Abstract
Certain aspects of lipid metabolism have been examined in denervated muscle from normal mice and in dystrophic muscle from mice of the Bar Harbor strain 129. A number of parameters show no change or similar changes. For example, the utilization of palmitate-[1-14C] and palmitylcarnitine by mitochondria from denervated and dystrophic hind leg skeletal muscle showed parallel decreased in the oxidation of palmitate (30-42%) and palmitylcarnite (37-66%). A comparable study with acetylcarnitine showed a striking difference with no change evident in mitochondria from denervated muscle and 80-85% decrease in dystrophic muscle. The study of succinate dehydrogenase and the enzymes of beta-oxidation in the above mitochondrial preparation showed similar findings except for acyl CoA dehydrogenase activity (an enzyme with a regulatory role in beta-oxidation) which was significantly diminished (29%) in denervated muscle, whereas no change was observed in dystrophic muscle. The findings show a close parallel in a number of parameters but distinct differences were observed in denervated as compared with dystrophic muscle. It is unlikely that the muscular disorder in murine muscular dystrophy can be explained solely on the basis of denervation or the loss of a neural trophic factor.
Full text
PDF

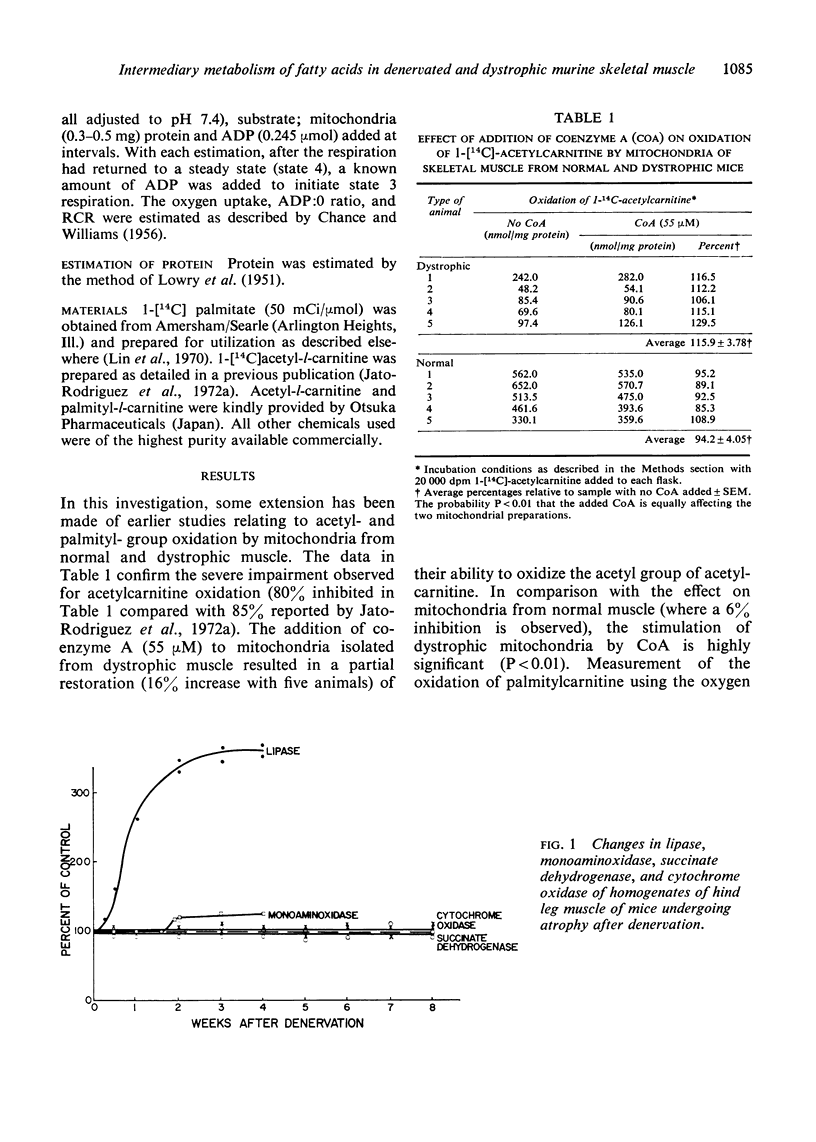
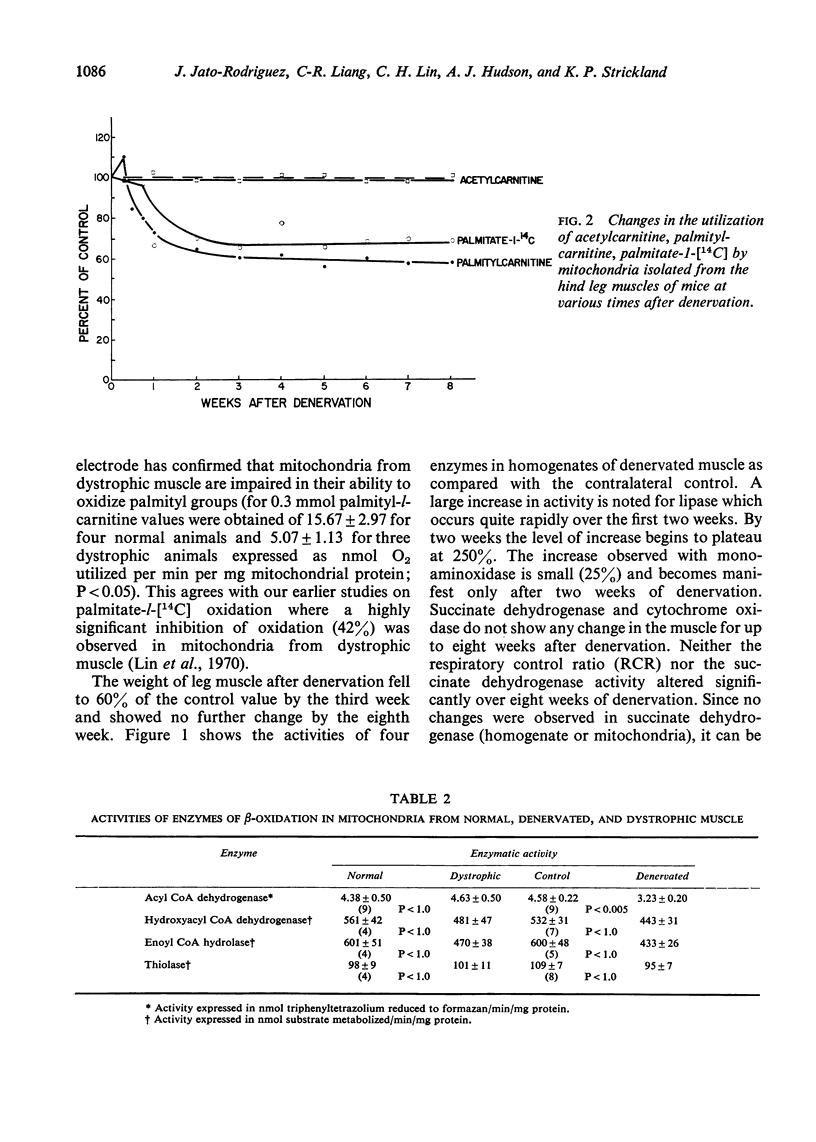



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado Rigault M. Y., Blanchaer M. C. Respiration and oxidative phosphorylation by mitochondria of red and white skeletal muscle. Can J Biochem. 1970 Jan;48(1):27–32. doi: 10.1139/o70-005. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Engel W. K., Karpati G. Impaired skeletal muscle maturation following neonatal neurectomy. Dev Biol. 1968 Jun;17(6):713–723. doi: 10.1016/0012-1606(68)90015-8. [DOI] [PubMed] [Google Scholar]
- Guth L., Watson P. K., Brown W. C. Effects of cross-reinnervation on some chemical properties of red and white muscles of rat and cat. Exp Neurol. 1968 Jan;20(1):52–69. doi: 10.1016/0014-4886(68)90124-6. [DOI] [PubMed] [Google Scholar]
- HUMOLLER F. L., HATCH D., MCINTYRE A. R. Cytochrome oxidase activity in muscle following neurotomy. Am J Physiol. 1952 Aug;170(2):371–374. doi: 10.1152/ajplegacy.1952.170.2.371. [DOI] [PubMed] [Google Scholar]
- Jato-Rodriguez J. J., Hudson A. J., Strickland K. P. Activities of enzymes of the citric acid cycle and electron transport chain in the skeletal muscle of normal and dystrophic mice (strain 129). Enzyme. 1972;13(5-6):286–292. doi: 10.1159/000459676. [DOI] [PubMed] [Google Scholar]
- Jato-Rodriguez J. J., Hudson A. J., Strickland K. P. Triglyceride metabolism in skeletal muscle from normal and dystrophic mice. Biochim Biophys Acta. 1974 Apr 26;348(1):1–13. doi: 10.1016/0005-2760(74)90087-3. [DOI] [PubMed] [Google Scholar]
- Jato-Rodriguez J. J., Lin C. H., Hudson A. J., Strickland K. P. Acetyl-1- 14 C-l-carnitine oxidation, carnitine acetyltransferase activity, and CoA content in skeletal muscle mitochondria from normal and dystrophic mice (strain 129). Can J Biochem. 1972 Jul;50(7):749–754. doi: 10.1139/o72-104. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin C. H., Hudson A. J., Strickland K. P. Fatty acid metabolism in dystrophic muscle in vitro. Life Sci. 1969 Jan 15;8(2):21–26. doi: 10.1016/0024-3205(69)90112-x. [DOI] [PubMed] [Google Scholar]
- Lin C. H., Hudson A. J., Strickland K. P. Palmitic acid-1- 14 C oxidation by skeletal muscle mitochondria of dystrophic mice. Can J Biochem. 1970 May;48(5):566–572. doi: 10.1139/o70-093. [DOI] [PubMed] [Google Scholar]
- MARTIN H. F., PEERS F. G. Oat lipase. Biochem J. 1953 Oct;55(3):523–529. doi: 10.1042/bj0550523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Comas A. J., Sica R. E., Currie S. Muscular dystrophy: evidence for a neural factor. Nature. 1970 Jun 27;226(5252):1263–1264. doi: 10.1038/2261263a0. [DOI] [PubMed] [Google Scholar]
- McComas A. J., Sica R. E., Campbell M. J. "Sick" motoneurones. A unifying concept of muscle disease. Lancet. 1971 Feb 13;1(7694):321–326. doi: 10.1016/s0140-6736(71)91045-2. [DOI] [PubMed] [Google Scholar]
- McComas A. J., Sica R. E., Currie S. An electrophysiological study of Duchenne dystrophy. J Neurol Neurosurg Psychiatry. 1971 Aug;34(4):461–468. doi: 10.1136/jnnp.34.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetropoulos T. A., Bradley W. G. Spinal motor neurones in murine muscular dystrophy and spinal muscular atrophy. A quantitative histological study. J Neurol Neurosurg Psychiatry. 1972 Feb;35(1):60–65. doi: 10.1136/jnnp.35.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN J. R., DEL CAMPILLO A., RAW I. Enzymes of fatty acid metabolism. I. General introduction; crystalline crotonase. J Biol Chem. 1956 Feb;218(2):971–983. [PubMed] [Google Scholar]
- Sica R. E., McComas A. J. An electrophysiological investigation of limb-girdle and facioscapulohumeral dystrophy. J Neurol Neurosurg Psychiatry. 1971 Aug;34(4):469–474. doi: 10.1136/jnnp.34.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKIL S. J., GREEN D. E., MII S., MAHLER H. R. Studies on the fatty acid oxidizing system of animal tissues. VI. beta-Hydroxyacyl coenzyme A dehydrogenase. J Biol Chem. 1954 Apr;207(2):631–638. [PubMed] [Google Scholar]
- WAKIL S. J., GREEN D. E., MII S., MAHLER H. R. Studies on the fatty acid oxidizing system of animal tissues. VI. beta-Hydroxyacyl coenzyme A dehydrogenase. J Biol Chem. 1954 Apr;207(2):631–638. [PubMed] [Google Scholar]
- WURTMAN R. J., AXELROD J. A SENSITIVE AND SPECIFIC ASSAY FOR THE ESTIMATION OF MONOAMINE OXIDASE. Biochem Pharmacol. 1963 Dec;12:1439–1441. doi: 10.1016/0006-2952(63)90215-6. [DOI] [PubMed] [Google Scholar]
- Ward C. W., Fairbairn D. Enzymes of beta-oxidation and their function during development of Ascaris lumbricoides eggs. Dev Biol. 1970 Jun;22(2):366–387. doi: 10.1016/0012-1606(70)90159-4. [DOI] [PubMed] [Google Scholar]
- Weeks G., Shapiro M., Burns R. O., Wakil S. J. Control of fatty acid metabolism. I. Induction of the enzymes of fatty acid oxidation in Escherichia coli. J Bacteriol. 1969 Feb;97(2):827–836. doi: 10.1128/jb.97.2.827-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


