Introduction
Stevens-Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) are severe idiosyncratic adverse drug reactions characterized by a low incidence but high mortality. The incidence of SJS is approximately six cases per million persons per year, and that of TEN is approximately two cases per million persons per year [1]. The most frequently incriminated drugs are antibiotics, non-steroidal anti-inflammatory drugs and anti-convulsants. Other causes include infections and immunizations. The typical interval between the onset of drug therapy and SJS/TEN is between one and three weeks. Drugs with long half-lives are more likely to have a fatal outcome than those with short half-lives. In the past, attempts have been made to decrease mortality through improved supportive care as there was no specific therapy. Recently intravenous immunoglobulin (IVIG) has emerged as a promising therapeutic option [2]. We report a case of TEN following allopurinol and cefixime administration that was managed successfully with IVIG.
Case Report
A 40 year old male patient from Nepal presented with five days history of fever, cough, sore throat followed by erythematous macular and bullous rashes over the face, trunk, extremities, and genitalia. He had severe erosive stomatitis and esophagitis with marked difficulty in swallowing liquids and saliva. He had received oral cefixime for fever and sore throat two days prior to development of the rash. Prior to this he was receiving allopurinol for last three months with a provisional diagnosis of gout by the local physician. There was no history of any other drug intake, high risk behavior and malignancy. He did not have any joint pains at the time of admission. Examination showed extensive exfoliating erythematous macular and bullous rashes involving more than 80% of total body area (Fig. 1) involving scalp, face, trunk, extremities and glans penis. The bulbar and palpebral parts of the conjunctivae were congested bilaterally. He had constant drooling of saliva due to extensive erosive stomatitis. Rest of the general and systemic examination was unremarkable. Initially palms and the soles were spared but after a few days fresh crops of rash appeared over palm and soles as well. Investigations at the time of admission were included hemoglobin of 13.7 g/dl, total leukocyte count 6300/ mm3, neutrophils 67%, lymphocytes 28%, eosinophils 5%, serum bilirubin 0.23 mg/dl, serum creatinine 1.2 mg/dl and blood sugar of 169 mg/dl. Urinalysis showed plenty of red blood cells and pus cells. During the hospital stay total leukocyte counts rose to 13,600/mm3 whereas rest of the hemogram, renal and liver function tests remained within normal limits. Serum uric acid was 5.6 mg/dl. Serology for antinuclear antibodies, antibodies against the extractable nuclear antigens and antineutrophilic cytoplasmic antibodies was negative. Multiple blood, urine and fluid from blister cultures were sterile.
Fig. 1.
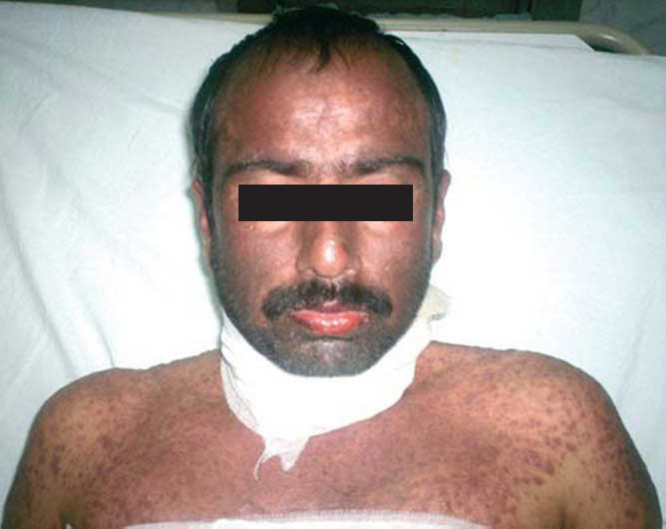
Generalized erythematous maculo-papular rash involving scalp, face, neck and front of chest with mucositis
He was managed with fluid supplements, total parenteral nutrition (TPN), tazobactum, piperacillin, amikacin and daily dressing of the involved body surface. In view of extensive involvement of the body surface area, a high SCORTEN score and likelihood of poor outcome, decision was taken to treat him with IVIG. IVIG in a dose of 2g/kg was given over five days. Ten days later, mucosal ulcers and rashes gradually started healing with improvement in the general condition and complete recovery in three weeks.
Discussion
The classic presentation in the form of acute onset, extensive and severe mucocutaneous blistering lesions involving more than 80% of the body surface area following a recently introduced antibiotic therapy was diagnostic of TEN in this case. The recent insight into the pathogenesis of TEN has suggested that there is increased rate of keratinocyte apoptosis in lesional skin of patients due to increased expression of keratinocyte membrane bound Fas and FasL [3, 4, 5]. IVIG is known to contain antibodies against Fas that block its binding to FasL [4], thus preventing apoptosis of the keratinocytes. There appears to be a strong rationale for its use in management of TEN. There are a number of studies, though small and non-controlled, that had documented the efficacy of IVIG in TEN. The study by Campione et al [6] describes 10 patients suffering from TEN with a mean total body surface area (TBSA) of 44% that were treated with 400 mg/kg of IVIG per day on five consecutive days (2 g/kg total dose), starting on average three days after disease onset. According to the calculated SCORTEN, the predicted mortality rate was 35% at the time of admission, however the observed mortality after IVIG therapy was 10%. The authors reported that in nine of their patients, clinical improvement could be observed as early as after the first infusion of IVIG. However, two studies did not confirm the beneficial effect of IVIG. It should be noted that in the two studies that showed no benefit on mortality, the total dose of IVIG used was of 2 g/kg or less, whereas in five of the seven studies showing a benefit of IVIG on mortality, the total dose of IVIG used was greater than 2 g/kg.
Recently, a scoring system, SCORTEN, has been proposed on the basis of seven clinico-biologic parameters to grade the severity of TEN and predict the risk of mortality (Table 1) [6]. A SCORTEN score of 3, that has a predicted mortality risk of 35%, prompted us to consider the use of IVIG, besides the usual supportive care in our patient.
Table 1.
SCORTEN scoring for severity of TEN [6]
| Variable | SCORTEN | Predicted mortality (%) |
|---|---|---|
| 1. Age >40 years | 0-1 | 3.2 |
| 2. Malignancy | 2 | 12.1 |
| 3. Tachycardia (>120/min) | ||
| 4. Initial surface of epidermal | 3 | 35.3 |
| detachment >10% | ||
| 5. Blood urea nitrogen >10 mmol/L | 4 | 58.3 |
| 6. Serum glucose >14 mmol/L | ||
| 7. Bicarbonate <20 mmol/L | > 5 | 90 |
Presence of variable parameter is scored as 1 whereas its absence is scored as 0. The sum total of all the individual scores predict the risk of mortality
Offending drugs in our patient were allopurinol and cefixime in combination. Temporally, cefixime appeared to be more closely related to TEN as he was already receiving allopurinol for last three months without any adverse reactions. However allopurinol has been one of the most common drugs implicated in TEN. Review of literature showed that genetic susceptibility may also play a role, as evidenced by the increased incidence of HLA-B12 in individuals affected by TEN [7]. In SJS and TEN due to allopurinol, a genetic predisposition in Han Chinese with the HLA-B*5801 allele has been recently described [8].
Conflicts of Interest
None identified
References
- 1.Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994;331:1272–1285. doi: 10.1056/NEJM199411103311906. [DOI] [PubMed] [Google Scholar]
- 2.Tan A, Thong BY, Yip LW. High dose intravenous immunoglobulin in the treatment of toxic epidermal necrolysis: an Asian series. J Dermatol. 2005;32:1–6. doi: 10.1111/j.1346-8138.2005.tb00704.x. [DOI] [PubMed] [Google Scholar]
- 3.Nassif A, Moslehi H, Le Gouvello S. Evaluation of the potential role of cytokines in toxic epidermal necrolysis. J Invest Dermatol. 2004;123:850–855. doi: 10.1111/j.0022-202X.2004.23439.x. [DOI] [PubMed] [Google Scholar]
- 4.Viard I, Wehrli P, Bullani R. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998;282:490–493. doi: 10.1126/science.282.5388.490. [DOI] [PubMed] [Google Scholar]
- 5.Ito K, Hara H, Okada T, Shimojima H, Suzuki H. Toxic epidermal necrolysis treated with low-dose intravenous immunoglobulin: immunohistochemical study of Fas and Fasligand expression. Clin Exp Dermatol. 2004;29:679–680. doi: 10.1111/j.1365-2230.2004.01635.x. [DOI] [PubMed] [Google Scholar]
- 6.Campione E, Marulli GC, Carrozzo AM, Chimenti MS, Costanzo A, Bianchi L. High dose intravenous immunoglobulin for severe drug reactions: Efficacy in toxic epidermal necrolysis. Acta Derm Venereol. 2003;83:430–432. doi: 10.1080/0001550310005852. [DOI] [PubMed] [Google Scholar]
- 7.Ueta M, Sotozono C, Inatomi T, Kojima K, Tashiro K, Hamuro J. Toll-like receptor 3 gene polymorphisms in Japanese patients with Stevens-Johnson syndrome. Br J Ophthalmol. 2007;91:962–965. doi: 10.1136/bjo.2006.113449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung SI, Chung WH, Liou LB. HLA-B*5801 allele as genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci USA. 2005;102:4134–4139. doi: 10.1073/pnas.0409500102. [DOI] [PMC free article] [PubMed] [Google Scholar]


