Abstract
Background
Evidence hints at the ability of β-cells to emerge from non-β-cells upon genetic or pharmacological interventions. However, their quantitative contributions to the process of autonomous β-cell regeneration without genetic or pharmacological manipulations remain to be determined.
Methods & results
Using PANIC-ATTAC mice, a model of titratable, acute β-cell apoptosis capable of autonomous, and effective islet mass regeneration, we demonstrate that an extended washout of residual tamoxifen activity is crucial for β-cell lineage tracing studies using the tamoxifen-inducible Cre/loxP systems. We further establish a doxycycline-inducible system to label different cell types in the mouse pancreas and pursued a highly quantitative assessment to trace adult β-cells after various metabolic challenges. Beyond proliferation of pre-existing β-cells, non-β-cells contribute significantly to the post-challenge regenerated β-cell pool. α-cell trans-differentiation is the predominant mechanism upon post-apoptosis regeneration and multiparity. No contributions from exocrine acinar cells were observed. During diet-induced obesity, about 25% of α-cells arise de novo from β-cells. Ectopic expression of Nkx6.1 promotes α-to-β conversion and insulin production.
Conclusions
We identify the origins and fates of adult β-cells upon post-challenge upon autonomous regeneration of islet mass and establish the quantitative contributions of the different cell types using a lineage tracing system with high temporal resolution.
Keywords: Lineage tracing, Adult β-cell origins, Nkx6.1, Tamoxifen artifacts
Highlights
-
•
Insufficient washout for tamoxifen leads to β-cell lineage tracing artifacts.
-
•
Inter-conversion between α- and β-cells after differential metabolic challenges.
-
•
Developmental distance and precursor population determine conversion efficiency.
-
•
Nkx6.1 promotes α-to-β conversion.
1. Introduction
Both type 1 and type 2 diabetes can be attributed to a failure of insulin-producing β-cells. For the development of regenerative therapies, the reconstitution of β-cell mass under metabolically challenging conditions has therefore attracted great research interest. Initial lineage tracing studies concluded that self-replication accounts for nearly all of the turnover and regeneration of adult β-cells [1], [2]. Other investigators reported non-β-cell precursors for adult β-cells in specific contexts, such as pancreatic duct ligation [3], [4], [5], pregnancy [6], extreme β-cell ablation [7], [8], or induced differentiation [9], [10], [11], [12], [13], [14], [15]. The concept of interconversion of different pancreatic cell types is emerging, but its quantitative contribution to the cell-autonomous process, i.e. without any genetic or pharmacological manipulations, of β-cells turnover upon metabolic challenges remains largely unknown. Furthermore, most of these studies used the tamoxifen-inducible CreER/loxP system to label specific pools of cells in a temporally controlled manner. However, recent observations have cast doubt on the ability of temporal control of gene expression/elimination with tamoxifen [16]. In adipocytes, the residual tamoxifen enables nuclear translocation of the CreER recombinase beyond two months of a washout period, along with significant metabolic side effects due to the pharmacological nature of tamoxifen as an estrogen receptor antagonist [17]. It is therefore essential to gain a better understanding of the actions of tamoxifen in the context of β-cell lineage tracing studies, compare it to other systems, and re-visit the data interpretation if necessary. As an alternative, the doxycycline-inducible system has proven to generate reliable lineage tracing results in adipose tissue, with fewer side effects and better temporal control on Cre recombinase activity [17], [18].
As a common strategy for regenerative studies, β-cell ablation can be induced in adult rodents through a variety of methods, including pancreatectomy, pancreatic duct ligation, and administration of streptozotocin, alloxan, targeted delivery of diphtheria toxin, or targeted mutations in critical genes [19]. These approaches have disadvantages such as unspecific ablation [20], poor control over the extent of the ablation, unspecific toxicity, limited regenerative capacity post ablation [21], or irreversibly impaired functionality or viability of surviving β-cells. In contrast, the PANIC-ATTAC (“pancreatic islet β-cell apoptosis through targeted activation of caspase 8”) transgenic mouse has been characterized as a model of inducible, titratable, acute β-cell apoptosis [22], [23]. Administration of a chemical dimerizer, which is nontoxic and has a short half-life of around 5 h in mice, activates the intracellular apoptosis signaling in β-cells. In adult mice, the dimerizer-induced β-cell death peaks by 2 weeks post treatment, without any detectable inflammation (F4/80 or CD3 immunostaining, data not shown) or other side effects. More importantly, a moderate ablation, as indicated by a peak fed blood glucose level of 250–600 mg/dL accompanied by 50–75% β-cell loss, allows for autonomous recovery of β-cell mass and blood glucose by 10 weeks post treatment.
Here, we utilized the PANIC-ATTAC model to trace the regenerated β-cells with a tamoxifen-inducible labeling system. After a short washout of 1 week, the nuclear Cre was sustained by the residual tamoxifen activity and labeled most of the insulin+ cells during the chase phase. In contrast, a much more extensive washout period of 4 months revealed ∼20% β-cells with a non-β-cell origin. This was further confirmed independently with a doxycycline-inducible lineage tracing system. Such a significant contribution from non-β-cells was also evident after multiparity or high-fat diet-induced obesity. Labeling other pancreatic cells with the doxycycline system identified the glucagon-producing α-cell as a predominant source of β-cell trans-differentiation in the cases of post-apoptosis regeneration and multiparity. In contrast, in diet-induced obesity, β-cell conversion contributed to about one fourth of the expanded α-cell pool, explaining the disorganized distribution of α-cells post high-fat diet. In fact, de novo and/or trans-differentiation were active mechanisms to replenish α-cells. We failed to detect any acinar-to-β-cell trans-differentiation. Ectopic expression of Nkx6.1, a key transcription factor for β-cell differentiation [24] and identity [25], promotes α-cell trans-differentiation and systemic insulin production. Here, we provide comprehensive and highly quantitative measurements of the autonomous contributions from multiple pancreatic cell types to the adult β-cell pool upon different metabolic challenges. Our results suggest that adult β-cells preferentially originate from cells with relatively small developmental distance and high pre-existing abundance, and the relative contribution can be changed by metabolic insults or pharmacological interventions. We demonstrate the general usefulness of our lineage tracing system for the comprehensive and quantitative analysis of pancreatic cell fate and for the development of regenerative therapies.
2. Materials and methods
2.1. Mice
The transgenic mouse strains MIP-CreERT2, MIP-rtTA, PPG-rtTA [26], and TRE-Nkx6.1 were generated and recently characterized by our laboratory. The transgene constructs were generated by subcloning the coding DNA sequence (CDS) into a plasmid containing the promoter: MIP-CreERT2, the 1983-bp CreERT2 CDS following a 8.3-kb mouse insulin 1 promoter; MIP-rtTA, the 747-bp rtTA.M2 CDS following a 8.3-kb mouse insulin 1 promoter; PPG-rtTA, the 747-bp rtTA.M2 CDS following a 1.7-kb mouse preproglucagon promoter; TRE-Nkx6.1, the 1098-bp golden hamster Nkx6.1 CDS following a 0.3-kb TRE-tight promoter. The PANIC-ATTAC transgenic mouse was generated by our laboratory as previously described [22]. The mouse strains TRE-Cre (#006234), RIP-rtTA (#008250), Rosa26-LacZ (Rosa26-loxP-STOP-loxP-LacZ, #003474), Rosa26-Tomato (Rosa26-loxP-STOP-loxP-tdTomato Ai9, #007909), and Ptf1a-rtTA (#018070) were purchased from the Jackson Laboratory. All mice were bred in the C57BL/6 genetic background. Mice were fed on regular (LabDiet #5058), high-fat (60%, Research #D12492), or doxycycline chow diet (600 mg/kg, Bio-Serv #S4107). Mice were maintained in 12-h dark/light cycles, with ad libitum access to diet and water. All protocols for mouse use and euthanasia were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center.
2.2. Genotyping PCR
Approximately 3 mm of mouse tail tip was incubated in 80 μL 50 mM NaOH at 95 °C for 1.5 h. 8 μL 1 M Tris–HCl (pH 8.0) was added for neutralization. After vortexing and a short spin down, 0.5–1 μL of supernatant was used as PCR template. Primer sequences for genotyping PCR are listed in Table S1. The PCR program was: 95 °C for 5 min, followed by 35 cycles of 95 °C for 15 s, 62 °C for 30 s, and 72 °C for 30 s, and ended with 72 °C for 3 min.
2.3. Tamoxifen administration
A 25-mg tamoxifen citrate pellet with a release time of 21 days (Innovative Research #E-351) was implanted subcutaneously, and the mice were housed individually during the release period.
2.4. Dimerizer administration
Mice were subjected to one intraperitoneal injection of the dimerizer AP20187 (Clontech #635059) at the dose of 0.3–0.5 μg/g body weight/day. The dimerizer stock solution was stored at −80 °C, and freshly diluted in 2% Tween-20 with 10% polyethylene glycol 400 before injection.
2.5. Multiparity
Adult female mice were mated to be pregnant at least three times and sacrificed for pancreas paraffin sections during the last pregnancy, at around 15.5 days post-coitus.
2.6. β-gal staining
Mice were subjected to isoflurane anesthesia and cardiac perfusion of 0.2% glutaraldehyde in PBS (10–15 mL per mouse). Tissues were immediately dissected, transferred to 20-mL scintillation vials with 0.2% glutaraldehyde in PBS, and minced into 1–3 mm wide slices. Tissue slices were washed with rinse buffer (0.1 M sodium phosphate, 2 mM MgCl2, 0.01% sodium deoxycholate, and 0.02% NP-40, pH 7.3) three times for 30 min and incubated with X-gal staining buffer (1 g/L X-galactoside, 5 mM K3[Fe(CN)6], and 5 mM K4[Fe(CN)6] in rinse buffer) in the dark, at room temperature, with shaking at 100 rpm, for 24 h. Tissues slices were then fixed in 10% formalin overnight and briefly rinsed three times with 50% ethanol. In the University of Texas Southwestern Medical Center Molecular Pathology Core, tissue slices were embedded in paraffin blocks, and the sections were counterstained with Nuclear Fast Red. Bright field images were acquired on a Nikon Coolscope digital microscope or an Olympus FSX100 all-in-one microscope.
2.7. Immunohistochemistry
Mouse tissues were collected and processed for paraffin sections as previously described [23]. Primary antibodies and dilution for immunostaining or immunofluorescence were: β-galactosidase (1:50, Abcam #ab9361), insulin (1:500, Dako #A0564), Cre (1:100, Millipore # 69050-3), RFP for tdTomato (rabbit polyclonal, 1:100, Abcam #ab34771), RFP for tdTomato (mouse monoclonal, 1:250, ThermoFisher #MA5-15257), glucagon (1:500, Invitrogen #18-0064), Nkx6.1 (1:50, Santa Cruz Biotechnology #sc-15027), and Amylase (1:800, Cell Signaling #3796). Images were acquired on a Nikon Coolscope digital microscope, a Zeiss Axio Observer Z1 inverted microscope, or an Olympus FSX100 all-in-one microscope.
2.8. Assay of metabolites
Tail blood was collected with a heparinized Microhematocrit capillary tube (Fisher Scientific #22-362-566) and prepared for plasma. Glucose was measured with a glucose meter or colorimetric assays with PGO enzymes (Sigma #P7119) plus o-dianisidine (Sigma #F5803). Insulin was measured with an ELISA kit (Crystal Chem #90080).
2.9. RT-qPCR on pancreatic islets
Mouse pancreatic islets of Langerhans were isolated as previously described [23]. Total RNA was extracted with an RNeasy Mini kit (Qiagen #74106). cDNA was synthesized with a SuperScript II reverse transcriptase (Invitrogen # 18064-014) plus the RNaseOUT recombinant ribonuclease inhibitor (Invitrogen # 10777-019). Quantitative real-time PCR (qPCR) was performed with the Power SYBR Green PCR master mix (Applied Biosystems # 4368708) on a 7900HT Fast Real-Time PCR System (Applied Biosystems # 4329001). Primer sequences for qPCR are listed in Table S2.
2.10. Glucose tolerance test
Mice were fasted for 4–6 h and subjected to an oral gavage of dextrose (2 mg/g body weight). Tail blood was collected at 0, 15, 60, and 120 min, prepared for plasma, and assayed for glucose and insulin.
2.11. Data analysis
Immunofluorescence-stained cell numbers, as well as islet areas, were quantitated with ImageJ. Cell percentages in individual islets were presented as bubble charts, with the bubble size proportional to the denominator cell number. For an individual mouse, every cell population was summed from all the examined islets respectively, and calculated for percentages. The area under curve (AUC) in tolerance tests was calculated for individual mouse by the trapezoidal method. Two-tailed student's t-test was applied for all paired and unpaired comparisons. Statistical significance was accepted at P < 0.05.
3. Results
3.1. Only extended washout periods for tamoxifen unmask the non-β-cell origins of autonomous β-cell regeneration
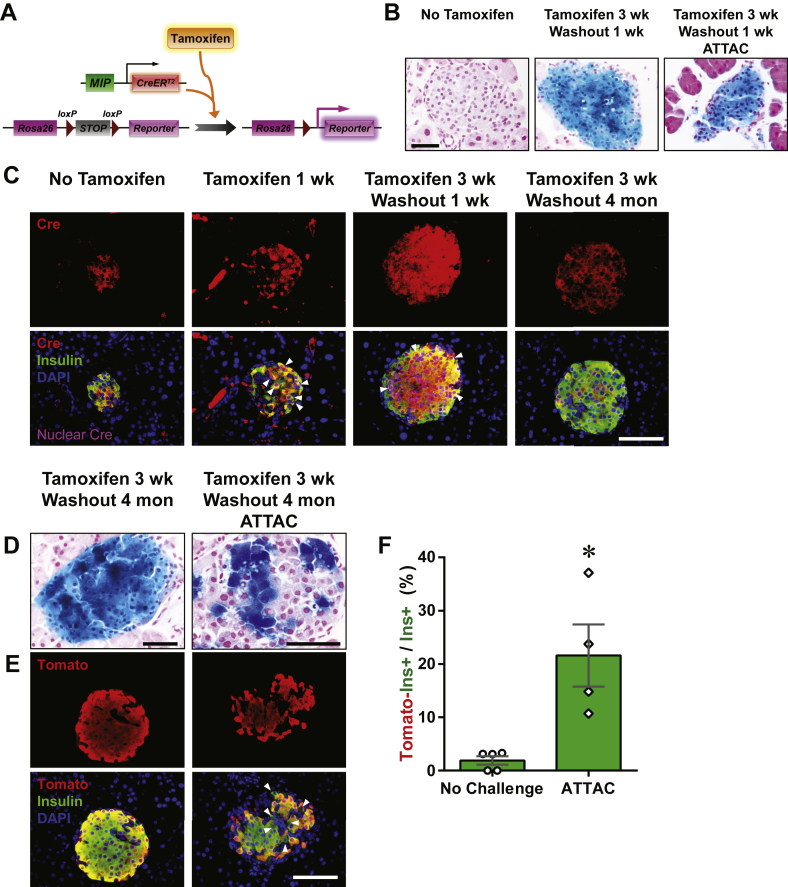
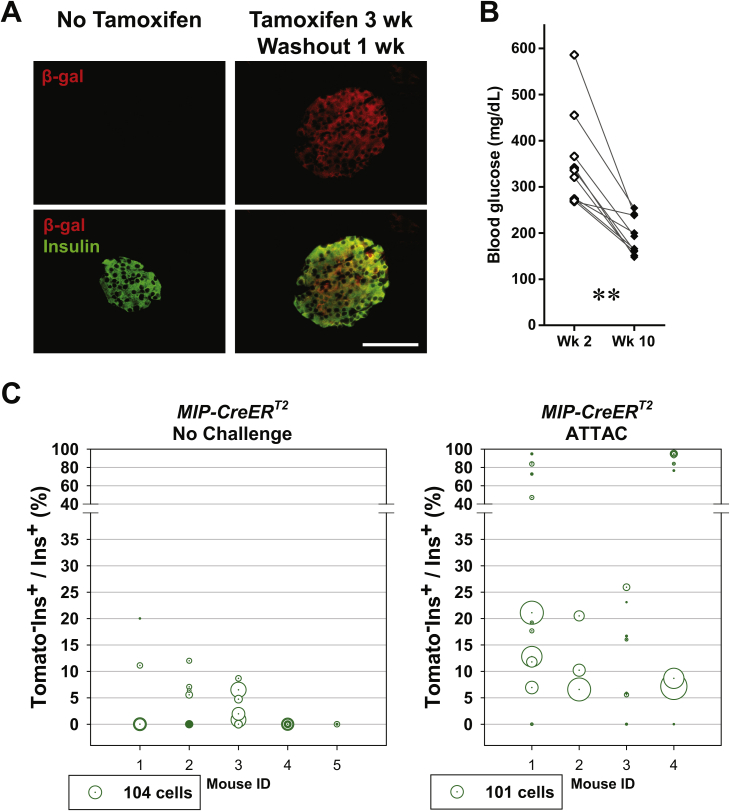
We employed a tamoxifen-inducible system to label the pre-existing β-cells in adult mice. A 25-mg tamoxifen pellet was subcutaneously implanted to the [MIP-CreERT2; Rosa26-LacZ] mouse, and released tamoxifen for a 21-day period. Only in the presence of tamoxifen, the CreERT2 protein translocated into the nucleus, removed the floxed stop codon in the Rosa26-LacZ locus, and activated the expression of β-galactosidase (β-gal) permanently (Figure 1A). After the 3-week release and 1-week washout of tamoxifen, the insulin+ cells were nearly 100% β-gal+, as displayed by both β-gal staining (Figure 1B) and immunofluorescence (Figure S1A). At this point, the labeled mice carrying the PANIC-ATTAC transgene were subjected to dimerizer-induced β-cell apoptosis, followed by two months of autonomous regeneration. Consistent with our previous report [22], the β-cell ablation was evident by hyperglycemia two weeks after dimerizer administration, and a significant decrease in blood glucose 10 weeks post dimerizer as the result of the recovery of β-cell mass (Figure S1B). In the regenerated islets, there was no apparent evidence of β-cells originating from any precursors other than β-cells (Figure 1B).
Figure 1.
Tamoxifen-pulsed linage tracing of regenerated β-cells. (A) Tamoxifen-inducible labeling of β-cells in mice. The transgene MIP-CreERT2 expresses exclusively in the insulin-producing β-cells a Cre recombinase fused with ERT2, a ligand-binding domain of mutated estrogen receptor. Tamoxifen binds the CreERT2 protein and transports it into the nucleus, where it excises the loxP-flanked stop codon in the Rosa26 knock-in locus and activates the reporter gene (LacZ or tdTomato) expression irreversibly and heritably. (B) X-gal staining (blue) of pancreas from [MIP-CreERT2; Rosa26-LacZ] mice without tamoxifen treatment (left), after 3-week release from the subcutaneously implanted tamoxifen pellet and 1-week washout (middle), and [MIP-CreERT2; Rosa26-LacZ; PANIC-ATTAC] mice after 3-week tamoxifen release, 1-week washout, dimerizer-induced β-cell apoptosis, and 2-month autonomous regeneration (the ATTAC challenge, right). Paraffin sections of the X-gal stained pancreas were counterstained with nuclear fast red (NFR, red). Scale bar: 50 μm. (C) Immunofluorescence of Cre recombinase on pancreas sections from MIP-CreERT2 mice without tamoxifen treatment, after 1-week tamoxifen release, after 3-week tamoxifen release and 1-week washout, and after 3-week tamoxifen release and 4-month washout, as indicated. Top panels: Cre (red) signal only. Bottom panels: Cre merged with insulin (green) and DAPI (blue) signals. Arrowheads: the nuclear Cre signal co-localized with DAPI (purple). Scale bar: 100 μm. (D–F) [MIP-CreERT2; Rosa26-LacZ; PANIC-ATTAC] and [MIP-CreERT2; Rosa26-Tomato; PANIC-ATTAC] mice were subjected to 3-week tamoxifen release and 4-month washout. Before (left) or after (right) the ATTAC challenge, (D) X-gal (blue) staining of pancreas from the Rosa26-LacZ mice. Scale bars: 50 μm. (E) Immunofluorescence of tdTomato (red) and insulin (green) on pancreas sections from the Rosa26-Tomato mice. Top panels: Tomato signal only. Bottom panels: Tomato merged with insulin and DAPI (blue). Arrowheads: Tomato−insulin+ cells (green). Scale bar: 100 μm. (F) In each mouse from (E) the total numbers of Tomato−insulin+ cells and insulin+ cells were counted and calculated for percentage. Data are presented as mean ± SEM. Dots: individual mice. *P < 0.05 by unpaired t-test.
Tamoxifen has a prolonged activity in CreERT2 nuclear translocation, which may confound the results of lineage tracing experiments [16], [17]. To establish a time point when the β-cells are functionally free of tamoxifen activity, we examined the subcellular location of the CreERT2 protein by immunofluorescence (Figure 1C). Importantly, the nuclear CreERT2 signal was sustained beyond the 1-week washout period and became undetectable after 4 months of washout. At this point, the labeled islet cells remained mostly β-gal+ (Figure 1D).
To quantitatively trace the origins of autonomously regenerated β-cells, we employed another reporter, tdTomato, and generated the [MIP-CreERT2; Rosa26-Tomato; PANIC-ATTAC] mouse model (Figures 1E, F and S1C). After a 4-month washout of tamoxifen, 98.1 ± 0.8% insulin+ cells remained as Tomato+. When mice were subjected to dimerizer-induced ablation and autonomous regeneration of β-cells only after the 4 month washout, they displayed 21 ± 6% of Tomato− cells in the insulin+ cell population after recovery. The significant increase (P = 0.04) in Tomato−insulin+ cells suggested an important contribution of non-β-cells to adult β-cell regeneration. This is also independently supported by the β-gal− cells in the islets of [MIP-CreERT2; Rosa26-LacZ; PANIC-ATTAC] mice (Figure 1D).
3.2. Tet-On lineage tracing quantitates the non-β-cell contribution to adult β-cell turnover
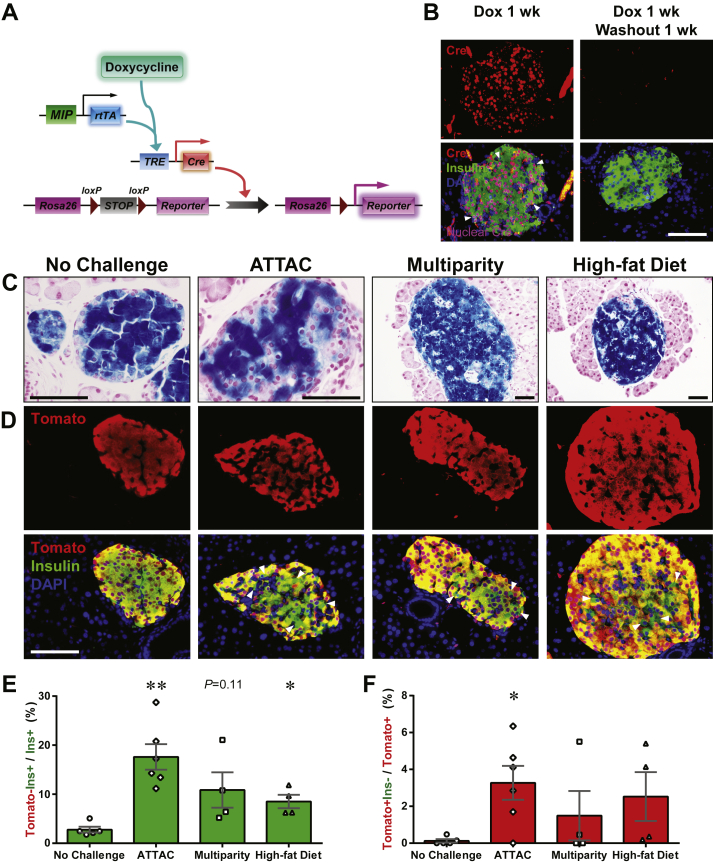
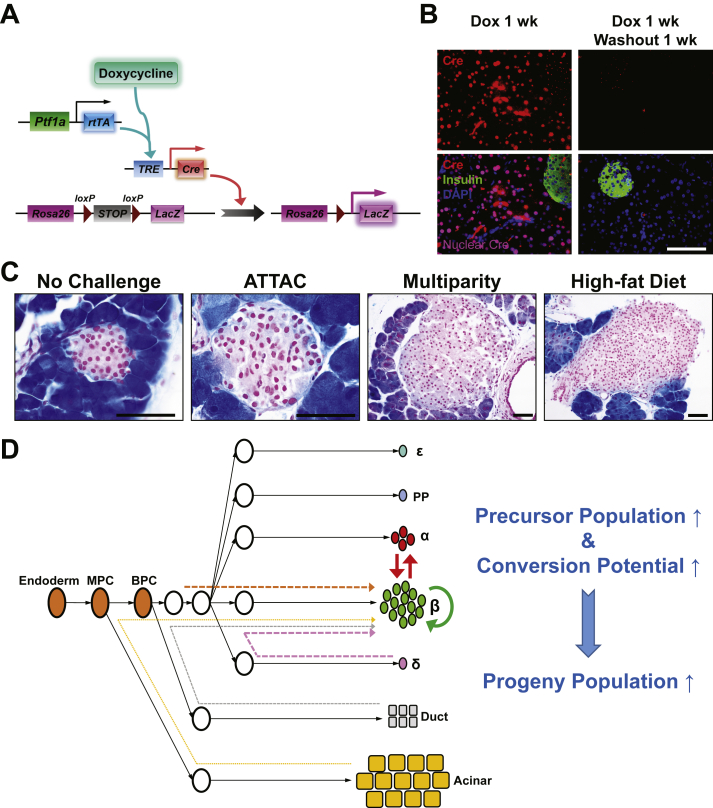
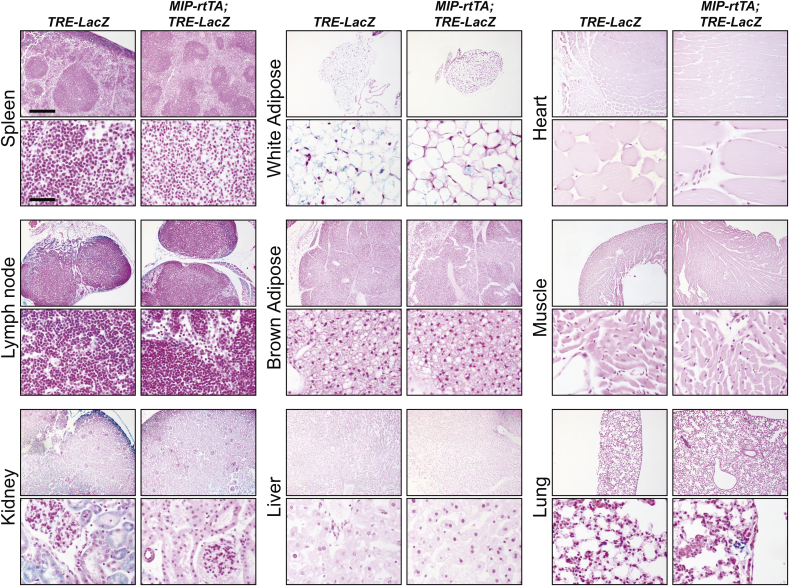
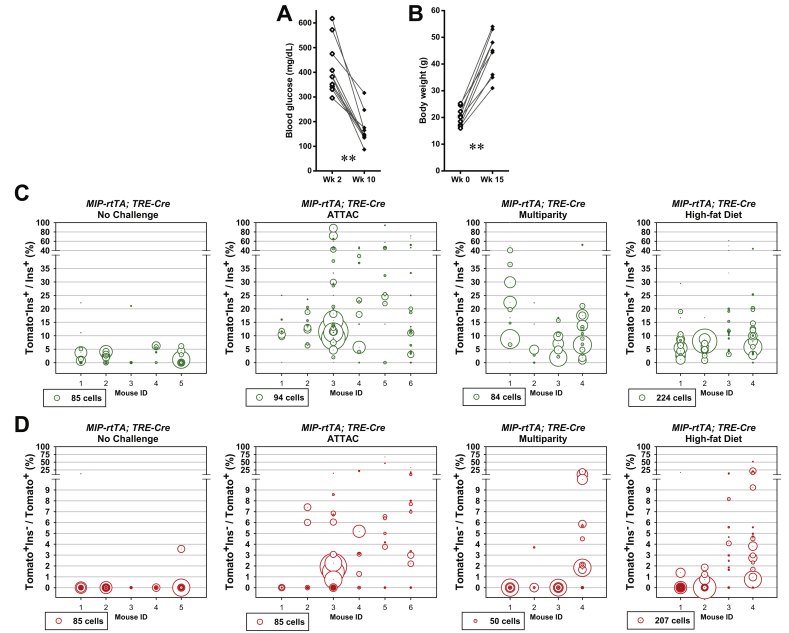
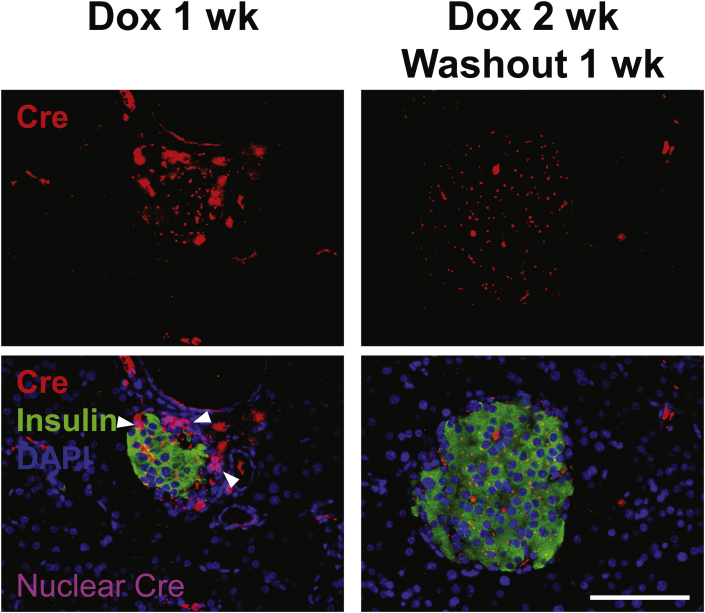
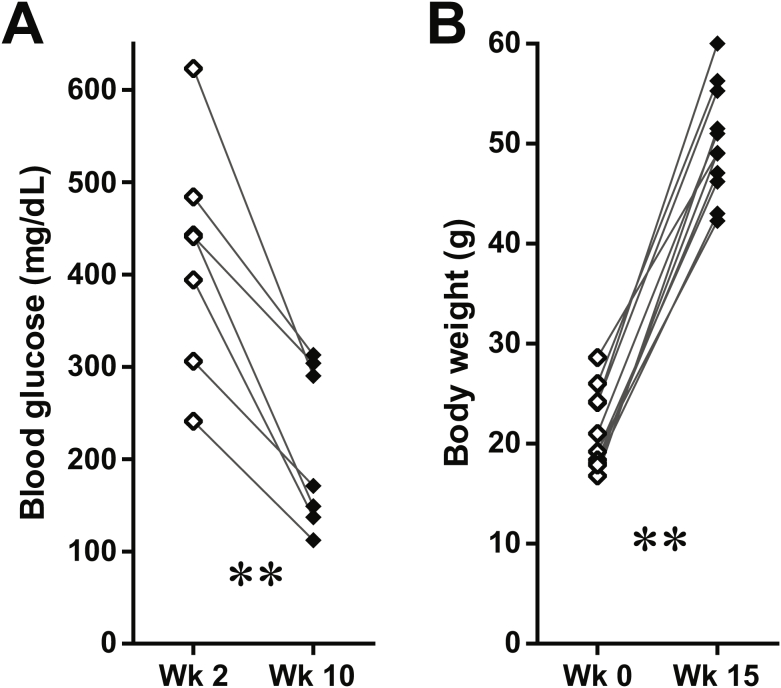
To improve the temporal control in lineage tracing experiments, we employed the doxycycline-inducible gene expression system (“Tet-On”). We created a MIP-rtTA transgenic mouse strain, which showed no activity in tissues (Figure S2) other than pancreatic β-cells [26] and crossed the mice to generate the [MIP-rtTA; TRE-Cre; Rosa26-LacZ; PANIC-ATTAC] and [MIP-rtTA; TRE-Cre; Rosa26-Tomato; PANIC-ATTAC] mice. The rtTA protein is expressed exclusively in insulin+ β-cells and activates the local expression of Cre only in the presence of doxycycline. The nuclear localization sequence-fused Cre protein translocates into the nucleus and irreversibly activates the expression of reporter genes (LacZ or tdTomato) in the cell and its progeny (Figure 2A). After 1 week of doxycycline diet, the Cre protein was detected in the nuclei of nearly all insulin+ cells, and could no longer be detected in the nucleus within 1 week after doxycycline withdrawal (Figure 2B). In agreement with the qualitative β-gal reporter expression (Figure 2C), after dimerizer-induced β-cell ablation and autonomous regeneration (with the ATTAC challenge) (Figure S3A), the Tomato−insulin+/insulin+ cell ratio increased from 2.8 ± 0.6% to 18 ± 3% (P = 0.002) (Figure 2D and E). Hence, a significant contribution of non-β-cells to the autonomous regeneration of adult β-cells is supported by the Tet-On system, consistent with the observations in the tamoxifen system after prolonged washout period (Figure 1E and F).
Figure 2.
Doxycycline-inducible linage tracing of adult β-cells. (A) Doxycycline-inducible labeling of β-cells in mice. The transgene MIP-rtTA expresses a reverse tetracycline transactivator (rtTA) specifically in β-cells. In the presence of doxycycline, rtTA activates the transcription of the TRE-Cre transgene. The Cre recombinase in turn activates the β-cell-specific expression of the reporter gene (LacZ or tdTomato) in an irreversible and heritable manner. (B) Immunofluorescence of Cre recombinase on pancreas sections from [MIP-rtTA; TRE-Cre] mice after 1-week doxycycline diet, before (left) and after (right) 1-week washout. Top panels: Cre (red) signal only. Bottom panels: Cre merged with insulin (green) and DAPI (blue) signals. Arrowheads: the nuclear Cre signal co-localized with DAPI (purple). Scale bar: 100 μm. (C–E) [MIP-rtTA; TRE-Cre; Rosa26-LacZ; PANIC-ATTAC] and [MIP-rtTA; TRE-Cre; Rosa26-Tomato; PANIC-ATTAC] mice were subjected to 1-week of doxycycline diet and 1-week washout. Subsequently, mice were subjected to no challenge, an ATTAC challenge, multiple pregnancies, or high-fat diet-induced obesity. (C) X-gal (blue) staining of pancreas from the Rosa26-LacZ mice. Scale bars: 50 μm (“no challenge” and “ATTAC”) or 100 μm (“multiparity” and “high-fat diet”). (D) Immunofluorescence of tdTomato (red) and insulin (green) on pancreas sections from the Rosa26-Tomato mice. Top panels: Tomato signal only. Bottom panels: Tomato merged with insulin and DAPI (blue). Arrowheads: Tomato−insulin+ cells (green). Scale bar: 100 μm. (E and F) In each mouse from (D), the total numbers of Tomato−insulin+ cells and insulin+ cells (E), or the total numbers of Tomato+insulin− cells and Tomato+ cells (F), were counted and calculated for percentage. Data are presented as mean ± SEM. Dots: individual mice. *P < 0.05, **P < 0.01 versus the no challenge control, by unpaired t-test.
We utilized the Tet-On system to trace the lineages of adult β-cells upon other metabolic challenges (Figure 2C, D and E). We allowed female mice to undergo at least 3 pregnancies, which implements cycles of β-cell hyperplasia and apoptosis at low frequency [27]. Under these conditions, about 11 ± 4% of insulin+ cells were Tomato−, i.e. multiparous female mice display slightly more than one in ten β-cells that originate from non-β-cells. In male mice, after 15 weeks of high-fat diet-induced obesity (Figure S3B), we detected a 9 ± 1% contribution (P = 0.02) to the β-cell population from non-β-cells. Despite the heterogeneity among individual islets, the distribution of their cell ratios was consistent with the cell population ratio seen amongst different mice (Figure S3C). These results suggest that upon a variety of challenges, a contribution of non-β-cells to the adult β-cell pool is routinely seen to a significant extent, and may reach as much as 20% in systems with a high reliance on β-cell apoptosis, such as the PANIC-ATTAC mouse.
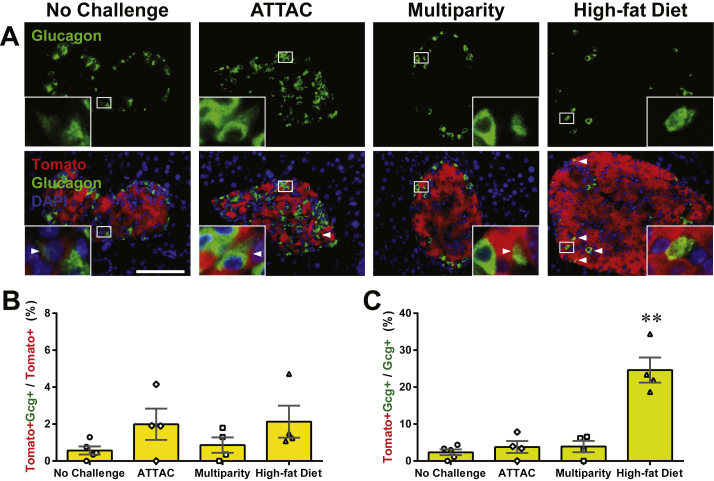
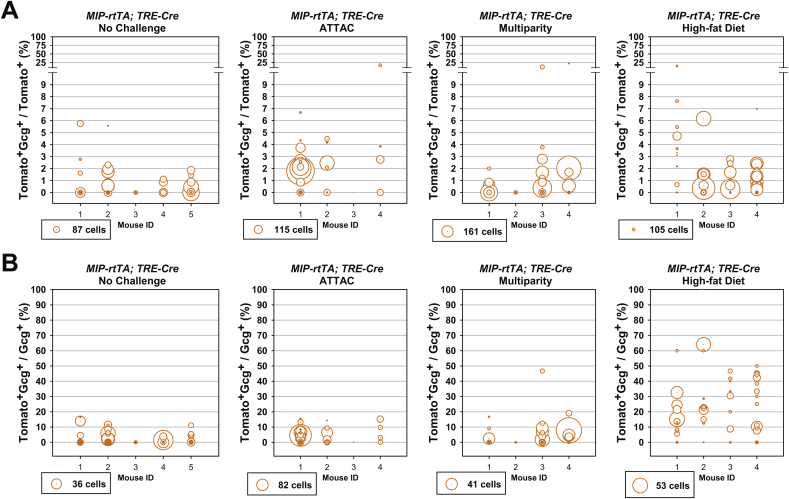
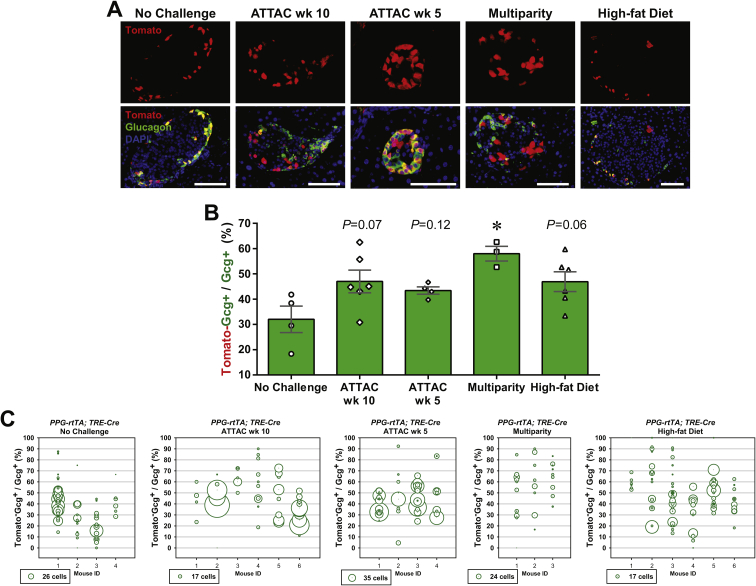
Importantly, we also examined the dedifferentiation and/or trans-differentiation of adult β-cells under the different conditions by quantitating the Tomato+insulin−/Tomato+ cell ratio (Figures 2F and S3D). This process was significant only after the ATTAC challenge (3.3 ± 0.9% versus 0.12 ± 0.09% in controls, P = 0.02), and no significant change was observed in the cases of multiparity or diet-induced obesity. We further investigated whether these former β-cells trans-differentiate into glucagon-producing α-cells in the islets (Figure 3A). The Tomato+glucagon+ cells accounted for <3% of the Tomato-tagged cells (Figures 3B and S4A), consistent with the Tomato+insulin− population (Figure 2F). However, they composed 25 ± 3% of the α-cell pool after the challenge with diet-induced obesity, but not upon exposure to the ATTAC treatment or multiparity (Figures 3C and S4B). This finding suggests the chronic β-cell hyperplasia induces active β-to-α conversion and provides an explanation for the disorganized α-cell distribution in obese mouse islets.
Figure 3.
Active β-to-α conversion in diet-induced obese mice. [MIP-rtTA; TRE-Cre; Rosa26-Tomato; PANIC-ATTAC] mice were subjected to 1-week of doxycycline diet and 1-week washout. Subsequently, mice were subjected to no challenge, an ATTAC challenge, multiple pregnancies, or high-fat diet-induced obesity. (A) Immunofluorescence of tdTomato (red) and glucagon (green) on pancreas sections. Top panels: glucagon signal only. Bottom panels: Tomato merged with glucagon and DAPI (blue). Arrowheads: Tomato+glucagon+ cells (yellow). Insets: representative Tomato+glucagon+ cells in the boxed area. Scale bar: 100 μm. (B and C) In each mouse from (A), the total numbers of Tomato+glucagon+ cells were counted, and calculated for percentage in the population of Tomato+ (B) or glucagon+ (C) cells. Data are presented as mean ± SEM. Dots: individual mice. **P < 0.01 versus the no challenge control, by unpaired t-test.
3.3. Glucagon-producing α-cells are the main sources of newly converted adult β-cells
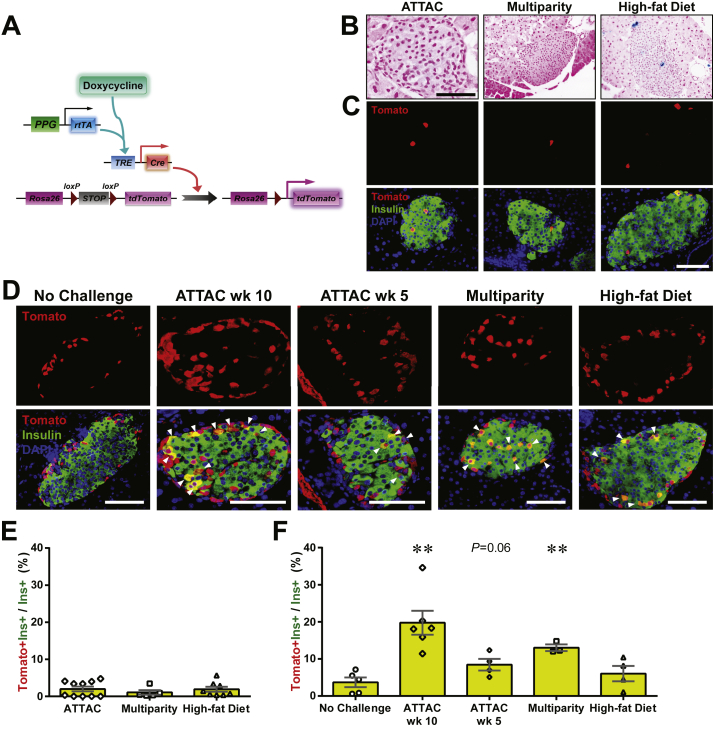
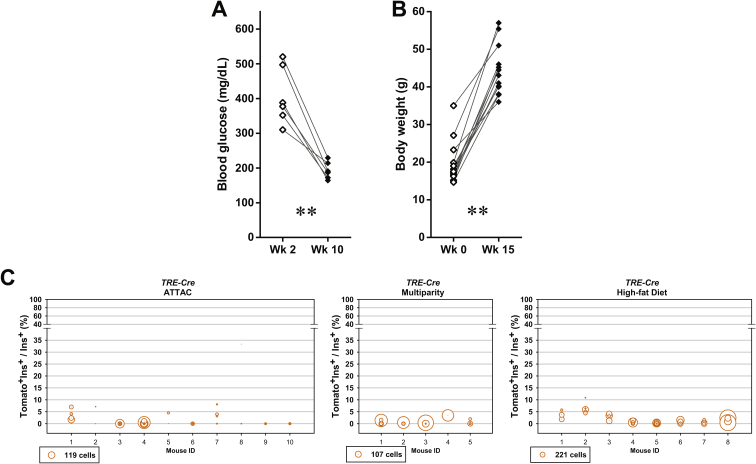
The newly differentiated β-cells can originate from de novo differentiation of precursor cells and/or trans-differentiation of other mature cell types in the pancreas. Previous studies have shown the progressive α-to-β cell conversion upon extreme β-cell loss accompanied by insulin treatment in adult or aged mice [7], [8]. By generating the [PPG-rtTA; TRE-Cre; Rosa26-Tomato; PANIC-ATTAC] mouse model, we measured the contribution of glucagon-producing α-cells to the autonomous β-cell regeneration after moderate β-cell ablation by 50–75% (data not shown). Expression of the rtTA protein is directed by the promoter for the preproglucagon gene, and the tdTomato reporter is specifically expressed in α-cells after doxycycline treatment (Figure 4A). Washout of doxycycline for 1 week completely abolished Cre protein expression in α-cells (Figure S5). In [TRE-Cre; Rosa26-Tomato; PANIC-ATTAC] mice lacking rtTA, <2.0% insulin+ cells were reporter-tagged after the ATTAC challenge, reflecting only negligible activity caused by the leaky expression of the TRE-Cre gene (Figures 4B, C, E and S6).
Figure 4.
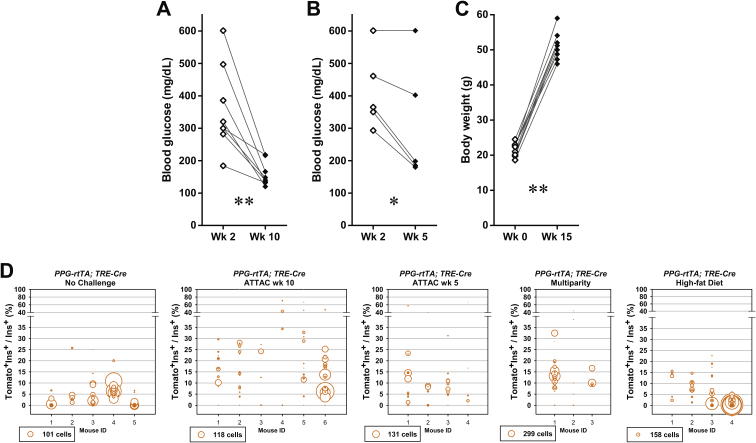
The contribution of α-cells to the adult β-cell turnover. (A) Doxycycline-inducible labeling of glucagon-producing α-cells in mice. The transgene PPG-rtTA expresses the rtTA protein specifically in α-cells. In the presence of doxycycline, rtTA activates the expression of Cre, and consequently the reporter tdTomato irreversibly and heritably in α-cells. (B and C) [TRE-Cre; Rosa26-LacZ; PANIC-ATTAC] and [TRE-Cre; Rosa26-Tomato; PANIC-ATTAC] control mice were subjected to mice were subjected to 2-week doxycycline diet, 1-week washout, and subsequent challenges of ATTAC, multiparity, or diet-induced obesity. (B) X-gal (blue) staining of pancreas from the Rosa26-LacZ mice. Scale bars: 50 μm. (C) Immunofluorescence of tdTomato (red) and insulin (green) on pancreas sections from the Rosa26-Tomato mice. Top panels: Tomato signal only. Bottom panels: Tomato merged with insulin and DAPI (blue). Scale bar: 100 μm. (D) [PPG-rtTA; TRE-Cre; Rosa26-Tomato; PANIC-ATTAC] mice were subjected to 2-week doxycycline diet and 1-week washout. Subsequently, mice were subjected to no challenge, an ATTAC challenge, multiparity, or diet-induced obesity. The ATTAC-challenged mice were examined at 10 or 5 weeks after dimerizer administration as indicated. Pancreas sections were subjected to immunofluorescence of tdTomato (red) and insulin (green). Top panels: Tomato signal only. Bottom panels: Tomato merged with insulin and DAPI (blue). Arrowheads: Tomato+insulin+ cells (yellow). Scale bar: 100 μm. (E and F) Every [TRE-Cre; Rosa26-Tomato; PANIC-ATTAC] (E) and [PPG-rtTA; TRE-Cre; Rosa26-Tomato; PANIC-ATTAC] (F) mouse examined in (C) and (D), respectively, was counted for the total numbers of Tomato+insulin+ cells and insulin+ cells, and calculated for percentage. Data are presented as mean ± SEM. Dots: individual mice. **P < 0.01 versus the no challenge control, by unpaired t-test.
We allowed the ATTAC-challenged [PPG-rtTA; TRE-Cre; Rosa26-Tomato; PANIC-ATTAC] mice more than 2 months of regeneration (week 10). The Tomato+insulin+/insulin+ cell ratio was increased from 4 ± 1% to 20 ± 4% (P = 0.003), indicating a very significant conversion of α-to-β trans-differentiation (Figures 4D, F and S7A). At an earlier time point (week 5) of the regeneration time course (Figure S7B), there were fewer Tomato+ cells within the insulin+ cell population (8 ± 4%, P = 0.06 versus the no challenge control) (Figure 4D and F), supporting a mechanism with progressive α-to-β conversion. In multiparous females, the Tomato+insulin+/insulin+ cell ratio was also prominently increased to 13.0 ± 0.9% (P = 0.001). However, the obese males (Figure S7C) did not show a significant α-to-β conversion (6 ± 2%, P = 0.4), consistent with the active reverse β-to-α conversion under high-fat diet (Figures 3C and S4B).
Assuming the reporter expression did not affect cell fates, an increase in the Tomato−glucagon+/glucagon+ cell ratio would suggest a replenishment of α-cells through de novo or trans-differentiation. Such trends were indeed observed in all the challenged groups, most significantly in the multiparous females (Figure S8). In the case of diet-induced obesity, β-cell trans-differentiation contributed to one fourth of the α-cell pool, which represents a major source of the newly differentiated α-cells (Figures 3C and S4B). All of the post-challenge Tomato+insulin+/insulin+ cell ratios were significantly higher than those observed in the [TRE-Cre; Rosa26-Tomato; PANIC-ATTAC] mice (Figure 4E), indicating that these labels are not artifacts from a leaky TRE-Cre expression.
Taken together, these results demonstrate quantitatively the inter-conversion plasticity of adult α-cells and β-cells. Upon the challenges of induced (ATTAC) or repeated (multiparity) β-cell-apoptosis followed by regeneration, α-cells make an important contribution to adult β-cell pool and are the major sources of the newly differentiated β-cells. In contrast, diet-induced obesity, insulin resistance and the consequent chronic β-cell hyperplasia do not stimulate the α-to-β conversion. Instead, a minor portion (<3%) of the enriched β-cell pool trans-differentiates into a significant portion (∼25%) of the α-cells.
3.4. Pancreatic acinar cells do not contribute to adult β-cell regeneration
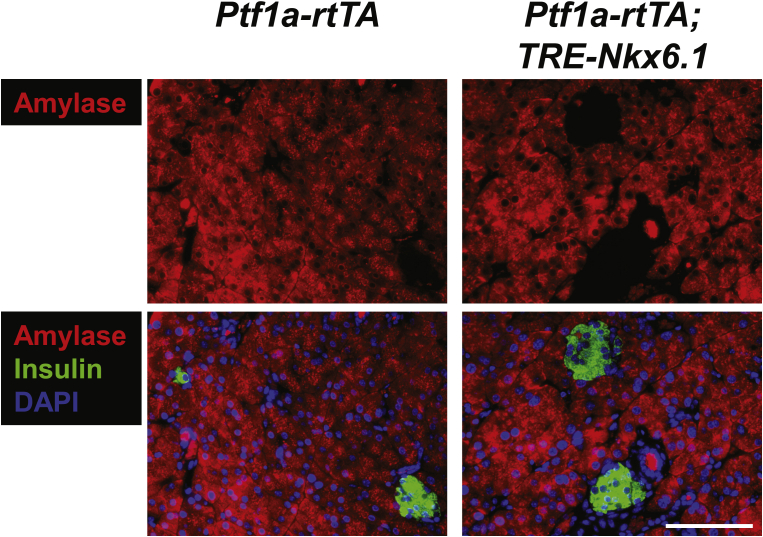
We also assessed the contribution of the exocrine pancreatic acinar cells towards regeneration of β-cell mass by generating the [Ptf1a-rtTA; TRE-Cre; Rosa26-LacZ; PANIC-ATTAC] mice (Figure 5A). The specific expression of Cre in acinar cells responded promptly to the administration and withdrawal of doxycycline (Figure 5B). With complete labeling of the surrounding acinar cells, few β-gal+ islet cells were observed under normal or challenged conditions (Figures 5C and S9), suggesting the contribution of autonomous acinar-to-β trans-differentiation is marginal under the conditions examined.
Figure 5.
No detectable contribution of exocrine acinar cells to adult β-cell turnover. (A) Doxycycline-inducible labeling of pancreatic acinar cells in mice. The transgene Ptf1a-rtTA expresses a reverse tetracycline transactivator (rtTA) specifically in acinar cells. In the presence of doxycycline, rtTA activates the transcription of the TRE-Cre transgene. The Cre recombinase in turn activates the expression of the LacZ reporter in acinar cells irreversibly and heritably. (B) Immunofluorescence of Cre recombinase in pancreas from [Ptf1a-rtTA; TRE-Cre] mice after 1-week doxycycline diet, before (left) and after (right) 1-week washout. Top panels: Cre (red) signal only. Bottom panels: Cre merged with insulin (green) and DAPI (blue) signals. The nuclear Cre signal is co-localized with DAPI (purple). Scale bar: 100 μm. (C) [Ptf1a-rtTA; TRE-Cre; Rosa26-LacZ; PANIC-ATTAC] mice were subjected to 1-week of doxycycline diet and 1-week washout. Subsequently, mice were subjected to no challenge, an ATTAC challenge, multiparity, or diet-induced obesity. Pancreas were subjected to X-gal (blue) staining and processed for paraffin sections. Scale bars: 50 μm (“no challenge” and “ATTAC”) or 100 μm (“multiparity” and “high-fat diet”). (D) A kinetic model of adult β-cell generation. The contribution of certain cell type to the β-cell pool depends on its number and “differentiation barrier”. Self-replication of pre-existing β-cells is the most readily approach, and is the predominant source unless β-cells are extremely ablated [7]. As the second abundant endocrine cell type in the islet, α-cell can contribute significantly upon β-cell loss, presumably by switching on/off some key transcription factors. Conversely, β-to-α conversion is active during chronic β-cell hyperplasia, e.g. in diet-induced obesity. Differentiation from endocrine δ-cells [8], endogenous progenitors [4], or ductal cells [3] were observed before adulthood or with certain induction, due to the limited number or developmental distance. As the most abundant pancreatic cell type, the exocrine acinar cell has a great potential in artificially induced β-trans-differentiation [10], [11], but its autonomous conversion is rare in adulthood [28], [29].
Combining our results with previous studies [3], [4], [5], [7], [8], [9], [10], [11], [12], [13], [14], [19], [28], [29], we proposed a “kinetic” model of adult β-cell generation (Figure 5D), generally applicable to the conversion of cell identities in vivo. Analogous to the stochastic models of chemical reactions [30], the kinetics of the conversion from certain pancreatic cell types to β-cells depends on (1) the “energy barrier”, i.e. the difficulty for the conversion to happen. This step positively correlates with the developmental distance between the precursor and the progeny. Self-replication has the lowest barrier and the highest potential. Cells closely related to the β-cells, such as α-cells and δ-cells [31], may readily undergo de-differentiation and re-differentiation, or even direct interconversion by regulating key transcription factors. Exocrine acinar cells share a distant progenitor with β-cells, and the conversion happens extremely slowly, if at all. An endogenous progenitor cell may lie somewhere in-between these cell types. The conversion barriers are likely to be lower at the embryonic and postnatal stages than in adulthood, conferring more plasticity to differentiated cell types. Exogenous induction or metabolic challenges may also catalyze some subtypes of conversions. (2) The conversion from certain pancreatic cell types to β-cells also depends on the population size of the precursor cells. Like the concentration of chemical reactants, the more abundant a precursor cell type is, the more it contributes to the progeny population. Hence, β-cell self-replication [23], [32], [33] plays a predominant role unless the original population of mature β-cells is depleted to an extreme extent (>99%) [7], [8] or with irreversibly impaired viability [19]. In the setting of moderate (70–85% in PAINC-ATTAC, data not shown) or repeated (multiparity) β-cell apoptosis, α-cells stand out from the other non-β endocrine cells due to their relative abundance [34]. Their conversion can be induced by genetic manipulation of transcription factors [12], [13], [15] or a tumor suppressor [14]. The contributions from δ-cells [8], Ngn3+ endogenous progenitor cells [4], and ductal cells [3], [13] are context-dependent or hardly detectable, because of their limited number and/or more distantly-related genetic programs. Considering the very large population size, acinar cells could be a potent target for induced conversion [10], [11], but, in the models used here, there is no detectable contribution to the β-cell pool under any circumstances. When the β-cell population is chronically enriched, e.g. during diet-induced obesity, the process drives the reverse conversion into α-cells.
3.5. Nkx6.1 promotes α-to-β trans-differentiation
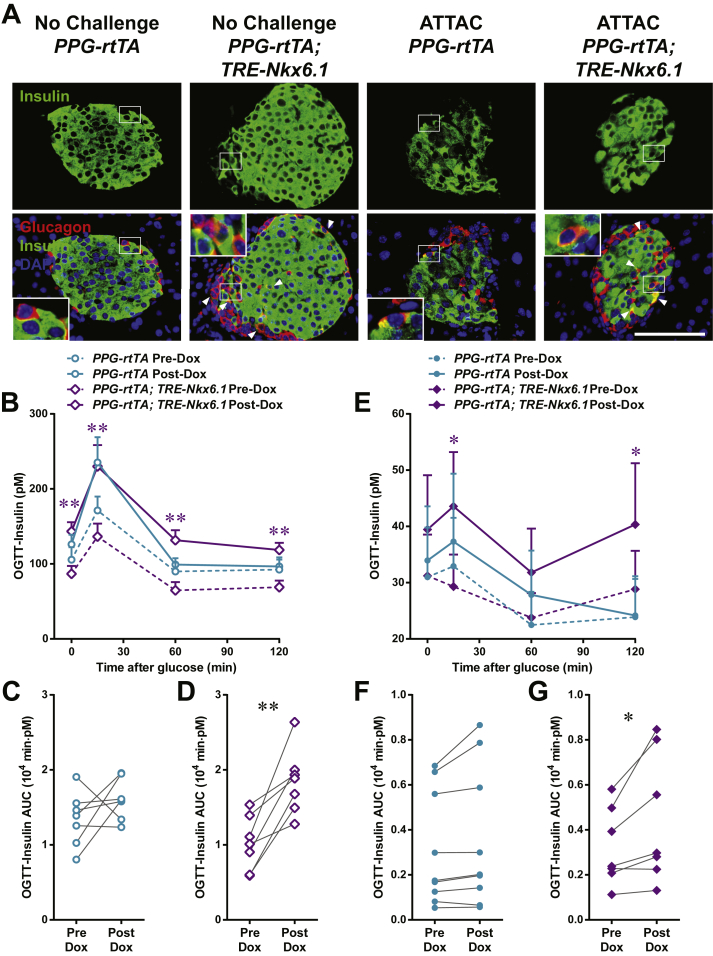
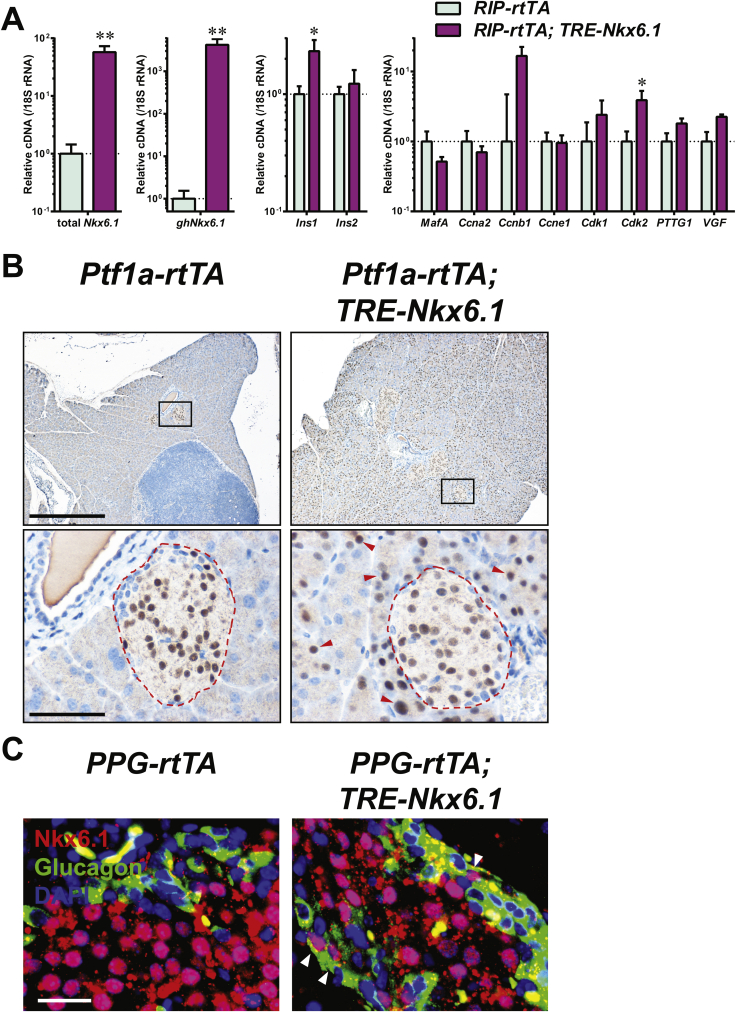
A validation of this model can be to decrease the barriers of cell conversion to examine whether such steps can promote trans-differentiation. Since the α-cell is the major precursor of adult β-cell regeneration post apoptosis in our PANIC-ATTAC mouse, we investigated whether this process can be promoted by overexpression of a key transcription factor for β-cell differentiation, such as Nkx6.1. This is a transcription factor that plays a key role in maintaining identity and function of the β-cell [24], [25], [35]. We generated a TRE-Nkx6.1 transgenic mouse that enabled us to take advantage of doxycycline-inducible overexpression in adults. When crossed with the RIP-rtTA transgenic mouse, the exogenous Nkx6.1 is overexpressed and functional in pancreatic islets, as demonstrated by moderately increased expression of insulin and cell cycle regulators such as Cdk2 (Figure S10A). After doxycycline treatment on the [Ptf1a-rtTA; TRE-Nkx6.1] mice, ectopic expression of Nkx6.1 emerged in a majority of pancreatic acinar cells, and showed a conspicuous nuclear localization (Figure S10B). We also detected nuclear Nkx6.1 in glucagon+ islet cells of the doxycycline-treated [PPG-rtTA; TRE-Nkx6.1] mice, though at a lower proportion (Figure S10C).
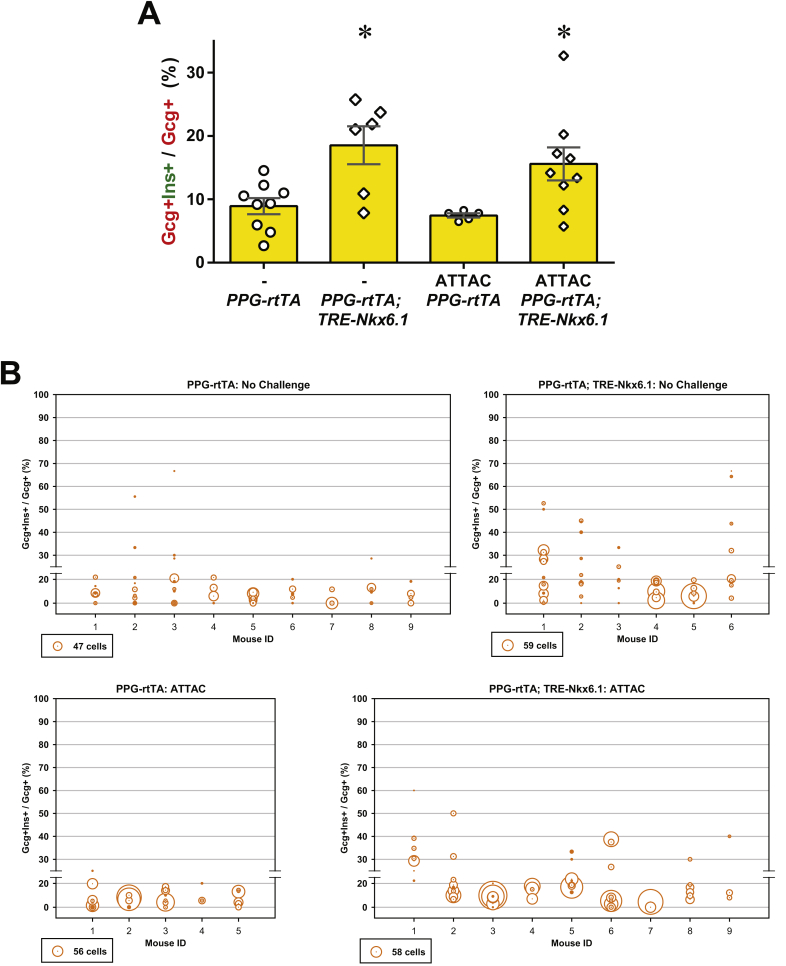
In the [Ptf1a-rtTA; TRE-Nkx6.1] mice, we did not detect any amylase+ cells expressing insulin (Figure S11). In contrast, in the [PPG-rtTA; TRE-Nkx6.1] mice, ectopic expression of Nkx6.1 in α-cells significantly increased the glucagon+insulin+ cell proportion in glucagon+ cells (P = 0.005) (Figures 6A and S12), suggesting active α-to-β conversion. To investigate whether this conversion can be further enhanced after β-cell apoptosis, we generated the [PPG-rtTA; TRE-Nkx6.1; PANIC-ATTAC] mice and subjected them to dimerizer administration. These hyperglycemic mice also showed a significant increase in the glucagon+insulin+/glucagon+ cell ratio compared to the [PPG-rtTA; PANIC-ATTAC] controls (P = 0.01), and the fold change was similar to the unchallenged group (Figures 6A and S12). Interestingly, the glucagon+ cell proportion in the pancreatic islets significantly increased in the groups post β-cell ablation (Figure S13). Hence there were more glucagon+insulin+ cells per unit area of the islets (Figure S14).
Figure 6.
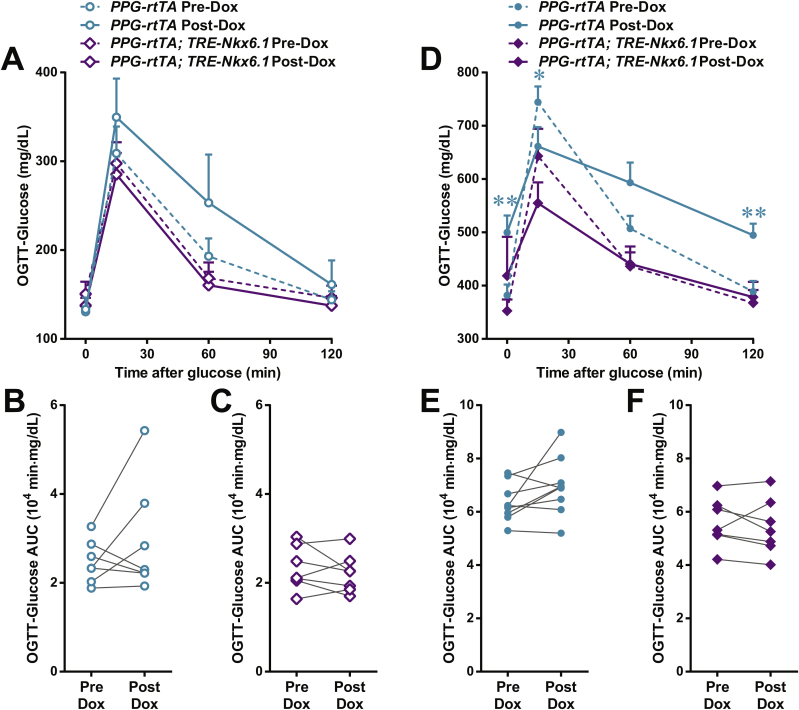
Nkx6.1 ectopic expression in α-cells promotes insulin production. PPG-rtTA and [PPG-rtTA; TRE-Nkx6.1] mice were fed on doxycycline diet for 1 week. [PPG-rtTA; PANIC-ATTAC] and [PPG-rtTA; TRE-Nkx6.1; PANIC-ATTAC] mice were subjected to dimerizer-induced β-cell apoptosis (ATTAC) and, 2 weeks later, doxycycline diet regimen for another week. (A) Immunofluorescence of insulin (green) and glucagon (red) on pancreas sections. Top panels: insulin signal only. Bottom panels: insulin merged with glucagon and DAPI (blue). Arrowheads: glucagon+insulin+ cells (yellow). Insets: representative glucagon+insulin+ cells in the boxed area. Scale bar: 100 μm. (B–G) Plasma insulin during oral glucose tolerance test on the unchallenged (B–D) and ATTAC-challenged (E–G) mice before and after doxycycline diet. n ≥ 7 mice per condition. (B and E) Plasma insulin concentrations at the indicated time points are presented as mean ± SEM. *P < 0.05, **P < 0.01 for [PPG-rtTA; TRE-Nkx6.1] vs PPG-rtTA mice, by unpaired t-test. (C, D, F and G) Areas under curve of individual mice before and after doxycycline are presented as dots and connected by line. *P < 0.05, **P < 0.01 by paired t-test.
To evaluate if the ectopic Nkx6.1-promoted α-to-β cell conversion can improve insulin production, we performed oral glucose tolerance test. In both the unchallenged (Figure 6B, C, D) and ATTAC-challenged (Figure 6E, F, G) groups, the in vivo glucose-stimulated insulin secretion was significantly increased in the Nkx-6.1-overexpressing mice, but not in the control cohort. Although this increase was not sufficient to improve glucose tolerance (Figure S15), it provides evidence, in principle, that the trans-differentiated β-cells are functional.
Collectively, our results suggest that ectopic expression of Nkx6.1 can promote β-cell conversion in α-cells but not in exocrine acinar cells. This forced α-to-β conversion improves insulin production and release in both normal and diabetic mice.
4. Discussion
The tamoxifen-inducible Cre/loxP system was almost exclusively used in the previous lineage tracing studies for the insulin-producing β-cells. While these experiments were very elegant and state-of-the-art at the time, they may have provided somewhat misleading results. In experiments labeling pre-existing β-cells, no evidence of a non-β-cell contribution to adult β-cell regeneration was observed when the washout period between the tamoxifen pulse and the turnover chase was short, such as less than 1 week [1], [2]. However, such evidence was more easily found when the washout period was extended to months [6]. Powers and colleagues reported tamoxifen-driven nuclear translocation of Cre recombinase lasting beyond 1 month in β-cells [16]. We recently demonstrated a prolonged presence of residual tamoxifen in adipocyte lineage tracing experiments, leading to artifacts [17]. In previous studies [1], [2], [6], tamoxifen treatment via intraperitoneal injections labeled <50% β-cells. To quantitate the minor contribution from non-β-cells, we optimized the tamoxifen administration protocol to achieve nearly 100% labeling of pre-existing β-cells, as the doxycycline system does. Here we demonstrate how the short and long washout periods for tamoxifen lead to different results and opposite conclusions in the context of β-cell lineage tracing. The contribution from non-β-cells to the post-apoptosis regeneration of β-cells is evident if the chase starts after the confirmed clearance of nuclear Cre (4 months), but it is completely concealed if the washout period is merely 1 week. This difference is not attributable to the non-β-cell differentiation during the extended washout period, since the ratio of reporter-positive β-cells remains close to 100%. In the latter case, the residual tamoxifen activity and the presence of nuclear Cre continue to label the insulin+ cells, including those originating from the non-β-cell, and create false-positive results for this lineage tracing setting. Taken together, our findings call for caution in the usage of tamoxifen and data interpretation in lineage tracing experiments and suggest the measurement of nuclear Cre recombinase as an indicator of tamoxifen activity.
We further established doxycycline-inducible systems to label β-cells, glucagon-producing α-cells or exocrine acinar cells with high specificity and high efficiency. This system allows us to quantitatively assess their contributions to the adult β-cell pool under various pathophysiological conditions, such as post-apoptosis autonomous regeneration in adult mice. Consistent with the tamoxifen system with proper washout time, the Tet-On system highlights in the regenerated islets ∼20% insulin+ cells not originating from pre-existing β-cells. Such contributions are also evident in mice after multiple pregnancies, i.e. cycles of β-cell hyperplasia and apoptosis [27], as well as diet-induced obesity, although to a lesser extent. Labeling the glucagon-producing α-cell in the pulse stage reveals it to be a major source of the newly differentiated β-cells in the cases of ATTAC regeneration and multiparity. Of note, our results do not exclude minor contributions from other differentiated cell types [3], [8] or endogenous progenitors [4]. Conversely, we observe no β-cell conversion from the exocrine acinar cells in adult mice, in line with previous reports [28], [29]. However, this can be seen at a significant degree by cytokine treatment [11] or transcriptional manipulation [10]. In fact, our results support a “kinetic” model for β-cell replenishment as an analogy to the kinetic theory of chemical reactions [30]. The stochastic models of microscopic reactant particles/precursor cells suggest their reaction/conversion kinetics depends on both their abundance and the energy/developmental barriers. The more developmentally related cells are more readily used: first, self-replication; and second, trans-differentiation from related cell types, such as the endocrine α-cells or δ-cells [8]. The less related cell types, e.g. the exocrine acinar cells, make minimal contributions.
We provide the first, precise characterization of the contribution of α-cells to the cell-autonomous processes of adult β-cell turnover, which is supported with direct lineage tracing evidence. As the two most abundant cell types in the endocrine pancreatic islets, adult α-cells and β-cells have the plasticity to compensate for each other under specific metabolic conditions. The progressive α-to-β conversion is a predominant mechanism of the limited β-cell regeneration in adult mice subjected to diphtheria toxin-induced, extreme β-cell loss (>99%) and kept alive by insulin treatment [7], [8]. In this setting, the initial β-cell population was ablated too much to make a significant contribution. Similarly, β-cells with irreversible loss of TIF-IA fail to survive or proliferate and solicit non-β-cells as the primary source of regeneration [19]. Artificial induction of α-to-β conversion can be achieved by overexpression of Pax4 [12], [13] or Pdx1 [15] in progenitor cells or α-cells, as well as endocrine tumorigenesis [14]. Other studies support the α-to-β mechanism with observations of glucagon+insulin+ bihormonal cells [5], [19]. In the PANIC-ATTAC model with more moderate β-cell ablation followed by autonomous regeneration without any exogenous support or interference, the remnant β-cell population renders their self-replication as the primary source of regeneration, while α-cell trans-differentiation emerged as a significant, but secondary mechanism. Interestingly, α-to-β conversion is also relevant in the context of multiparity. The postnatal β-cell apoptosis may trigger the conversion, which subsequently contributes to the acute β-cell hyperplasia during the next pregnancy. The signal transduction may involve the decrease in vicinal insulin concentration or other metabolites, which awaits future investigation. Contrarily, in diet-induced obesity, the chronic β-cell hyperplasia favors the reverse β-to-α conversion.
On the other hand, we provide proof of principle that ectopic expression of Nkx6.1 in α-cells can promote α-to-β conversion and improve insulin production in adult mice. In contrast, no trans-differentiation was observed in the exocrine acinar cells with forced expression of Nkx6.1. Compared to the high frequency of Nkx6.1+ but absence of insulin+ acinar cells, the relatively rare Nkx6.1+glucagon+ and insulin+glucagon+ cells on the pancreas section may suggest a rapid conversion of identity and/or an α-cell-specific suppression on Nkx6.1. The underlying mechanisms, such as whether this conversion is reversible, and whether it triggers new α-cell generation, remain to be studied. Alternatively, an overexpression cocktail of Nkx6.1 and other β-cell transcription factors, such as Pdx1 [15], MafA, and NeuroD, may further potentiate the α-to-β conversion. Interestingly, in β-cell-ablated mice, the ectopic Nkx6.1 drives the trans-differentiation at a similar percentage to α-cells as in the euglycemic mice. However, the bihormonal cell population increases along with the increased α-cell population. It further highlights the clinical potential for α-to-β conversion, considering the much higher proportion of α-cells in human islets [36].
The Tet-On system for lineage tracing of pancreatic cells has multiple advantages. First, based on the similar labeling efficiency (∼100%), prompt (<1 week) and complete washout of doxycycline-driven Cre expression grants much better temporal control than the tamoxifen system. Second, administration of the water-soluble doxycycline produces fewer side effects than that of the oil-based tamoxifen, which triggers an acute lipoatrophy [17] and chronic lesions [16]. These side effects can be especially confounding in mouse models with impaired β-cells or other metabolic disorders. Third, specific cell types can be labeled by adopting an appropriate promoter-driven rtTA transgene. We have successfully labeled the β-cell, α-cell, and pancreatic acinar cell. Other cell types such as pancreatic ductal cells, endocrine δ-cells, PP cells, ε-cells, and endogenous progenitor cells can be targeted and studied for their potential to emerge as β-cells. In fact, this system can also be used to validate new rtTA transgenic strains. Fourth, it can be adapted to other reporters, e.g. mTmG and HPAP, and facilitate the quantitation of different cell populations. Last but not least, this system can be readily subjected to various physiological/pathological conditions, or induction of differentiation, and studied for the developmental mechanisms.
5. Conclusions
In summary, we establish an efficient and adaptable lineage tracing system for pancreatic cells, quantitatively identify the sources of adult β-cells under multiple metabolic conditions, and propose a kinetic model of cell conversion. Our data also reconcile the discrepant findings generated by the tamoxifen-inducible system.
Author contributions
P.E.S. conceptualized the study. R.Y. designed the experiments. R.Y. and M.W. performed the experiments and analyzed the data. Q.A.W., S.B.S., Z.V.W. and K.S. generated essential materials. R.Y. and P.E.S. wrote the manuscript.
Acknowledgments
We thank Dr. Rana Gupta for helpful advice and discussion. We thank the University of Texas Southwestern Medical Center Molecular Pathology Core for tissue processing, H&E staining, and Nuclear Fast Red counterstaining. This study was supported by the Juvenile Diabetes Research Foundation (JDRF 17-2012-36), National Institutes of Health (NIH) grants R01-DK55758, R01-DK099110 and P01-DK088761 (to P.E.S.); a Naomi Berrie Research Fellowship from Naomi Berrie Diabetes Center, Columbia University Medical Center (to R.Y.); an American Diabetes Association postdoctoral fellowship 7-11-MN-47 (to Q.A.W.).
Footnotes
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.molmet.2016.05.001.
Conflict of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
The following are the Supplementary data related to this article:
Figure S1.
Tamoxifen-induced lineage tracing of adult β-cell regeneration. (A) Immunofluorescence of β-galactosidase (β-gal) on pancreas sections from [MIP-CreERT2; Rosa26-LacZ] mice without tamoxifen treatment (left), and after 3-week tamoxifen release followed by 1-week washout (right). Top panels: β-gal (red) signal only. Bottom panels: β-gal merged with insulin (green). Scale bar: 100 μm. (B) Fed blood glucose of [MIP-CreERT2; Rosa26-LacZ; PANIC-ATTAC] and [MIP-CreERT2; Rosa26-Tomato; PANIC-ATTAC] mice 2 and 10 weeks after dimerizer administration. Dots connected by line represent individual mice. **P < 0.01 by paired t-test. (C) [MIP-CreERT2; Rosa26-Tomato; PANIC-ATTAC] mice were subjected to 3-week tamoxifen release and 4-month washout. Before and after the ATTAC challenge, pancreas sections were subjected to immunofluorescence of tdTomato and insulin. The examined islets of individual mice are plotted as circles, which area represents the number of insulin+ cells in the islet, and the center position marks the percentage of Tomato− cells in the insulin+ cell population.
Figure S2.
Characterization of the MIP-rtTA transgenic mouse. [MIP-rtTA; TRE-LacZ] and TRE-LacZ control mice were fed on doxycycline diet for 10 days. The indicated tissues were subjected to X-gal staining (blue), paraffin sectioning, and nuclear fast red (NFR, red) counterstaining. Scale bars: 400 μm (top panels) or 50 μm (bottom panels).
Figure S3.
Doxycycline-induced lineage tracing of adult β-cells. [MIP-rtTA; TRE-Cre; Rosa26-LacZ; PANIC-ATTAC] and [MIP-rtTA; TRE-Cre; Rosa26-Tomato; PANIC-ATTAC] mice were subjected to 1-week of doxycycline diet and 1-week washout. Subsequently, mice were subjected to no challenge, dimerizer-induced β-cell apoptosis and autonomous regeneration (ATTAC), multiple pregnancies, or high-fat diet-induced obesity. (A) Fed blood glucose of ATTAC-challenged mice 2 and 10 weeks after dimerizer administration. Dots connected by line represent individual mice. **P < 0.01 by paired t-test. (B) Fed body weight of male mice before and after 15 weeks of high-fat diet regimen. Dots connected by line represent individual mice. **P < 0.01 by paired t-test. (C and D) Mouse pancreas sections were subjected to immunofluorescence of tdTomato and insulin. The examined islets of individual mice are plotted as circles, which area represents the total number of insulin+ (C) or Tomato+ (D) cells in the islet, and the center position marks the percentage of Tomato−insulin+ (green) cells (C) or Tomato+insulin− (red) cells (D) in the corresponding population.
Figure S4.
Adult α-cells converted from β-cells. [MIP-rtTA; TRE-Cre; Rosa26-Tomato; PANIC-ATTAC] mice were subjected to 1-week of doxycycline diet and 1-week washout. Subsequently, mice were subjected to no challenge, an ATTAC challenge, multiparity, or high-fat diet-induced obesity. Pancreas sections were subjected to immunofluorescence of tdTomato and glucagon. The examined islets of individual mice are plotted as circles, which area represents the total number of Tomato+ (A) or glucagon+ (B) cells in the islet, and the center position marks the percentage of Tomato+glucagon+ (yellow) cells in the corresponding population.
Figure S5.
Rapid washout of doxycycline-induced Cre expression in pancreatic α-cells. Immunofluorescence of Cre recombinase on pancreas sections from [PPG-rtTA; TRE-Cre] mice after 1-week doxycycline diet (left) or after 2-week doxycycline diet and 1-week washout (right). Top panels: Cre (red) signal only. Bottom panels: Cre merged with insulin (green) and DAPI (blue) signals. Arrowheads: the nuclear Cre signal co-localized with DAPI (purple). Scale bar: 100 μm.
Figure S6.
Background labeling from TRE-Cre leaky expression. [TRE-Cre; Rosa26-Tomato; PANIC-ATTAC] mice were subjected to 2-week doxycycline diet, 1-week washout, and subsequent challenges of ATTAC, multiparity, or diet-induced obesity. (A) Fed blood glucose of ATTAC-challenged mice 2 and 10 weeks after dimerizer administration. Dots connected by line represent individual mice. **P < 0.01 by paired t-test. (B) Fed body weight of male mice before and after 15 weeks of high-fat diet regimen. Dots connected by line represent individual mice. **P < 0.01 by paired t-test. (C) Mouse pancreas sections were subjected to immunofluorescence of tdTomato and insulin. The examined islets of individual mice are plotted as circles, which area represents the number of insulin+ cells in the islet, and the center position marks the percentage of Tomato+ cells in the insulin+ cell population.
Figure S7.
The contribution of α-cells to adult β-cells. [PPG-rtTA; TRE-Cre; Rosa26-Tomato; PANIC-ATTAC] mice were subjected to 2-week doxycycline diet followed by 1-week washout. Subsequently, mice were subjected to no challenge, an ATTAC challenge, multiparity, or diet-induced obesity. The ATTAC-challenged mice were examined at 10 or 5 weeks after dimerizer administration. (A and B) Fed blood glucose of ATTAC-challenged mice 2 and 10 weeks (A) or 2 and 5 weeks (B) after dimerizer administration. Dots connected by line represent individual mice. *P < 0.05, **P < 0.01 by paired t-test. (C) Fed body weight of male mice before and after 15 weeks of high-fat diet regimen. Dots connected by line represent individual mice. **P < 0.01 by paired t-test. (D) Mouse pancreas sections were subjected to immunofluorescence of tdTomato and insulin. The examined islets of individual mice are plotted as circles, which area represents the number of insulin+ cells in the islet, and the center position marks the percentage of Tomato+ cells in the insulin+ cell population.
Figure S8.
Newly generated α-cells. [PPG-rtTA; TRE-Cre; Rosa26-Tomato; PANIC-ATTAC] mice were subjected to 2-week doxycycline diet followed by 1-week washout. Subsequently, mice were subjected to no challenge, an ATTAC challenge, multiparity, or diet-induced obesity. The ATTAC-challenged mice were examined at 10 or 5 weeks after dimerizer administration. (A) Pancreas sections were subjected to immunofluorescence of tdTomato (red) and glucagon (green). Top panels: Tomato signal only. Bottom panels: Tomato merged with glucagon and DAPI (blue). Scale bar: 100 μm. (B) Every mouse was counted for the total numbers of Tomato−glucagon+ cells and glucagon+ cells, and calculated for percentage. Data are presented as mean ± SEM. Dots: individual mice. *P < 0.05 versus the no challenge control, by unpaired t-test. (C) The examined islets of individual mice are plotted as circles, which area represents the number of glucagon+ cells in the islet, and the center position marks the percentage of Tomato− cells in the glucagon+ cell population.
Figure S9.
Metabolic parameters of challenged mice after acinal cell-labeling. [Ptf1a-rtTA; TRE-Cre; Rosa26-LacZ; PANIC-ATTAC] mice were subjected to 1-week of doxycycline diet and 1-week washout. (A) Fed blood glucose of ATTAC-challenged mice 2 and 10 weeks after dimerizer administration. Dots connected by line represent individual mice. **P < 0.01 by paired t-test. (B) Fed body weight of male mice before and after 15 weeks of high-fat diet regimen. Dots connected by line represent individual mice. **P < 0.01 by paired t-test.
Figure S10.
Ectopic expression of functional Nkx6.1. (A) RIP-rtTA and [RIP-rtTA; TRE-Nkx6.1] mice were fed on doxycycline diet for 3 days, and subjected to pancreatic islet isolation. The isolated islets were subjected to RNA extraction and RT-qPCR analysis on gene expression. Primers were designed to detect both the endogenous and exogenous Nkx6.1 (total Nkx6.1), the exogenous golden hamster Nkx6.1 only (ghNkx6.1), and other indicated genes. Data are presented as mean ± SEM in logarithmic scale. n = 3 mice per genotype. *P < 0.05, **P < 0.01 by unpaired t-test. (B) Ptf1a-rtTA and [Ptf1a-rtTA; TRE-Nkx6.1] mice were fed on doxycycline diet for 10 days. Pancreas sections were immunostained for Nkx6.1 (brown) and counterstained with hematoxylin (blue). Bottom panels (scale bar: 50 μm) show the boxed area in the top panels (scale bar: 400 μm). Dash line: islet area. Arrowheads: nuclear Nkx6.1 in exocrine acinar cells. (C) PPG-rtTA and [PPG-rtTA; TRE-Nkx6.1] mice were fed on doxycycline diet for 2 days. Pancreas sections were subjected to immunofluorescence of Nkx6.1 (red) merged with glucagon (green) and DAPI (blue). Arrowheads: nuclear Nkx6.1 (purple) in glucagon+ (green) cells.
Figure S11.
Undetectable insulin expression in Nkx6.1-expressing acinar cells. [Ptf1a-rtTA; TRE-Nkx6.1] mice and Ptf1a-rtTA controls were fed on doxycycline diet for 10 days. Pancreas sections were subjected to immunofluorescence of amylase (red) and insulin (green). Top panels: amylase signal only. Bottom panels: amylase merged with glucagon and DAPI (blue). Scale bar: 100 μm.
Figure S12.
Quantitation of insulin-expressing α-cells in [PPG-rtTA; TRE-Nkx6.1] mice. PPG-rtTA and [PPG-rtTA; TRE-Nkx6.1] mice were fed on doxycycline diet. [PPG-rtTA; PANIC-ATTAC] and [PPG-rtTA; TRE-Nkx6.1; PANIC-ATTAC] mice were subjected to dimerizer-induced β-cell apoptosis (ATTAC) followed by doxycycline diet regimen. Pancreas sections were subjected to immunofluorescence of glucagon and insulin. (A) Every mouse was counted for the total numbers of glucagon+insulin+ cells and glucagon+ cells, and calculated for percentage. Data are presented as mean ± SEM. Dots: individual mice. *P < 0.05 versus the TRE-Nkx6.1 negative control, by unpaired t-test. (B) The examined islets of individual mice are plotted as circles, which area represents the number of glucagon+ cells in the islet, and the center position marks the percentage of insulin+ cells in the glucagon+ cell population.
Figure S13.
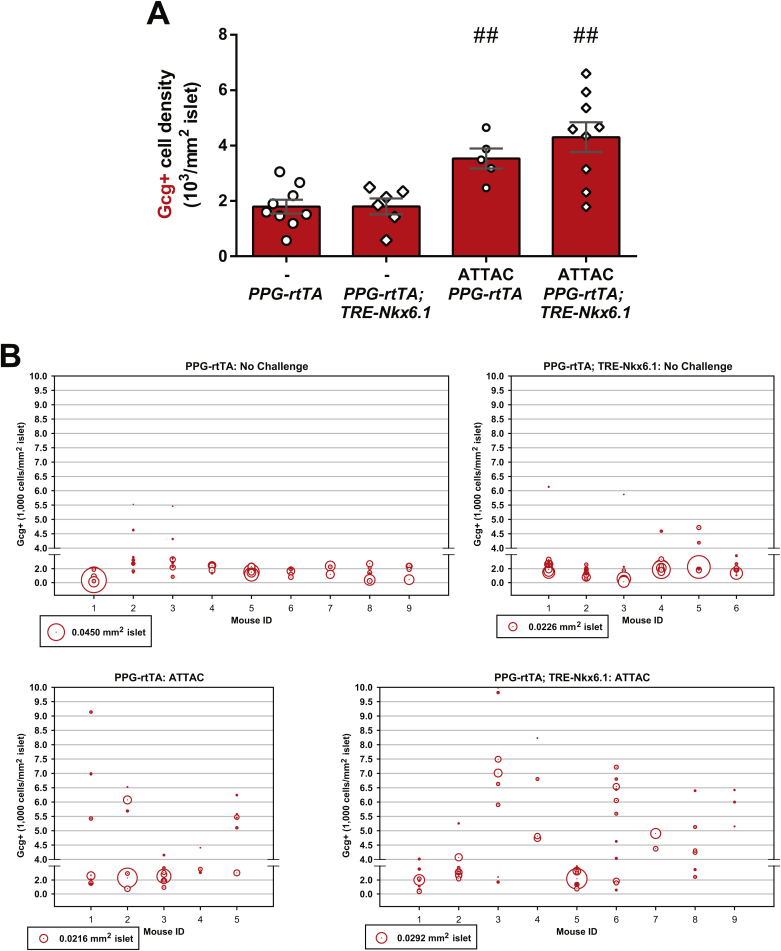
Quantitation of α-cells in [PPG-rtTA; TRE-Nkx6.1] mice. PPG-rtTA and [PPG-rtTA; TRE-Nkx6.1] mice were fed on doxycycline diet for 1 week. [PPG-rtTA; PANIC-ATTAC] and [PPG-rtTA; TRE-Nkx6.1; PANIC-ATTAC] mice were subjected to dimerizer-induced β-cell apoptosis (ATTAC) and, 2 weeks later, doxycycline diet regimen for another week. Pancreas sections were subjected to immunofluorescence of glucagon and insulin. (A) Every mouse was counted for the total number of glucagon+ cells, and normalized against the total islet area. Data are presented as mean ± SEM. Dots: individual mice. ##P < 0.01 versus the no challenge control, by unpaired t-test. (B) The examined islets of individual mice are plotted as circles, which area represents the islet size, and the center position marks the density of glucagon+ cells in the islet.
Figure S14.
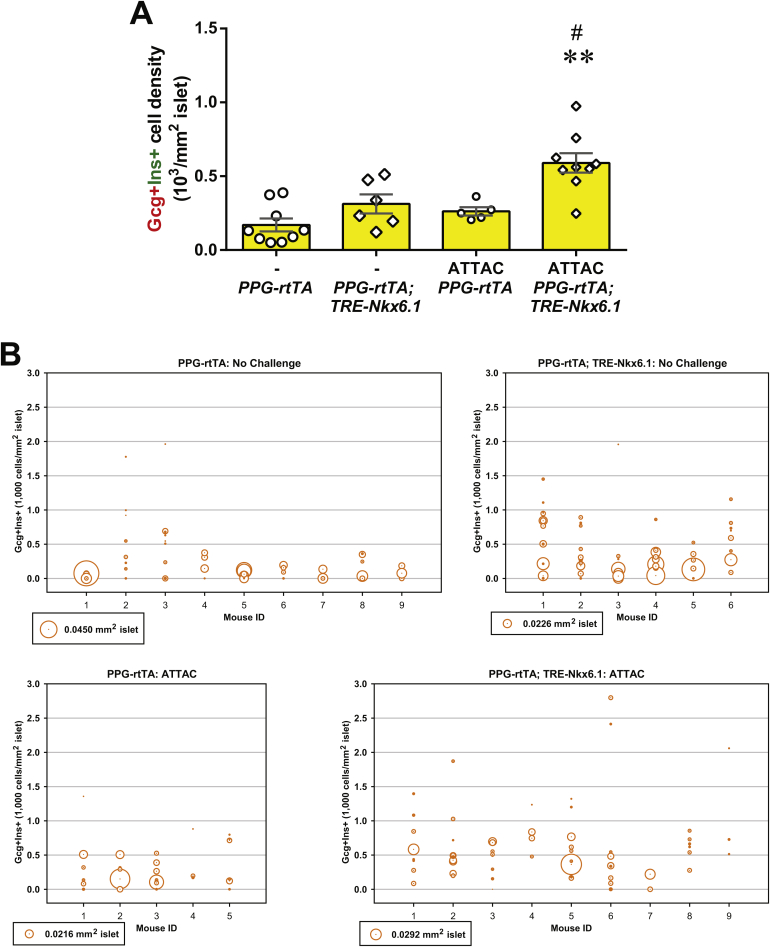
Quantitation of bi-hormonal cells in [PPG-rtTA; TRE-Nkx6.1] mice. PPG-rtTA and [PPG-rtTA; TRE-Nkx6.1] mice were fed on doxycycline diet for 1 week. [PPG-rtTA; PANIC-ATTAC] and [PPG-rtTA; TRE-Nkx6.1; PANIC-ATTAC] mice were subjected to dimerizer-induced β-cell apoptosis (ATTAC) and, 2 weeks later, doxycycline diet regimen for another week. Pancreas sections were subjected to immunofluorescence of glucagon and insulin. (A) Every mouse was counted for the total number of glucagon+insulin+ cells, and normalized against the total islet area. Data are presented as mean ± SEM. Dots: individual mice. **P < 0.01 versus the TRE-Nkx6.1 negative control, #P < 0.05 versus the no challenge control, by unpaired t-test. (B) The examined islets of individual mice are plotted as circles, which area represents the islet size, and the center position marks the density of glucagon+insulin+ cells in the islet.
Figure S15.
Glucose tolerance in [PPG-rtTA; TRE-Nkx6.1] mice. Before and after doxycycline diet, [PPG-rtTA; TRE-Nkx6.1] mice and the PPG-rtTA controls (A–C), or ATTAC-challenged [PPG-rtTA; TRE-Nkx6.1; PANIC-ATTAC] mice and the [PPG-rtTA; PANIC-ATTAC] controls (D–F), were subjected to oral glucose tolerance test and assayed for blood glucose. n ≥ 7 mice per condition. (A and D) Blood glucose concentrations at the indicated time points are presented as mean ± SEM. *P < 0.05, **P < 0.01 versus the TRE-Nkx6.1 negative controls, by unpaired t-test. (B, C, E and F) Areas under curve of individual mice before and after doxycycline are presented as dots and connected by line.
References
- 1.Dor Y., Brown J., Martinez O.I., Melton D.A. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 2.Nir T., Melton D.A., Dor Y. Recovery from diabetes in mice by β cell regeneration. Journal of Clinical Investigation. 2007;117(9):2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inada A., Nienaber C., Katsuta H., Fujitani Y., Levine J., Morita R. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(50):19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu X., D'Hoker J., Stangé G., Bonné S., De Leu N., Xiao X. β cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132(2):197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Chung C.-H., Hao E., Piran R., Keinan E., Levine F. Pancreatic β-cell neogenesis by direct conversion from mature α-cells. Stem Cells. 2010;28(9):1630–1638. doi: 10.1002/stem.482. [DOI] [PubMed] [Google Scholar]
- 6.Abouna S., Old R.W., Pelengaris S., Epstein D., Ifandi V., Sweeney I. Non-β-cell progenitors of β-cells in pregnant mice. Organogenesis. 2010;6(2):125–133. doi: 10.4161/org.6.2.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorel F., Népote V., Avril I., Kohno K., Desgraz R., Chera S. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature. 2010;464(7292):1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chera S., Baronnier D., Ghila L., Cigliola V., Jensen J.N., Gu G. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature. 2014;514(7523):503–507. doi: 10.1038/nature13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Q., Brown J., Kanarek A., Rajagopal J., Melton D.A. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature. 2008;455(7213):627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W., Cavelti-Weder C., Zhang Y., Clement K., Donovan S., Gonzalez G. Long-term persistence and development of induced pancreatic beta cells generated by lineage conversion of acinar cells. Nature Biotechnology. 2014;32(12):1223–1230. doi: 10.1038/nbt.3082. [DOI] [PubMed] [Google Scholar]
- 11.Baeyens L., Lemper M., Leuckx G., De Groef S., Bonfanti P., Stange G. Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nature Biotechnology. 2014;32(1):76–83. doi: 10.1038/nbt.2747. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Collombat P., Xu X., Ravassard P., Sosa-Pineda B., Dussaud S., Billestrup N. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into α and subsequently β cells. Cell. 2009;138(3):449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Hasani K., Pfeifer A., Courtney M., Ben-Othman N., Gjernes E., Vieira A. Adult duct-lining cells can reprogram into β-like cells able to counter repeated cycles of toxin-induced diabetes. Development Cell. 2013;26(1):86–100. doi: 10.1016/j.devcel.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Lu J., Herrera P.L., Carreira C., Bonnavion R., Seigne C., Calender A. α cell-specific men1 ablation triggers the transdifferentiation of glucagon-expressing cells and insulinoma development. Gastroenterology. 2010;138(5) doi: 10.1053/j.gastro.2010.01.046. 1954–1965.e8. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y.-P., Thorel F., Boyer D.F., Herrera P.L., Wright C.V.E. Context-specific α-to-β-cell reprogramming by forced Pdx1 expression. Genes Development. 2011;25(16):1680–1685. doi: 10.1101/gad.16875711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinert R.B., Kantz J., Misfeldt A.A., Poffenberger G., Gannon M., Brissova M. Tamoxifen-induced Cre-loxP recombination is prolonged in pancreatic islets of adult mice. PLoS ONE. 2012;7(3):e33529. doi: 10.1371/journal.pone.0033529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye R., Wang Q.A., Tao C., Vishvanath L., Shao M., McDonald J.G. Impact of tamoxifen on adipocyte lineage tracing: inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Molecular Metabolism. 2015;4(11):771–778. doi: 10.1016/j.molmet.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q.A., Tao C., Gupta R.K., Scherer P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nature Medicine. 2013;19(10):1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shamsi F., Parlato R., Collombat P., Mansouri A. A genetic mouse model for progressive ablation and regeneration of insulin producing beta-cells. Cell Cycle. 2014;13(24):3948–3957. doi: 10.4161/15384101.2014.952176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rankin M.M., Wilbur C.J., Rak K., Shields E.J., Granger A., Kushner J.A. β-cells are not generated in pancreatic duct ligation-induced injury in adult mice. Diabetes. 2013;62(5):1634–1645. doi: 10.2337/db12-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouwens L., Rooman I. Regulation of pancreatic beta-cell mass. Physiological Reviews. 2005;85(4):1255–1270. doi: 10.1152/physrev.00025.2004. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z.V., Mu J., Schraw T.D., Gautron L., Elmquist J.K., Zhang B.B. PANIC-ATTAC: a mouse model for inducible and reversible β-cell ablation. Diabetes. 2008;57(8):2137–2148. doi: 10.2337/db07-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye R., Holland W.L., Gordillo R., Wang M., Wang Q.A., Shao M. Adiponectin is essential for lipid homeostasis and survival under insulin deficiency and promotes β-cell regeneration. eLife. 2014;3:e03851. doi: 10.7554/eLife.03851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sander M., Sussel L., Conners J., Scheel D., Kalamaras J., Dela Cruz F. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127(24):5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- 25.Schaffer A.E., Taylor B.L., Benthuysen J.R., Liu J., Thorel F., Yuan W. Nkx6.1 controls a gene regulatory network required for establishing and maintaining pancreatic beta cell identity. PLOS Genetics. 2013;9(1):e1003274. doi: 10.1371/journal.pgen.1003274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kusminski C.M., Chen S., Ye R., Sun K., Wang Q.A., Spurgin S.B. MitoNEET-Parkin effects in pancreatic α- and β-cells, cellular survival and intra-insular crosstalk. Diabetes. 2016 doi: 10.2337/db15-1323. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rieck S., Kaestner K.H. Expansion of β-cell mass in response to pregnancy. Trends in Endocrinology and Metabolism. 2010;21(3):151–158. doi: 10.1016/j.tem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai B.M., Oliver-Krasinski J., De Leon D.D., Farzad C., Hong N., Leach S.D. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet β cell, regeneration. Journal of Clinical Investigation. 2007;117(4):971–977. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopinke D., Murtaugh L.C. Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Developmental Biology. 2010;10(1):38. doi: 10.1186/1471-213X-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartholomay A.F. Stochastic models for chemical reactions: I. Theory of the unimolecular reaction process. The Bulletin of Mathematical Biophysics. 1958;20(3):175–190. [Google Scholar]
- 31.Romer A.I., Sussel L. Pancreatic islet cell development and regeneration. Current Opinion in Endocrinology, Diabetes and Obesity. 2015;22(4):255–264. doi: 10.1097/MED.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H., Toyofuku Y., Lynn F.C., Chak E., Uchida T., Mizukami H. Serotonin regulates pancreatic beta cell mass during pregnancy. Nature Medicine. 2010;16(7):804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellenbroek J.H., Töns H.A., de Graaf N., Loomans C.J., Engelse M.A., Vrolijk H. Topologically heterogeneous beta cell adaptation in response to high-fat diet in mice. PLoS ONE. 2013;8(2):e56922. doi: 10.1371/journal.pone.0056922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steiner D.J., Kim A., Miller K., Hara M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets. 2010;2(3):135–145. doi: 10.4161/isl.2.3.11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor Brandon L., Liu F.-F., Sander M. Nkx6.1 is essential for maintaining the functional state of pancreatic beta cells. Cell Reports. 2013;4(6):1262–1275. doi: 10.1016/j.celrep.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cabrera O., Berman D.M., Kenyon N.S., Ricordi C., Berggren P.-O., Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(7):2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.