Abstract
Purpose
Hypersensitivity to fungi is associated with rhinoconjunctivitis and asthma. For some fungi, such as Alternaria alternata (A. alternata), the symptoms of asthma are persistent, increasing disease severity and the risk of fatal outcomes. There are a large number of species of fungi but knowledge of them remains limited. This, together with the difficulties in obtaining adequate standardized extracts, means that there remain significant challenges in the diagnosis and immunotherapy of allergy associated with fungi. The type of indoor fungi related to asthma/allergy varies according to geographic, climatic, and seasonal factors, making their study difficult. The aim of this study was to determine hypersensitivity to indoor fungi in a population from Cuenca, Spain.
Methods
Thirty-five patients with symptoms compatible with rhinitis or asthma who showed clear worsening of their symptoms in their homes or workplace were included. In vivo and in vitro tests were made with a battery of fungal allergens, including the species isolated in the home or workplace.
Results
Ulocladium botrytis (U. botrytis) and A. alternata were the most representative species as a source of home sensitization. These species showed very high concordance in skin tests, specific IgE, and histamine release. The allergen Alt a 1, which was recognized in all patients, was detected in A. alternata, U. botrytis, and Stemphylium botryosum (S. botryosum).
Conclusions
U. botrytis and A. alternata were the most representative species as a source of home sensitization. Alt a 1 was recognized in all patients and may be considered a non-species-specific allergen that could be used as a diagnostic source of sensitization to some species of the Pleosporaceae family.
Keywords: Alternaria alternata, Ulocladium botrytis, Alt a 1, orthologous allergens, biological standardization, pleosporaceae
INTRODUCTION
Hypersensitivity to fungi is associated with rhinoconjunctivitis and asthma.1,2 This association was described in 1,726 by Floyer in asthmatic patients visiting a winery.3 Other allergic diseases are allergic fungal sinusitis, allergic bronchopulmonary aspergillosis, and extrinsic allergic alveolitis.
In the case of A. alternata, the symptoms of asthma are persistent, thus increasing disease severity and the risk of fatal outcomes.4 This has implications for the clinical management of patients, and these diseases have substantial social and economic repercussions.
A. alternata spores and mycelium are the primary source of sensitization, although other fungal particles derived from them may also reach the respiratory system of the allergic patient.5 Their almost permanent presence, both in the atmosphere and in dwellings6 depends primarily on the environmental, climatic, and topographic conditions, and the sources that the fungus colonizes.7
The reported prevalence of A. alternata varies widely according to the geographical location (ranging from 3% in the Nordic countries to 20% in the Mediterranean environment) and the methodology used.8 The prevalence is gradually increasing, with children being especially affected.9
In dwellings and interior environments, species such as Stachybotrys chartarum (S. chartarum) are generally receiving increasing scientific interest due to the severity of the symptoms caused by the mycotoxins released10 and as the cause of the so-called sick building syndrome. The aims of this study was to determine hypersensitivity to fungi in dwellings in patients from Cuenca, Spain, and to study polysensitization and cross-reactivity due to the known sensitization to various species, and the recognition of allergens belonging or not to a same group species from the same taxonomic family.11
MATERIALS AND METHODS
Patients
Patients were selected randomly and prospectively. Inclusion criteria were clinical demonstration of the signs and symptoms of rhinitis or rhinoconjunctivitis and/or associated mild-to-moderate asthma of ≥1 year of evolution and a positive skin prick test (>3 mm wheal) and positive specific IgE levels (≥0.35 kU/L, class 1; ThermoFisher Scientific, last Phadia, Uppsala, Sweden) to various species of fungal extracts. All patients included provided signed informed consent to participate in the study.
Home collection and identification of fungal samples
Fungal samples were obtained from each dwelling (home or workplace suspected of involvement in the symptoms). Samples were identified by the Department of Microbiology, Immunology and Parasitology (University of the Basque Country, Vitoria, Spain) (Fig. 1).
Fig. 1. Home collection and identification of fungal samples.
Allergenic sources and extracts
The natural sources of raw materials containing spores and mycelia of Candida albicans (C. albicans), U. botrytis, Mucor mucedo, Fusarium sp, Trichophyton rubrum, Aspergillus niger, A. alternata, S. chartarum, S. botryosum, Penicillium notatum (P. notatum), Rhizopus sp, Aspergillus fumigatus (A. fumigatus), Botrytis sp, and Cladosporium herbarum (C. herbarum) were supplied by Allergon (Allergon, Ängelholm, Sweden), Greer Lab., (Lenoir. USA) and the Departments of Microbiology, Immunology, and Parasitology (University of the Basque Country, Vitoria, Spain).
Proteins from the above-mentioned raw materials were obtained after prior homogenization and subsequent extraction in Coca buffer, under magnetic agitation at 4℃ for 5 hours. The soluble fractions were centrifuged at 8,400 g×30 minutes. at 4℃. The supernatants were subjected to filtration, dialysis (7,000 Da), and subsequent lyophilization.
Skin prick tests
All patients underwent a skin prick test according to standard procedures and a standard battery of fungal extracts (Diater Laboratories, Madrid, Spain).
Standardization of U. botrytis extract
The activity of the allergenic extract of U. botrytis was measured in biological units (10,000 BU/mL), after obtaining the median skin reactivity produced at different concentrations, and compared with a reference solution of histamine dihydrochloride at 54.3 mmol/L (10 mg/mL) in at least 20 sensitized patients, according to the method of Aas12 modified by Malling13 and after conforming to the assumptions of the methodological study by Dreborg14 which, together, are the method recommended by the recent EMEA Guideline on Allergen Products: Production and Quality Issues (EMEACHMP/BWP/304831/2007) which regulates this type of products in the EU.
Briefly, the histamine equivalent prick (HEP) value from the selected population was obtained according to the application of four 10-fold concentrations of allergen together with the histamine reference solution, on the volar surface of the forearm of each patient by duplicate. The area of the wheal was measured with a digitizer by following the contour lines. The dose-response relationship of the allergen was estimated by linear regression analysis using the geometric mean of the 2 wheal areas obtained with each concentration, in a double-logarithmic system. The concentration of allergen estimated to provoke a response with the same wheal area as the histamine reaction (geometric mean of the 2 wheal responses caused by histamine) is then calculated from the regression line formula. The median value based on all patients tested represents the concentration of the allergen preparation corresponding to 10,000 BU/mL.
Specific IgE and histamine release tests
The levels of specific IgE to U. botrytis and Alt a 1 were measured using the CAP system (ThermoFisher Scientific, last Phadia, Uppsala, Sweden). The levels ≥0.35 kU/L were considered positive. The binding capacity for specific IgE protein extracts of C. albicans, U. botrytis, Mucor mucedo, Fusarium sp, Trichophyton rubrum, Aspergillus niger, A. alternata , S. chartarum, S. botryosum, P. notatum, Rhizopus sp, A. fumigatus, Botrytis sp, and C. herbarum were determined by IgE immunoblotting and Dot blot analysis.
Histamine release was made after stimulating blood basophils with the extracts of U. botrytis and Alt a 1 coupled to the CM-strip of the histamine release test (Reflab, Denmark) according to the method described by Per Stahl Skov et al.15,16
Quantification of Alt a 1 and cross reactivity tests
The presence and content of Alt a 1 in extracts of A. alternata, U. botrytis, S. botryosum, A. fumigatus, C. herbarum, and P. notatum were measured using the ELISA quantification kit for Alt a 1 (INDOOR Biotechnologies, Charlottesville, VA, USA). For this quantification, a polyclonal antiserum Alt a 1 obtained by immunization of a New Zealand rabbit17 with rAlt a 1 in complete Freund's adjuvant18 according to the protocol of Gallart et al. was also used.17 Polysensitization and/or cross-reactivity of the extracts tested was measured by ELISA inhibition assays, according to the method of Ceska and Lundqvist19 with modifications.
2D-electrophoresis, 2D-immunoblotting, and sequencing
The orthologous allergen of Alt a 1 in the extract of U. botrytis was identified by choosing the closest to the reference point in terms of the molecular weight and the isoelectric point after performing 2D electrophoresis and IgE-2D-immunoblotting. The point recognized by the sera of patients was subjected to sequence analysis by peptide mass fingerprinting (MALDI-TOF-MS) and MS/MS analysis (Proteomics Center, Faculty of Pharmacy, University Complutense of Madrid, Spain). Trypsin digestion was made using the method of Shevchenko.20 The peptide fingerprint was obtained using MALDI-TOF-MS21 and de novo sequencing by MS/MS according to the method of Gautam et al.22
RESULTS
Study population and selection of the most prevalent species
There were 35 male or female patients. They had (1) a mean age of 15 years (7-32 years), (2) symptoms compatible with rhinitis or asthma showing clear worsening of their symptoms in their dwellings or workplaces where damp areas were identified in walls or ceilings, and (3) positive skin prick tests and specific IgE (≥0.35 kU/L) to U. botrytis, A. alternata, A. fumigatus, and C. notatum (Table 1).
Table 1. Clinical description of patients.
| Number | Age* | Sex | Pathology† | Time‡ | Sensitization§ |
|---|---|---|---|---|---|
| 1 | 7 | M | Rhinitis and asthma | 2 | Alt and Ulo |
| 2 | 8 | M | Rhinitis and asthma | 2 | Alt and Ulo |
| 3 | 16 | M | Rhinitis | 4 | Alt, Asp, and Ulo |
| 4 | 15 | F | Rhinitis and asthma | 6 | Alt and Ulo |
| 5 | 8 | M | Rhinitis and asthma | 3 | Alt, Asp, Clad, and Ulo |
| 6 | 15 | F | Rhinitis and asthma | 4 | Alt, Asp, Clad, and Ulo |
| 7 | 11 | F | Rhinitis and asthma | 5 | Alt, Asp, Clad, and Ulo |
| 8 | 12 | M | Rhinitis and asthma | 4 | Alt and Ulo |
| 9 | 11 | M | Rhinitis and asthma | 2 | Alt and Ulo |
| 10 | 14 | M | Rhinitis and asthma | 7 | Alt and Ulo |
| 11 | 14 | M | Rhinitis and asthma | 5 | Alt, Asp, Clad, and Ulo |
| 12 | 19 | M | Rhinitis and asthma | 7 | Alt, Asp, and Ulo |
| 13 | 14 | F | Rhinitis | 3 | Alt and Ulo |
| 14 | 17 | M | Rhinitis | 4 | Alt and Ulo |
| 15 | 14 | F | Rhinitis and asthma | 7 | Alt, Asp, Clad, and Ulo |
| 16 | 18 | F | Rhinitis and asthma | 3 | Alt, Asp, and Ulo |
| 17 | 18 | M | Rhinitis and asthma | 6 | Alt and Ulo |
| 18 | 20 | M | Rhinitis and asthma | 7 | Alt and Ulo |
| 19 | 11 | M | Rhinitis and asthma | 7 | Alt and Ulo |
| 20 | 11 | F | Rhinitis and asthma | 5 | Alt, Asp, Clad, and Ulo |
| 21 | 16 | F | Rhinitis and asthma | 4 | Alt and Ulo |
| 22 | 15 | F | Rhinitis and asthma | 3 | Alt and Ulo |
| 23 | 22 | M | Rhinitis and asthma | 4 | Alt and Ulo |
| 24 | 11 | M | Rhinitis and asthma | 2 | Alt and Ulo |
| 25 | 32 | M | Rhinitis and asthma | 5 | Alt, Asp, and Ulo |
| 26 | 10 | M | Rhinitis and asthma | 3 | Alt and Ulo |
| 27 | 12 | F | Rhinitis and asthma | 3 | Alt and Ulo |
| 28 | 21 | F | Rhinitis and asthma | 4 | Alt, Asp, Clad, and Ulo |
| 29 | 16 | M | Rhinitis and asthma | 3 | Alt and Ulo |
| 30 | 20 | M | Rhinitis | 5 | Alt and Ulo |
| 31 | 19 | F | Rhinitis and asthma | 3 | Alt and Ulo |
| 32 | 8 | M | Rhinitis and asthma | 2 | Alt and Ulo |
| 33 | 26 | F | Rhinitis | 3 | Alt and Ulo |
| 34 | 16 | F | Rhinitis and asthma | 5 | Alt, Asp, Clad, and Ulo |
| 35 | 11 | M | Rhinitis and asthma | 3 | Alt, Asp, Clad, and Ulo |
*Patient age in years; †Clinical entity; ‡Years of disease progression; §Fungal species to which the patient has a positive skin test (prick test) and/or specific IgE >0.70 kU/L.
Alt, Alternaria alternata; Asp, Aspergillus fumigatus; Clad, Cladosporium herbarum; Ulo, Ulocladium brotrytis, M, Male; F, Female.
U. botrytis and A. alternata were the most representative species as sources of sensitization in these dwellings.
Skin tests and biological standardization of U. botrytis
To determine the conventional parameters of biological activity in vivo and allergenic potency in vitro of extracts of U. botrytis, biological standardization was carried out. Of the 35 patients included, 26 were selected according to the inclusion criteria regarding the papules obtained with different concentrations of the allergen extracts used. The 95% confidence limits of the population were determined between the values of HEP of patients 8 and 19 (0.88 and 2.02 mg/mL, respectively).
The HEP value obtained from the U. botrytis extract corresponded to the median individual HEP values in the 26 selected patients and was 1.25 mg/mL (Table 2).
Table 2. Biological standardization: HEP values for Ulocladium botrytis extract and levels of specific IgE of U. botrytis, Ulocladium chartarum and Alt a 1.
| patient | HEP/patient | HEP/patient | IgE Ulocladium botrytis | IgE Ulocladium chartarum | IgE Alt a 1 |
|---|---|---|---|---|---|
| In increasing order | kU/L | kU/L | kU/L | ||
| 1 | 1.255 | 0.052 | 5.36 | 1.10 | 28.90 |
| 2 | 5.927 | 0.234 | 5.15 | 0.79 | 62.80 |
| 3 | 0.234 | 0.548 | 1.33 | 0.90 | 13.00 |
| 4 | 0.548 | 0.635 | 5.54 | 0.91 | 30.70 |
| 5 | 0.872 | 0.693 | 7.39 | 1.55 | 38.40 |
| 6 | 1.251 | 0.740 | 1.21 | 1.01 | 9.56 |
| 7 | 0.635 | 0.872 | 0.89 | 0.90 | 1.45 |
| 9 | 1.676 | 0.881 | 6.64 | 2.43 | 33.90 |
| 10 | 0.693 | 0.885 | 1.01 | 0.70 | 7.96 |
| 11 | 0.881 | 1.006 | 1.22 | 0.97 | 8.93 |
| 14 | 4.494 | 1.194 | 1.85 | 2.09 | 40.90 |
| 15 | 1.194 | 1.203 | 2.24 | 3.86 | 13.20 |
| 16 | 3.569 | 1.251 | 3.24 | 1.45 | 21.60 |
| 17 | 2.080 | 1.255 | 1.14 | 0.79 | 6.28 |
| 18 | 1.006 | 1.281 | 0.75 | 0.74 | 6.69 |
| 19 | 2.436 | 1.306 | 3.77 | 7.74 | 3.75 |
| 20 | 2.839 | 1.403 | 5.70 | 2.48 | 87.00 |
| 21 | 0.740 | 1.676 | 0.81 | 0.92 | 7.30 |
| 24 | 0.885 | 2.025 | 21.20 | 3.11 | >100 |
| 25 | 1.203 | 2.080 | 1.52 | 0.71 | 0.91 |
| 26 | 1.306 | 2.436 | 24.10 | 9.81 | >100 |
| 27 | 1.403 | 2.839 | 2.65 | 2.10 | 34.30 |
| 28 | 4.118 | 3.569 | 2.43 | 2.03 | 13.80 |
| 30 | 1.281 | 4.118 | 8.06 | 8.52 | 79.10 |
| 32 | 2.025 | 4.494 | 6.33 | 7.40 | 24.80 |
| 35 | 0.052 | 5.927 | 3.81 | 7.23 | 11.00 |
Allergenic profiles
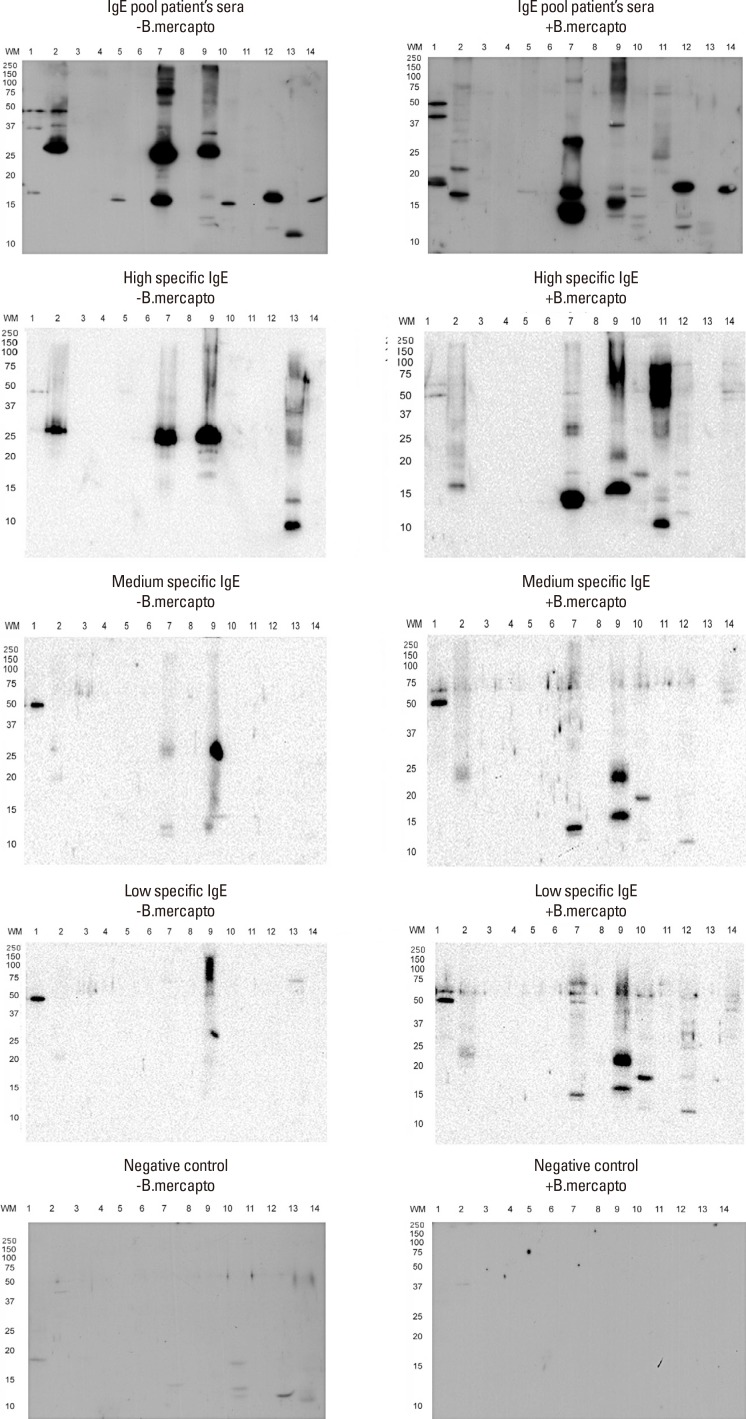
Fig. 2 shows the allergogram of the selected allergen extracts under reducing and nonreducing conditions, revealed by pooled sera, high, medium and low specific IgE of selected patients in the standardization of U. botrytis. The main bands observed, with an approximate weight of 29 kDa (under nonreducing conditions), appeared in the extracts of U. botrytis, A. alternata, and S. botryosum.
Fig. 2. Allergogram of allergenic extracts of fungi vs pooled sera, high, medium and low specific IgE of selected patients under reducing and non-reducing conditions. Lane 1: Candida albicans; Lane 2: Ulocladium botrytis; Lane 3: Mucor mucedo; Lane 4: Fusarium sp.; Lane 5: Trichophyton rubrum; Lane 6: Aspergillus niger; Lane 7: Alternaria alternata; Lane 8: Stachybotrys chartarum; Lane 9: Stemphylium botryosum; Lane 10: Penicillium notatum; Lane 11: Rhizopus sp.; Lane 12: Aspergillus fumigatus; Lane 13: Botrytis sp.; Lane 14: Cladosporium herbarum.
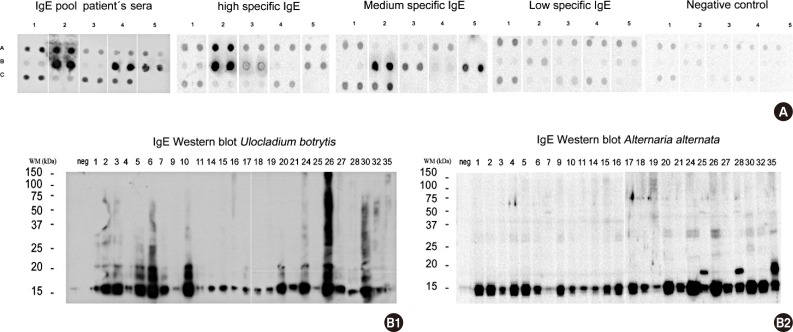
The Dot blot in Fig. 3A shows the IgE-binding capacity to recognize different species of fungi, with a positive reaction to samples of U. botrytis, A. alternata, and S. botryosum and, to a lesser degree, A. fumigatus and C. herbarum. Fig. 3B shows the IgE-binding capacity of U. botrytis and A. alternata in each patient, and shows a band of about 15-17 kDa that appeared in all the samples.
Fig. 3. (A) Dot blot of selected extracts vs pooled sera, high, medium and low specific IgE of selected patients. (duplicate samples are shown) Row A: Candida albicans, Ulocladium botrytis, Mucor mucedo, Fusarium oxysporum, Trichophyton rubrum/Row B: Aspergillus niger, Alternaria alternata, Stachybotrys chartarum, Stemphylium botryosum, Rhizopus nigricams/Row C: Aspergillus fumigatus, Botrytis cinerea, Cladosporium herbarum, Penicillium notatum, (B1) Immunoblotting of allergenic extract of Ulocladium botrytis. (B2) Immunoblotting of allergenic extract of Alternaria alternata.
Allergenic potency and cross-reactivity
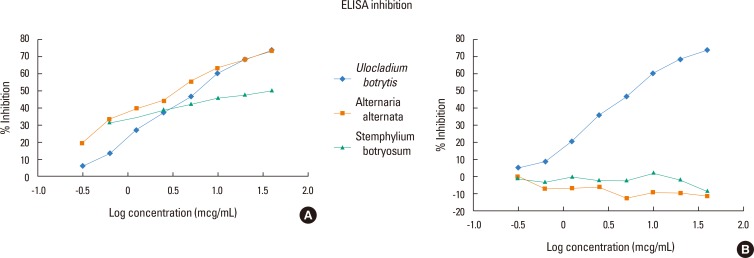
To determine whether the reactivity of sera to different fungal species is due to cross-reactivity or polysensitization, we determined Ag50 values for each fungal extract by ELISA inhibition (see Fig. 4A). The Ag50 value of U. botrytis extract was 6.029 µg/mL, while those for A. alternata and S. botryosum were 3.604 and 25.373 mg/mL, respectively. No inhibition was observed for A. fumigatus or C.herbarum (Fig. 4B).
Fig. 4. (A) ELISA inhibition of allergenic extract of Ulocladium botrytis versus Ulocladium botrytis, Alternaria alternata, Stemphylium botryosum and (B) ELISA inhibition allergenic extract of Ulocladium botrytis versus Ulocladium botrytis, Aspergillus fumigatus and Cladosporium herbarum.
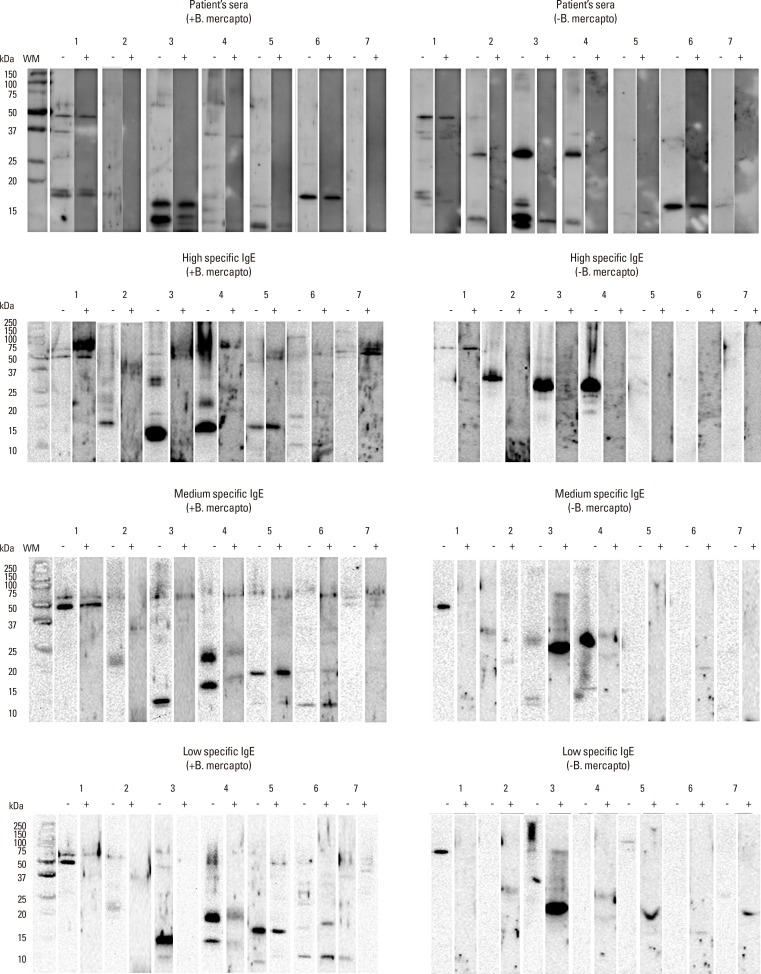
Taking into account the fact that the allergen Alt a 1 is the most important allergen described in A. alternata and is possibly what most patients react to, we decided to study its capacity to inhibit binding of IgE to extracts of various fungi. Western blot analysis in Fig. 5 shows the inhibitory capacity of Alt a 1 on the specific IgE versus the allergens of the extracts of C. albicans, U. botrytis, A. alternata, S. botryosum, P. notatum, A. fumigates, and C. herbarum. Alt a 1 inhibited its homologue in the extracts of A. alternata, U. botrytis, and S. botryosum.
Fig. 5. Inhibition of immunoblot with pooled sera, high, medium and low specific IgE of selected patients under reducing and non-reducing conditions: (-) No inhibition with Alt a 1; (+) Inhibition with Alt a 1; Lane 1: Candida albicans, Lane 2: Ulocladium botrytis, Lane 3: Alternaria alternata, Lane 4: Stemphylium botryosum, Lane 5: Penicillium notatum, Lane 6: Aspergillus fumigatus, Lane 7: Cladosporium herbarum.
2D-electrophoresis, 2D-immunoblotting, and sequencing
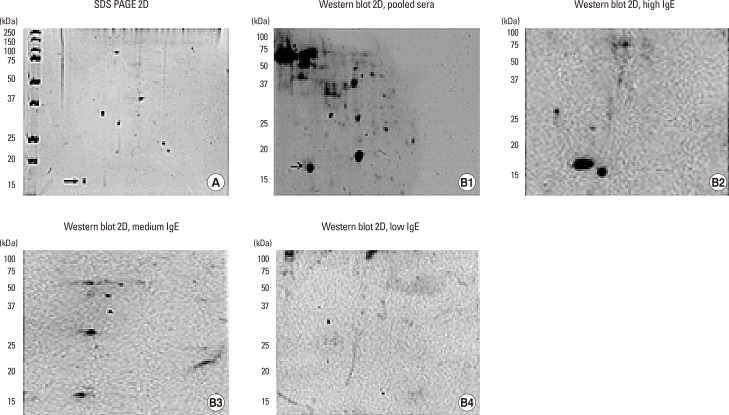
To confirm the presence of Alt a 1 in the extract of U. botrytis, we carried out 2D-electrophoresis, which showed the presence of a protein of 15 kDa and a pI around 4.5 (Fig. 6A), compatible with the orthologue of Alt a 1 in U. botrytis. Immunoblotting showed the presence of a protein of approximately 17 kDa with a pI of around 4.5, compatible with the orthologous allergen of Alt a 1 in the extract of U. botrytis, recognized by the pooled sera (Fig. 6B1), and a high, medium and low specific IgE values of selected patients (Fig. 6B2, B3, and 6B4).
Fig. 6. (A) 2D electrophoresis of Ulocladium botrytis extract. (B1) immunoblotting with pooled sera of selected patients. Arrows indicate the possible orthologue of Alt a 1. (B2) immunoblotting with high level of specific IgE to Ulocladium botrytis extract. (B3) immunoblotting with medium level of specific IgE to Ulocladium botrytis extract. (B4) immunoblotting with low level of specific IgE to Ulocladium botrytis extract.
To identify the possible homologous protein of Alt a 1, this was extracted from SDS PAGE gel and analyzed by MS sequencing. The amino acid sequence coincided in 24% (sequence coverage) with the orthologue allergen of Alt a 1 expressed below (in red):
1 MQFTTIASLF AAAGLAAAAP LESRQDNASC PVTTK 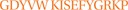
51
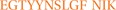 ATNGGTL DFTCSAQADK LEDHKWYSCG ENSFMDFSFD
ATNGGTL DFTCSAQADK LEDHKWYSCG ENSFMDFSFD
101 SDRSGLLLKQ KVSDE
Histamine release
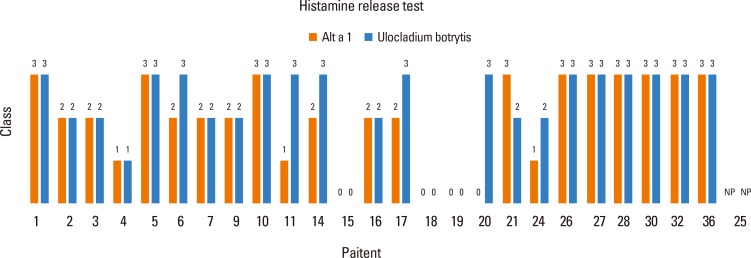
To evaluate the ex vivo behavior of the allergenic extract used in the skin tests, histamine release tests were made using samples from the 26 patients selected in the skin tests. Basophils were stimulated with extracts of U. botrytis and Alt a 1. The results (see Fig. 7) showed a good correlation between the results obtained with both stimuli.
Fig. 7. Histamine release test. Results obtained after stimulation with Alt a 1 and Ulocladium botrytis.
Quantification of Alt a 1
We then determined the presence and content in Alt a 1 in the extracts of A. alternata, U. botrytis, S. botryosum, A. fumigatus, C. herbarum, and P. notatum by ELISA. The content of Alt a 1 in extracts of A. alternata, U. botrytis, and S. botryosum was 895 ng/mg, 85 ng/mg, and 18.29 ng/mg, respectively, of the total extract. Alt a 1 was not detected in A. fumigatus, C. herbarum, and P. notatum.
DISCUSSION
Currently, more than 3 billion people worldwide are infected by parasites (worms) or suffering from allergic diseases that affect the respiratory tract (asthma and rhinitis), skin (dermatitis, urticaria, and eczema), and different expressions of sensitivity to food and medicine that are considered impaired Th2-(Th2) immune responses.23
The diagnosis of these disorders is essentially based on a combination of (1) etiological identification tests that show the presence of the source of sensitization in the patient's environment and the possible cause and effect, (2) the patient's medical records, and (3) in vivo and in vitro diagnostic tests.24 Diagnostic tests require allergenic extracts, and the results obtained will depend on their quality.25 Allergenic extracts are therefore indispensable tools for the diagnosis and treatment of allergic diseases and research on processes involved in this inflammatory phenomenon.26
In the specific case of allergy to fungi, the problem is compounded due to the wide variety and polymorphisms of species, and the high variability of these organisms which is manifested in different compositions and potency of allergenic extracts obtained, even from those of the same strain. Therefore, the diagnosis is complicated because, in many cases, there is no correlation between the source of sensitization and the possible etiologic diagnosis: this means that diagnostic tests often do not show the efficacy, in terms of sensitivity and specificity, obtained with other allergens.8
The present study analyzed a sample of patients with suspected sensitization to fungi in the home or work environment. The allergenic extracts of fungi used in the study were selected according to aerobiological and incidence studies of the most important reported species,8,27,28,29 including some emerging species, such as S. chartarum.30
The allergenic extracts used underwent the internal procedures of biological standardization and quality control recommended by accepted guidelines.12 The results of serological titration of specific IgE of both Ulocladium species (U. botrytis and U. chartarum) were almost the same, implying that allergenic variation at the species level does not show significant differences.31
Our results suggest that the allergen Alt a 1 of A. alternata could be considered a non-species-specific allergen which could be used as a diagnostic source of sensitization to some species of the pleosporaceae family.
A study by Twaroch et al.32,33 of 80 patients allergic to A. alternata showed recognition of rAlt 1 in 98% of cases, a percentage similar to that found by Asturias et al.34 in a cohort of 42 patients, most of whom recognized the major allergen of U. botrytis, the homologue of Alt a 1, similar to the already described Ste b 1 of S. botryosum, suggesting that Alt a 1 could be used as a marker for sensitization to A. alternata and other species of the Pleosporaceae family, and could replace whole extracts of these species.
Patients who do not recognize Alt a 1 may be recognizing other reported orthologue allergens, such as Alt a 6 and Alt a 14 (MnSOD),35 which like other allergens from fungal species, such as Asp f 4 and Asp f 6, are discriminators of bronchopulmonary aspergillosis and sensitization to Aspergillus, and which could like Alt a 1 and homologues, report and discriminate aspects, such as the greater frequency of asthma in patients sensitized to A. alternata compared to those not sensitised.36
The biological standardization of the allergenic extract of U. botrytis made in this study allows correct etiologic diagnosis and concordance between the identification of the presence of the source of sensitization in dwellings and the perennial, non-seasonal nature of these sources to be established.
Another factor in determining allergenic sources, especially fungi, is the importance of recognizing an allergen in sensitized patients. However, reactivity to IgE does not necessarily reflect its ability to induce allergic symptoms. Hence, the incorporation of the results obtained can apply for the technique of allergen-specific histamine release and identify their concordance with the results of the skin tests and the detection of specific IgE.
ACKNOWLEDGMENTS
We sincerely thank our patients for their courage and interest and David Buss for his editorial assistance.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Salo PM, Arbes SJ, Jr, Sever M, Jaramillo R, Cohn RD, London SJ, et al. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J Allergy Clin Immunol. 2006;118:892–898. doi: 10.1016/j.jaci.2006.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calabria CW, Dice J. Aeroallergen sensitization rates in military children with rhinitis symptoms. Ann Allergy Asthma Immunol. 2007;99:161–169. doi: 10.1016/S1081-1206(10)60640-0. [DOI] [PubMed] [Google Scholar]
- 3.Floyer J. Violent asthma after visiting a wine cellar: a treatise on asthma. London: Innys and Parker; 1745. [Google Scholar]
- 4.Black PN, Udy AA, Brodie SM. Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy. 2000;55:501–504. doi: 10.1034/j.1398-9995.2000.00293.x. [DOI] [PubMed] [Google Scholar]
- 5.Breitenbach M, Crameri R, Lehrer SB. Impact of current genome projects on the study of pathogenic and allergenic fungi. Chem Immunol. 2002;81:5–9. doi: 10.1159/000058860. [DOI] [PubMed] [Google Scholar]
- 6.Horner WE, Lehrer SB, Salvaggio JE. Indoor air pollution. Fungi. Immunol Allergy Clin North Am. 1994;14:551–566. [Google Scholar]
- 7.Rodríguez-Rajo FJ, Iglesias I, Jato V. Variation assessment of airborne Alternaria and Cladosporium spores at different bioclimatical conditions. Mycol Res. 2005;109:497–507. doi: 10.1017/s0953756204001777. [DOI] [PubMed] [Google Scholar]
- 8.D'Amato G, Chatzigeorgiou G, Corsico R, Gioulekas D, Jäger L, Jäger S, et al. Evaluation of the prevalence of prick skin test positivity to Alternaria and Cladosporium in patients with suspected respiratory allergy. Allergy. 1997;52:711–716. doi: 10.1111/j.1398-9995.1997.tb01227.x. [DOI] [PubMed] [Google Scholar]
- 9.Perzanowski MS, Sporik R, Squillace SP, Gelber LE, Call R, Carter M, et al. Association of sensitization to Alternaria allergens with asthma among school-age children. J Allergy Clin Immunol. 1998;101:626–632. doi: 10.1016/S0091-6749(98)70170-8. [DOI] [PubMed] [Google Scholar]
- 10.Lorenz AR, Lüttkopf D, May S, Scheurer S, Vieths S. The principle of homologous groups in regulatory affairs of allergen products--a proposal. Int Arch Allergy Immunol. 2009;148:1–17. doi: 10.1159/000151243. [DOI] [PubMed] [Google Scholar]
- 11.Fung F, Clark R, Williams S. Stachybotrys, a mycotoxin-producing fungus of increasing toxicologic importance. J Toxicol Clin Toxicol. 1998;36:79–86. doi: 10.3109/15563659809162592. [DOI] [PubMed] [Google Scholar]
- 12.Aas K, Backman A, Belin L, Weeke B. Standardization of allergen extracts with appropriate methods. The combined use of skin prick testing and radio-allergosorbent tests. Allergy. 1978;33:130–137. doi: 10.1111/j.1398-9995.1978.tb01522.x. [DOI] [PubMed] [Google Scholar]
- 13.Malling HJ, Dreborg S, Weeke B. Diagnosis and immunotherapy of mould allergy. VI. IgE-mediated parameters during a one-year placebo-controlled study of immunotherapy with Cladosporium. Allergy. 1987;42:305–314. doi: 10.1111/j.1398-9995.1987.tb02214.x. [DOI] [PubMed] [Google Scholar]
- 14.Skin tests used in type I allergy testing Position paper. Sub-Committee on Skin Tests of the European Academy of Allergology and Clinical Immunology. Allergy. 1989;44(Suppl 10):1–59. [PubMed] [Google Scholar]
- 15.Stahl Skov P, Norn S, Weeke B. A new method for detecting histamine release. Agents Actions. 1984;14:414–416. doi: 10.1007/BF01973840. [DOI] [PubMed] [Google Scholar]
- 16.Wenande EC, Skov PS, Mosbech H, Poulsen LK, Garvey LH. Inhibition of polyethylene glycol-induced histamine release by monomeric ethylene and diethylene glycol: a case of probable polyethylene glycol allergy. J Allergy Clin Immunol. 2013;131:1425–1427. doi: 10.1016/j.jaci.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 17.Gallart T, Bladé J, Martínez-Quesada J, Sierra J, Rozman C, Vives J. Multiple myeloma with monoclonal IgG and IgD of lambda type exhibiting, under treatment, a shift from mainly IgG to mainly IgD. Immunology. 1985;55:45–57. [PMC free article] [PubMed] [Google Scholar]
- 18.Pineda F. Expresión y purificación del alérgeno Alt a 1 de Alternaria alternata. Implicación en la hipersensibilidad tipo I [master's thesis] Madrid: Universidad Complutense de Madrid; 2005. [Google Scholar]
- 19.Ceska M, Lundkvist U. A new and simple radioimmunoassay method for the determination of IgE. Immunochemistry. 1972;9:1021–1030. doi: 10.1016/0019-2791(72)90112-7. [DOI] [PubMed] [Google Scholar]
- 20.Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 21.Suckau D, Resemann A, Schuerenberg M, Hufnagel P, Franzen J, Holle A. A novel MALDI LIFT-TOF/TOF mass spectrometer for proteomics. Anal Bioanal Chem. 2003;376:952–965. doi: 10.1007/s00216-003-2057-0. [DOI] [PubMed] [Google Scholar]
- 22.Gautam P, Sundaram CS, Madan T, Gade WN, Shah A, Sirdeshmukh R, et al. Identification of novel allergens of Aspergillus fumigatus using immunoproteomics approach. Clin Exp Allergy. 2007;37:1239–1249. doi: 10.1111/j.1365-2222.2007.02765.x. [DOI] [PubMed] [Google Scholar]
- 23.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patterson R. Allergic diseases. Philadelphia (PA): Lippincott Co.; 1985. [Google Scholar]
- 25.Kurth R. Regulatory control and standardization of allergenic extracts. New York (NY): Gustav Fisher Verlag; 1990. [Google Scholar]
- 26.Allergen immunotherapy: therapeutic vaccines for allergic diseases. Geneva: January 27-29 1997. Allergy. 1998;53:1–42. doi: 10.1111/j.1398-9995.1998.tb04930.x. [DOI] [PubMed] [Google Scholar]
- 27.Heinzerling L, Frew AJ, Bindslev-Jensen C, Bonini S, Bousquet J, Bresciani M, et al. Standard skin prick testing and sensitization to inhalant allergens across Europe--a survey from the GALEN network. Allergy. 2005;60:1287–1300. doi: 10.1111/j.1398-9995.2005.00895.x. [DOI] [PubMed] [Google Scholar]
- 28.Rivera-Mariani FE, Nazario-Jiménez S, López-Malpica F, Bolaños-Rosero B. Skin test reactivity of allergic subjects to basidiomycetes’ crude extracts in a tropical environment. Med Mycol. 2011;49:887–891. doi: 10.3109/13693786.2011.574238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JH, Lee HS, Park MR, Lee SW, Kim EH, Cho JB, et al. Relationship between indoor air pollutant levels and residential environment in children with atopic dermatitis. Allergy Asthma Immunol Res. 2014;6:517–524. doi: 10.4168/aair.2014.6.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson BD. APS Features. Stachybotrys chartarum: the toxic indoor mold. St. Paul (MN): American Phytopathological Society; 2001. pp. 101–130. [Google Scholar]
- 31.Gutiérrez-Rodríguez A, Postigo I, Guisantes JA, Suñén E, Martínez J. Identification of allergens homologous to Alt a 1 from Stemphylium botryosum and Ulocladium botrytis. Med Mycol. 2011;49:892–896. doi: 10.3109/13693786.2011.576350. [DOI] [PubMed] [Google Scholar]
- 32.Twaroch TE, Focke M, Fleischmann K, Balic N, Lupinek C, Blatt K, et al. Carrier-bound Alt a 1 peptides without allergenic activity for vaccination against Alternaria alternata allergy. Clin Exp Allergy. 2012;42:966–975. doi: 10.1111/j.1365-2222.2012.03996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Twaroch TE, Curin M, Valenta R, Swoboda I. Mold allergens in respiratory allergy: from structure to therapy. Allergy Asthma Immunol Res. 2015;7:205–220. doi: 10.4168/aair.2015.7.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asturias JA, Ibarrola I, Ferrer A, Andreu C, López-Pascual E, Quiralte J, et al. Diagnosis of Alternaria alternata sensitization with natural and recombinant Alt a 1 allergens. J Allergy Clin Immunol. 2005;115:1210–1217. doi: 10.1016/j.jaci.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Postigo I, Gutiérrez-Rodríguez A, Fernández J, Guisantes JA, Suñén E, Martínez J. Diagnostic value of Alt a 1, fungal enolase and manganese-dependent superoxide dismutase in the component-resolved diagnosis of allergy to Pleosporaceae. Clin Exp Allergy. 2011;41:443–451. doi: 10.1111/j.1365-2222.2010.03671.x. [DOI] [PubMed] [Google Scholar]
- 36.Katotomichelakis M, Anastassakis K, Gouveris H, Tripsianis G, Paraskakis E, Maroudias N, et al. Clinical significance of Alternaria alternata sensitization in patients with allergic rhinitis. Am J Otolaryngol. 2012;33:232–238. doi: 10.1016/j.amjoto.2011.07.004. [DOI] [PubMed] [Google Scholar]