Abstract
Microbial colonization of the infant gut is unstable and shows a wide range of diversity between individuals. Gut microbiota play an important role in the development of the immune system, and an imbalance in these organisms can affect health, including an increased risk of allergic diseases. Microbial colonization of young infants is affected by the delivery mode at birth and the consequent alterations of gut microbiota in early life affect the development of allergic diseases. We investigated the effects of the delivery mode on the temporal dynamics of gut microbiota in healthy Korean infants. Fecal samples were collected at 1-3 days, 1 month, and 6 months after birth in six healthy infants. Microbiota were characterized by 16S rRNA shotgun sequencing. At the first and third days of life, infants born by vaginal delivery showed a higher richness and diversity of gut microbiota compared with those born by cesarean section. However, these differences disappeared with age. The Bacteroides genus and Bacteroidetes phylum were abundant in infants born by vaginal delivery, whereas Bacilli and Clostridium g4 were increased in infants born by cesarean section. The Firmicutes phylum and Bacteroides genus showed convergent dynamics with age. This study demonstrated the effect of delivery mode on the dynamics of gut microbiota profiles in healthy Korean infants.
Keywords: Delivery mode, gut, microbiota
INTRODUCTION
The prevalence of immune-mediated chronic diseases, such as allergic diseases, has increased over the last 50 years.1 Changes in life-style and environmental factors may have contributed to an imbalance in gut microbiota,2,3,4 which can affect the host immune responses, nutritional status and metabolic status, resulting in systemic chronic inflammation.5 This chronic low-grade inflammation can cause various noncommunicable diseases, including allergic diseases.2
The composition of gut microbiota is influenced by various factors, such as age, ethnicity, and environmental factors.6,7 Given that gut microbiota profiles tend to fluctuate until 3 years of age,8,9 and that allergic diseases occur earlier in life compared with other non-communicable diseases, it is essential to characterize early gut microbiota profiles for identification of the mechanisms underlying their relationship to immune-mediated diseases.
Environmental factors, such as perinatal antibiotic administration, mode of delivery, type of infant feeding, gestational age, and probiotic administration affect the composition of the gut microbiota.10,11,12 Among the modifying factors, mode of delivery has been reported to strongly affect the development of allergic diseases.13,14,15,16 In particular, the risk of allergic diseases, including asthma and allergic rhinitis, is increased in infants born by cesarean section.13,14,15,16 This association may be attributable to the lack of contact with maternal gut microbiota during cesarean section.10 However, studies on the effects of delivery mode on the gut microbiota have been limited by confounding factors such as antibiotic usage and a single specimen collection.10,17
The aim of our current study was to identify the effects of delivery mode on the composition of gut microbiota in infants after controlling for confounding factors such as perinatal antibiotics, perinatal probiotics, and feeding type.
MATERIALS AND METHODS
Study population
The study population consisted of six healthy infants enrolled from January 2012 to December 2013. Fecal samples were collected 1-3 days, 1 month, and 6 months after birth. Three of the six infants were born by caesarean section and the other three infants were born vaginally (Table 1). To control for external factors that might influence the composition of gut microbiota, we selected subjects with no history of antibiotic use, probiotic use, or infection during the first six months of life and no history of antibiotics during pregnancy. All subjects were fed with a combination of breastmilk and formula during the study period.
Table 1. Characteristics of the study infants.
| Sample ID | Gestational age (week) | Sex | Mode of delivery | Use of antibiotics & probiotics during the first 6 months of age | Use of antibiotics & probiotics during pregnancy | Maternal sensitization on skin prick tests | Paternal sensitization on skin prick tests | Family history of allergic diseases |
|---|---|---|---|---|---|---|---|---|
| CD 1 | 40.0 | F | CD | No | No | No | Not done | No |
| CD 2 | 38.5 | F | CD | No | No | Yes | No | No |
| CD 3 | 40.3 | F | CD | No | No | No | Yes | Yes |
| VD 1 | 38.5 | M | VD | No | No | No | Yes | No |
| VD 2 | 38.2 | F | VD | No | No | Not done | Not done | No |
| VD 3 | 39.2 | M | VD | No | No | No | No | No |
CD, cesarean delivery; F, female; M, male; VD, vaginal delivery.
This study protocol was approved by the institutional review boards (IRBs) of the Asan Medical Center (IRB No. 2012-1137). Informed consent forms were confirmed by each IRB and obtained from the parents of each infant.
Genomic DNA extraction
Parents collected fecal samples into feces tubes and immediately placed them at -20℃. The samples were then rapidly delivered to Asan Institute for Life Science and stored at -70℃. Metagenomic DNA was isolated from fecal samples using a DNA extraction kit (MP Biomedicals, Santa Ana, CA, USA) in accordance with the manufacturer's instructions. DNA was eluted in 50 µL of elution buffer and stored at -20℃ prior to use. Genomic DNA concentration and purity was assessed by spectrophotometry. After removing humic acid with the Power-Clean® DNA Clean-Up Kit (MO BIO Laboratories, Carlsbad, CA, USA), polymerase chain reaction (PCR) was performed on each fecal specimen.
Sequencing of the 16S rRNA Gene
16S rRNA gene analysis was performed by 454 pyrosequencing of the V1-V3 regions. Details of this gene analysis are described elsewhere.18
Statistical analysis
The CLcommunity™ software program was used to analyze the pyrosequencing sequences of the gut microbiota. Comparisons of the richness, diversity, and relative abundance of gut microbiota between infants born by caesarean section and those born vaginally were performed using the Mann-Whitney U test. All statistical analyses were performed with SAS version 9.3 for Windows (SAS Inc., Cary, NC, USA). P values ≤0.05 were considered statistically significant.
RESULTS
General characteristics of the study population
The general characteristics of the study population are summarized in Table 1. There were no significant differences in gestational age or birth weight between infants born by vaginal delivery and those born by cesarean section.
Pyrosequences of gut microbiota
Operational taxonomic units (OTUs) were significantly increased in infants born by vaginal delivery compared with those born by caesarean section at 1-3 days of life (P=0.024). OTUs continuously increased over time in infants delivered by caesarean section. However, OTUs decreased at 1 month of age in infants born by vaginal delivery, although they subsequently increased (Table 2).
Table 2. Comparisons of the number of sequences analyzed and operational taxonomic units (OTUs) in the study series.
| Variables | Sampling periods | Infants born by vaginal delivery (n=3) | Infants born by cesarean section (n=3) | P value* | ||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |||
| Total bacterial sequence | 1-3 days | 1,097.0 | 0.0 | 4,389.0 | 3,292.0 | 0.317 |
| 1 mo | 5,312.0 | 0.0 | 5,312.0 | 0.0 | 1.000 | |
| 6 mo | 15,495.0 | 6,024.2 | 7,497.7 | 2,079.4 | 0.050 | |
| OTUs | 1-3 days | 129.3 | 22.5 | 33.0 | 15.2 | 0.024 |
| 1 mo | 79.7 | 4.3 | 67.7 | 14.9 | 0.482 | |
| 6 mo | 169.3 | 30.2 | 104.0 | 4.4 | 0.161 | |
| Good's coverage | 1-3 days | 0.948 | 0.015 | 0.997 | 0.015 | 0.050 |
| 1 mo | 0.996 | 0.000 | 0.995 | 0.001 | 0.513 | |
| 6 mo | 0.997 | 0.001 | 0.996 | 0.001 | 0.050 | |
*Mann-Whitney U test.
mo, months; OTUs, operational taxonomic units; SEM, standard error of mean.
Comparisons of gut microbiota richness and diversity
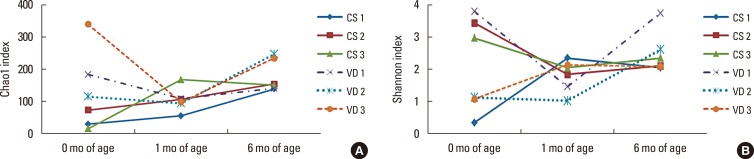
The Chao1 and Shannon indexes, which represent alpha-diversity and microbiota richness, decreased from 1-3 days to 1 month of life in infants born by vaginal delivery, with the exception of one infant that showed an increase in the Shannon index (Fig. 1). Conversely, infants born by cesarean section showed a pattern of continuous increases in these indexes with age.
Fig. 1. Comparisons of the (A) Chao1 and (B) Shannon diversity index of the gut microbiota according to age between infants born by vaginal delivery and those born by cesarean delivery.
Comparisons of gut microbiota composition
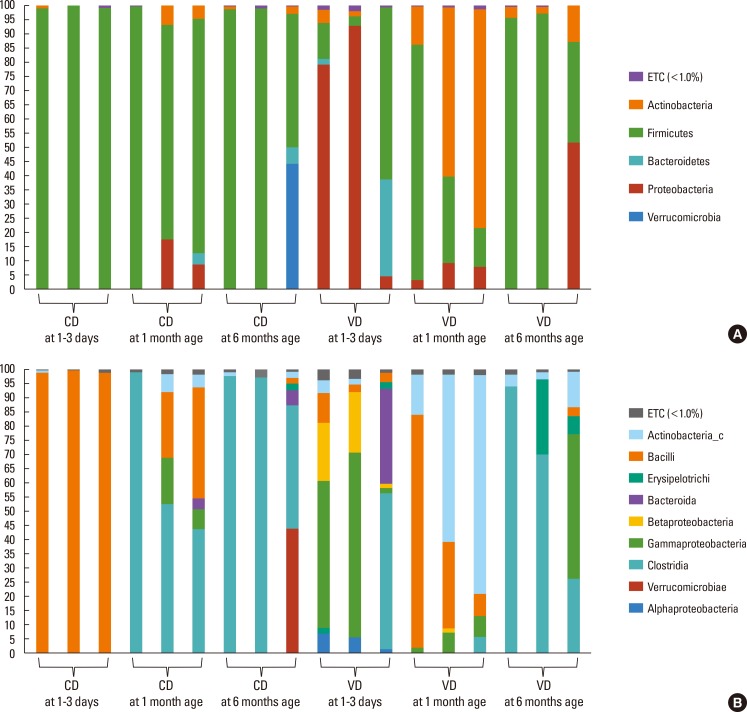
In both study groups, the most dominant bacteria for the first six months of life were of the Firmicutes phylum (cesarean section, 78.96%; vaginal delivery, 48.07%; P=0.005) and the Clostridia class (cesarean section, 48.52%; vaginal delivery, 28.30%; P=0.627) (Figs. 2 and 3).
Fig. 2. Relative abundance of bacterial 16S rRNA genes from fecal samples of the three vaginally delivered and 3 cesarean delivered infants at the phylum (A) and class (B) level by age. Each column represents 1 infant, as described in Table 1. CD, cesarean section delivery; VD, vaginal delivery.
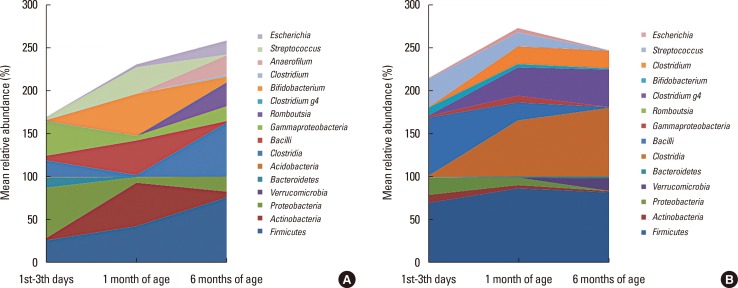
Fig. 3. Microbiota composition at 1st-3rd day of life, 1 month, and 6 months of age in vaginally delivered infants (A) and those born by caesarean section (B).
At birth, levels of the Bacilli class were significantly higher in infants born by cesarean section (Table 3). However, the Bacilli class consistently decreased over time in these infants. The Bacteroidetes phylum was nearly undetectable at 1-3 days of life in infants born by cesarean section, but increased with age, while it showed fluctuation with age in those born by vaginal delivery.
Table 3. Comparisons of fecal microbiota in the infant subjects by the mode of delivery.
| Sampling period | Class/Genus/Species | Microbiota | Vaginal delivery (n=3) | Cesarean section delivery (n=3) | P value* | ||
|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | ||||
| 1-3 days | |||||||
| Class | Bacilli | 5.409 | 4.564 | 99.441 | 0.529 | <0.001 | |
| Genus | Clostridium | 0.547 | 0.598 | 0.030 | 0.053 | 0.210 | |
| Lactobacillus | 0.365 | 0.190 | 33.300 | 33.300 | 0.817 | ||
| Staphylococcus | 1.459 | 0.329 | 4.430 | 3.717 | 0.507 | ||
| Species | Bacteroidetes | 11.942 | 19.186 | 0.003 | 0.005 | 0.046 | |
| Uncultured Bifidobacterium | NA | NA | NA | NA | NA | ||
| Bifidobacterium longum | 0.699 | 0.699 | 0.334 | 0.334 | 0.796 | ||
| Clostridium difficile | 0.000 | 0.000 | 0.030 | 0.030 | 0.317 | ||
| Staphylococcus epidermidis | 0.729 | 0.329 | 33.090 | 33.090 | 0.507 | ||
| 1 month | |||||||
| Class | Bacilli | 40.412 | 38.307 | 20.858 | 19.632 | 0.475 | |
| Genus | Clostridium | 0.038 | 0.065 | 33.082 | 57.300 | 0.423 | |
| Lactobacillus | 4.474 | 2.567 | 4.154 | 4.126 | 0.513 | ||
| Staphylococcus | 4.430 | 3.717 | 0.100 | 0.060 | 0.275 | ||
| Species | Bacteroidetes | 0.408 | 0.462 | 1.255 | 2.174 | 0.507 | |
| Uncultured Bifidobacterium | 0.038 | 0.019 | 0.000 | 0.000 | 0.026 | ||
| Bifidobacterium longum | 48.739 | 18.810 | 2.297 | 2.297 | 0.046 | ||
| Clostridium difficile | 0.000 | 0.000 | 33.020 | 33.020 | 0.317 | ||
| Staphylococcus epidermidis | 3.765 | 3.450 | 0.044 | 0.044 | 0.246 | ||
| 6 months | |||||||
| Class | Bacilli | 1.583 | 1.747 | 0.931 | 0.788 | 0.587 | |
| Genus | Clostridium | 0.121 | 0.139 | 43.236 | 16.409 | 0.045 | |
| Lactobacillus | 0.037 | 0.033 | 0.000 | 0.000 | 0.121 | ||
| Staphylococcus | 0.011 | 0.006 | 0.004 | 0.004 | 0.246 | ||
| Species | Bacteroidetes | 0.007 | 0.007 | 1.885 | 3.248 | 0.376 | |
| Uncultured Bifidobacterium | 0.019 | 0.032 | 0.004 | 0.006 | 0.510 | ||
| Bifidobacterium longum | 5.203 | 1.999 | 0.798 | 0.278 | 0.050 | ||
| Clostridium difficile | 0.093 | 0.093 | 19.250 | 10.017 | 0.246 | ||
| Staphylococcus epidermidis | 0.003 | 0.003 | 0.000 | 0.000 | 0.317 | ||
*Mann-Whitney U test.
SEM, standard error of mean; NA, not applicable.
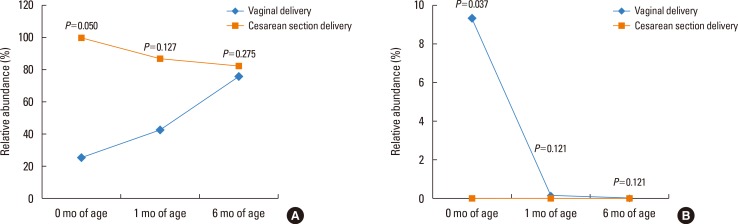
The relative proportion of both the uncultured Bifidobacterium and Bifidobacterium longum species was higher in infants born by vaginal delivery for the first six months of life. The Clostridium difficile species was higher in infants born by cesarean section compared with those born by vaginal delivery. The Clostridium g4 genus increased with age in infants born by cesarean section, but remained at low levels in infants born by vaginal delivery. The Firmicutes phylum and Bacteroides genus showed a convergent pattern with age between the 2 groups (Fig. 4).
Fig. 4. Temporal patterns of convergent gut microbiota including (A) Firmicutes phylum and (B) Bacteroides genus.
DISCUSSION
In our present study, we evaluated the temporal pattern of the diversity and composition of gut microbiota according to delivery mode in healthy Korean infants during the first six months of life after controlling for confounding factors. The richness and diversity of gut microbiota in vaginally delivered infants were higher at birth, decreased at 1 month, and then subsequently increased. In infants born by cesarean section, the richness and diversity of gut microbiota were low at birth, but continuously increased with age. This chronological pattern of gut microbiota may reflect differences in the maturation course of gut microbiota according to delivery mode.
Previous studies have shown a lower diversity and lower abundance of gut microbiota during early life, especially in infants born by cesarean section.17 Under the influence of the mother's gut microbiota during delivery, the diversity and richness of gut microbiota after birth were greater in infants born vaginally but subsequently decreased by 1 month of age, which was also observed in the previous studies.17 Although the underlying meaning has not been established, the increased diversity immediately after birth may be attributable to the transmission of maternal gut microbiota, even including noncolonizable microbiota in vaginally delivered infants. After 1 month of life, the diversity and richness of the gut microbiota increased by six months of age. Although there was a wide variety between individuals, the variability in richness and diversity of gut microbiota during early life might reflect the transitional state between the sterile infant gut and the mature adult gut microbiota.
Differences in the composition of gut microbiota immediately after birth are attributable to mother-related factors such as intestinal microflora, especially in infants born vaginally. The representative gut microbiota in infants delivered vaginally include Lactobacillus, Prevotella, and Sneathia species, which are abundantly present in the mother's vagina.19 On the other hand, the Staphylococcus, Corynebacterium, and Propionibacterium species, which are present on the skin surface, have been identified as the main gut microbiota in infants born by cesarean section.19 In our present study, the relative proportions of the Bacteroides genus and the Bacteroidetes phylum at 1-3 days after birth were higher in infants born vaginally. Considering that the Bacteroides is thought to be the main component of the adult gut microbiota,20 the increased proportion of Bacteroides in infants born vaginally might be partially attributable to the maternal transmission of gut microbiota during delivery. Some species of the Bacteroides genus can activate T cell-dependent immune responses with homeostasis of the host immune response and thereby contribute to health.21
The Clostridium difficile species and Clostridium g4 genus increased with age in the infants in our study born by cesarean section, while consistently lower levels were observed in infants born vaginally during the first 6 months of life. This result is in agreement with the previous findings of a Netherlands birth cohort study performed on infants at 1 month of age.12 Our present study provides new evidence for a common and characteristic composition pattern of gut microbiota during early life in infants born by cesarean section, regardless of ethnicity.
Clostridium has been reported to be increased in infants born by cesarean section.22 Also, colonization with Clostridium in the gut was shown to be associated with increased risk of wheeze and eczema.23 Based on these previous findings as well as our own, we conclude that the delivery mode may affect the development of allergic diseases through immune-modulatory effects resulting from changes in the composition of gut microbiota.
Even at 6 months of age, the Bacteroides genus and Clostridia class, which are abundant in gut microbiota of mature adults,24 did not dominate either infant group in our current study. The relative abundance of the Firmicutes phylum and Bacteroides genus seemed to converge towards a similar level with age in both groups. As these bacteria are dominant in the adult gut,24 this convergent pattern might reflect a maturation process in the gut microbiota of healthy infants.
One strength of our study is that we attempted to avoid confounding factors that affect the composition of gut microbiota, such as administration of probiotics and antibiotics in both infants and mothers. We also enrolled infants who were undergoing mixed feeding of breastmilk and formula during the first six months of life. Differences in the concentration and composition of gut microbiota might be partially affected by different ratios between breast milk and formula.
Our study was limited by the small number of participants and short follow-up period. However, it has value in its presentation of chronological changes in the diversity and composition of gut microbiota during early life in healthy Korean infants after controlling for several important confounding factors. Also, our current findings provide fundamental new information on the dynamics of gut microbiota in healthy Korean infants for further studies.
In conclusion, the increased richness and diversity of gut microbiota at birth gradually decrease at 1 month of age in infants born by vaginal delivery. Thereafter, an increasing pattern of diversity is observed with age. The richness and diversity of gut microbiota steadily increase with age in infants born by cesarean section. The convergent temporal pattern of specific gut microbiota with age in infants according to delivery mode might suggest effects on human health via several mechanisms, including immune-modulation early in life.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (NRF-2012R1A1A1015308).
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Kim WK, Kwon JW, Seo JH, Kim HY, Yu J, Kim BJ, et al. Interaction between IL13 genotype and environmental factors in the risk for allergic rhinitis in Korean children. J Allergy Clin Immunol. 2012;130:421–426.e5. doi: 10.1016/j.jaci.2012.04.052. [DOI] [PubMed] [Google Scholar]
- 2.West CE, Renz H, Jenmalm MC, Kozyrskyj AL, Allen KJ, Vuillermin P, et al. The gut microbiota and inflammatory noncommunicable diseases: associations and potentials for gut microbiota therapies. J Allergy Clin Immunol. 2015;135:3–13. doi: 10.1016/j.jaci.2014.11.012. quiz 4. [DOI] [PubMed] [Google Scholar]
- 3.Kim BJ, Lee SY, Kim HB, Lee E, Hong SJ. Environmental changes, microbiota, and allergic diseases. Allergy Asthma Immunol Res. 2014;6:389–400. doi: 10.4168/aair.2014.6.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SY, Kang MJ, Kwon JW, Park KS, Hong SJ. Breastfeeding Might Have Protective Effects on Atopy in Children With the CD14C-159T CT/CC Genotype. Allergy Asthma Immunol Res. 2013;5:239–241. doi: 10.4168/aair.2013.5.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renz H, von Mutius E, Brandtzaeg P, Cookson WO, Autenrieth IB, Haller D. Gene-environment interactions in chronic inflammatory disease. Nat Immunol. 2011;12:273–277. doi: 10.1038/ni0411-273. [DOI] [PubMed] [Google Scholar]
- 6.Bisgaard H, Bønnelykke K, Stokholm J. Immune-mediated diseases and microbial exposure in early life. Clin Exp Allergy. 2014;44:475–481. doi: 10.1111/cea.12291. [DOI] [PubMed] [Google Scholar]
- 7.Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5:e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev. 2010;86(Suppl 1):13–15. doi: 10.1016/j.earlhumdev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Russell SL, Gold MJ, Reynolds LA, Willing BP, Dimitriu P, Thorson L, et al. Perinatal antibiotic-induced shifts in gut microbiota have differential effects on inflammatory lung diseases. J Allergy Clin Immunol. 2015;135:100–109. doi: 10.1016/j.jaci.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 13.Bager P, Wohlfahrt J, Westergaard T. Caesarean delivery and risk of atopy and allergic disease: meta-analyses. Clin Exp Allergy. 2008;38:634–642. doi: 10.1111/j.1365-2222.2008.02939.x. [DOI] [PubMed] [Google Scholar]
- 14.Pistiner M, Gold DR, Abdulkerim H, Hoffman E, Celedón JC. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J Allergy Clin Immunol. 2008;122:274–279. doi: 10.1016/j.jaci.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seo JH, Kim HY, Jung YH, Lee E, Yang SI, Yu HS, et al. Interactions between innate immunity genes and early-life risk factors in allergic rhinitis. Allergy Asthma Immunol Res. 2015;7:241–248. doi: 10.4168/aair.2015.7.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SY, Yu J, Ahn KM, Kim KW, Shin YH, Lee KS, et al. Additive effect between IL-13 polymorphism and cesarean section delivery/prenatal antibiotics use on atopic dermatitis: a birth cohort study (COCOA) PLoS One. 2014;9:e96603. doi: 10.1371/journal.pone.0096603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63:559–566. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 18.Park SH, Kim KA, Ahn YT, Jeong JJ, Huh CS, Kim DH. Comparative analysis of gut microbiota in elderly people of urbanized towns and longevity villages. BMC Microbiol. 2015;15:49. doi: 10.1186/s12866-015-0386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troy EB, Kasper DL. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front Biosci (Landmark Ed) 2010;15:25–34. doi: 10.2741/3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penders J, Gerhold K, Thijs C, Zimmermann K, Wahn U, Lau S, et al. New insights into the hygiene hypothesis in allergic diseases: mediation of sibling and birth mode effects by the gut microbiota. Gut Microbes. 2014;5:239–244. doi: 10.4161/gmic.27905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Nimwegen FA, Penders J, Stobberingh EE, Postma DS, Koppelman GH, Kerkhof M, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011;128:948–955.e3. doi: 10.1016/j.jaci.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 24.Andersson AF, Lindberg M, Jakobsson H, Bäckhed F, Nyrén P, Engstrand L. Comparative analysis of human gut microbiota by barcoded pyrosequencing. PLoS One. 2008;3:e2836. doi: 10.1371/journal.pone.0002836. [DOI] [PMC free article] [PubMed] [Google Scholar]