor for atherosclerosis.[3] Hcy was also suggested to be independently associated with severity and calcified plaque by CTA.[4] A free plasma Hcy level over 4.11 mmol/L was an independent risk factor for patients hospitalized for ACS.[5] Hcy is able to convert a stable plaque into an unstable one by promoting the expression of chemokine and oxidized low-density lipoprotein,[6] leading to endothelial cell injury.[7] Our study aims to clarify the relationship between serum Hcy level and the progression of NCCL in elderly ACS patients who have undergone PCI and to investigate whether Hcy is a potential risk factor for the recognition of NCCLs with high risks.
2. Methods
2.1. Study population
A total of 260 patients (≥ 65 years old) with ACS who successfully received percutaneous coronary intervention (PCI) with stents implantation and follow-up diagnostic coronary angiography (CAG) in the Chinese PLA General Hospital between August 1st, 2009 and March 31st, 2013 were enrolled. The median follow-up time was 12.2 months. The diagnosis of ACS was made by two experienced clinicians according to the European Society of Cardiology (ESC) guidelines. The values of Hcy were detected before baseline coronary intervention for all of the enrolled patients. STEMI patients were qualified to primary PCI within 12 h from the onset of chest pain. Follow-up CAG was performed from the 11th to the 13th month after PCI. Among those 260 patients, 37 patients were excluded including: four patients who could not be analyzed due to the lack of two angiographic projections; 13 patients who had a history of PCI or CABG; one patient who had acute myocardial infarction (AMI) within two weeks after the primary procedure; six patients who had severe renal or liver diseases or malignancies; and 17 patients who had > 24 months of follow-up. Finally, a total of 223 patients were analyzed in this study. They were classified into two groups according to the blood Hcy level: the hyper-Hcy group (H-Hcy, Hcy ≥ 15 mmol/L) and the low Hcy group (L-Hcy, Hcy < 15 mmol/L). During both hospitalizations, all clinical, laboratory and angiographic parameters were collected. All patients were given written informed consent for participation in the study, and the study protocol was approved by the Ethic Committees of the Chinese PLA General Hospital and complies with the Declaration of Helsinki.
2.2. PCI procedure and medication
PCI was performed by one of four interventionists using standard techniques. Drug-eluting stents were implanted as the first-line choice of stents. All patients were treated with optimal medical therapy after PCI, including statins, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, β-blockers, and antiplatelet agents, all of which have been proven to reduce the risk of adverse cardiovascular events.
2.3. Evaluating the progression of NCCL by 3D quantitative coronary angiography
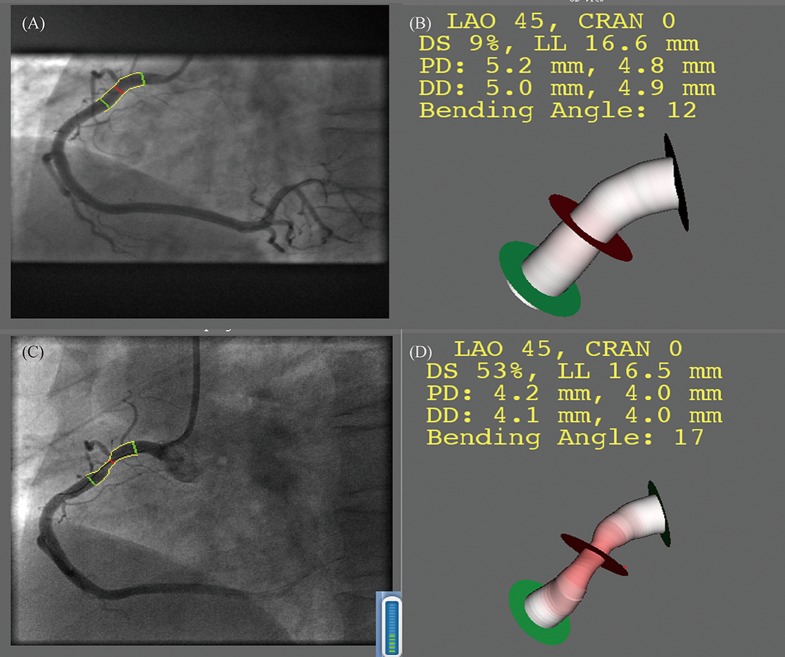
Angiographic images were recorded using different X-ray systems (Allura Xper, Philips Medical Systems, Best, the Netherlands; and Innova 3100, GE Medical Systems, USA). Coronary angiograms were reviewed separately by two independent observers who were blinded to all clinical data. When the two observers could not reach a consensus, a third experienced observer was asked to review the results to get a more accurate outcome. A recently developed three dimensional quantitative coronary angiography (3D QCA) software package (QAngio® XA 3D Research Edition 1.0, Medis Specials BV, Leiden, the Netherlands[8],[9]) was used for the 3D angiographic reconstruction and quantitative analysis. The entire process consisted of the following major steps: (1) two image sequences acquired at two arbitrary angiographic views with projection angles at least 25° apart were loaded; (2) properly contrast-filled end-diastolic frames of these angiographic image sequences were selected; (3) one to three anatomical markers, e.g., bifurcations, were identified as reference points in the two angiographic views for the automated correction of angiographic system distortions; (4) the lumen of the interrogated vessel segment was automatically delineated using an extensively validated edge detection algorithm; and (5) the lumen and reference surface, i.e., the normal lumen as if there was no obstruction, was reconstructed in 3D, and the relevant QCA parameters were derived. Minimal lumen diameter (MLD), percent diameter stenosis (DS), minimal lumen area (MLA), percent area obstruction (AS), and plaque volume were measured at primary PCI and at follow-up CAG. Figure 1 shows a comparison of the progression in the same lesion using 3D-QCA.
Figure 1. Assessment of plaque progression by 3D QCA.
(A&B): The interrogated lesion at baseline in 2D and 3D; (C&D): the same lesion at follow-up in 2D and in 3D. CRAN: cranial; DD: distal diameter; DS: diameter stenosis; LAO: left anterior oblique; LL: lesion longitude; PD: proximal diameter.
2.4. Definitions
NCCL was the de novo stenotic lesion that was not responsible for the ischemic symptoms or positive functional ischemic tests. When multiple NCCLs were present, the lesion that had the biggest increase in diameter stenosis in the follow-up CAG was identified as the index lesion for each patient. Patients were stratified into the angiographic NCCL progression group if ≥ 10% diameter reduction of a pre-existing stenosis ≥ 50%; ≥ 30% diameter reduction of a pre-existing stenosis < 50%; development of a new stenosis ≥ 30% diameter reduction in a segment that was normal at the first diagnostic CAG; or progression of any lesion to total occlusion at the second follow-up CAG.[10] Hypertension was defined as a history of a systolic blood pressure ≥ 140 mmHg, a diastolic blood pressure ≥ 90 mmHg, or the use of antihypertensive therapy. Diabetes mellitus was defined as a fasting plasma glucose concentration ≥ 126 mg/dL or the use of anti-diabetic therapy.
2.5. Statistical analysis
Statistical analyses were performed by SPSS 17.0. Continuous data were expressed as the mean ± SD or median (inter-quartile range). The Student's t-test or one way analysis of variance (ANOVA) was used when continuous variables were normally distributed; otherwise, the Wilcoxon two-sample test was performed. Categorical variables were compared via chi-square test. The influence of hyper-Hcy on the progression of NCCL was analyzed by unadjusted Kaplan-Meier curve along with the log-rank test. A Cox proportional hazards model was used to identify multivariate predictors of NCCL progression. Odds ratios (ORs) were reported with corresponding 95% confidence intervals (CI). A two-sided P-value of < 0.05 was considered statistically significant.
3. Results
3.1. The baseline characteristics
In this study, we enrolled a total of 223 elderly patients (≥ 65 years old) with ACS who underwent successful PCI with stent implantation. The median age was 71.1 years old, 75.8% were males and 40.8% of the patients suffered from diabetes mellitus. The median follow-up interval was 12.2 months. During the follow-up, there were 55 patients who had non-culprit coronary lesion progression. Patients were classified into two groups according to the level of plasma Hcy (≥ 15.0 µmol/L and < 15.0 µmol/L). There were 127 patients in H-Hcy group and 96 patients in L-Hcy group. The baseline clinical characteristics of the two groups are shown in Table 1. Compared with those in the L-Hcy group, higher ratios of males (85% vs. 63.5%, P < 0.05) and patients with smoking history (35.4% vs. 14.6%, P < 0.05) were seen in the H-Hcy group, while there were no significant differences in other characteristics including age, plasma glucose or hypertension. Hcy is associated with NCCL progression.
Table 1. Clinical, angiography and procedural data examined in different groups.
| Characteristics | H-Hcy (n = 127) | L-Hcy (n = 96) | P value |
| Age, yrs | 71.69 ± 7.26 | 70.73 ± 6.73 | 0.312 |
| Follow-up years | 12.06 ± 5.21 | 12.29 ± 5.76 | 0.749 |
| Male | 108 (85%) | 61 (63.5%) | < 0.001* |
| Hypertension | 41 (32.3%) | 26 (27.1%) | 0.402 |
| Smoking status | 45 (35.4%) | 14 (14.6%) | < 0.001* |
| Diabetes mellitus | 49 (38.6%) | 42 (43.7%) | 0.437 |
| AMI | 13 (10.2%) | 9 (9.4%) | 0.768 |
| BMI, kg/m2 | 25.46 ± 0.27 | 25.15 ± 0.32 | 0.462 |
| Physical findings on admission | |||
| Systolic blood pressure, mmHg | 132.11 ± 15.44 | 133.71 ± 16.14 | 0.458 |
| Diastolic blood pressure, mmHg | 72.41 ± 10.24 | 70.79 ± 10.42 | 0.249 |
| Heart rate, beats/min | 65 ± 10 | 68 ± 8 | 0.323 |
| Baseline blood feature | |||
| Plasma glucose, mmol/L | 6.01 ± 2.18 | 6.16 ± 2.21 | 0.608 |
| CRP, mg/d L | 1.6 ± 0.5 | 1.8 ± 0.8 | 0.434 |
| BNP, mmol/L | 104 ± 21 | 91 ± 17 | 0.165 |
| HDL, mmol/L | 1.12 ± 0.35 | 1.16 ± 0.26 | 0.308 |
| LDL, mmol/L | 2.36 ± 0.93 | 2.45 ± 0.84 | 0.442 |
| Triglycerides, mmol/L | 1.41 ± 0.63 | 1.43 ± 0.72 | 0.820 |
| Total cholesterol, mmol/L | 4.11 ± 1.18 | 4.28 ± 0.96 | 0.224 |
| Follow-up blood feature | |||
| Plasma glucose, mmol/L | 6.13 ± 1.93 | 6.06 ± 2.01 | 0.792 |
| CRP, mg/dL | 1.50 ± 0.43 | 1.61 ± 0.78 | 0.181 |
| BNP, mmol/L | 97.24 ± 19.54 | 96.15 ± 17.65 | 0.668 |
| HDL, mmol/L | 1.13 ± 0.34 | 1.17 ± 0.16 | 0.287 |
| LDL, mmol/L | 2.30 ± 0.83 | 2.35 ± 0.64 | 0.625 |
| Triglycerides, mmol/L | 1.31 ± 0.53 | 1.30 ± 0.72 | 0.905 |
| Total cholesterol, mmol/L | 4.09 ± 1.08 | 4.30 ± 0.94 | 0.130 |
| Aspirin | 127 (100%) | 94 (97.9%) | 0.184 |
| Clopidogrel | 117 (92.1%) | 91 (94.8%) | 0.674 |
| Statin | 127 (100%) | 92 (95.8%) | 0.395 |
| β-blocker | 82 (64.6%) | 59 (61.4%) | 0.737 |
| ACEI/ARB | 60 (47.2%) | 49 (51.0%) | 0.670 |
| Lesion' length, mm | 12.39 ± 3.06 | 12.08 ± 2.76 | 0.436 |
| MLD, mm | 1.86 ± 0.45 | 1.81 ± 0.48 | 0.426 |
| Percent diameter obstruction | 34.21% ± 11.24% | 35.83% ± 11.01% | 0.284 |
| MLA, mm2 | 3.37 ± 2.18 | 3.41 ± 2.55 | 0.900 |
| Percent area obstruction | 41.66% ± 18.54% | 42.45% ± 17.20% | 0.746 |
| Plaque volume, mm3 | 21.37 ± 16.60 | 20.06 ± 15.70 | 0.551 |
Data are expressed as the mean ± SD or n (%). *P < 0.05. ACEI: angiotensin-converting enzyme inhibitors; AMI: acute myocardial infarction; ARB: angiotensin receptor blocker; BMI: body mass index; BNP: brain natriuretic peptide; CRP: C-reactive protein; HDL: high-density lipoprotein cholesterol; H-Hcy: homocysteine ≥ 15 mmol/L; LDL: Low-density lipoprotein cholesterol; L-Hcy: homocysteine < 15 mmol/L; MLA: minimum lumen area; MLD: minimum lumen diameter.
At the 12 month angiography follow-up, NCCL progression was observed in 41 patients (32.3%) with baseline Hcy concentrations above 15 mmol/L and in 14 patients (14.6%) with Hcy concentrations below 15 mmol/L (P = 0.002).
3.2. No significant relationships exist between STEMI and progression of NCCL or plasma level of Hcy
Thirteen patients suffered from AMI in the H-Hcy group, while nine patients suffered from AMI in the L-Hcy group, and these results were not significantly different (10.2 vs. 9.4%, P = 0.768); Similarly, there were five patients with AMI in the progression group and 16 patients with AMI in the non-progression group, which still showed no significant difference (9.1 vs. 10.1%, P = 0.946).
3.3. Blood Hcy level is an independent risk factor for NCCL progression
We used the accumulated hazard curve derived from the Kaplan-Meier analysis, and it showed that there is a significant difference in the rates of NCCL progression between the H-Hcy group and the L-Hcy group (P < 0.001 by the log-rank test) (Figure 2). The multivariate Cox regression analysis (step-wise) including plasma level of Hcy, age, sex, BMI, SBP, DBP, serum lipids, blood glucose, smoking, drinking, hypertension, and diabetes mellitus revealed that Hcy [P = 0.007, hazard ratio (HR): 1.024, 95% CI: 1.007–1.042) and the history of diabetes mellitus (P = 0.013, HR: 1.992, 95% CI: 1.15–3.44) were the independent risk factors of NCCL progression (Table 3), while there were no significant differences in sex (P = 0.805), smoking (P = 0.389), and total cholesterol (P = 0.052).
Figure 2. The Kaplan-Meier curves of NCCL progression-free survivors in the groups separated based on the level of Hcy.
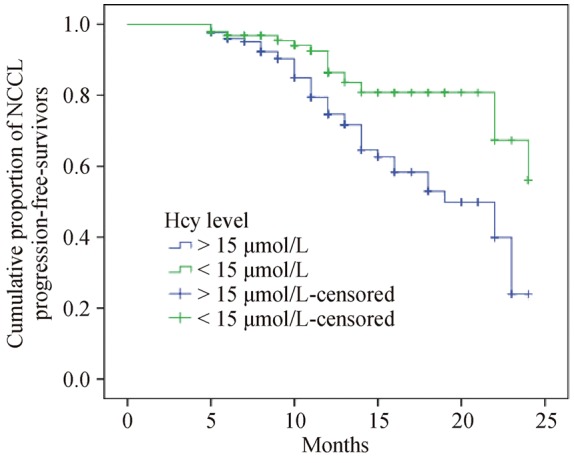
Log rank: P < 0.001. Hcy: homocysteine; NCCL: non-culprit coronary lesion.
Table 3. Association of Hcy and diabetes mellitus with progression of NCCL.
| H-Hcy (n = 127) | L-Hcy (n = 96) | Hcy |
Diabetes mellitus |
|||
| Crude HR (95% CI) | Adjusted HR (95% CI) | Crude HR (95% CI) | Adjusted HR (95% CI) | |||
| Progression | 41 (74.5%) | 14 (25.5%) | 1.056 (1.01–1.104) | 1.024 (1.007–1.042) | 1.855 (1.078–3.191) | 1.992 (1.15–3.44) |
| No progression | 86 (51.2%) | 82 (48.8%) | P = 0.015 | P = 0.007 | P = 0.026 | P = 0.013 |
Data are presented as n (%) unless other indicated. Crude HR and adjusted HR were obtained after controlling for age, sex, BMI, SBP, DBP, serum lipids, fasting blood glucose, smoking, drinking, hypertension, homocysteine, diabetes mellitus and lesion characteristics of NCCL by Cox proportional hazards regression analysis. BMI: body mass index; DBP: diastolic blood pressure; Hcy: homocysteine; H-Hcy: homocysteine ≥ 15 mmol/L; HR: hazard ratio; L-Hcy: homocysteine < 15 mmol/L; NCCL: non-culprit coronary lesion; SBP: systolic blood pressure.
Table 2. Angiography and preexisting stenosis data examined in different groups.
| Characteristics | H-Hcy (n = 127) | Normal Hcy (n = 96) | P value |
| Angiography | |||
| Multivessel disease | 92 (72.4%) | 73 (76%) | 0.213 |
| Non-culprit coronary lesion | |||
| Preexisting stenosis ≥ 50% | 53 (41.7%) | 41 (42.7%) | 0.096 |
| Preexisting stenosis < 50% | 39 (30.7%) | 32 (33.3%) | 0.143 |
| Normal | 35 (27.6%) | 23 (24%) | 0.328 |
Data are expressed as n (%). Hcy: homocysteine; H-Hcy: homocysteine ≥ 15 mmol/L.
4. Discussion
Our study demonstrates that Hcy can be an independent risk factor for the progression of NCCL in elderly patients. We evaluated the association between the levels of Hcy with the progressing degree of plaques of non-culprit arteries during the interval and the follow-up. Hcy is a type of amino acid that contains sulfur and is produced by methionine metabolism. Studies show that mild elevation of Hcy level in the range of 15–25 µmol/L is common in populations,[11] and it has been shown to be correlated with various diseases such as coronary artery disease, stroke, peripheral artery stenosis and venous thrombosis.[12]–[15] Furthermore, it has been suggested that the risk of coronary artery disease would be increased by 60% for men and 80% for women with an elevation of 5 µmol/L in plasma Hcy.[16] Although mounting evidence shows that elevated Hcy levels can predict the outcomes of patients with atherosclerosis or restenosis and can increase the risk of restenosis in patients undergoing PCI,[17] there are equal amounts of contradictory results that dispute the detrimental ability of HCY.[18]–[21] Its potential role as a marker or as a true cardiovascular risk factor is still up for debate, and its relationship with the progression of plaque after PCI, in particular, is confusing as well. In the present study, we thoroughly investigated, for the first time, the possible role of Hcy in the occurrence of progression of NCCL in elderly patients with ACS after successful coronary stenting.
Studies have shown that the progression of plaques could induce various symptoms such as pectoris and may result in restenosis or total myocardial infarction, which all put the patients at risk. The progression of plaques after percutaneous coronary interventions is a process caused by many factors including the formation of neointima, endothelial injury, smooth muscle cell proliferation, and oxidative stress, among others.[22] Several experimental studies have laid down a theoretical foundation for the possible mechanisms by which hyper-Hcy could lead to atherothrombotic disease and progression of coronary plaque.[23]–[26] First, it helps leukocyte recruitment via upregulation of monocyte chemoattractant protein-1 and interleukin-8 expression and secretion.[27] Second, it promotes the migration and proliferation of vascular smooth muscle cells, which plays a crucial role in the progression of coronary plaque.[28],[29] Finally, platelet accumulation and oxidative stress are strengthened when the level of Hcy is elevated.[30],[31] Taken together, hyper-Hcy accelerates endothelial dysfunction via downregulation of endothelia nitric oxide synthase and overexpression of reactive oxygen species, which exacerbates endoplasmic reticulum stress as well.
In this study, we focused on elderly people who underwent percutaneous intervention twice in our department. Because elderly people are more susceptible to more complex lesions, worse physical conditions, poorer prognoses and higher rates of mortality, it is necessary to determine risk factors that likely influence the progression of coronary disease. De Ruijter, et al.[32] have shown that the association of Hcy level with cardiovascular disease appears to be strong, and it outperforms the Framingham risk score and other classic risk factors in the ability to predict the risk of cardiovascular mortality in elderly people with no history of cardiovascular disease. Furthermore, the predictive abilities of the classic risk factors such as hypercholesterolemia and hypertension seem to weaken with age.[33] Fu, et al.[34] also found that elevated Hcy was an independent predictor of long-term mortality and major adverse cardiovascular events for elderly people with ACS. Our results are consistent with the above outcomes. Although we did not evaluate the final long-term outcome of old people with coronary disease, we paid attention to the development process, which allows us to prevent the adverse accidents occurring at the early phase. The underlying mechanisms may be attributed to the fact that older people suffer more severe oxidative stress and more accumulation of reactive oxygen species,[35],[36] which are all related to the elevated level of blood Hcy.
However, there are also many studies disputing the predictive value of Hcy. Kosokabe, et al.[37] explored the relationship between MTHFR genotypes, levels of Hcy and restenosis. Study showed that MTHFR genotypes have an influence on neointimal hyperplasia, while levels of Hcy have none. Schnyder, et al.[38] also observed that there is no relationship between Hcy and restenosis. Moreover, other clinical studies showed a lack of association between Hcy concentrations and prediction of restenosis or myocardial infarction prevention.[38]–[40] These results are conflict with our conclusion. However, the enrolled population of our study was different. We focused on elderly people who are more than 65 years old and whose basic physical conditions were worse than the subjects of some previous studies, which may result in different outcomes. What is more, we evaluated the influence of the basic level of plasma Hcy on the progression of NCCL, and we aimed to evaluate the culprit lesion instead of observing the occurrence of restenosis rate.
Recently, non-culprit coronary lesions have drawn much attention. It has been shown that dealing with the non-culprit lesions is associated with a lower 5-year mortality compared with the culprit-only PCI strategy in STEMI patients with multi-vessel vascular disease.[41] It is reasonable to hypothesize the non-culprit lesions play a significant role in acute accidents. Moreover, the non-culprit plaques are more vulnerable in ACS patients than in patients with stable angina pectoris.[42] Because the non-culprit lesions influence the prognosis of patients and may be responsible for the recurrence of acute accidents because of the vulnerability of the plaques, searching for the related risk factors has great significance. Our study focused on many risk factors including diabetes mellitus, smoking, hypertension, hyper-Hcy, and lipid levels via multivariate Cox analysis. We found that the level of Hcy and the history of diabetes mellitus can be independent risk factors for the progression of non-culprit lesions. Thus, we expect that a cure targeted at lowering hyper-Hcy will be effective for the prevention of non-culprit lesion progression.
4.1. Conclusions
Our study suggests that a high plasma level of Hcy is associated with a higher incidence of the progression of NCCL in elderly patients with ACS. Although diabetes mellitus also plays a significant role in the progression of NCCL, a high plasma level of Hcy is still an independent risk factor. Determining the concentrations of Hcy at the time of the acute event may allow for the selection of patients at higher risk for future new events.
4.2. Limitations
Our study was an observational, retrospective, and single-center study, which has some limitations. First, we included only patients undergoing PCI for culprit lesions. Second, we did not evaluate any Hcy-related chemokines or cytokines, which may play roles in promoting the increased level of Hcy. Third, due to the lack of follow-up angiography of a majority of patients in our center, the selection bias may have exaggerated the current findings. In conclusion, a further prospective study with larger cohorts is needed.
References
- 1.Mauri L. Nonculprit lesions–or guilty by association. N Engl J Med. 2013;369:1166–1167. doi: 10.1056/NEJMe1309383. [DOI] [PubMed] [Google Scholar]
- 2.Cutlip DE, Chhabra AG, Baim DS, et al. Beyond restenosis: five-year clinical outcomes from second-generation coronary stent trials. Circulation. 2004;110:1226–1230. doi: 10.1161/01.CIR.0000140721.27004.4B. [DOI] [PubMed] [Google Scholar]
- 3.McCully KS. Homocysteine and the pathogenesis of atherosclerosis. Expert Rev Clin Pharmacol. 2015;8:211–219. doi: 10.1586/17512433.2015.1010516. [DOI] [PubMed] [Google Scholar]
- 4.Sun Q, Jia X, Gao J, et al. Association of serum homocysteine levels with the severity and calcification of coronary atherosclerotic plaques detected by coronary CT angiography. Int Angiol. 2014;33:316–323. [PubMed] [Google Scholar]
- 5.van Oijen MG, Claessen BE, Clappers N, et al. Prognostic value of free plasma homocysteine levels in patients hospitalized with acute coronary syndrome. Am J Cardiol. 2008;102:135–139. doi: 10.1016/j.amjcard.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Wang G, Mao JM, Wang X, et al. Effect of homocysteine on plaque formation and oxidative stress in patients with acute coronary syndromes. Chin Med J (Engl) 2004;117:1650–1654. [PubMed] [Google Scholar]
- 7.Chen Z, Li CS, Zhang J, et al. Relationship between endothelial dysfunction and serum homocysteine in patients with coronary lesions. Chin Med Sci J. 2005;20:63–66. [PubMed] [Google Scholar]
- 8.Han YF, Jing J, Tu SX, et al. ST elevation acute myocardial infarction accelerates non-culprit coronary lesion atherosclerosis. Int J Cardiovasc Imaging. 2014;30:253–261. doi: 10.1007/s10554-013-0354-z. [DOI] [PubMed] [Google Scholar]
- 9.Tu S, Xu L, Ligthart J, et al. In vivo comparison of arterial lumen dimensions assessed by co-registered three-dimensional (3D) quantitative coronary angiography, intravascular ultrasound and optical coherence tomography. Int J Cardiovasc Imaging. 2012;28:1315–1327. doi: 10.1007/s10554-012-0016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zouridakis E, Avanzas P, Arroyo-Espliguero R, et al. Markers of inflammation and rapid coronary artery disease progression in patients with stable angina pectoris. Circulation. 2004;110:1747–1753. doi: 10.1161/01.CIR.0000142664.18739.92. [DOI] [PubMed] [Google Scholar]
- 11.Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 12.Arnesen E, Refsum H, Bonaa KH, et al. Serum total homocysteine and coronary heart disease. Int J Epidemiol. 1995;24:704–709. doi: 10.1093/ije/24.4.704. [DOI] [PubMed] [Google Scholar]
- 13.Collaboration HS. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 14.Graham IM, Daly LE, Refsum HM, et al. Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project. JAMA. 1997;277:1775–1781. doi: 10.1001/jama.1997.03540460039030. [DOI] [PubMed] [Google Scholar]
- 15.Cattaneo M. Hyperhomocysteinemia and venous thromboembolism. Semin Thromb Hemost. 2006;32:716–723. doi: 10.1055/s-2006-951456. [DOI] [PubMed] [Google Scholar]
- 16.Boushey CJ, Beresford SA, Omenn GS, et al. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 17.Stubbs PJ, Al-Obaidi MK, Conroy RM, et al. Effect of plasma homocysteine concentration on early and late events in patients with acute coronary syndromes. Circulation. 2000;102:605–610. doi: 10.1161/01.cir.102.6.605. [DOI] [PubMed] [Google Scholar]
- 18.Schnyder G, Flammer Y, Roffi M, et al. Plasma homocysteine levels and late outcome after coronary angioplasty. J Am Coll Cardiol. 2002;40:1769–1776. doi: 10.1016/s0735-1097(02)02481-6. [DOI] [PubMed] [Google Scholar]
- 19.Morita H, Kurihara H, Kuwaki T, et al. Homocysteine as a risk factor for restenosis after coronary angioplasty. Thromb Haemost. 2000;84:27–31. [PubMed] [Google Scholar]
- 20.Hodish I, Matetzky S, Sela BA, et al. Effect of elevated homocysteine levels on clinical restenosis following percutaneous coronary intervention. Cardiology. 2002;97:214–217. doi: 10.1159/000063113. [DOI] [PubMed] [Google Scholar]
- 21.Ortolani P, Marzocchi A, Marrozzini C, et al. Clinical relevance of homocysteine levels in patients receiving coronary stenting for unstable angina. Ital Heart J. 2004;5:189–196. [PubMed] [Google Scholar]
- 22.Koch W, Ndrepepa G, Mehilli J, et al. Homocysteine status and polymorphisms of methylenetetrahydrofolate reductase are not associated with restenosis after stenting in coronary arteries. Arterioscler Thromb Vasc Biol. 2003;23:2229–2234. doi: 10.1161/01.ATV.0000105055.68038.29. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Chen J, Li YS, et al. Folic acid reduces adhesion molecules VCAM-1 expession in aortic of rats with hyperhomocysteinemia. Int J Cardiol. 2006;106:285–288. doi: 10.1016/j.ijcard.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Gruntzig Lecture ESC 2014. Eur Heart J. 2015;36:3320–3331. doi: 10.1093/eurheartj/ehv511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Luca G, Suryapranata H, Gregorio G, et al. Homocysteine and its effects on in-stent restenosis. Circulation. 2005;112:e307–e311. doi: 10.1161/CIRCULATIONAHA.105.573923. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Cui L, Joseph J, et al. Homocysteine induces cardiomyocyte dysfunction and apoptosis through p38 MAPK-mediated increase in oxidant stress. J Mol Cell Cardiol. 2012;52:753–760. doi: 10.1016/j.yjmcc.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang XC, Sun WT, Yu CM, et al. ER stress mediates homocysteine-induced endothelial dysfunction: Modulation of IKCa and SKCa channels. Atherosclerosis. 2015;242:191–198. doi: 10.1016/j.atherosclerosis.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 28.Poddar R, Sivasubramanian N, DiBello PM, et al. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular disease. Circulation. 2001;103:2717–2723. doi: 10.1161/01.cir.103.22.2717. [DOI] [PubMed] [Google Scholar]
- 29.Majors A, Ehrhart LA, Pezacka EH. Homocysteine as a risk factor for vascular disease. Enhanced collagen production and accumulation by smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997;17:2074–2081. doi: 10.1161/01.atv.17.10.2074. [DOI] [PubMed] [Google Scholar]
- 30.Jiang C, Zhang H, Zhang W, et al. Homocysteine promotes vascular smooth muscle cell migration by induction of the adipokine resistin. Am J Physiol Cell Physiol. 2009;297:C1466–C1476. doi: 10.1152/ajpcell.00304.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coppola A, Davi G, De Stefano V, et al. Homocysteine, coagulation, platelet function, and thrombosis. Semin Thromb Hemost. 2000;26:243–254. doi: 10.1055/s-2000-8469. [DOI] [PubMed] [Google Scholar]
- 32.De Ruijter W, Westendorp RG, Assendelft WJ, et al. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ. 2009;338:a3083. doi: 10.1136/bmj.a3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guilland JC, Favier A, Potier de Courcy G, et al. Hyperhomocysteinemia: an independent risk factor or a simple marker of vascular disease? Pathol Biol (Paris) 2003;51:101–110. doi: 10.1016/s0369-8114(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 34.Fu Z, Qian G, Xue H, et al. Hyperhomocysteinemia is an independent predictor of long-term clinical outcomes in Chinese octogenarians with acute coronary syndrome. Clin Interv Aging. 2015;10:1467–1474. doi: 10.2147/CIA.S91652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikhed Y, Daiber A, Steven S. Mitochondrial oxidative stress, mitochondrial DNA damage and their role in age-related vascular dysfunction. Int J Mol Sci. 2015;16:15918–15953. doi: 10.3390/ijms160715918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camici GG, Savarese G, Akhmedov A, et al. Molecular mechanism of endothelial and vascular aging: implications for cardiovascular disease. Eur Heart J. 2015;36:3392–3403. doi: 10.1093/eurheartj/ehv587. [DOI] [PubMed] [Google Scholar]
- 37.Kosokabe T, Okumura K, Sone T, et al. Relation of a common methylenetetrahydrofolate reductase mutation and plasma homocysteine with intimal hyperplasia after coronary stenting. Circulation. 2001;103:2048–2054. doi: 10.1161/01.cir.103.16.2048. [DOI] [PubMed] [Google Scholar]
- 38.Schnyder G, Roffi M, Flammer Y, et al. Association of plasma homocysteine with restenosis after percutaneous coronary angioplasty. Eur Heart J. 2002;23:726–733. doi: 10.1053/euhj.2001.2962. [DOI] [PubMed] [Google Scholar]
- 39.Marti-Carvajal AJ, Sola I, Lathyris D, et al. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2013;1:CD006612. doi: 10.1002/14651858.CD006612.pub4. [DOI] [PubMed] [Google Scholar]
- 40.Wong CK, Hammett CJ, The R, et al. Lack of association between baseline plasma homocysteine concentrations and restenosis rates after a first elective percutaneous coronary intervention without stenting. Heart. 2004;90:1299–1302. doi: 10.1136/hrt.2003.020701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toyota T, Shiomi H, Taniguchi T, et al. Culprit vessel-only vs. staged multivessel percutaneous coronary intervention strategies in patients with multivessel coronary artery disease undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Circ J. 2016;80:371–378. doi: 10.1253/circj.CJ-15-0493. [DOI] [PubMed] [Google Scholar]
- 42.Sudo M, Hiro T, Takayama T, et al. Tissue characteristics of non-culprit plaque in patients with acute coronary syndrome vs. stable angina: a color-coded intravascular ultrasound study. Cardiovasc Interv Ther. 2016;31:42–50. doi: 10.1007/s12928-015-0345-1. [DOI] [PubMed] [Google Scholar]