Abstract
Objective
A branched-chain amino acid (BCAA)-related metabolic signature is strongly associated with insulin resistance and predictive of incident diabetes and intervention outcomes. To better understand the role that this metabolite cluster plays in obesity-related metabolic dysfunction, we studied the impact of BCAA restriction in a rodent model of obesity in which BCAA metabolism is perturbed in ways that mirror the human condition.
Methods
Zucker-lean rats (ZLR) and Zucker-fatty rats (ZFR) were fed either a custom control, low fat (LF) diet, or an isonitrogenous, isocaloric LF diet in which all three BCAA (Leu, Ile, Val) were reduced by 45% (LF-RES). We performed comprehensive metabolic and physiologic profiling to characterize the effects of BCAA restriction on energy balance, insulin sensitivity, and glucose, lipid and amino acid metabolism.
Results
LF-fed ZFR had higher levels of circulating BCAA and lower levels of glycine compared to LF-fed ZLR. Feeding ZFR with the LF-RES diet lowered circulating BCAA to levels found in LF-fed ZLR. Activity of the rate limiting enzyme in the BCAA catabolic pathway, branched chain keto acid dehydrogenase (BCKDH), was lower in liver but higher in skeletal muscle of ZFR compared to ZLR and was not responsive to diet in either tissue. BCAA restriction had very little impact on metabolites studied in liver of ZFR where BCAA content was low, and BCKDH activity was suppressed. However, in skeletal muscle of LF-fed ZFR compared to LF-fed ZLR, where BCAA content and BCKDH activity were increased, accumulation of fatty acyl CoAs was completely normalized by dietary BCAA restriction. BCAA restriction also normalized skeletal muscle glycine content and increased urinary acetyl glycine excretion in ZFR. These effects were accompanied by lower RER and improved skeletal muscle insulin sensitivity in LF-RES fed ZFR as measured by hyperinsulinemic-isoglycemic clamp.
Conclusions
Our data are consistent with a model wherein elevated circulating BCAA contribute to development of obesity-related insulin resistance by interfering with lipid oxidation in skeletal muscle. BCAA-dependent lowering of the skeletal muscle glycine pool appears to contribute to this effect by slowing acyl-glycine export to the urine.
Keywords: Obesity, BCAA, Insulin sensitivity, Metabolism
Highlights
-
•
Feeding a BCAA restricted diet improves skeletal muscle insulin sensitivity in Zucker fatty rats.
-
•
BCKDH activity is decreased in liver and increased in skeletal muscle in Zucker fatty versus lean rats.
-
•
High BCAA levels drive the obesity-associated decline in circulating and muscle glycine levels.
-
•
BCAA-driven glycine depletion restricts formation of acyl-glycine adducts for excretion in urine.
-
•
High BCAA/low glycine reduces efficiency of fat oxidation in muscle leading to acyl CoA buildup.
1. Introduction
Aberrant amino acid metabolism has long been recognized as a feature of obesity and accompanying metabolic disease. In 1969 Felig, Marliss, and Cahill [1] made the seminal observation that obese persons have higher levels of the branched chain (BCAA; Leucine, isoleucine and valine) and aromatic (phenylalanine and tyrosine) amino acids and lower levels of glycine in blood compared to lean individuals. More recently, unbiased metabolic profiling studies performed by our group [2], [3], [4] and others [5] have revived interest in perturbed amino acid metabolism as a potential contributor to development of metabolic diseases by revealing that a cluster of circulating metabolites comprising these same branched-chain and aromatic amino acids, as well as glutamate/glutamine, methionine, alanine, and the C3 and C5 acylcarnitines is strongly associated with insulin sensitivity [2], cardiometabolic health [6], future diabetes risk [5], and metabolic outcomes of weight loss interventions [7], [8].
Current evidence suggests that the obesity-related rise in circulating BCAA is the product of multiple metabolic perturbations related to their synthesis and catabolism, rather than being driven by increased intake of these essential amino acids [4], [9]. One potential contributing factor has emerged from studies of the microbiota from monozygotic twins discordant for obesity, which revealed that obesity-driven shifts in microbial communities results in higher production and lower catabolism of BCAA by the intestinal flora [10]. Indeed, transfer of gut microbiota from obese or lean twins to gnotobiotic mice is sufficient to raise circulating BCAA in animals that received the microbiota of the obese twin by a magnitude similar to that reported for obese versus lean humans.
In addition, hepatic activity of the branched chain keto acid dehydrogenase (BCKDH) complex, which is responsible for the first irreversible and rate limiting step in BCAA metabolism, is low in obese and insulin resistant animals [11], [12]. This is due to increased expression of the BCKDH kinase, BDK, and decreased expression of the BCKDH phosphatase, PPM1K, causing BCKDH to be in a hyperphosphorylated and inhibited state. Since liver is considered the primary site for catabolism of branched chain keto acids (BCKA) this represents a considerable systemic impairment. Demonstrating the control strength of this reaction, hypothalamic insulin and leptin lower circulating BCAA by up to 50% by reducing the phosphorylation state of hepatic BCKDH [13]. Furthermore, expression of BDK and PPM1K is regulated by adiponectin through an AMPK-dependent signal, and adiponectin knockout mice have lower PPM1K expression in liver accompanied by higher circulating BCAA [12]. Together, these data suggest that obesity-related changes in the hormonal milieu likely drive the inactivation of hepatic BCKDH by influencing the balance of the BDK/PPM1K regulatory node.
In adipose tissue, decreased BCAA metabolism in response to obesity appears to occur via global regulation of multiple enzymes in the catabolic pathway at a transcriptional level, rather than by post-translational modification as in liver [14], [15]. Interestingly, interventions that reverse obesity-associated metabolic dysregulation, including bariatric surgery or treatment with thiazolidinedione drugs, restore expression of the BCAA catabolic enzymes in adipose tissue in concert with improved glucose homeostasis [11], [15]. Remarkably, obesity-regulated post-translational or transcriptional regulation of the BCAA catabolic pathway has not been reported in skeletal muscle. Taken together, these findings highlight tissue-specific differences in BCAA catabolism in response to obesity.
Although our understanding of factors regulating the levels of circulating BCAA in obesity has evolved as described, the role of these metabolites in obesity-associated metabolic disorders remains to be defined [16]. In healthy humans, acute infusion of a complete amino acid mixture results in skeletal muscle and hepatic insulin resistance accompanied by activation of the mammalian target of rapamycin/S6 kinase 1 pathway [17], [18], [19]. However, various rodent studies involving specific supplementation of BCAA have yielded diverse findings, probably due in part to differences in study design. These have ranged from supplementation of all three BCAA in HF diets, resulting in exacerbation of insulin resistance [2], to addition of leucine alone in the drinking water, which is reported to have either no effect [20] or a beneficial effect on glucose homeostasis [21], [22]. Less studied is the impact of BCAA restriction on metabolic control in models in which endogenous BCAA metabolism is perturbed in ways that mirror the human condition.
Herein we describe the results of a nutritional intervention study in which we restricted BCAA dietary supply by 45% in Zucker-lean (ZLR) and Zucker-fatty rats (ZFR), the latter being a model of insulin resistance and impaired BCAA metabolism [23]. We performed comprehensive metabolic and physiologic profiling to characterize the role of BCAA restriction on energy balance, insulin sensitivity, and glucose, lipid, and amino acid metabolism. Our findings demonstrate a clear cause and effect relationship between BCAA supply and insulin sensitivity and highlight underlying biochemical mechanisms.
2. Materials & methods
2.1. Animals and diets
Six week-old male Zucker-lean (ZLR) and Zucker-fatty rats (ZFR) from Charles River Laboratories were placed on either a custom control low fat (LF) diet (A11072001, Research Diets, New Brunswick, NJ) or a LF BCAA restricted diet (LF-RES; A11072002, Research Diets, New Brunswick, NJ) in which 45% of the BCAA component of the LF diet was removed and replaced by a small increment in all other amino acids (except phenylalanine and tyrosine) such that nitrogen content was equal in both diets (Table 1). Rats were individually housed in a 12 h light:dark cycle with ad libitum access to water and food for up to 15 weeks. Food intake and weight gain were monitored weekly. A non-fasting blood sample was taken after 9 weeks on the diets at 9AM to determine the genotype and dietary effect on circulating amino acid concentrations. Plasma and tissue samples used for biochemical analysis were derived from rats that were euthanized in the fed state at week 15 by exsanguination following intraperitoneal administration of Nembutal (80 mg/kg). Tissues were rapidly excised, weighed, and freeze clamped in liquid nitrogen and then stored at −80 °C until further analysis. All animal procedures were approved and carried out in accordance with the directions of the Duke University Institutional Animal Care and Use Committee.
Table 1.
Diet amino acid content.
| % | LF: Research Diets A11072001 |
LF-RES: Research Diets A11072002 |
||
|---|---|---|---|---|
| grams | kcal | grams | kcal | |
| Macronutrients | ||||
| Protein | 33.6 | 33 | 33.6 | 33 |
| Carbohydrate | 49.3 | 49 | 49.3 | 49 |
| Fat | 8.0 | 18 | 8.0 | 18 |
| Fiber | 4.0 | 0 | 4.0 | 0 |
| Total | 90.9 | 100 | 90.9 | 100 |
| kcal/gm | 4.03 | 4.03 | ||
| Amino acids | ||||
| Isoleucine | 17.88 | 72 | 9.83 | 39 |
| Leucine | 31.63 | 127 | 17.40 | 70 |
| Valine | 23.38 | 94 | 12.86 | 51 |
| Phenylalanine | 17.88 | 72 | 17.88 | 72 |
| Tyrosine | 12.38 | 50 | 12.38 | 50 |
| Cysteine | 5.50 | 22 | 6.29 | 25 |
| Lysine | 24.75 | 99 | 28.28 | 113 |
| Methionine | 6.88 | 28 | 7.86 | 31 |
| Tryptophan | 5.50 | 22 | 5.50 | 22 |
| Histidine | 8.25 | 33 | 9.43 | 38 |
| Alanine | 12.38 | 50 | 14.15 | 57 |
| Arginine | 27.50 | 110 | 31.43 | 126 |
| Aspartic acid | 20.63 | 83 | 23.58 | 94 |
| Glutamic acid | 37.13 | 149 | 42.43 | 170 |
| Glycine | 38.50 | 154 | 44.00 | 176 |
| Proline | 17.88 | 72 | 20.43 | 82 |
| Serine | 15.13 | 61 | 17.29 | 69 |
2.2. Glucose tolerance tests
After 10 weeks on the respective diets, rats were fasted for 16 h with free access to water and a 1 g/kg intraperitoneal glucose tolerance test was performed. Blood samples were obtained from the tail vein before the glucose injection and at the indicated time points after the glucose load. Glucose levels were measured with a BD Logic glucose meter and insulin concentrations were determined in plasma samples using a Millipore EMD Rat insulin ELISA.
2.3. Indirect calorimetry
Indirect calorimetry was performed in weeks 11 and 12 of feeding using an eight-chamber Oxymax system (Columbus Instruments; Columbus, OH). Rats were acclimatized to the system for 24 h prior to measurement. Recordings were made while food and water was freely accessible. Urine was also collected from the Oxymax chambers for analysis of urinary acetylglycine content.
2.4. Hyperinsulinemic isoglycemic clamps
A cohort of ZFR was subjected to hyperinsulinemic-isoglycemic clamps after 14–15 weeks of LF or LF-RES diet feeding in order to assess insulin sensitivity. Seven days prior to clamp experiments, animals were anesthetized with 2% isoflurane (Butler Schein, Dublin OH), and polyethylene catheters were aseptically placed in the left carotid artery (advanced to the aortic arch) or the right jugular vein. Catheters were filled with 3% heparinized saline to maintain patency and exteriorized in the intrascapular region. Buprenorphine (0.03 mg/kg, s.c.) was postsurgically administered for pain control, and animals recovered to preoperative weight prior to experiments. Buprenorphine was given to all animals in the first 3 days following surgery, and then removed for 4 days until clamps were carried out on day 7.
Clamps were performed in conscious unrestrained animals using swivels and tethers (Instech) to allow uninterrupted movement of the animals without disruption of infusion lines. Hyperinsulinemia was achieved by a primed (3 min 60 mU/kg/min) continuous 10 mU/kg/min intravenous insulin infusion. Blood was sampled from arterial lines at 5 min intervals and analyzed with a BD Logic glucose meter. Isoglycemia, defined as blood glucose within 10% of individual fasting glycemia, was restored and maintained by variable infusion of 30% dextrose. Steady state was achieved approximately 90 min after initiating hyperinsulinemia and maintained for at least 45 min. Once steady state had been maintained for 45 min, a [U-14C] glucose (45 μCi/rat) and [3H]2-deoxyglucose (67.6 μCi/rat) bolus was administered to determine tissue glucose uptake and rates of glucose incorporation into glycogen. After tracer injection, blood was collected at 2, 5, 10, 15, 20, 30 and 40 min and 50 μL plasma was transferred into tubes containing 1 mL of ZnSO4 (2.75%w/v). 350 μL of saturated BaOH was then added to the ZnSO4-plasma. Samples were centrifuged and total 3H and 14C in the supernatant was counted in Uniscint BD Scintillation fluid in a Beckman Coulter LS6500 liquid scintillation counter to determine total tracer disappearance. Immediately following the clamp procedure, rats were euthanized with Euthasol, and gastrocnemius muscle and epididymal white adipose tissue (eWAT) were quickly harvested and freeze clamped. Samples were later pulverized under liquid nitrogen and stored at −80 °C for further biochemical analysis.
Tissue content of 3H-2-deoxyglucose-6-phosphate was used to determine glucose uptake in gastrocnemius and epididymal adipose samples. Briefly, tissue samples were deproteinated, loaded onto columns packed with a 0.75 mL AG-1 resin bed (BioRad #140-1453), and washed with 4 mL of distilled water. The 3H-2-DG-6-phosphate was then eluted off the column with 4 mL of 1 M ammonium acetate (Sigma #431311). Specific activity of the eluate was determined by counting 3H in Uniscint BD Scintillation fluid in a Beckman Coulter LS6500 liquid scintillation counter. To evaluate 14C glucose incorporation into glycogen, we extracted glycogen by a modified version of the Pfluger method. Briefly, 200 mg of tissue was dissolved in 1.5 mL of a 30% KOH solution at 60 °C for 1 h. Samples were vortexed gently, and 250 μL of saturated Na2SO4 and 2 mL of 100% ethanol were added to each sample. After gentle vortexing, samples were incubated at 85 °C until boiling point was reached and then immediately placed on ice to precipitate glycogen. Samples were centrifuged at 2500 RPM at 4 °C, and the supernatant was removed. The pellet was washed three times by suspension in 2 mL of distilled water and precipitation in 100% ethanol. The washed pellet was then resuspended in 1 mL of distilled water, and 14C was counted in Uniscint BD Scintillation fluid in a Beckman Coulter LS6500 liquid scintillation counter. Rates of glucose uptake and incorporation into glycogen were calculated as described [24].
2.5. Metabolite profiling
Amino acid profiles were derived from whole blood, liver, and gastrocnemius muscle. Organic acid, acylcarnitine and acyl CoA profiles were derived from liver and gastrocnemius muscle. Methods of sample handling and extraction have been described previously [25], [26]. Amino acid, acylcarnitine, acyl CoA and acetylglycine profiling was performed by tandem mass spectrometry (MS/MS) [2], [25], [26], [27]. Organic acids in liver and gastrocnemius muscle samples were analyzed by capillary gas chromatography-mass spectrometry (GS-MS) on a TRACE ISQ instrument (Thermo Electron Corporation) [2]. All MS analyses employed stable-isotope-dilution with internal standards from Isotec, Cambridge Isotopes Laboratories, and CDN Isotopes. A list of all internal standards used in these studies has been published previously [2], [25].
Plasma concentrations of the alpha-keto acids of leucine (α-keto-isocaproate, KIC), isoleucine (α-keto-β-methylvalerate, KMV) and valine (α-keto-isovalerate, KIV) were measured by LC-MS as previously described [28], [29]. Other plasma analytes were measured on a Beckman DxC600 autoanalyzer, using reagents for lactate, total cholesterol, and triglycerides from Beckman, and non-esterified fatty acids (NEFA) and ketones (total and 3-hydroxybutyrate) from Wako (Richmond, VA). Glycerol was measured using reagents from TG-B by Roche Diagnostics (Indianapolis, IN).
2.6. BCKDH activity assay
Tissue BCKDH activity was determined as previously described [13]. Briefly, frozen tissue samples were pulverized in liquid nitrogen, then homogenized using a QIAGEN TissueLyser II in 250 ul of ice cold buffer I (30 mM KPi pH 7.5, 3 mM EDTA, 5 mM DTT, 1 mM α-ketoisovalerate, 3% FBS, 5% Triton X-100, 1 μM Leupeptin). Samples were then centrifuged for 10 min at 10 000 ×g and 50 μL of supernatant was added to 300 μL of buffer II (50 mM HEPES pH 7.5, 30 mM KPi pH 7.5, 0.4 mM CoA, 3 mM NAD+, 5% FBS, 2 mM Thiamine Pyrophosphate, 2 mM MgCl2, 7.8 μM α-keto [1-14C] isovalerate) in a polystyrene test tube containing a raised 1 M NaOH CO2 trap. Tubes were capped and placed in a shaking water bath at 37 °C for 30 min. The reaction mixture was acidified by injection of 70% perchloric acid followed by shaking on an orbital shaker for 1 h. The 14CO2 contained in the trap was counted in a liquid scintillation counter.
2.7. Statistical analysis
All data are reported as mean ± the standard error of the mean. Statistical outliers determined as 2.5 standard deviations outside of the mean were excluded prior to statistical analysis. For plasma metabolites, there was a single outlier detected for hydroxybutyrate and two detected for glycerol. In liver, there was a single outlier detected for succinate, phenylalanine, Glx, citrulline, C6, C5-OH/C3-DC, C10, and C18:1 acylcarnitine; and two detected for leucine/isoleucine. In skeletal muscle there was a single outlier detected for pyruvate, glycine, serine, proline, valine, leucine/isoleucine, C4/Ci4, C5, C6, and C14 acylcarnitine and two for lactate, succinate, C16, C18, and C18:1 acylcarnitine. For studies comparing all four experimental groups, a full factorial analysis of variance (ANOVA) was performed in JMP Pro 12 to evaluate diet and genotype effects followed by Tukey's HSD post-hoc test for all pairwise multiple comparisons. A Pearson's correlation test was performed in JMP Pro 12 to assess the association between urinary acetylglycine and skeletal muscle glycine levels. For clamp and metabolic cage studies where only ZFR fed the two diets were compared, an unpaired two-sided student's t-test was performed in excel. In all cases, a P-value less than 0.05 was considered significant.
3. Results
3.1. Influence of Zucker-fatty genotype and BCAA restriction on circulating amino acids
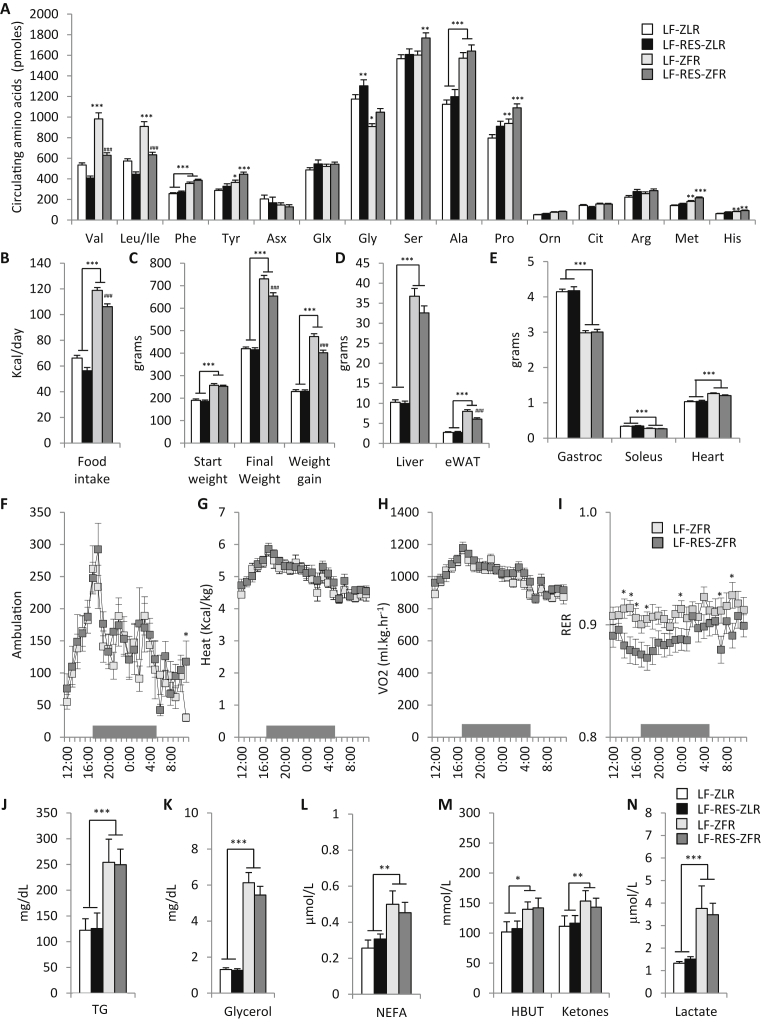
In this study, we employed a dietary approach to manipulate BCAA availability in Zucker-fatty rats (ZFR), which are hyperphagic due to a missense mutation in the leptin receptor (Lepr) gene, and also studied Zucker-lean rats (ZLR), which have at least one copy of normal Lepr. We fed these animals for 15 weeks with either a standard low fat (LF) diet, or a LF diet in which the BCAA content was reduced by 45% (LF-RES). The LF-RES diet was rendered isocaloric and isonitrogenous to the LF diet by increasing the levels of the non-BCAA amino acids evenly to offset the decrease in calories and nitrogen caused by BCAA restriction (Table 1). We first measured circulating amino acids in the two genotypes fed the two diets (Figure 1A). We found that ZFR fed the LF diet had approximately 50% higher levels of valine, leucine and isoleucine compared to ZLR on the LF diet (P < 0.001; Figure 1A). Thus, ZFR have similar increases in circulating BCAA to those found in obese and insulin resistant compared to lean and insulin sensitive humans [2], suggesting that they are a useful model for our studies. Feeding of ZFR with the LF-RES diet lowered circulating BCAA levels to those found in ZLR on the LF diet. Interestingly, preventing the rise in circulating BCAA in ZFR via dietary BCAA restriction did not lower phenylalanine or tyrosine levels in circulation even though these aromatic amino acids are consistently found to be increased in obese and insulin resistant humans in concert with the rise in BCAA [2], [5].
Figure 1.
Effect of Zucker-fatty genotype and BCAA restriction on circulating amino acids and energy balance. Male Zucker-lean rats (ZLR) or Zucker-fatty rats (ZFR) were fed a custom low fat (LF) diet or an isonitrogenous isocaloric LF diet in which BCAA were restricted by 45% (LF-RES). (A) Circulating amino acid profiles. (B) Daily food intake (kcal/day). (C) Starting weight, final weight, and total weight gain (grams). (D) Liver and epididymal adipose tissue (eWAT) weights (grams). (E) Gastrocnemius, soleus and heart tissue weights (grams). n = 12–28 per group (F) Ambulation (total x and y beam breaks), (G) Heat (kcal/kg), (H) VO2 (mL/kg/hr), and (I) Respiratory exchange ratio (RER) measured hourly during a 24 h cycle following a 24 h acclimation period in the metabolic cages, during which the dark period was from 5PM to 5AM as shown by the gray bar, n = 9–12 per group. (J) Plasma triglycerides (TG; mg/dL), (K) glycerol (mg/dL), (L) non-esterified fatty acids (NEFA; μM/L), (M) hydroxybutyrate and ketones (mmol/L), and (N) lactate (μM/L), n = 9–15 per group. Data represent mean ± SEM. Statistical differences indicated by: *: P < 0.05, **: P < 0.01, ***: P < 0.001 comparisons indicated by the connecting lines or in the absence of connecting lines vs LF-ZLR, and #: P < 0.05, ##: P < 0.01, ###: P < 0.001 vs LF-ZFR.
While neither diet nor genotype had a significant impact on circulating aspartate/asparagine (Asx) or glutamate/glutamine (Glx) (Figure 1A), LF-fed ZFR had lower circulating glycine levels than ZLR on the same diet (P < 0.05), consistent with differences in lean and obese humans [2], [30]. Notably, BCAA restriction raised circulating glycine in both ZLR and ZFR, revealing for the first time that the obesity-driven decline in circulating glycine is related to BCAA availability. In line with the partial restoration of the glycine pool in ZFR rats fed the LF-RES diet, we also observed higher levels of serine, derived from glycine by the bidirectional serine hydroxymethyltransferase reaction (P < 0.01).
We also note that circulating alanine was increased approximately 50% in ZFR compared to ZLR (P < 0.001), and diet did not influence this difference. Proline, methionine and histidine were each raised modestly by both the ZF genotype and the BCAA restriction diet (Figure 1A).
3.2. Dietary BCAA restriction influences food intake and weight gain
We found that feeding an isonitrogenous and isocaloric 45% BCAA restriction diet had a small yet significant (p < 0.001) effect on food intake in both ZLR and hyperphagic ZFR, with both groups consuming approximately 10% less kcal per day (Figure 1B). This decrease in food intake had no effect on weight gain over the feeding period or final weight at the end of feeding in ZLR but caused an approximate 15% decrease in weight gain and 10% decrease in final weight in the ZFR setting (Figure 1C). Nonetheless, on both the LF and LF-RES diets, ZFR were severely obese, as both groups gained approximately twice the weight gained by the ZLR during the 15-week feeding period (Figure 1C).
Furthermore, the livers of ZFR weighed 3.5 fold more than livers of ZLR (P < 0.001) and this was not significantly affected by BCAA restriction (Figure 1D). However, tissue weight of the eWAT was modestly yet significantly lower in the ZFR fed the LF-RES rats compared to the LF diet (P < 0.001; Figure 1D). This effect of the BCAA restriction diet was not present in ZLR, suggesting that elevated BCAA supply may contribute to the expansion of adipose mass in obese states. Notably, skeletal muscle and cardiac muscle mass were not significantly affected by BCAA restriction (Figure 1E).
3.3. Substrate preference is regulated by dietary BCAA supply
In order to better understand the metabolic effects of dietary BCAA restriction we studied ZFR in metabolic cages. Dietary BCAA restriction in ZFR had no effect on ambulation (Figure 1F), heat production (Figure 1G), or oxygen consumption (Figure 1H). However, BCAA restriction did cause a clear lowering of RER during the transitional period between the light and dark cycles (Figure 1I). These data are consistent with increased reliance on fatty acid oxidation in ZFR fed the BCAA restricted diet and suggest that decreased food intake rather than increased energy expenditure accounts for the small reduction in weight gain in LF-RES-fed ZFR.
Notably, the lower RER observed in ZFR fed the BCAA restricted diet was not associated with any changes in circulating triglycerides, glycerol, NEFA, ketones, or lactate (Figure 1J–N). This suggests that lower RER in BCAA restricted ZFR is not due to alterations in substrate supply.
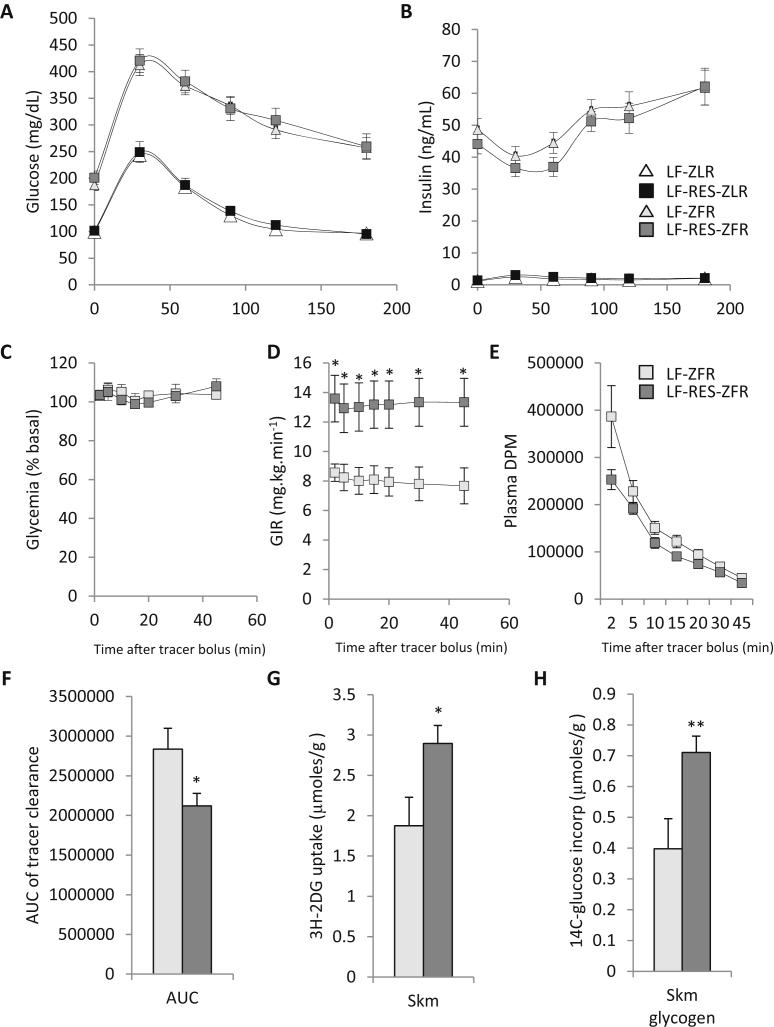
3.4. Glucose tolerance is not influenced by BCAA restriction
After 10 weeks on the diets, ZFR displayed severely impaired glucose tolerance that was characterized by elevated fasting glycemia, delayed return to fasting glycemia following the intraperitoneal (IP) glucose challenge, and extremely high insulin levels that displayed no acute response to glucose (Figure 2A and B). Dietary BCAA restriction had no significant impact on glucose or insulin responses during IP glucose tolerance testing in either ZFR or ZLR. However, there was a trend for lower insulin levels at 60 min in ZFR fed the LF-RES diet compared to LF feeding (P = 0.065; Figure 2B).
Figure 2.
BCAA restriction improves skeletal muscle insulin sensitivity in Zucker-fatty rats. Male Zucker-lean rats (ZLR) and Zucker-fatty rats (ZFR) were studied on a custom low fat (LF) diet or an isonitrogenous, isocaloric LF diet in which BCAA were restricted by 45% (LF-RES). Panels A–B show results of a 1 g/kg intraperitoneal glucose tolerance test (IPGTT) performed in week 10 on the diets, whereas panels C–G show results of a hyperinsulinemic-isoglycemic clamp experiment, carried out in weeks 14 and 15, during which [U-14C] glucose and 3H 2-deoxyglucose were included as tracers (clamp data shown only for ZFR). (A) Glucose (mg/dL) and (B) insulin (ng/mL) excursions during the IPGTT, n = 10–27 per group. (C) Blood glucose during the clamp, expressed as percent of starting value; (D) glucose infusion rate (GIR; mg/kg/min), and (E) tracer disappearance (Plasma DPM) during the steady state period following tracer injection of the clamp; n = 5–6 per group. (F) Area under the tracer disappearance curve (from panel E). (G) 3H-2-deoxyglucose uptake in gastrocnemius muscle (μmoles/g). (H) [U-14C]-glucose conversion to glycogen in gastrocnemius muscle (μmoles/g). Data represent mean ± SEM, statistical differences indicated by: *: P < 0.05, **: P < 0.01 vs LF-ZFR.
3.5. Skeletal muscle insulin sensitivity and glucose disposal is improved by BCAA restriction
Considering that the glucose-stimulated insulin secretory response was entirely absent in ZFR during the GTT and that these obese animals require pharmacologic concentrations of insulin to elicit effects on systemic glucose metabolism [31], we carried out hyperinsulinemic-isoglycemic clamps using a dose of insulin (10 mU·kg−1·min−1) found previously to be sufficient to inhibit hepatic glucose production in ZFR [31]. Under these conditions, ZFR fed the BCAA restricted diet required a higher glucose infusion rate (GIR) than LF-fed ZFR to maintain blood glucose at fasting levels in the steady state (P < 0.05; Figure 2C and D). [U-14C] glucose and [3H]-2-deoxyglucose were provided as a tracer bolus during the clamp experiments, allowing us to assess the effect of BCAA restriction on muscle glucose disposal and storage as glycogen. Consistent with the higher GIR, we observed a more rapid clearance of tracer from circulation after the bolus injection (P < 0.05; Figure 2E and F), accompanied by greater accumulation of [3H] 2-DG-6-phosphate in muscle (P < 0.05; Figure 2G) and increased [14C] glucose incorporation into muscle glycogen (P < 0.01; Figure 2H) in ZFR fed the LF-RES compared to LF diets. These data demonstrate improved whole-animal and skeletal muscle insulin sensitivity and glucose disposal in ZFR fed the BCAA restricted diet. We also observed a slight yet significant improvement in accumulation of 3H 2-DG-6-phosphate in the epididymal white adipose tissue (eWAT) of ZFR fed the BCAA restricted diet (P < 0.05; data not shown).
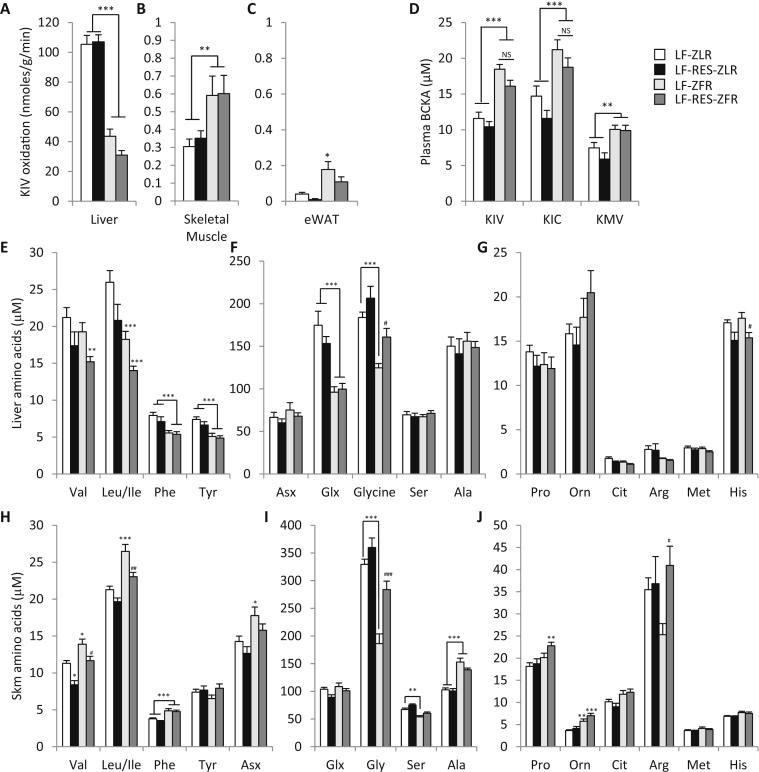
3.6. Tissue-specific effects of obesity and BCAA restriction on BCKDH activity
We next measured BCKDH activity in skeletal muscle, adipose tissue, and liver. Consistent with previous reports [11], [23], we found that ZFR have lower hepatic BCKDH activity compared to ZLR (P < 0.001, Figure 3A). We also found that BCAA restriction does not relieve this impairment in ZFR. Interestingly, skeletal muscle and eWAT BCDKH activities were increased in ZFR compared to ZLR (P < 0.05), but were unaffected by the diet consumed (Figure 3B and C). These data suggest that obesity-associated decreases in hepatic BCKDH activity are partially compensated by an increase in skeletal muscle and adipose tissue activity, although note the differences in scale in Figure 3A–C.
Figure 3.
Effect of Zucker-fatty genotype and BCAA restriction on tissue BCKDH activity and amino acid profiles and on plasma BCKA levels. Tissue branched chain keto acid dehydrogenase (BCKDH) activity, plasma branched chain keto acid content (BCKA) and liver and skeletal muscle amino acid profiles were evaluated in ZLR or ZFR fed the LF or LF-RES diets. (A) Liver, (B) skeletal muscle, and (C) adipose tissue BCKDH activity measured by 14CO2 produced by tissue extracts incubated with [1-14C] α-keto-isovalerate (KIV), n = 8–14 per group. (D) Plasma concentration of the branched chain keto acids (BCKA), α-keto-isovalerate (KIV), α-keto-isocaproate (KIC), and α-keto-methylvalerate (KMV), n = 9–15 per group. (E–G) Liver amino acid levels. (H–J) Skeletal muscle amino acid levels. n = 9–15 per group. Data represent mean ± SEM. Statistical differences indicated by: *: P < 0.05, **: P < 0.01, ***: P < 0.001 comparisons indicated by the connecting lines or in the absence of connecting lines vs LF-ZLR, and #: P < 0.05, ##: P < 0.01, ###: P < 0.001 vs LF-ZFR.
We next quantified levels of the circulating branched chain keto acids (BCKA): α-keto-isovalerate (KIV), α-keto-isocaproate (KIC), and α-keto-β-methylvalerate (KMV; Figure 3D). Here we observed that circulating BCKA reflect the impairment in hepatic BCKDH activity, with higher levels of all three BCKA in plasma of ZFR compared to ZLR (P < 0.001 for KIV and KIC, P < 0.01 for KMV). Importantly, despite the near complete normalization of circulating BCAA achieved by dietary BCAA restriction (Figure 1A), the diet intervention had no significant impact on any of the circulating BCKA in ZFR. Thus it appears that the compensatory rise in skeletal muscle and adipose tissue BCKDH activity is not sufficient to overcome the impairment of hepatic activity in ZFR.
3.7. Divergent effects of obesity and BCAA restriction on liver and skeletal muscle amino acid profiles
In light of the divergent effects of genotype on tissue BCKDH activities, we next performed amino acid profiling in liver and muscle by targeted MS/MS. Despite the elevated levels of BCAA found in circulation (Figure 1A), livers from LF-fed ZFR had slightly lower concentrations of valine and significantly lower levels of leucine and isoleucine compared to LF-fed ZLR (P < 0.001; Figure 3E). Dietary BCAA restriction tended to further lower hepatic BCAA content in both ZFR and ZLR. In concert with lower hepatic BCAA in ZFR we also observed lower hepatic content of the transamination product glutamate/glutamine compared to ZLR (P < 0.001; Figure 3F). In alignment with our measurements of BCKDH activity, we observed that hepatic glutamate/glutamine levels were not responsive to BCAA restriction. Interestingly, hepatic phenylalanine and tyrosine content was also lower in liver of ZFR compared to ZLR (P < 0.001; Figure 3E), and these aromatic amino acids were also not responsive to dietary BCAA restriction (Figure 3E). One interpretation of these data is that impaired hepatic BCKDH activity may be eliciting a feedback signal to inhibit transport of BCAA into the liver through the large neutral amino acid transporters (LAT1 and 2) that are also employed by tyrosine and phenylalanine, contributing to accumulation of BCAA, phenylalanine, and tyrosine in the circulation (Figure 1A and [2]).
In contrast to most other amino acids in liver, hepatic glycine levels were reflective of circulating glycine (Figure 1A). ZFR had lower hepatic glycine levels compared to ZLR (P < 0.001), and dietary BCAA restriction raised glycine levels in the ZFR compared to LF-fed ZFR (P < 0.05, Figure 3F). However, hepatic serine content was not affected by the fatty genotype or the BCAA restricted diet. In light of the minimal impact of BCAA restriction on hepatic BCAA metabolism, it is likely that changes in liver glycine reflect BCAA-dependent regulation of glycine metabolism at an extrahepatic site.
Hepatic histidine was not affected by the fatty genotype but was sensitive to dietary BCAA content in both ZLR and ZFR (Figure 3G). This may reflect elevated reliance on the histidine pool to maintain hepatic glutamate levels.
In contrast to our findings in liver, the skeletal muscle amino acid profile closely resembled that from circulation. Accordingly, LF-fed ZFR had higher levels of valine (P < 0.05), leucine and isoleucine (P < 0.001) in skeletal muscle than LF-fed ZLR, and these were almost completely normalized by dietary BCAA restriction (Figure 3H). Higher skeletal muscle phenylalanine levels in ZFR compared to ZLR were not responsive to dietary BCAA restriction (Figure 3H), as was also true in the circulation. Also aligned with BCAA supply to skeletal muscle, levels of the transamination products aspartate/asparagine (P < 0.05; Figure 3H) were higher in LF-fed ZFR compared to LF-fed ZLR, and were lowered in response to BCAA restriction. Alanine levels were higher in ZFR compared to ZLR (P < 0.001) but the trend for BCAA restriction to lower alanine in ZFR did not achieve statistical significance (P = 0.13; Figure 3I).
Skeletal muscle glycine levels were lower in LF-fed ZFR compared to ZLR (P < 0.001; Figure 3I) and were almost completely normalized in ZFR by BCAA restriction (P < 0.001; Figure 3I). Furthermore, in contrast to liver, changes in skeletal muscle glycine were mirrored by serine, with lower levels in LF-fed ZFR compared to ZLR (P < 0.01; Figure 3I), and a trend for increased serine levels in response to BCAA restriction in both genotypes. Arginine levels exhibited a similar pattern to glycine but with greater variability (Figure 3J). Skeletal muscle proline and ornithine content also resembled circulating levels and were raised modestly by both the fatty genotype and BCAA restriction (Figure 3J).
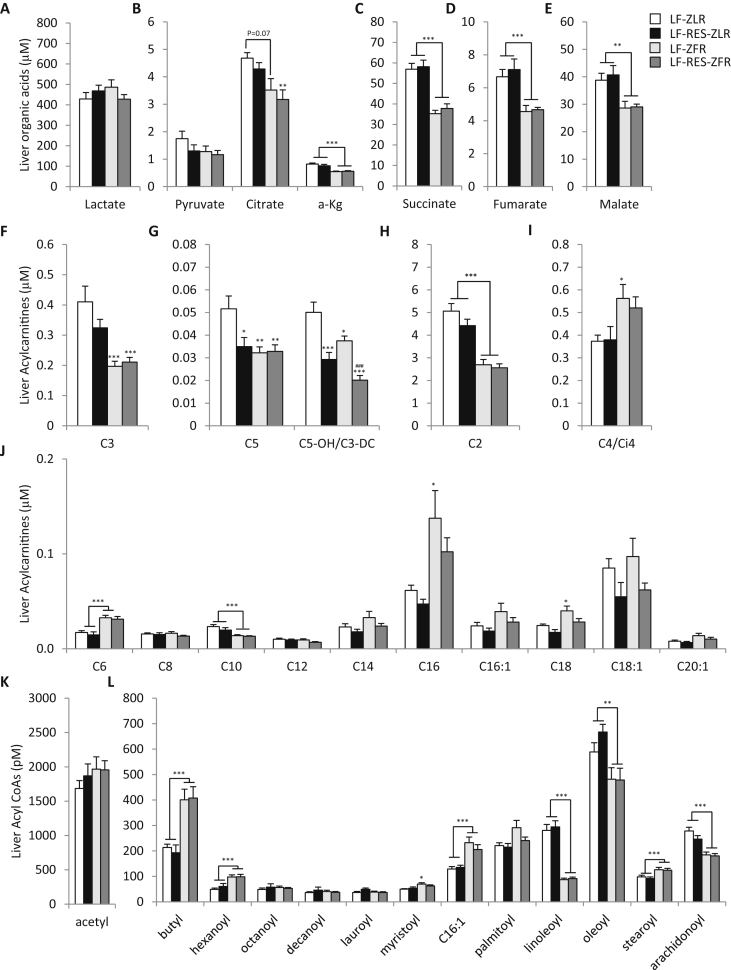
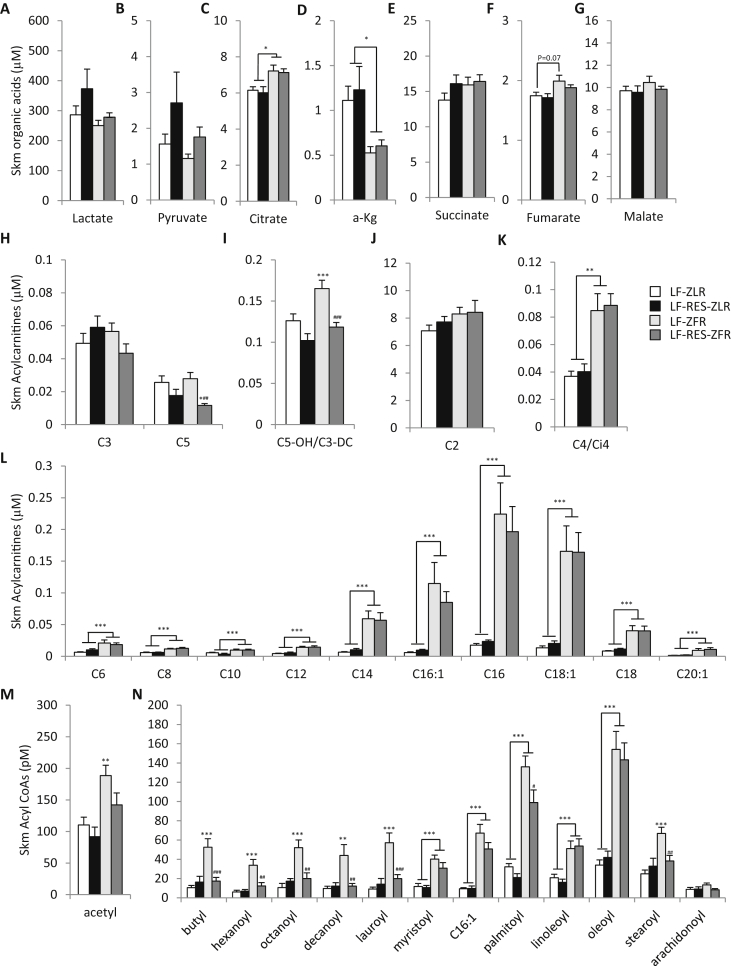
3.8. BCAA restriction has minimal effects on liver metabolites in ZFR
We next studied the impact of the fatty genotype and BCAA dietary restriction on liver organic acids, acylcarnitines, and acyl CoAs measured by targeted GC-MS and MS/MS. Notably, ZFR had lower levels of the organic acids α-ketoglutarate (α-KG), succinate, fumarate, and malate in liver compared to ZLR, but these metabolites were not responsive to dietary BCAA restriction (Figure 4A–E). Levels of C3 and C5 acylcarnitines, which are derived in part from BCAA catabolism, were also lower in ZFR, but were not further reduced by BCAA restriction (Figure 4F and G); BCAA restriction did lower C5 acylcarnitine in ZLR (P < 0.05; Figure 4G). Interestingly, the most BCAA responsive acylcarnitine metabolite in liver was C5-OH/C3-DC acylcarnitine, which was lower in LF-fed ZFR compared to LF-fed ZLR (P < 0.05; Figure 4G) and further decreased by BCAA restriction in both genetic backgrounds (P < 0.001; Figure 4G).
Figure 4.
BCAA restriction has little impact on hepatic metabolites in Zucker-fatty rats. Hepatic metabolites were measured in male ZFR or ZLR fed the LF or LF-Res diets. (A–E) Hepatic organic acid levels; (F–I) Hepatic short chain acylcarnitine levels; (J) Hepatic medium-long chain acylcarnitines. (K–L) Hepatic fatty acyl CoA's. n = 9–15 per group. Data represent mean ± SEM. Statistical differences indicated by: *: P < 0.05, **: P < 0.01, ***: P < 0.001 comparisons indicated by the connecting lines or in the absence of connecting lines vs LF-ZLR, and ###: P < 0.001 vs LF-ZFR.
Hepatic C2 (acetyl) acylcarnitine (Figure 4H) levels were lower in ZFR compared to ZLR and not responsive to diet, whereas C4 (Figure 4I) and C6 (Figure 4J) acylcarnitine levels were higher in ZFR than in ZLR. Similar increases in the C4 and C6 acyl CoA species, butyl CoA and hexanoyl CoA, were also observed (Figure 4L); whereas C2 acetyl CoA was not different among groups (Figure 4K). There was a trend for levels of the long chain C14, C16, C16:1, C18, and C18:1 acylcarnitines as well as myristoyl (C14) CoA and palmitoyl (C16) CoA to be higher in liver of ZFR compared to ZLR and lower in LF-RES diet-fed rats (Figure 4J and L).
3.9. BCAA restriction prevents short and medium even chain acyl CoA accumulation in skeletal muscle
We next studied the impact of the fatty genotype and BCAA restriction on skeletal muscle organic acids, acylcarnitines, and acyl CoA's. Dietary BCAA restriction tended to raise both lactate and pyruvate levels in skeletal muscle of ZFR with P-values for the diet effect of 0.067 and 0.023, respectively (Figure 5A and B). Dietary BCAA restriction also blunted the trend for ZFR to have higher levels of fumarate and malate in skeletal muscle compared to ZLR (Figure 5G and H). ZFR had clearly higher levels of citrate (P < 0.05; Figure 5C) and lower levels of α-KG (P < 0.05; Figure 5D) in skeletal muscle than ZLR, and these differences were not affected by BCAA restriction.
Figure 5.
BCAA restriction abrogates even-chain acyl CoA accumulation in skeletal muscle of Zucker-fatty rats. Metabolites were measured in gastrocnemius muscle from male ZFR or ZLR fed LF or LF-Res diets. (A–G) Organic acid levels. (H–K) Short chain acylcarnitine levels. (L) Medium-long chain acylcarnitine levels. (M–N) Fatty acyl CoA levels. n = 9–15 per group. Data represent mean ± SEM. Statistical differences indicated by: *: P < 0.05, **: P < 0.01, ***: P < 0.001 comparisons indicated by the connecting lines or in the absence of connecting lines vs LF-ZLR, and #: P < 0.05, ##: P < 0.01, ###: P < 0.001 vs LF-ZFR.
Interestingly, skeletal muscle C3 and C5 acylcarnitines were not different between genotypes and only C5 was responsive to dietary BCAA restriction (P < 0.01 for overall diet effect; Figure 5H). On the other hand, the concentration of C5-OH/C3-DC was clearly higher in skeletal muscle of LF-fed ZFR compared to LF-fed ZLR (P < 0.001; Figure 5I), with this difference completely abrogated by BCAA restriction. Taken together, with our findings in liver (Figure 4G), the C5-OH/C3-DC acylcarnitine metabolite appears to be the best tissue biomarker of BCKDH activity and BCAA availability. Importantly, further LC-MS/MS analysis identified the BCAA responsive isobar in the C5-OH/C3-DC peak as C5-OH, representing the leucine metabolite, 3-hydroxyisovaleryl carnitine, and the isoleucine metabolite, 3-hydroxy-2-methylbutyryl carnitine (Supplemental Figure 1).
ZFR had elevated levels of all even chain acylcarnitines from C4/Ci4 to C20:1 in skeletal muscle relative to ZLR, and none of these analytes was responsive to BCAA restriction in either background (Figure 5K and L). LF-fed ZFR also had higher levels of all even chain acyl CoA species in skeletal muscle relative to LF-fed ZLR, but in striking contrast to the acylcarnitines, BCAA restriction completely prevented the accumulation of all short and medium even chain acyl CoA's, ranging in chain length from acetyl (C2) to lauroyl (C12) CoA in skeletal muscle of ZFR (Figure 5M and N). BCAA restriction also partially lowered palmitoyl (C16) CoA (P < 0.05; Figure 5N) and completely lowered stearoyl (C18) CoA in skeletal muscle of ZFR (P < 0.01; Figure 5N). These data suggest that reduced BCAA intake relieves mitochondrial metabolic overload in skeletal muscle to facilitate efficient and complete oxidation of fatty acids, such that even chain acyl CoAs no longer accumulate. This interpretation aligns with the lower RER observed in response to BCAA restriction in the ZFR background (Figure 1I).
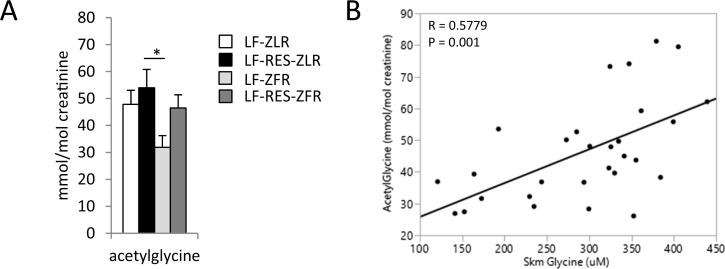
3.10. BCAA restriction reverses obesity-associated decreases in urinary acetyl-glycine
Acyl CoA metabolites do not readily cross biological membranes. One way of reducing acyl CoA burden in the mitochondria is to conjugate acyl groups with glycine to form membrane-permeant acyl-glycines that can be excreted in the urine. As an indicator we measured the urinary content of the most abundant acyl-glycine, acetylglycine. We found that urinary acetylglycine content was lower in LF-fed ZFR compared to ZLR (P = 0.084 vs LF-fed ZLR and P < 0.05 vs LF-RES-fed ZLR; Figure 6A). BCAA restriction in ZFR restored urinary acetylglycine to levels observed in ZLR. Accordingly, we found a significant linear association of urinary acetylglycine with skeletal muscle glycine levels (R = 0.5779 P = 0.001; Figure 6B). We interpret this to mean that BCAA-driven depletion of skeletal muscle glycine contributes to accumulation of acyl CoA species in ZFR by limiting the pool of glycine available for formation of acyl-glycine adducts that can be exported to the urine.
Figure 6.
Acyl-glycine levels in the urine correlate with skeletal muscle glycine content. We measured acetylglycine content in urine collected from male ZFR or ZLR fed LF or LF-RES diets. (A) Urinary acetylglycine (mmol/mol creatinine). (B) Scatterplot showing positive linear correlation between urinary acetylglycine and skeletal muscle glycine content. n = 8 per group. Data represent mean ± SEM. Statistical differences indicated by: *: P < 0.05, comparisons indicated by the connecting lines.
4. Discussion
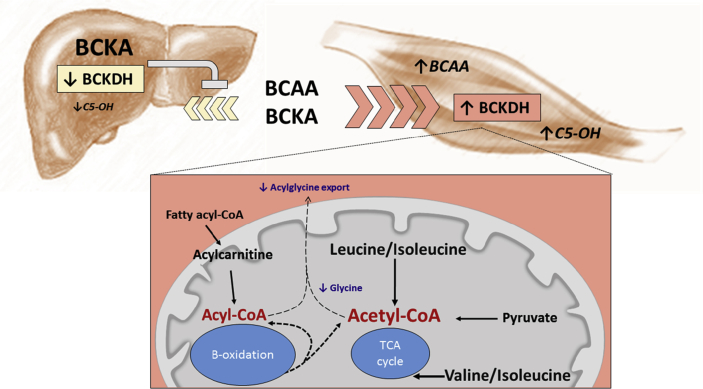
In an effort to elucidate the role played by circulating BCAA in obesity-related metabolic dysfunction, we restricted dietary BCAA supply and performed comprehensive metabolic and physiologic profiling in a rodent model of severe obesity that mirrors human defects in BCAA metabolism. Our findings indicate that impaired hepatic BCAA metabolism in obesity is linked to compensatory upregulation of the activity of the rate-limiting enzyme of BCAA oxidation in skeletal muscle. The resulting presumed increase in BCAA flux increases substrate load on the mitochondria, decreasing the efficiency of skeletal muscle fatty acid oxidation and promoting accumulation of lipid-derived short- and medium-chain acyl CoAs (Figure 7). Whereas it was previously shown that accumulation of incompletely oxidized lipids contributes to skeletal muscle insulin resistance [32], [33], this is a first demonstration that BCAA restriction affects the levels of these lipid mediators. We also demonstrate that the obesity-related rise in BCAA is responsible for the obesity-associated lowering of glycine levels observed in multiple human studies [1], [2], [5], [30]. Interestingly, skeletal muscle depletion of glycine appears to intersect with fatty acyl CoA accumulation by limiting export of acyl groups out of the skeletal muscle in the form of acyl-glycine adducts that are excreted in the urine. Consistent with a role of this process in regulation of insulin sensitivity, a recent study from our group demonstrated increased acyl-glycine levels in urine of obese subjects in which an exercise intervention was used to improve insulin sensitivity [29].
Figure 7.
Working model of metabolic mechanism of BCAA-driven skeletal muscle insulin resistance. Obesity-driven inhibition of hepatic BCKDH activity lowers hepatic uptake of BCAA leading to lower tissue levels of BCAA and their metabolic byproducts, particularly C5-OH carnitine, causing BCAA and their α-keto acids (BCKAs) to rise in circulation and accumulate in skeletal muscle. The obesity-driven increase in skeletal muscle BCKDH activity combined with higher tissue BCAA supply results in higher BCAA oxidative flux, reflected by increased C5-OH. This “BCAA overload” in skeletal muscle interferes with the complete oxidation of fatty acids, leading to accumulation of even chain fatty acyl CoAs in skeletal muscle. This effect is compounded by BCAA-driven depletion of skeletal muscle glycine levels, which limits acyl CoA excretion as acyl-glycines in the urine. BCAA restriction improves muscle insulin sensitivity by relieving substrate pressure and raising glycine levels, leading to normalization of muscle acyl CoA content.
RER was found to be lower in ZFR on the BCAA restricted diet in the absence of any change in oxygen consumption, heat production, or activity levels. These findings are consistent with a simple substrate supply model wherein increased availability and oxidation of BCAA in skeletal muscle of obese rats impedes the oxidation of fatty acids. An interesting finding of our study is the increase in a broad array of both acylcarnitine and acyl CoA species in muscle of ZFR compared to ZLR, but with a selective effect of BCAA restriction to lower the acyl CoA and not the acylcarnitine metabolites in the ZFR background. This suggests that BCAA restriction has no influence on fatty acid uptake or on the CPT1 mitochondrial acylcarnitine transport system for initiating fatty acid oxidation but rather relieves a block on oxidation of the inter-mitochondrial pool of acyl CoAs. These findings are in contrast to a recent paper, which proposed that BCAA increase vascular fatty acid transport into skeletal muscle through the paracrine actions of a valine metabolite, 3-hydroxyisobutyrate (3-HIB) [34]. Such a mechanism appears inconsistent with the uniformly high levels of long chain acylcarnitines observed in muscle of ZFR fed either the LF or LF-RES diets, and the lack of effect of BCAA restriction on circulating NEFA and triglycerides, suggesting no differences in lipid supply or uptake.
BCAA overload in skeletal muscle could conceivably promote the accumulation of short- and medium-chain acyl CoAs by competitively inhibiting lipid derived acetyl CoA entry into the TCA cycle. Indeed, both leucine and isoleucine oxidation can result in acetyl CoA formation. However, it is currently unclear what percentage of the total mitochondrial acetyl CoA pool of muscle is derived from leucine and isoleucine. Nonetheless, a study by Sunny et al. [35] recently demonstrated that BCAA infusion lowers TCA cycle flux in liver of healthy rats. Hence, it is possible that our data represent the additive effect of elevated acetyl CoA synthesis from BCAA and reduced overall TCA cycle flux.
As expected, ZFR rats were severely insulin resistant and glucose intolerant compared to ZLR (Figure 2A and B). In line with the strong lowering of skeletal muscle acyl CoAs, BCAA restriction caused a clear improvement in skeletal muscle insulin sensitivity in ZFR, as indicated by a higher glucose infusion rate required to control blood glucose during the hyperinsulinemic clamp. This was accompanied by enhanced skeletal muscle uptake of radiolabeled glucose and its incorporation into glycogen (Figure 2C–H). However, although clearly significant, the improvement in insulin sensitivity in response to BCAA restriction of ZFR rats was only partial relative to the insulin sensitivity of ZLR, which display a mean GIR of 37 during a 4 mU·kg−1·min−1 clamp in our lab (data not shown). Given that BCAA restriction had very little effect on hepatic metabolites measured in this study (Figure 3, Figure 4), we assume that insulin sensitivity in liver was not improved by BCAA restriction and that this obscured the effects of improved muscle glucose disposal on glucose tolerance. Nevertheless, our study suggests that BCAA restriction in ZFR promotes non-oxidative muscle glucose utilization, based on the following observations: 1) RER was decreased in response to BCAA restriction, indicative of an increased reliance on fatty acid oxidation; 2) glucose storage as glycogen, a non-oxidative pathway, was increased by BCAA restriction; 3) BCAA restriction tended to increase levels of the glycolytic intermediates pyruvate and lactate and significantly raised serine and glycine without affecting the levels of TCA cycle intermediates in skeletal muscle. Thus, increased non-oxidative glucose metabolism may contribute to normalization of both skeletal muscle fatty acyl CoA and glycine levels in response to BCAA restriction in ZFR (Figure 7).
The approach taken herein focused on the biochemical, metabolic changes that underlie BCAA-related insulin resistance rather than changes in the canonical pathway of insulin signal transduction. However, our observation that BCAA restriction not only lowers skeletal muscle BCAA content but also prevents acyl CoA accumulation suggests that lipid and amino acid triggered signals that converge on the insulin signaling pathway could have contributed to our findings. Work from our group and others has demonstrated that supplementation of BCAA, or infusion of a complete mixture of amino acids, activates the mTOR/S6K1 pathway leading to inhibitory serine phosphorylation of IRS1 [2], [17], [19]. Indeed, in our prior work, glucose intolerance caused by feeding of a BCAA-supplemented high fat diet was lessened by infusion of the mTOR inhibitor rapamycin [2]. Thus, altered insulin signaling could account for part of the improved skeletal muscle insulin sensitivity in ZFR fed the BCAA restricted diet. Furthermore, changes in protein acylation states secondary to changes in the acyl CoA pool and/or reduced formation of lipid derivatives such as diacylglycerol and ceramide could also have contributed to improved insulin sensitivity in BCAA-restricted ZFR [36], [37]. Future studies are warranted to delineate these and other possible signaling mechanisms linking BCAA to skeletal muscle insulin resistance.
Our comprehensive profiling of liver provides important insight into the poorly understood association of BCAA with the aromatic amino acids phenylalanine and tyrosine in obese persons [1], [2], [5]. Surprisingly, despite having elevated levels of each of these amino acids in circulation, we observed significantly lower levels of these amino acids in liver of ZFR rats. Since BCAA, phenylalanine, and tyrosine are all taken into cells by the LAT1/2 transporters [38], this observation suggests that impaired hepatic import may contribute to coordinate elevation of their levels in circulation. Future studies will investigate the hypothesis that accumulation of BCKA or other factors involved in suppression of BCDKH activity in ZFR elicit a feedback signal to downregulate activity of the LAT1/2 transporter.
We observed that the BCAA-related metabolite that was most responsive to genotype and BCAA restriction was C3-DC/C5-OH acylcarnitine. We performed targeted LC-MS/MS analysis to identify which isobar was responsible for the differences in the C3-DC/C5-OH acylcarnitine peak. Our analysis revealed that C5-OH, which represents the leucine intermediate, 3-hydroxyisovaleryl carnitine, and the isoleucine intermediate, 3-hydroxy-2-methylbutyryl carnitine, was the BCAA responsive metabolite (Supplemental Figure 1). Whereas 3-hydroxy-2-methylbutyryl carnitine is an intermediate from the classical isoleucine catabolic pathway, 3-hydroxyisovaleryl carnitine is a non-canonical leucine metabolite formed from β-methylcrotonyl CoA, by the actions of enoyl CoA hydratase and carnitine acyltransferase. 3-hydroxy-2-methylbutyryl carnitine and 3-hydroxyisovaleryl carnitine are circulating and urinary markers of 3-hydroxy-2-methylbutyryl-CoA dehydrogenase deficiency and 3-methylcrotonyl CoA carboxylase (3-MCC) deficiency, respectively [39]. Since 3-MCC is a biotin-dependent enzyme, 3-hydroxyisovaleryl carnitine has also been studied as a marker of biotin deficiency [40]. Our data from liver and skeletal muscle suggest that C5-OH carnitine levels might also be used as a tissue biomarker for BCAA catabolism (Figure 4, Figure 5). Given the well appreciated role of enoyl CoA hydratase in beta oxidation, future studies will be undertaken to determine whether accumulation of 3-hydroxyisovaleryl carnitine or its precursor β-methylcrotonyl CoA might contribute to metabolic dysfunction in response to skeletal muscle BCAA overload.
We observed a slight but significant reduction in food intake in ZFR rats in response to feeding of the BCAA restricted diet, resulting in reduced adipose mass. This effect on food intake was unanticipated given our previous report that BCAA supplementation of high fat diets decreased food intake in normal rats [2] and findings of others that hypothalamic activation of mTOR by ICV administration of leucine reduces food intake [41]. While it is likely that reduced adipose mass is mostly driven by reduced food intake in ZFR fed the BCAA restriction diet, it is noteworthy that BCAA oxidation was recently shown be induced and required for adipocyte differentiation and lipogenesis [42]. Thus, our data showing increased BCKDH activity in ZFR compared to ZLR adipose tissues, and the reduction in adipose mass caused by BCAA restriction of ZFR, might also support the notion that obesity-associated elevations in BCAA contribute to adipose tissue expansion.
We recognize that one interpretation of our findings is that the multiple metabolic changes observed in skeletal muscle of ZFR fed the BCAA restricted diet are driven by the slightly lesser weight gain in this group compared to ZFR fed the BCAA replete diet, even though both groups of ZFR rats were massively obese compared to ZLR. Nevertheless, several lines of evidence support the idea that the metabolic effects in skeletal muscle are in fact regulated by BCAA availability: (1) BCAA restriction lowered RER but did not alter circulating levels of glycerol, NEFA, triglycerides, ketones, and lactate as one would expect if the drop in RER was simply explained by generalized nutrient restriction; (2) The metabolic effects of BCAA restriction on lipid accumulation were most dramatic in skeletal muscle. Importantly, this tissue-selective effect does not align with a mechanism for lower RER driven by reduced food intake, which would be expected to produce more global changes across tissues; (3) BCAA restriction completely prevented the accumulation of short and medium chain acyl-CoAs in skeletal muscle without modifying even chain acyl-carnitines or the long chain palmitoyl CoA. We maintain that this metabolite profile speaks to an improvement in clearance or complete oxidation of fatty acids in skeletal muscle rather than an alteration in substrate availability. Indeed, altering lipid availability should coordinately influence both acyl-carnitines and acyl-CoAs, as has in fact been observed in prior studies from our group [2], [32]. (4) In a prior study, we performed the opposite manipulation to that described in the current paper—namely, BCAA supplementation. We found that, despite lower food consumption and body weight, normal rats fed a HF diet supplemented with BCAA accumulated as much or more incompletely oxidized lipids in muscle than animals fed the unsupplemented HF diet [2]. This finding coupled with the current work appears to demonstrate an effect of BCAA to influence lipid oxidation in muscle that is independent of body weight and food intake.
Finally, the current study employed ZFR to model defects present in BCAA metabolism in human obesity. While human obesity is not commonly caused by a mutation in the leptin receptor, ZFR do replicate key metabolic features of the human condition without the requirement for dietary manipulation. Most importantly, ZFR display spontaneous elevations in circulating BCAA and aromatic amino acids in concert with development of obesity as observed for obese humans [1], [2], [5], accompanied by similar perturbations in expression of enzymes required for BCAA oxidation in adipose tissue and liver [11], [23]. This is in contrast to normal rats fed on high-fat diets, which do not exhibit increases in circulating BCAA unless supplementation is used [2]. ZFR also have lower circulating glycine levels as observed in obese humans. Finally, and again in contrast to rodents fed on high fat diets, ZFR have an RER of approximately 0.9, similar to that reported for obese humans [2]. All of these considerations support the use of ZFR for studies of BCAA metabolism.
In conclusion, our data support a model in which obesity-driven inhibition of hepatic BCAA oxidation drives BCAA, phenylalanine, and tyrosine into skeletal muscle, where increases in BCAA oxidation interfere with the complete oxidation of lipid, leading to accumulation of fatty acyl CoAs, depletion of glycine/serine, and skeletal muscle insulin resistance. Our data further suggest that BCAA spillover into adipose tissue may promote obesity-related adipose expansion. Future studies will be directed at determining the physiological impact of restoring hepatic BCKDH activity and raising skeletal muscle glycine on obesity-related metabolic dysfunction.
Acknowledgments
The authors thank Huaxia Cui for expert technical assistance. The work was supported by National Institutes of Health grant NIDDK PO1-DK58398 (to C.B.N.), a “Pathways” Initiator Award from the American Diabetes Association (to P.J.W.), and a sponsored research agreement with Pfizer (to C.B.N.).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.04.006.
Conflict of interest statement
C.B.N. is a paid consultant for the CVMED group at Pfizer, and was also the lead investigator of a now completed sponsored research agreement with Pfizer that supported a portion of the studies described here.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Felig P., Marliss E., Cahill G.F. Plasma amino acid levels and insulin secretion in obesity. The New England Journal of Medicine. 1969;281(15):811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- 2.Newgard C.B., An J., Bain J.R., Muehlbauer M.J., Stevens R.D., Lien L.F. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metabolism. 2009;9(4):311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huffman K.M., Shah S.H., Stevens R.D., Bain J.R., Muehlbauer M., Slentz C.A. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32(9):1678–1683. doi: 10.2337/dc08-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tai E.S., Tan M.L.S., Stevens R.D., Low Y.L., Muehlbauer M.J., Goh D.L.M. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53(4):757–767. doi: 10.1007/s00125-009-1637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang T.J., Larson M.G., Vasan R.S., Cheng S., Rhee E.P., McCabe E. Metabolite profiles and the risk of developing diabetes. Nature Medicine. 2011;17(4):448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah S.H., Bain J.R., Muehlbauer M.J., Stevens R.D., Crosslin D.R., Haynes C. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circulation. Cardiovascular Genetics. 2010;3(2):207–214. doi: 10.1161/CIRCGENETICS.109.852814. [DOI] [PubMed] [Google Scholar]
- 7.Laferrère B., Reilly D., Arias S., Swerdlow N., Gorroochurn P., Bawa B. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Science Translational Medicine. 2011;3(80) doi: 10.1126/scitranslmed.3002043. 80re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah S.H., Crosslin D.R., Haynes C.S., Nelson S., Turer C.B., Stevens R.D. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012;55(2):321–330. doi: 10.1007/s00125-011-2356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newgard C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metabolism. 2012;15(5):606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridaura V.K., Faith J.J., Rey F.E., Cheng J., Duncan A.E., Kau A.L. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science (New York, N.Y.) 2013;341(6150):1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.She P., Van Horn C., Reid T., Hutson S.M., Cooney R.N., Lynch C.J. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. American Journal of Physiology. Endocrinology and Metabolism. 2007;293(6):E1552–E1563. doi: 10.1152/ajpendo.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lian K., Du C., Liu Y., Zhu D., Yan W., Zhang H. Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mice. Diabetes. 2015;64(1):49–59. doi: 10.2337/db14-0312. [DOI] [PubMed] [Google Scholar]
- 13.Shin A.C., Fasshauer M., Filatova N., Grundell L.A., Zielinski E., Zhou J.-Y. Brain insulin lowers circulating BCAA levels by inducing hepatic BCAA catabolism. Cell Metabolism. 2014 doi: 10.1016/j.cmet.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herman M.A., She P., Peroni O.D., Lynch C.J., Kahn B.B. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. The Journal of Biological Chemistry. 2010;285(15):11348–11356. doi: 10.1074/jbc.M109.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsiao G., Chapman J., Ofrecio J.M., Wilkes J., Resnik J.L., Thapar D. Multi-tissue, selective PPARγ modulation of insulin sensitivity and metabolic pathways in obese rats. American Journal of Physiology. Endocrinology and Metabolism. 2011;300(1):E164–E174. doi: 10.1152/ajpendo.00219.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch C.J., Adams S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nature Reviews Endocrinology. 2014;10(12):723–736. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krebs M., Krssak M., Bernroider E., Anderwald C., Brehm A., Meyerspeer M. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes. 2002;51(3):599–605. doi: 10.2337/diabetes.51.3.599. [DOI] [PubMed] [Google Scholar]
- 18.Krebs M., Brehm A., Krssak M., Anderwald C., Bernroider E., Nowotny P. Direct and indirect effects of amino acids on hepatic glucose metabolism in humans. Diabetologia. 2003;46(7):917–925. doi: 10.1007/s00125-003-1129-1. [DOI] [PubMed] [Google Scholar]
- 19.Tremblay F., Krebs M., Dombrowski L., Brehm A., Bernroider E., Roth E. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005;54(9):2674–2684. doi: 10.2337/diabetes.54.9.2674. [DOI] [PubMed] [Google Scholar]
- 20.Nairizi A., She P., Vary T.C., Lynch C.J. Leucine supplementation of drinking water does not alter susceptibility to diet-induced obesity in mice. The Journal of Nutrition. 2009;139(4):715–719. doi: 10.3945/jn.108.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macotela Y., Emanuelli B., Bång A.M., Espinoza D.O., Boucher J., Beebe K. Dietary leucine–an environmental modifier of insulin resistance acting on multiple levels of metabolism. PloS One. 2011;6(6):e21187. doi: 10.1371/journal.pone.0021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo K., Yu Y.-H., Hou J., Zhang Y. Chronic leucine supplementation improves glycemic control in etiologically distinct mouse models of obesity and diabetes mellitus. Nutrition & Metabolism. 2010;7:57. doi: 10.1186/1743-7075-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.She P., Olson K.C., Kadota Y., Inukai A., Shimomura Y., Hoppel C.L. Leucine and protein metabolism in obese Zucker rats. PloS One. 2013;8(3):e59443. doi: 10.1371/journal.pone.0059443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James D.E., Jenkins A.B., Kraegen E.W. Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. The American Journal of Physiology. 1985;248(5 Pt 1):E567–E574. doi: 10.1152/ajpendo.1985.248.5.E567. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara C.T., Wang P., Neto E.C., Stevens R.D., Bain J.R., Wenner B.R. Genetic networks of liver metabolism revealed by integration of metabolic and transcriptional profiling. PLoS Genetics. 2008;4(3):e1000034. doi: 10.1371/journal.pgen.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ronnebaum S.M., Ilkayeva O., Burgess S.C., Joseph J.W., Lu D., Stevens R.D. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. The Journal of Biological Chemistry. 2006;281(41):30593–30602. doi: 10.1074/jbc.M511908200. [DOI] [PubMed] [Google Scholar]
- 27.An J., Muoio D.M., Shiota M., Fujimoto Y., Cline G.W., Shulman G.I. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nature Medicine. 2004;10(3):268–274. doi: 10.1038/nm995. [DOI] [PubMed] [Google Scholar]
- 28.Olson K.C., Chen G., Lynch C.J. Quantification of branched-chain keto acids in tissue by ultra fast liquid chromatography-mass spectrometry. Analytical Biochemistry. 2013;439(2):116–122. doi: 10.1016/j.ab.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glynn E.L., Piner L.W., Huffman K.M., Slentz C.A., Elliot-Penry L., AbouAssi H. Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia. 2015;58(10):2324–2335. doi: 10.1007/s00125-015-3705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thalacker-Mercer A.E., Ingram K.H., Guo F., Ilkayeva O., Newgard C.B., Garvey W.T. BMI, RQ, diabetes, and sex affect the relationships between amino acids and clamp measures of insulin action in humans. Diabetes. 2014;63(2):791–800. doi: 10.2337/db13-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terrettaz J., Assimacopoulos-Jeannet F., Jeanrenaud B. Severe hepatic and peripheral insulin resistance as evidenced by euglycemic clamps in genetically obese fa/fa rats. Endocrinology. 1986;118(2):674–678. doi: 10.1210/endo-118-2-674. [DOI] [PubMed] [Google Scholar]
- 32.Koves T.R., Ussher J.R., Noland R.C., Slentz D., Mosedale M., Ilkayeva O. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metabolism. 2008;7(1):45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Muoio D.M., Noland R.C., Kovalik J.-P., Seiler S.E., Davies M.N., DeBalsi K.L. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metabolism. 2012;15(5):764–777. doi: 10.1016/j.cmet.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jang C., Oh S.F., Wada S., Rowe G.C., Liu L., Chan M.C. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nature Medicine. 2016 doi: 10.1038/nm.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sunny N.E., Kalavalapalli S., Bril F., Garrett T.J., Nautiyal M., Mathew J.T. Cross-talk between branched-chain amino acids and hepatic mitochondria is compromised in nonalcoholic fatty liver disease. American Journal of Physiology. Endocrinology and Metabolism. 2015;309(4):E311–E319. doi: 10.1152/ajpendo.00161.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies M.N., Kjalarsdottir L., Thompson J.W., Dubois L.G., Stevens R.D., Ilkayeva O.R. The acetyl group buffering action of carnitine acetyltransferase offsets macronutrient-induced lysine acetylation of mitochondrial proteins. Cell Reports. 2016;14(2):243–254. doi: 10.1016/j.celrep.2015.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coen P.M., Goodpaster B.H. Role of intramyocelluar lipids in human health. Trends in Endocrinology and Metabolism: TEM. 2012;23(8):391–398. doi: 10.1016/j.tem.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernstrom J.D. Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino Acids. 2013;45(3):419–430. doi: 10.1007/s00726-012-1330-y. [DOI] [PubMed] [Google Scholar]
- 39.van Hove J.L., Rutledge S.L., Nada M.A., Kahler S.G., Millington D.S. 3-Hydroxyisovalerylcarnitine in 3-methylcrotonyl-CoA carboxylase deficiency. Journal of Inherited Metabolic Disease. 1995;18(5):592–601. doi: 10.1007/BF02436004. [DOI] [PubMed] [Google Scholar]
- 40.Horvath T.D., Stratton S.L., Bogusiewicz A., Pack L., Moran J., Mock D.M. Quantitative measurement of plasma 3-hydroxyisovaleryl carnitine by LC-MS/MS as a novel biomarker of biotin status in humans. Analytical Chemistry. 2010;82(10):4140–4144. doi: 10.1021/ac1003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cota D., Proulx K., Smith K.A.B., Kozma S.C., Thomas G., Woods S.C. Hypothalamic mTOR signaling regulates food intake. Science (New York, N.Y.) 2006;312(5775):927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 42.Green C.R., Wallace M., Divakaruni A.S., Phillips S.A., Murphy A.N., Ciaraldi T.P. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nature Chemical Biology. 2016;12(1):15–21. doi: 10.1038/nchembio.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.