Abstract
Breast cancer (BC) is diagnosed in ≥ 65 year old women in about half of cases. Experts currently recommend that systemic therapy is offered to elderly patients with BC, if, based on their overall conditions and life expectancy, it can be reasonably anticipated that the benefits will outweigh the risks of treatment. Like for young subjects, the monoclonal antibody against human epidermal growth factor receptor-2 (HER-2), trastuzumab, represents a valid therapeutic option when BC over-expresses this receptor. Unfortunately, administration of trastuzumab is associated with the occurrence of left ventricular dysfunction and chronic heart failure (CHF), possibly because of interference with the homeostatic functions of HER-2 in the heart. Registry-based, retrospective analyses have reported an incidence of CHF around 25% in elderly women receiving trastuzumab compared with 10%–15% in those not given any therapy for BC, and the risk of CHF has been estimated to be two-fold higher in > 60–65 year old trastuzumab users vs. non-users. Extremely advanced age and preexisting cardiac disease have been shown to predispose to trastuzumab cardiotoxicity. Therefore, selection of older patients for treatment with trastuzumab should be primarily based on their general status and the presence of comorbidities; previous chemotherapy, especially with anthracyclines, should be also taken into account. Once therapy has started, efforts should be made to ensure regular cardiac surveillance. The role of selected biomarkers, such as cardiac troponin, or new imaging techniques (three-dimension, tissue Doppler echocardiography, magnetic resonance imaging) is promising, but must be further investigated especially in the elderly. Moreover, additional studies are needed in order to better understand the mechanisms by which trastuzumab affects the old heart.
Keywords: Cardiotoxicity, Elderly, Heart failure, HER-2, Trastuzumab
1. Introduction
Dramatic improvements in socioeconomic conditions and health care resources have led to a substantial increase in life expectancy in industrialized countries, which are nowadays largely composed by elderly people. This demographical change has also impacted the medical field, and old patients have become common in any setting, including cardiology and oncology. This is especially the case with breast cancer (BC): according to the latest statistics, around half of BC are diagnosed in women aged 65 years or more.[1]
BC of the elderly most often displays favorable biological characteristics, i.e., luminal molecular subtype, presence of hormone receptors (HR), low mitotic rate and nuclear grade, absence of p53 mutations, and no overexpression of epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor-2 (HER-2).[2]–[5] However, these features have not historically translated into better outcomes in older vs. younger patients: in fact, mortality is higher in the former than in the latter ones.[6] Various reasons have been proposed to explain this paradox, such as competing risks of death from other diseases or the possibility that tumors exhibiting the same markers, but arising at young vs. old age, behave differently.[4],[5] It has also been argued that adherence to treatment guidelines is poor and, particularly, that systemic chemotherapy is frequently not delivered in advanced age because of the concern of toxicity.[7] The fact that elderly subjects have been underrepresented in clinical trials and, thereby, few evidence-based data exist has probably strengthened the tendency to omit chemotherapy in this population.
Nevertheless, in the last years there has been a reappraisal of the balance between benefits and side effects of chemotherapy for old patients with BC. Retrospective analyses have shown that it may be effective and improve prognosis. This conclusion has also been reached by a limited number of dedicated trials of chemotherapy in the elderly and is expected to be confirmed by other ongoing clinical studies.[8]–[10] Hence, it is now recommended not to consider age as an exclusion criterion for cancer treatment as long as survival for a significant period of time is likely and the burden of comorbidity is low.[11] On the other hand, it holds true that toxicity of chemotherapy is enhanced at older ages.[8],[9],[12]
As aforementioned, HER-2 positivity is relatively uncommon in BC of the elderly. Nonetheless, there is already quite a large experience with the use of the HER-2 targeting antibody, trastuzumab, in the geriatric population with HER-2 expressing tumors.[13],[14] At present, trastuzumab may be indicated for old subjects with early stage BC—either HER-2 positive/HR negative or HER-2 positive/HR positive with lymph node invasion—or HER-2 positive metastatic BC.[11] The current opinion that adjuvant systemic therapy should be systematically considered for all eligible patients, regardless of age, is likely to foster the prescription of trastuzumab in the elderly in the next future. Cardiotoxicity has been, and will remain, the main safety issue of this treatment.[15]
2. Search strategy
PubMed and Embase were searched for articles written in English and including the following words in the title and/or abstract: trastuzumab, HER-2, ErbB-2, breast cancer, elderly, old.
3. Mode of action of trastuzumab and pathogenesis of its cardiotoxicity
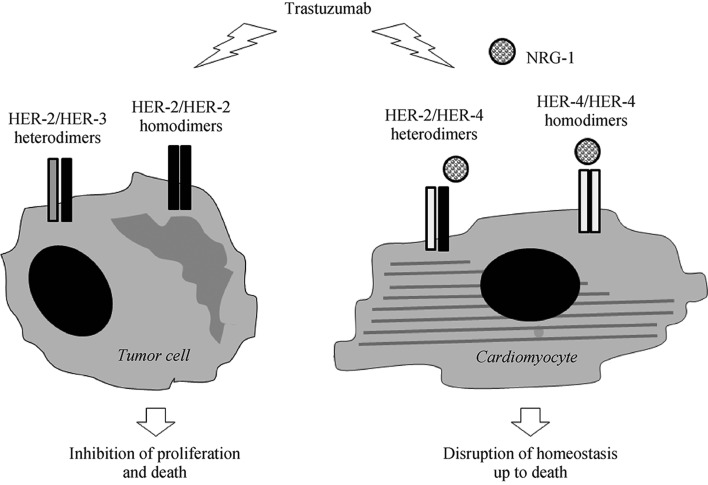
HER-2 (ErbB2 in rodents) is a membrane tyrosine kinase receptor belonging to the EGFR family, which also comprises HER-1 (also known as EGFR), HER-3, and HER-4 (ErbB1, ErbB3, and ErbB4 in rodents). No ligand is known for HER-2. In normal tissues, ligand-stimulated EGFR, HER-3,and HER-4 form homodimers (a pair of the same receptors, e.g., EGFR/EGFR) or combine with HER-2 in heterodimers, and elicit a number of physiological cellular responses. In 20%–30% of BC, HER-2 is overexpressed and instead capable of undergoing ligand-independent homodimerization and intracellular signal transduction; moreover, the formation of heterodimers with HER-1 and HER-3 is facilitated. This leads to the activation of signaling pathways promoting proliferation and survival of tumor cells (Figure 1). As a consequence, BC with amplified HER-2 carries a dismal prognosis.[16],[17] On the other hand, the existence of a subset of BC with high membrane levels of HER-2 has allowed the development of HER-2 directed therapies.[18] Trastuzumab was the first drug in this class.[19] It is a humanized monoclonal antibody against HER-2 that binds to subdomain IV of the extracellular domain of the receptor, halting the growth and causing the death of cancer cells likely through a number of mechanisms, such as induction of antibody-dependent immune cell-mediated cytotoxicity,[20] disruption of ligand-independent HER-2/HER-3 interaction and downstream signaling,[21] and enhanced activity of cell cycle inhibitor, p27.[22] Another FDA approved anti-HER-2 humanized monoclonal antibody is pertuzumab, which also targets the extracellular portion of HER-2, but at a different epitope in subdomain II.[23] Like trastuzumab, pertuzumab triggers antibody-dependent cellular cytotoxicity; in addition—and differently than trastuzumab—it prevents ligand-initiated heterodimerization of HER-2 and other HER family members, especially HER-3.[24] Since trastuzumab and pertuzumab recognize different sites of HER-2 and act in a complementary way, their combination results in greater antitumor efficacy.[25]
Figure 1. Schematic representation of the NRG-1/HER-2 paradigm for trastuzumab cardiotoxicity.
In oncology, trastuzumab is used to treat breast cancer in which HER-2 is overexpressed and spontaneously homodimerizes or forms heterodimers with other HER receptors, especially HER-3. As this ligand-independent activation of HER-2 sustains tumor growth and survival, trastuzumab halts the proliferation and causes the death of tumor cells (left side). In the heart, HER-2 functions as a dimerization partner of HER-4 after this latter is recruited by NRG-1 and regulates homeostatic cell responses. Off-target inhibition of cardiac HER-2 results in the disruption of part of NRG-1 dependent signaling and, eventually, in alterations of structure and function that may be lethal to cardiomyocytes (right side). HER: human epidermal growth factor receptor; NRG-1: neuregulin.
Small-molecule tyrosine kinase inhibitors constitute a second group of anti-HER-2 agents. The prototype, which has already entered clinical practice, is lapatinib, a dual blocker of both constitutive and ligand-induced EGFR and HER-2 phosphorylation and signaling.[26] Notably, lapatinib retains effectiveness in BC that has become resistant to trastuzumab.[27] Other HER tyrosine kinase inhibitors have been evaluated in early-phase clinical studies and are now in phase III trials.[28]
As soon as trastuzumab was tested in patients, left ventricular (LV) dysfunction turned out to be the main side effect of this drug and the leading cause of its discontinuation.[29] Cardiotoxicity secondary to exposure to trastuzumab is dose-independent and, thus, relatively unpredictable, and may culminate in overt congestive heart failure (CHF). On the other hand, it is reversible most of the time.[15],[29] It has been reported that, after resolution of cardiac complications that have arisen following a first administration of trastuzumab, rechallenge with the drug may be safely attempted while maintaining standard medications for CHF.[30] Chemotherapy with anthracyclines, which also have long been known to be cardiotoxic, predisposes to trastuzumab cardiac side effects, whose incidence is highest when anthracyclines and trastuzumab are given at the same time and decreases as the interval between anthracycline and trastuzumab infusion extends.[31] Surprisingly, the addition of pertuzumab to trastuzumab does not seem to raise the risk of LV dysfunction and CHF.[32],[33] As far as lapatinib is concerned, the data collected so far about its cardiac safety are quite reassuring.[34]
The exact mechanism of trastuzumab cardiotoxicity is yet to be pinpointed. Nonetheless, blockade of the action of neuregulin-1 (NRG-1), the ligand of ErbB4/HER-4, within the heart is believed to play a major role.[35] Studies based on animal models and primary cell cultures have revealed that NRG-1-induced ErbB4/ErbB4 homodimers and ErbB4/ErbB2 heterodimers mediate protective responses of cardiomyocytes to stress, including the one provoked by anthracyclines.[36] It is therefore assumed that trastuzumab and other ErbB2 antagonists make the myocardium more vulnerable to the damage caused by anthracyclines—and, in principle, by other noxious factors—as they impede the formation of NRG-1-triggered ErbB4/ErbB2 heterodimers (Figure 1). Indeed, cardiomyocytes of transgenic mice in which ErbB2 expression is deleted specifically in the heart are more susceptible to anthracycline toxicity than wild-type cells,[37] and treatment of rat ventricular myocytes with an antibody against ErbB2 significantly worsens myofibrillar disarray due to incubation with anthracycline.[38] This is likely to happen also in humans, but it still has to be confirmed. In fact, the few data available about the expression of HER-2 in human hearts after anthracycline chemotherapy are discordant.[39],[40] Interestingly, a downregulation of HER-2 has been observed in the cardiac tissue of mice given trastuzumab, but not in cancer cells.[41]–[43]
Some authors have pointed out that there is a discrepancy between the predicted pharmacodynamics of anti-HER-2 agents and the reported rates of cardiotoxicity.[44] Trastuzumab should theoretically inhibit only the constitutive activity of tumor HER-2 and not affect HER-2 in the heart, where HER-2 is assumed to function exclusively as a dimerization partner of ligand-occupied HER-4; clearly, this is not true. By contrast, off-target effects on the heart are anticipated for lapatinib, as it inhibits both ligand-independent and ligand-dependent signaling of HER-2, but they seem to be actually less frequent than with trastuzumab. This inconsistency might be due to differences in recruitment criteria between trastuzumab and lapatinib clinical trials, as well as to incomplete blockade of HER-2 and/or activation of rescue pathways by lapatinib.[44] Nevertheless, it sheds doubts on the validity of the NRG-1/HER-2 paradigm to explain the cardiotoxicity of HER-2 antagonists.
Trastuzumab cardiotoxicity is commonly viewed as transient, since it has no or only minor histological correlates and most often resolves after treatment withdrawal.[15],[29] However, there are patients in whom LV function does not recover. This may happen because trastuzumab causes the death of a substantial proportion of cardiomyocytes, as suggested by the finding that plasma concentrations of troponin I above 0.08 ng/mL after anthracyclines and trastuzumab therapy are associated with an almost three fold higher risk of suffering from a sustained reduction in LV ejection fraction (EF).[45] Alternatively, impairment of cardiac progenitor cells may be the substrate for long-lasting trastuzumab cardiotoxicity. In the last decade, apoptosis and senescence—i.e., inhibition of proliferation—of the endogenous pool of cardiac progenitor cells have been described as pivotal in the pathogenesis of persisting anthracycline-induced cardiomyopathy.[35],[46] Cardiac progenitor cells ensure an intrinsic, although limited capability of the heart to regenerate following injury. Therefore, their depletion may render the myocardium prone to accumulate minor lesions until symptoms appear.[47] Interestingly, trastuzumab has recently been shown to hinder the cardiomyogenic and angiogenic capacities of cardiac explants enriched for progenitor cells.[48]
4. Features of trastuzumab cardiotoxicity in elderly patients
4.1. Frequency
In the pivotal trial evaluating standard chemotherapy with or without the addition of trastuzumab for metastatic BC, NYHA class III or IV cardiac dysfunction was recorded in 16% of patients receiving anthracycline, cyclophosphamide, and trastuzumab vs. 3% of those treated only with anthracycline and cyclophosphamide, and in 2% of women given paclitaxel and trastuzumab vs. 1% of those assigned to paclitaxel alone.[49] Remarkably, no cardiac safety criteria were applied during enrollment.
In subsequent trastuzumab trials, the incidence of cardiac side effects was smaller, probably because study design and patient selection were different. For instance, the National Surgical Adjuvant Breast and Bowel Project trial (NSABP) B-31, comparing anthracycline and cyclophosphamide followed by paclitaxel with anthracycline and cyclophosphamide followed by trastuzumab and paclitaxel, recruited subjects with node-positive, HER-2-positive BC and normal post-chemotherapy LVEF on multiple-gated acquisition (MUGA) scan. Source documents were reviewed by a panel of cardiologists for: (1) NYHA III or IV CHF with either a drop in LVEF of more than 10% to < 55% or a decrease of > 5% to less than the lower limit of normality; (2) possible/probable cardiac death. The cumulative incidence of cardiac events was 4.1% in the trastuzumab group vs. 0.8% in the control one at three years and 4% vs. 1.3% at 7 years.[49],[51] In a joint analysis of the NSABP B-31 trial and of the North Central Cancer Treatment Group (NCCTG) N9831 trial, which also assessed the efficacy and safety of trastuzumab in addition to paclitaxel after anthracycline (doxorubicin) and cyclophosphamide, the rates of CHF in the trastuzumab or control arms were 3.8% vs. 1.3% and 2.3% vs. 0.9%, respectively.[52]
In a meta-analysis of eight major randomized clinical trials of trastuzumab for early and locally advanced BC, in which symptomatic CHF incidence ranged from 0.8% to 14.2% in trastuzumab-treated patients vs. 0.2%–4.1% in control women, the relative risk of CHF associated with use of trastuzumab was 5.11 (90% CI:3.00–8.72).[53] The relative risk of LVEF decline was 1.83 (90% CI: 1.36–2.47), although heterogeneity for this outcome was substantial.[53]
Elderly women participated in these trastuzumab clinical trials only to a minor extent. Keeping the example of NSABP B-31, only 16% of about 1600 evaluable subjects were ≥ 60 year old.[50] Nevertheless, in these studies age was found to predispose to cardiotoxicity of trastuzumab.[51],[54] Real-world data confirm this finding. In an Italian registry in which 32.6% of patients were aged 60 years or more, the cumulative risk of trastuzumab-related cardiotoxicity for those younger or older than 70 years was 1.3% vs. 6.4% after 1 year, 2% vs. 9.8% after 2 years, and 2.2% vs. 9.8% after 3 years, respectively.[14] In another US registry including cases of trastuzumab-treated HER-2 positive metastatic BC, the incidence of grade ≥ 3 LV dysfunction was 4.8%, 2.8%, and 1.5% for subjects ≥ 75, 65-74, or < 65 year old, respectively; and that of CHF 3.2%, 1.9%, and 1.5%, respectively.[55] In a multicentric cohort of German women receiving adjuvant trastuzumab the frequency of a drop in LVEF or CHF was 3.7% in the subgroup with < 65 years, 3.9% in the one between 65 and 69 years, and 5.7% in the one with ≥ 70 years.[56]
Table 1 summarizes the results of a series of retrospective analyses carried out to determine the frequency of trastuzumab cardiotoxicity specifically in cohorts of old women with BC. Overall, these investigations have shown that trastuzumab with or without anthracyclines confers a risk of suffering from CHF that can be roughly quantified as two times the one faced by trastuzumab non-users.[57]–[61] The most robust data come from Medicare records, with hazard ratios of HF from 1.95 (95% CI: 1.75–2.17) to 2.37 (95% CI: 1.76–3.19).[57],[58] The limitations of the retrospective approach and the data source used, which do not allow a strict control of confounding factors, must be acknowledged. On the other hand, these community practice-based observational studies take into account a sizable subset of patients who would have not been eligible for clinical trials of trastuzumab, but are actually treated in real-life.
Table 1. Studies of the incidence of trastuzumab cardiac side effects in elderly patients with breast cancer.
| Ref. No. | Study population | Treatment subgroups | CHF | Asymptomatic LVEF drop |
| 55 | Medicare 1998–2005 45,997 women; ≥ 65 yrs Stage II-IV BC No previous CVD |
TZ: 414 (0.9%) | 24.2%* | NA |
| A/TZ: 460 (1%) | 15.5% | |||
| A: 5979 (13%) | 9.1% | |||
| Non-A: 4922 (10.7%) | 10% | |||
| No therapy: 34,222 (74.4%) | 9.6% | |||
| 56 | Medicare 2005–2009 9535 women; ≥ 66 yrs stage I-III BC No previous CHF |
TZ: 2203 (23.1%) | 29.4% | NA |
| No TZ: 7332 (76.9%) | 18.9% | |||
| 57 | Medicare 2000–2007 45,537 women; ≥ 67 yrs Stage I-III BC |
TZ: 431 (1%) | 26.7%# | NA |
| A/TZ: 431 (1%) | 28.2% | |||
| A: 5257 (11.5%) | 15.3% | |||
| Non-A: 2712 (5.9%) | 17% | |||
| No therapy: 36,700 (80.6%) | 16.9% | |||
| 58 | Single center 45 women; ≥ 70 yrs Stage I-IV BC |
NS | 8.9% | 17.8% |
| 59 | Single center 2005–2010 59 women; ≥ 70 yrs Stage I-IV BC No previous cardiac disease and basal LVEF ≥ 50% |
NS | 1.9% | 10.7% |
Data are presented as n (%). *5-year cumulative incidence; #3-year cumulative incidence. A: anthracycline; A/TZ: anthracycline and trastuzumab; BC: breast cancer; CHF: chronic heart failure; CVD: cardiovascular disease; LVEF: left ventricular ejection fraction: NA: not assessed; Non-A: non-anthracycline chemotherapy; NS: not specified; TZ: trastuzumab.
4.2. Risk factors and management
In the Medicare population, the incidence of trastuzumab-related CHF in old women increased across age categories—i.e., it occured more often in ≥ 80 than > 65 year old women.[58] Additional variables associated with trastuzumab cardiotoxicity in elderly subjects are black race, presence of cardiac comorbidities, such as coronary artery disease, and hypertension.[58] These factors likely operate by independently affecting the heart and making it susceptible to the toxicity of trastuzumab. Recent work, however, has revealed that inappropriate prescription of trastuzumab to frail subjects or without demonstration of HER-2 overexpression also enhances the risk of side effects. A significantly higher rate of cardiac events has been reported in elderly patients who do not complete trastuzumab treatment because of very advanced age and/or too many comorbidities.[62] Based on billing codes in Medicare claims between 2000 and 2010, close to 5% of ≥ 65 years old women given trastuzumab did not receive prior HER-2 testing; they had similar survival to those who received chemotherapy and no trastuzumab, but experienced more often CHF.[63]
There is general consensus that cardiac function should be monitored during trastuzumab treatment and that a reduction of LVEF of at least 10% to < 55% is clinically relevant, even if asymptomatic.[64] LVEF has mainly been evaluated by conventional two-dimensional (2D) transthoracic echocardiography or radionuclide ventriculography (MUGA scans), which however may miss subtle abnormalities that might allow immediate recognition of trastuzumab cardiotoxicity and institution of a cardioprotective therapy, before cardiac function further declines or symptoms appear. Furthermore, the reliability of LVEF measurement by 2D echocardiography has been questioned. An elegant work by Thavendiranathan and colleagues has shown that the intrinsic variability of 2D EF values over time, secondary to physiological and technical aspects, may be > 0.10, which ironically corresponds to the > 0.10 cutoff defining asymptomatic cardiotoxicity.[65]
Hence, a number of alternative strategies, relying on circulating biomarkers and/or imaging techniques, have been suggested for early and accurate detection of trastuzumab toxicity.[15]
Cardiac troponin may be a good marker of trastuzumab cardiac side effects,[45],[66] although this conclusion has not been reached by all authors.[67] In a multi-center cohort of BC patients undergoing treatment with doxorubicin and trastuzumab, it has been found that myeloperoxidase also predicts cardiotoxicity of these drugs and that the combination of troponin I and myeloperoxidase may offer additive information.[68] This same study and another one have shown no correlation between levels of high-sensitivity C-reactive protein (CRP) and LVEF decline, in contrast with a third smaller investigation in which, instead, high-sensitivity CRP proved to be a valuable marker of trastuzumab-induced cardiotoxicity.[69],[70] B-type natriuretic peptide (BNP) or N-terminal proBNP seem to have no utility either.[66],[67]
Among imaging tools, newer echocardiography methods and cardiac magnetic resonance hold promise for improving the diagnosis of LV remodeling and dysfunction due to trastuzumab. Three-dimensional echocardiography has been suggested to perform better than 2D echocardiography in measuring LVEF and volumes and to be more reproducible over time and between different examiners.[65],[71] In addition, LV strain and tissue Doppler parameters may allow early identification of trastuzumab cardiotoxicity, before LVEF changes.[67],[72] The same goal may be achieved with cardiac magnetic resonance,[73] which may also reveal a peculiar linear pattern of late gadolinium enhancement in the subepicardial region of the LV lateral wall.[67],[74]
However, it must be taken into consideration that the studies addressing the role of the latest echocardiography techniques and cardiac magnetic resonance in trastuzumab cardiomyopathy are still few and small-sized; moreover, the performance and feasibility of these imaging modalities in old and especially very old BC patients have not yet been explored, as well as their cost-effectiveness. In addition, an approach that integrates the use of biomarkers and imaging results to detect chemotherapy cardiotoxicity has been presented,[75] but no data exist as far as trastuzumab is concerned.
Unfortunately, even cardiologic consultation and cardiac monitoring of trastuzumab therapy with standard echocardiography or ventriculography are often disregarded in the elderly. Among over 2,000 Medicare > 65 year old patients, who received adjuvant trastuzumab for stage I-III invasive BC between 2005 and 2009 and did not have history of CHF, baseline cardiac evaluation was performed in 78.8% and subsequent follow-up only in 42.6% (32% of the entire cohort).[76] Interestingly, after adjustment for multiple confounders, a positive association was found between being monitored for cardiac safety over time and being diagnosed in more recent years or being referred to a physician of female gender or graduated after 1990, highlighting that awareness of trastuzumab cardiotoxicity, or anyway attention to it, is not the same in the entire medical community.
Besides trastuzumab discontinuation, initiation of beta-blocker and ACE inhibitor is recommended if cardiotoxicity develops.[15],[29],[35] Even though these drugs may favorably influence the cardiac HER system,[77] their use is at present empirical and prompted by the knowledge of their positive effects on cardiac remodeling and on the maladaptive neurohormonal responses occurring in CHF of any etiology. The ongoing Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research Trial (MANTICORE 101—Breast) is evaluating the effectiveness of perindopril or bisoprolol in primary prevention of trastuzumab-induced modifications in LV geometry.[78] Since the LV will be evaluated by magnetic resonance imaging, important information about the role of this latter in the cardiologic follow-up after trastuzumab therapy will be also obtained.
5. Conclusions
Trastuzumab therapy carries a significant risk of cardiotoxicity in the elderly like at a younger age, with an incidence of CHF in trastuzumab recipients that may be twice as high as it is in women not given trastuzumab.
Careful selection of patients is mandatory to avoid unnecessary prescription of trastuzumab and, therefore, unjustified risk of cardiac side effects. Treatment should be offered only to subjects who can be reasonably assumed to be able to complete it, keeping in mind that extremely advanced age and preexisting cardiac disease predispose to trastuzumab cardiotoxicity. Efforts should be then made to ensure regular cardiac surveillance.
Future studies are needed to determine whether selected biomarkers or new imaging techniques may be employed in the geriatric population for timely detection and treatment of trastuzumab cardiac side effects. At the preclinical level, a better understanding of the mechanisms by which trastuzumab specifically affects the old heart is warranted.
References
- 1.DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Eppenberger-Castori S, Moore DH, Jr, Thor AD, et al. Age-associated biomarker profiles of human breast cancer. Int J Biochem Cell Biol. 2002;34:1318–1330. doi: 10.1016/s1357-2725(02)00052-3. [DOI] [PubMed] [Google Scholar]
- 3.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 4.de Kruijf EM, Bastiaannet E, Rubertá F, et al. Comparison of frequencies and prognostic effect of molecular subtypes between young and elderly breast cancer patients. Mol Oncol. 2014;8:1014–1025. doi: 10.1016/j.molonc.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebner F, van Ewijk R, Wöckel A, et al. Tumor biology in older breast cancer patients—what is the impact on survival stratified for guideline adherence? A retrospective multi-centre cohort study of 5378 patients. Breast. 2015;24:256–262. doi: 10.1016/j.breast.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Rosso S, Gondos A, Zanetti R, et al. Up-to-date estimates of breast cancer survival for the years 2000–2004 in 11 European countries: the role of screening and a comparison with data from the United States. Eur J Cancer. 2010;46:3351–3357. doi: 10.1016/j.ejca.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Angarita FA, Chesney T, Elser C, et al. Treatment patterns of elderly breast cancer patients at two Canadian cancer centres. Eur J Surg Oncol. 2015;41:625–634. doi: 10.1016/j.ejso.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 8.Muss HB, Woolf S, Berry D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293:1073–1081. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 9.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early–stage breast cancer. N Engl J Med. 2009;360:2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawaki M, Tokudome N, Mizuno T, et al. Evaluation of trastuzumab without chemotherapy as a post-operative adjuvant therapy in HER2-positive elderly breast cancer patients: randomized controlled trial [RESPECT (N-SAS BC07)] Jpn J Clin Oncol. 2011;41:709–712. doi: 10.1093/jjco/hyr011. [DOI] [PubMed] [Google Scholar]
- 11.Muss HB, Berry DA, Cirrincione C, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. J Clin Oncol. 2007;25:3699–3704. doi: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 12.Tew WP, Muss HB, Kimmick GG, et al. Breast and ovarian cancer in the older woman. J Clin Oncol. 2014;32:2553–2561. doi: 10.1200/JCO.2014.55.3073. [DOI] [PubMed] [Google Scholar]
- 13.Tarantini L, Gori S, Faggiano P, et al. Adjuvant trastuzumab cardiotoxicity in patients over 60 years of age with early breast cancer: a multicenter cohort analysis. Ann Oncol. 2012;23:3058–3063. doi: 10.1093/annonc/mds127. [DOI] [PubMed] [Google Scholar]
- 14.Bonifazi M, Franchi M, Rossi M, et al. Trastuzumab-related cardiotoxicity in early breast cancer: a cohort study. Oncologist. 2013;18:795–801. doi: 10.1634/theoncologist.2013-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tocchetti CG, Ragone G, Coppola C, et al. Detection, monitoring, and management of trastuzumab-induced left ventricular dysfunction: an actual challenge. Eur J Heart Fail. 2012;14:130–137. doi: 10.1093/eurjhf/hfr165. [DOI] [PubMed] [Google Scholar]
- 16.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–178. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 17.Witton CJ, Reeves JR, Going JJ, et al. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200:290–297. doi: 10.1002/path.1370. [DOI] [PubMed] [Google Scholar]
- 18.Brufsky A. Trastuzumab-based therapy for patients with HER2-positive breast cancer: from early scientific development to foundation of care. Am J Clin Oncol. 2010;33:186–195. doi: 10.1097/COC.0b013e318191bfb0. [DOI] [PubMed] [Google Scholar]
- 19.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 20.Arnould L, Gelly M, Penault-Llorca F, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006:9. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junttila TT, Akita RW, Parsons K, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Le XF, Claret FX, Lammayot A, et al. The role of cyclin-dependent kinase inhibitor p27Kip1 in anti-HER2 antibody-induced G1 cell cycle arrest and tumor growth inhibition. J Biol Chem. 2003;278:23441–23450. doi: 10.1074/jbc.M300848200. [DOI] [PubMed] [Google Scholar]
- 23.Lynce F, Swain SM. Pertuzumab for the treatment of breast cancer. Cancer Invest. 2014;32:430–438. doi: 10.3109/07357907.2014.922570. [DOI] [PubMed] [Google Scholar]
- 24.Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 25.Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rusnak DW, Lackey K, Affleck K, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85–94. [PubMed] [Google Scholar]
- 27.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 28.Eroglu Z, Tagawa T, Somlo G. Human epidermal growth factor receptor family-targeted therapies in the treatment of HER2-overexpressing breast cancer. Oncologist. 2014;19:135–150. doi: 10.1634/theoncologist.2013-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suter TM, Cook-Bruns N, Barton C. Cardiotoxicity associated with trastuzumab (Herceptin) therapy in the treatment of metastatic breast cancer. Breast. 2004;13:173–183. doi: 10.1016/j.breast.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Ewer MS, Vooletich MT, Durand JB, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–7826. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 31.Bowles EJ, Wellman R, Feigelson HS, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104:1293–1305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swain SM, Ewer MS, Cortés J, et al. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: a randomized, double-blind, placebo-controlled phase III study. Oncologist. 2013;18:257–264. doi: 10.1634/theoncologist.2012-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24:2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 34.Perez EA, Koehler M, Byrne J, et al. Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc. 2008;83:679–686. doi: 10.4065/83.6.679. [DOI] [PubMed] [Google Scholar]
- 35.Molinaro M, Ameri P, Marone G, et al. Recent advances on pathophysiology, diagnostic and therapeutic insights in cardiac dysfunction induced by antineoplastic drugs. BioMed Res Int. 2015;2015:138148. doi: 10.1155/2015/138148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odiete O, Hill MF, Sawyer DB. Neuregulin in cardiovascular development and disease. Circ Res. 2012;111:1376–1385. doi: 10.1161/CIRCRESAHA.112.267286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crone SA, Zhao YY, Fan L, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 38.Sawyer DB, Zuppinger C, Miller TA, et al. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1β and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105:1551–1554. doi: 10.1161/01.cir.0000013839.41224.1c. [DOI] [PubMed] [Google Scholar]
- 39.Fuchs IB, Landt S, Bueler H, et al. Analysis of HER2 and HER4 in human myocardium to clarify the cardiotoxicity of trastuzumab (Herceptin) Breast Cancer Res Treat. 2003;82:23–28. doi: 10.1023/b:brea.0000003916.39959.73. [DOI] [PubMed] [Google Scholar]
- 40.de Korte MA, de Vries EG, Lub-de Hooge MN, et al. 111Indium-trastuzumab visualises myocardial human epidermal growth factor receptor 2 expression shortly after anthracycline treatment but not during heart failure: a clue to uncover the mechanisms of trastuzumab-related cardiotoxicity. Eur J Cancer. 2007;43:2046–2051. doi: 10.1016/j.ejca.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 41.Riccio G, Esposito G, Leoncini E, et al. Cardiotoxic effects, or lack thereof, of anti-ErbB2 immunoagents. FASEB J. 2009;23:3171–3178. doi: 10.1096/fj.09-131383. [DOI] [PubMed] [Google Scholar]
- 42.Milano G, Raucci A, Scopece A, et al. Doxorubicin and trastuzumab regimen induces biventricular failure in mice. J Am Soc Echocardiogr. 2014;27:568–579. doi: 10.1016/j.echo.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Austin CD, De Mazière AM, Pisacane PI, et al. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell. 2004;15:5268–5282. doi: 10.1091/mbc.E04-07-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Keulenaer GW, Doggen K, Lemmens K. The vulnerability of the heart as a pluricellular paracrine organ: lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ Res. 2010;106:35–46. doi: 10.1161/CIRCRESAHA.109.205906. [DOI] [PubMed] [Google Scholar]
- 45.Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–3916. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 46.Altieri P, Barisione C, Lazzarini E, et al. Testosterone antagonizes doxorubicin-induced senescence of cardiomyocytes. J Am Heart Assoc. 2015;5:pii: e002383. doi: 10.1161/JAHA.115.002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang C, Zhang X, Ramil JM, et al. Juvenile exposure to anthracyclines impairs cardiac progenitor cell function and vascularization resulting in greater susceptibility to stress-induced myocardial injury in adult mice. Circulation. 2010;121:675–683. doi: 10.1161/CIRCULATIONAHA.109.902221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barth AS, Zhang Y, Li T, et al. Functional impairment of human resident cardiac stem cells by the cardiotoxic antineoplastic agent trastuzumab. Stem Cells Transl Med. 2012;1:289–297. doi: 10.5966/sctm.2011-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 50.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23:7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 51.Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node–positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;4:CD006243. doi: 10.1002/14651858.CD006243.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaufman PA, Brufsky AM, Mayer M, et al. Treatment patterns and clinical outcomes in elderly patients with HER2-positive metastatic breast cancer from the registHER observational study. Breast Cancer Res Treat. 2012;135:875–883. doi: 10.1007/s10549-012-2209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dall P, Lenzen G, Göhler T, et al. Trastuzumab in the treatment of elderly patients with early breast cancer: Results from an observational study in Germany. J Geriatr Oncol. 2015;6:462–469. doi: 10.1016/j.jgo.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 57.Du XL, Xia R, Burau K, et al. Cardiac risk associated with the receipt of anthracycline and trastuzumab in a large nationwide cohort of older women with breast cancer, 1998–2005. Med Oncol. 2011;28(Suppl. 1):S80–S90. doi: 10.1007/s12032-010-9717-7. [DOI] [PubMed] [Google Scholar]
- 58.Chavez-MacGregor M, Zhang N, Buchholz TA, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol. 2013;31:4222–4228. doi: 10.1200/JCO.2013.48.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J, Long JB, Hurria A, et al. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60:2504–2512. doi: 10.1016/j.jacc.2012.07.068. [DOI] [PubMed] [Google Scholar]
- 60.Serrano C, Cortés J, De Mattos-Arruda L, et al. Trastuzumab-related cardiotoxicity in the elderly: a role for cardiovascular risk factors. Ann Oncol. 2012;23:897–902. doi: 10.1093/annonc/mdr348. [DOI] [PubMed] [Google Scholar]
- 61.Adamo V, Ricciardi GR, Adamo B, et al. The risk of toxicities from trastuzumab, alone or in combination, in an elderly breast cancer population. Oncology. 2014;86:16–21. doi: 10.1159/000353450. [DOI] [PubMed] [Google Scholar]
- 62.Vaz-Luis I, Keating NL, Lin NU, et al. Duration and toxicity of adjuvant trastuzumab in older patients with early-stage breast cancer: a population-based study. J Clin Oncol. 2014;32:927–934. doi: 10.1200/JCO.2013.51.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shih YC, Xu Y, Dong W, et al. First do no harm: population-based study shows non-evidence-based trastuzumab prescription may harm elderly women with breast cancer. Breast Cancer Res Treat. 2014;144:417–425. doi: 10.1007/s10549-014-2874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 65.Thavendiranathan P, Grant AD, Negishi T, et al. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 66.Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fallah-Rad N, Walker JR, Wassef A, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57:2263–2270. doi: 10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 68.Ky B, Putt M, Sawaya H, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–816. doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morris PG, Chen C, Steingart R, et al. Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clin Cancer Res. 2011;17:3490–3499. doi: 10.1158/1078-0432.CCR-10-1359. [DOI] [PubMed] [Google Scholar]
- 70.Onitilo AA, Engel JM, Stankowski RV, et al. High-sensitivity C-reactive protein (hs-CRP) as a biomarker for trastuzumab-induced cardiotoxicity in HER2-positive early-stage breast cancer: a pilot study. Breast Cancer Res Treat. 2012;134:291–298. doi: 10.1007/s10549-012-2039-z. [DOI] [PubMed] [Google Scholar]
- 71.Walker J, Bhullar N, Fallah-Rad N, et al. Role of three-dimensional echocardiography in breast cancer: comparison with two-dimensional echocardiography, multiple-gated acquisition scans, and cardiac magnetic resonance imaging. J Clin Oncol. 2010;28:3429–3436. doi: 10.1200/JCO.2009.26.7294. [DOI] [PubMed] [Google Scholar]
- 72.Jassal DS, Han SY, Hans C, et al. Utility of tissue Doppler and strain rate imaging in the early detection of trastuzumab and anthracycline mediated cardiomyopathy. J Am Soc Echocardiogr. 2009;22:418–424. doi: 10.1016/j.echo.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 73.Lawley C, Wainwright C, Segelov E, et al. Pilot study evaluating the role of cardiac magnetic resonance imaging in monitoring adjuvant trastuzumab therapy for breast cancer. Asia Pac J Clin Oncol. 2012;8:95–100. doi: 10.1111/j.1743-7563.2011.01462.x. [DOI] [PubMed] [Google Scholar]
- 74.Fallah-Rad N, Lytwyn M, Fang T, et al. Delayed contrast enhancement cardiac magnetic resonance imaging in trastuzumab induced cardiomyopathy. J Cardiovasc Magn Reson. 2008;10:5. doi: 10.1186/1532-429X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kang Y, Xu X, Cheng L, et al. Two-dimensional speckle tracking echocardiography combined with high-sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine-based chemotherapy. Eur J Heart Fail. 2014;16:300–308. doi: 10.1002/ejhf.8. [DOI] [PubMed] [Google Scholar]
- 76.Chavez-MacGregor M, Niu J, Zhang N, et al. Cardiac monitoring during adjuvant trastuzumab-based chemotherapy among older patients with breast cancer. J Clin Oncol. 2015;33:2176–2183. doi: 10.1200/JCO.2014.58.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hahn VS, Lenihan DJ, Ky B. Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J Am Heart Assoc. 2014;3:e000665. doi: 10.1161/JAHA.113.000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pituskin E, Haykowsky M, Mackey JR, et al. Rationale and design of the Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research Trial (MANTICORE 101—Breast): a randomized, placebo-controlled trial to determine if conventional heart failure pharmacotherapy can prevent trastuzumab-mediated left ventricular remodeling among patients with HER2+ early breast cancer using cardiac MRI. BMC Cancer. 2011;11:318. doi: 10.1186/1471-2407-11-318. [DOI] [PMC free article] [PubMed] [Google Scholar]