Abstract
Asthma research is shifting from studying symptoms and lung functions to the narrow-focus cellular profiles protein analysis, biomarkers, and genetic markers. The transmembrane glycoprotein CD93 is involved in endothelial cell migration, angiogenesis, leukocytes extravasation, apoptosis, innate immunity and inflammation. Relationships between the serum level of soluble CD93 (sCD93) and acute myocardial infarction/premature MI/inflammatory arthritis/skin sclerosis have recently been reported. We hypothesized that sCD93 would be elevated during the acute phase of asthma. We measured the serum level of sCD93 in 57 patients with asthma exacerbation and 57 age-and gender-matched healthy controls. Additionally, sCD93 was reassessed at the time of discharge from the hospital. Clinical characteristics and peak expiratory flow (PEF) of the patients were assessed. The primary outcome was the comparison of serum level of sCD93 between asthmatics and healthy subjects. The sCD93 values ranged from 128 to 789 ng/mL in asthmatics (345.83±115.81) and from 31 to 289 ng/mL in control subjects (169.46±62.43). The difference between the 2 groups was statistically significant (P<0.001). The association between sCD93 and asthma remained significant after adjusting for age, sex, and BMI. The differences between asthmatics and controls remained significant on the last day of hospital stay. The association between sCD93 and PEF was not significant. In conclusion, the serum level of soluble CD93 is increased in patients with asthma exacerbation. It also showed that serum levels of sCD93 decreased with treatment of asthma attack. The clinical usefulness of determination of sCD93 as a biomarker of asthma requires further studies.
Keywords: Asthma, biomarker, CD93
INTRODUCTION
Asthma affects over than 300 million individuals worldwide, making it one of the most prevalent diseases. Asthma is managed according to consensus guidelines.1,2 It is a chronic immunological disorder of the lungs characterized by reversible airway obstruction, airway inflammation, and increased airway hyperresponsiveness in response to provocative challenges.3 The diagnosis and management of asthma is generally based on symptoms, often combined with pulmonary function test results. However, these criteria may not reflect underlying airway inflammation. Asthma research is shifting from studying symptoms and lung functions to the narrow-focus cellular profiles, protein analysis, biomarkers, and genetic markers. Asthma biomarkers include biomolecules that undergo cellular, biochemical, or molecular alterations in asthmatic patients versus healthy subjects that are measurable in biological samples, such as lung tissue, bronchoalveolar lavage fluid, nasal fluid, or blood.4 Noninvasive and reliable biomarkers would ideally be standard in the daily clinical routine, but are currently unavailable in asthma.
The type 1 transmembrane glycoproteine CD93(C1qRP) is encoded by the CD93 gene found on chromosome 20.5 It is expressed early on the surface of myeloid cells during B-cell differentiation in the bone marrow and on the surface of hematopoietic stem cells, natural killer cells, endothelial cells, platelets, and microglia.6,7,8 The predicted molecular weight of CD93 is 120 kD and consist of a c-type carbohydrate-recognition domain, a fine epidermal growth factor-like domain, a single transmembrane domain, a main domain, and an intracellular domain.5,9,10
CD93 has been implicated in regulating adhesive processes and expressions on the endothelial and circulating cells consistent with the role of CD93 in cellular homing to the sites of inflammation.11 It was hypothesized that CD93 could be involved in endothelial cell migration, angiogenesis, and leukocytes extravasation,12 and it was reported that CD93 plays a role in the C1q/MBL/SP-A-mediated removal of immune complexes and pathogens via phagocytosis.13 Other studies have demonstrated a role of CD93 in apoptosis, innate immunity, and inflammation.14,15
Soluble CD93 was detected in normal human plasma, suggesting that the cleavage event is physiologically relevant.16 In addition, production of sCD93 was induced by the inflammatory mediators TNF-α and LPS, indicating physiologic pathways that trigger this event.17
Based on previous studies that identified a soluble form of CD93 in human plasma in response to activation with inflammatory stimuli, we hypothesized that sCD93 would be elevated during the acute phase of asthma. To test this hypothesis, we investigated whether the concentration of sCD93 in asthmatic patients is higher than in age-and gender-matched healthy subjects. In addition, we investigated the correlation between sCD93 and peak expiratory flow, and some other clinical properties of asthmatics.
MATERIALS AND METHODS
All the adult patients who were admitted with acute exacerbation of asthma at Tohid Hospital (a 400 bed referral university hospital, Sanandaj, Iran) between June 2013 and July 2014 were enrolled. Inclusion criteria included male and female subjects with (1) a history of physician- diagnosed asthma who may or may not take inhaled corticosteroids (ICSs), (2) stay at the hospital for more than 2 days, and (3) ability to provide informed consent. Patients underwent a complete physical examination, measurement of baseline peak expiratory flow (PEF), and leukocyte count and differential cell count at the time of initial enrollment. Plasma sCD93 concentration was measured using ELISA (eBioscience, San Diego, CA, USA) at the time of admission and repeated on the morning of discharge day.
Patients with lung diseases other than asthma, history of chronic inflammatory or autoimmune disorders, and history of upper or lower respiratory infections within 4 weeks of the study were excluded from the study.
Age-and gender-matched healthy subjects were selected from volunteers. None of the control subjects had a history of asthma, allergic rhinitis, or other inflammatory disorders.
All the patients and control subjects provided written informed consent, and the study protocol was approved by the Ethics Review Board of the Kurdistan University of Medical Sciences.
For continuous variables, data are presented as mean (standard deviation). For multiple comparisons, 1-way analysis of variance was used. Correlations between 2 variables were evaluated using Pearson's correlation coefficient. Multiple linear regression analysis was performed to determine whether sCD93 levels would be associated with asthma after adjustment for other variables. A P value of <0.05 was considered significant.
RESULTS
During the study period, 57 adult patients (32 women and 25 men) with acute exacerbation of asthma and the same number of age-and gender-matched healthy controls were cross-sectionally studied. According to the Global Initiative for Asthma guidelines,1 specialists rated asthma control status as well-controlled in 10 (17.5%), partially controlled in 25 (44%) and uncontrolled in 22 (38%) of patients before current exacerbation. Regular use of ICSs had been stopped by 59% of patients prior to admission to the hospital. The characteristics of the patients are summarized in Table 1.
Table 1. Comparison of CD93 levels between the asthma and control groups after adjustment for sex, age, and smoking using multiple regression analysis.
| Characteristics | Asthmatics n=57 |
Healthy subjects n=57 |
P value |
|---|---|---|---|
| Age (year), mean (SD) | 46.98 (12.09) | 48.37 (8.04) | 0.47 |
| Gender (M/F) | 25/32 | 24/33 | <0.01 |
| BMI (kg/m2) | 28/50 (6.15) | 27.76 (5.52) | 0.62 |
| Duration of disease (year) (SD) | 8.46 (7.31) | - | |
| ICS negative before admission (no/percent) | 32 (59/4) | - | |
| Length of hospital stay (day) | - | ||
| mean (SD) | 4.7 (1.93) | - | |
| PEF at admission day (l/min) (SD) | 261.7 (90.3) | - | <0.01 |
| PEF at discharge day (l/min) (SD) | 316.1 (87.7) | - | 0.01 |
| Active smoking (no/percent) | 13 (22.6) | 13 (22.6) | <0.01 |
BMI, body mass index; IC, inhaled corticosteroid; PEF, peak expiratory flow.
The sCD93 values ranged from 128 to 789 ng/mL in asthmatics (mean±SD, 345.83±115.81) and from 31 to 289 ng/mL in control subjects (169.46±62.43). The difference between the 2 groups was statistically significant (P<0.001). The association between sCD93 and asthma remained significant after adjusting for age, sex, and smoking (P=0.001) (Table 2). In asthmatic patients, there was no significant difference in the sCD93 levels between men and women (men, 375.84±151.39 ng/mL; women, 322.37±70.37 ng/mL; P=0.473).
Table 2. Comparison of CD93 levels between the asthma and control groups after adjustment for sex, age, and smoking using multiple regression analysis.
| Unstandardized coefficients | Standardized coefficients | P value | 95.0% Confidence interval for beta | |||
|---|---|---|---|---|---|---|
| Beta | Std. Error | Beta | Lower bound | Upper bound | ||
| Constant | 99.302 | 44.889 | - | 0.029 | 10.334 | 188.269 |
| Group (Asthma/Control) | 179.944 | 17.502 | 0.705 | <0.001 | 145.255 | 214.633 |
| Sex (Male/Female) | 16.403 | 17.483 | 0.064 | 0.350 | -18.248 | 51.055 |
| Age (year) | 1.120 | 0.857 | 0.090 | 0.194 | -0.578 | 2.818 |
| Smoking (Yes/No) | 29.666 | 20.787 | 0.098 | 0.156 | -11.534 | 70.866 |
The difference in CD93 levels between the asthma and control groups is significant (P<0.001) after adjusting for sex (P=0.35), age (P=0.194), and smoking (P=0.156) in multiple regression analysis (R2=0.497).
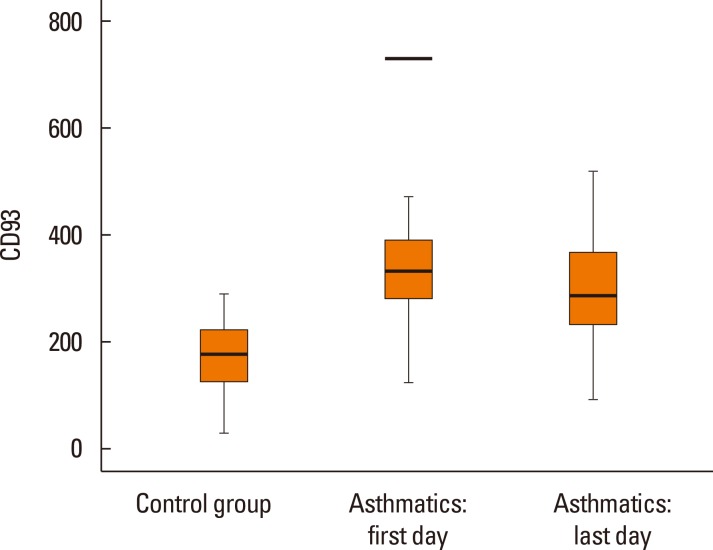
The mean (SD) length of hospital stay was 4.7 (1.97) days. The serum levels of sCD93 in asthmatics decreased to 303.54 (110.75) ng/mL in response to therapy. The difference in sCD93 between the first and last day of admission was significant (P=0.004) (Fig. 1). A marginal difference in the levels of sCD93 between patients with use of ICS and those without- was observed, but it was not statistically significant (ICS+, 377.52±129.06 ng/mL; ICS-, 327.01±99.44 ng/mL; P=0.067).
Fig. 1. Comparison of serum levels of CD93 between healthy control subjects and asthmatic patients. The mean serum level of CD93 in control group was 169.45 (± 62.43) and in the first and last day of hospital stay of asthmatics were 345.83 (±115.81) and 303.54 (±110.75) respectively. The differences between control subjects and both groups of asthmatics were significant.(P=0.001, P=0.001). The difference between first and last day of hospital stay in asthmatics remain significant (P=0.004).
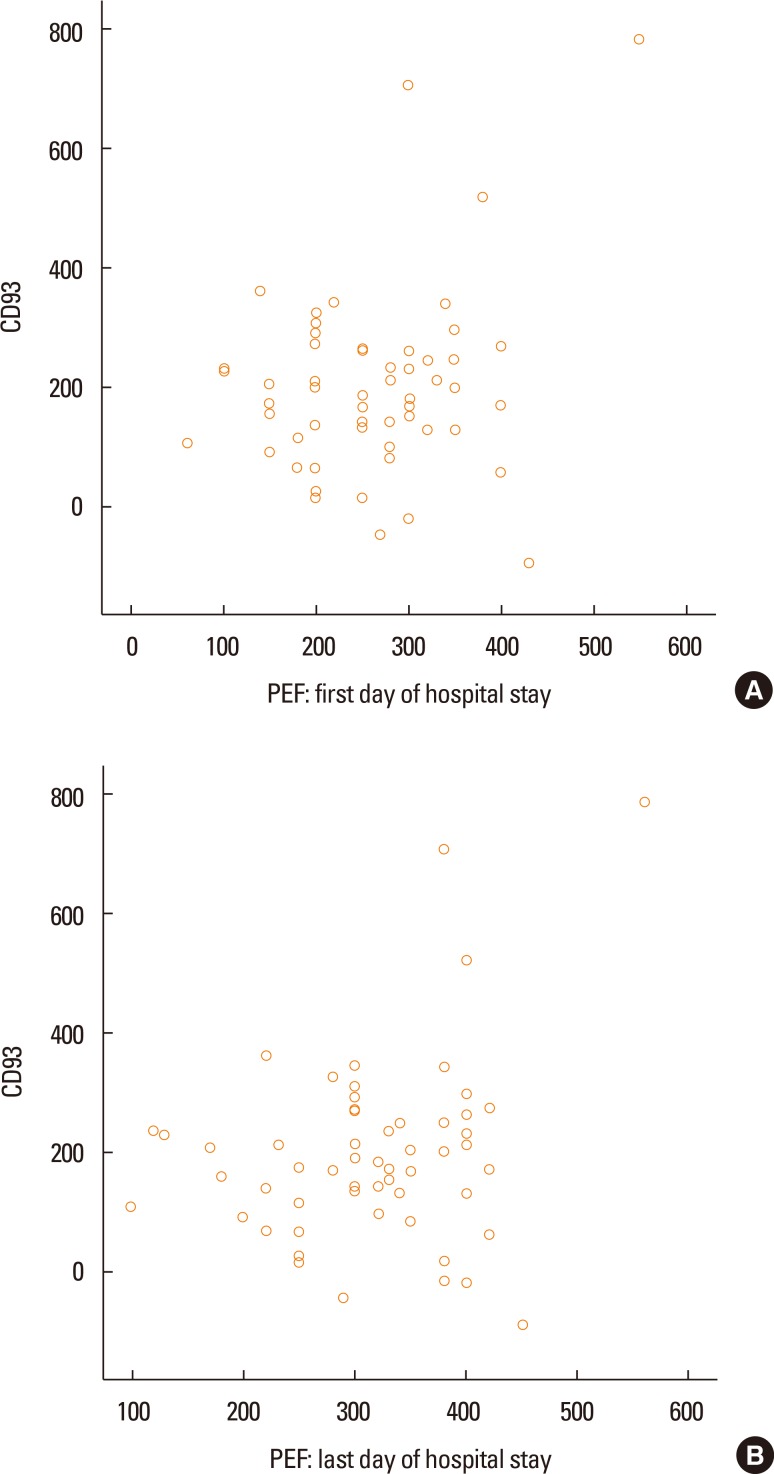
The mean (SD) of PEF on the first day of admission was 261.7 (90.3) L/min and increased to 316.1 (87.7) L/min on the last day of hospital stay (P<0.001). The association between sCD93 and PEF was not significant (Fig. 2A and B). There were no positive correlations between sCD93 and family history of asthma (P=0.38), age of patients (P=0.249), eosinophil count (P=0.735), neutrophil count (P=0.975), or duration of hospital stay (P=0.05). The duration of disease had a positive correlation with sCD93 (P=0.006). Twenty-five out of 57 patients had blood eosinophil count of >250 cells/µL. The difference in serum levels of sCD93 was not statistically significant between asthmatic patients with higher eosinophil (357.72±136 ng/mL) and those with lower levels of eosinophil (335.54±97.52 ng/mL) (P=0.467). Thirteen (22.6%) out of 57asthmatics were current smokers. Serum levels of sCD93 were higher in smokers than in nonsmokers (391.15±15 vs 332.44±103.21ng/mL), but the difference was not statistically significant (P=0.296).
Fig. 2. The association between PEF at the first day of hospital stay and CD93 was negative (A). This association in the last day of hospital staying remained negative (B).
DISCUSSION
The results of the present study showed that the mean level of sCD93 was 2-fold higher in asthmatic patients with acute exacerbation than in healthy subjects. This difference remained significant after treatment of asthma attack. To the best of our knowledge, this is the first study in which the serum levels of sCD93 in patients with asthma were investigated.
Symptoms and lung function tests may not reflect underlying airway inflammation of asthmatics. Even in its mildest form, asthma displays all the features of chronic inflammatory disease involving persistent activation of mast cell, eosinophil, and T-lymphocyte.18,19 Tissue-specific diagnostic methods, such as bronchoalveolar lavage, sputum induction, and bronchial biopsy, are used to evaluate airway inflammation. However, the invasiveness of these diagnostic procedures limits the use of these methods for daily clinical practice.20 Peripheral blood is relatively easy and less invasive than bronchoscopy or sputum induction. Several studies have evaluated whether inflammatory soluble mediators would be used as biomarkers of asthma phenotypes.21
Studies on asthma have shown that inflammatory cells, such as granulocytes and monocytes, respond with up-regulation of several markers, such as CD63, CD66, CD67, and CD11b/CD18 in response to inflammatory signals.22 The process of homing of the cells toward the tissue compartments is sufficient to activate the cells in homeostasis as well as in disease.23 Expression of CD93 on the endothelial and circulatory cells is consistent with the role of CD93 in cellular homing to the site of inflammation.11 The role of CD93 in apoptosis has been demonstrated. Defects in the removal of apoptotic cells during inflammation have been associated with inflammatory phenotypes, such as reduced recovery from lung inflammation.14 These mechanisms may play a role in prolonging airway inflammation in asthmatics.
In experimental studies of animals, Liu et al.6 showed alterations in expression, localization, and involvement of CD93 in central nervous system inflammation. Harhausen et al.7 demonstrated a significant up-regulation of CD93 mRNA and neuroprotective effects of this gene in specific animal models of cerebral ischemia. In mouse models of peritonitis, soluble CD93 expression is detected, and CD93 secretion is strongly associated with leukocyte migration, complement activation, macrophage phagocytosis, and clearance of apoptotic cells.14,24
A previous investigation demonstrated a potential clinical implication of CD93. Malarstig et al.25 demonstrated a significant association between plasma sCD93 levels, premature MI, and the incidence of coronary artery disease. Recently, Youn et al.26 showed in a case-control study that CD93 levels are significantly higher in patients with acute myocardial infarction than in control subjects and associated with adverse clinical outcomes. Joen et al.5 reported that the concentration of soluble CD93 is significantly higher in synovial fluid of patients with rheumatoid arthritis than in synovial fluid of those with osteoarthritis. They also investigated a possible role of sCD93 in the pathogenesis of chronic inflammation. Yanaba et al.27 reported a positive correlation between serum sCD93 and severity of skin sclerosis in patients with systemic sclerosis.
It has been reported that soluble CD93 induces differentiation of monocytes to macrophage-like cells, resulting in increased pro-inflammatory cytokines production.5 The mechanism behind elevated sCD93 levels in asthmatics may be the increased shedding of CD93 from monocytes. A recently published study demonstrated that activated monocytes (CD16+ CD4dim) are higher in obese and normal-weight asthmatics compare to healthy controls.28 A in vitro study by Zaslona et al.29 demonstrated that circulating monocytes promote early events in allergic lung inflammation. Based on these results, it could be speculated that the increased levels of sCD93 in asthmatics represent a more pronounced inflammatory activity at high levels of sCD93.
The present study has several limitations. Several factors other than the presence of asthma may affect serum levels of sCD93. Not only the presence of cardiovascular diseases and connective tissue disorders but also infections are associated with increased sCD93.5,24,25,26 Furthermore, in contrast to some asthma biomarkers, sCD93 levels were unstable and variable during the treatment. The sCD93 levels were negatively correlated with PEF and significantly higher at admission than at discharge. These findings suggest that sCD93 may not be a specific biomarker for asthma and could be a surrogate predictor of treatment response. However, further studies are required to better elucidate the clinical implications of the association between sCD93 and asthma. Inflammatory markers other than sCD93 were not measured in our study. Another limitation of the present study is no inclusion of patients in the controlled stage of asthma, which will help interpret the role of sCD93 as an inflammatory marker.
In conclusion, the results of this study suggest that soluble CD93 can be increased in the serum of patients with asthma exacerbation and that serum levels of sCD93 can be decreased with treatment of asthma attack. Further studies are needed to confirm the clinical usefulness of sCD93 determination as a biomarker of asthma.
ACKNOWLEDGMENTS
The present study was financially supported by the Research Council of Kurdistan University of Medical Sciences.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention [Internet] place unknown: Global Initiative for Asthma (GINA); 2015. [cited 2016 Jan]. Available from: http://www.ginasthma.org/documents/4. [Google Scholar]
- 2.National Institutes of Health, U.S; Department of Health and Human Services; National Heart, Lung, and Blood Institute (US) Guidelines for the diagnosis and management of asthma (EPR-3) [Internet] National Heart, Lung, and Blood Institute; 2007. [cited 2016 Jan]. Available from: http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines. [Google Scholar]
- 3.Zhao CN, Fan Y, Huang JJ, Zhang HX, Gao T, Wang C, et al. The Association of GSDMB and ORMDL3 gene polymorphisms with asthma: a meta-analysis. Allergy Asthma Immunol Res. 2015;7:175–185. doi: 10.4168/aair.2015.7.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sircar G, Saha B, Bhattacharya SG, Saha S. Allergic asthma biomarkers using systems approaches. Front Genet. 2014;4:308. doi: 10.3389/fgene.2013.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeon JW, Jung JG, Shin EC, Choi HI, Kim HY, Cho ML, et al. Soluble CD93 induces differentiation of monocytes and enhances TLR responses. J Immunol. 2010;185:4921–4927. doi: 10.4049/jimmunol.0904011. [DOI] [PubMed] [Google Scholar]
- 6.Liu C, Cui Z, Wang S, Zhang D. CD93 and GIPC expression and localization during central nervous system inflammation. Neural Regen Res. 2014;9:1995–2001. doi: 10.4103/1673-5374.145383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harhausen D, Prinz V, Ziegler G, Gertz K, Endres M, Lehrach H, et al. CD93/AA4.1: a novel regulator of inflammation in murine focal cerebral ischemia. J Immunol. 2010;184:6407–6417. doi: 10.4049/jimmunol.0902342. [DOI] [PubMed] [Google Scholar]
- 8.Chevrier S, Genton C, Kallies A, Karnowski A, Otten LA, Malissen B, et al. CD93 is required for maintenance of antibody secretion and persistence of plasma cells in the bone marrow niche. Proc Natl Acad Sci U S A. 2009;106:3895–3900. doi: 10.1073/pnas.0809736106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nepomuceno RR, Henschen-Edman AH, Burgess WH, Tenner AJ. cDNA cloning and primary structure analysis of C1qR(P), the human C1q/MBL/SPA receptor that mediates enhanced phagocytosis in vitro. Immunity. 1997;6:119–129. doi: 10.1016/s1074-7613(00)80419-7. [DOI] [PubMed] [Google Scholar]
- 10.McGreal EP, Ikewaki N, Akatsu H, Morgan BP, Gasque P. Human C1qRp is identical with CD93 and the mNI-11 antigen but does not bind C1q. J Immunol. 2002;168:5222–5232. doi: 10.4049/jimmunol.168.10.5222. [DOI] [PubMed] [Google Scholar]
- 11.Steinberger P, Szekeres A, Wille S, Stöckl J, Selenko N, Prager E, et al. Identification of human CD93 as the phagocytic C1q receptor (C1qRp) by expression cloning. J Leukoc Biol. 2002;71:133–140. [PubMed] [Google Scholar]
- 12.Norsworthy PJ, Fossati-Jimack L, Cortes-Hernandez J, Taylor PR, Bygrave AE, Thompson RD, et al. Murine CD93 (C1qRp) contributes to the removal of apoptotic cells in vivo but is not required for C1q-mediated enhancement of phagocytosis. J Immunol. 2004;172:3406–3414. doi: 10.4049/jimmunol.172.6.3406. [DOI] [PubMed] [Google Scholar]
- 13.Jakel A, Qaseem AS, Kishore U, Sim RB. Ligands and receptors of lung surfactant proteins SP-A and SP-D. Front Biosci (Landmark Ed) 2013;18:1129–1140. doi: 10.2741/4168. [DOI] [PubMed] [Google Scholar]
- 14.Greenlee MC, Sullivan SA, Bohlson SS. Detection and characterization of soluble CD93 released during inflammation. Inflamm Res. 2009;58:909–919. doi: 10.1007/s00011-009-0064-0. [DOI] [PubMed] [Google Scholar]
- 15.Ikewaki N, Tamauchi H, Yamada A, Mori N, Yamao H, Inoue H, et al. A unique monoclonal antibody mNI-11 rapidly enhances spread formation in human umbilical vein endothelial cells. J Clin Immunol. 2000;20:317–324. doi: 10.1023/a:1006623905019. [DOI] [PubMed] [Google Scholar]
- 16.Park M, Tenner AJ. Cell surface expression of C1qRP/CD93 is stabilized by O-glycosylation. J Cell Physiol. 2003;196:512–522. doi: 10.1002/jcp.10332. [DOI] [PubMed] [Google Scholar]
- 17.Bohlson SS, Silva R, Fonseca MI, Tenner AJ. CD93 is rapidly shed from the surface of human myeloid cells and the soluble form is detected in human plasma. J Immunol. 2005;175:1239–1247. doi: 10.4049/jimmunol.175.2.1239. [DOI] [PubMed] [Google Scholar]
- 18.Holgate ST. Mechanisms of asthma and implications for its prevention and treatment: a personal journey. Allergy Asthma Immunol Res. 2013;5:343–347. doi: 10.4168/aair.2013.5.6.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sigari N, Ghasri H. Correlation between hs-CRP and asthma control indices. Tanaffos. 2013;12:44–48. [PMC free article] [PubMed] [Google Scholar]
- 20.Vijverberg SJ, Hilvering B, Raaijmakers JA, Lammers JW, Maitland-van der Zee AH, Koenderman L. Clinical utility of asthma biomarkers: from bench to bedside. Biologics. 2013;7:199–210. doi: 10.2147/BTT.S29976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhakta NR, Woodruff PG. Human asthma phenotypes: from the clinic, to cytokines, and back again. Immunol Rev. 2011;242:220–232. doi: 10.1111/j.1600-065X.2011.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Fortunati E, Kazemier KM, Grutters JC, Koenderman L, Van den Bosch J. Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin Exp Immunol. 2009;155:559–566. doi: 10.1111/j.1365-2249.2008.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenlee-Wacker MC, Briseño C, Galvan M, Moriel G, Velázquez P, Bohlson SS. Membrane-associated CD93 regulates leukocyte migration and C1q-hemolytic activity during murine peritonitis. J Immunol. 2011;187:3353–3361. doi: 10.4049/jimmunol.1100803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mälarstig A, Silveira A, Wågsäter D, Öhrvik J, Bäcklund A, Samnegård A, et al. Plasma CD93 concentration is a potential novel biomarker for coronary artery disease. J Intern Med. 2011;270:229–236. doi: 10.1111/j.1365-2796.2011.02364.x. [DOI] [PubMed] [Google Scholar]
- 26.Youn JC, Yu HT, Jeon JW, Lee HS, Jang Y, Park YW, et al. Soluble CD93 levels in patients with acute myocardial infarction and its implication on clinical outcome. PLoS One. 2014;9:e96538. doi: 10.1371/journal.pone.0096538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yanaba K, Asano Y, Noda S, Akamata K, Aozasa N, Taniguchi T, et al. Augmented production of soluble CD93 in patients with systemic sclerosis and clinical association with severity of skin sclerosis. Br J Dermatol. 2012;167:542–547. doi: 10.1111/j.1365-2133.2012.11020.x. [DOI] [PubMed] [Google Scholar]
- 28.Raedler D, Ballenberger N, Klucker E, Böck A, Otto R, Prazeres da Costa O, et al. Identification of novel immune phenotypes for allergic and nonallergic childhood asthma. J Allergy Clin Immunol. 2015;135:81–91. doi: 10.1016/j.jaci.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 29.Zasłona Z, Przybranowski S, Wilke C, van Rooijen N, Teitz-Tennenbaum S, Osterholzer JJ, et al. Resident alveolar macrophages suppress, whereas recruited monocytes promote, allergic lung inflammation in murine models of asthma. J Immunol. 2014;193:4245–4253. doi: 10.4049/jimmunol.1400580. [DOI] [PMC free article] [PubMed] [Google Scholar]